Ultrasound-Assisted Salt Penetration in Sauced Duck: Insights from LF-NMR and MRI Combined Analysis
Abstract
1. Introduction
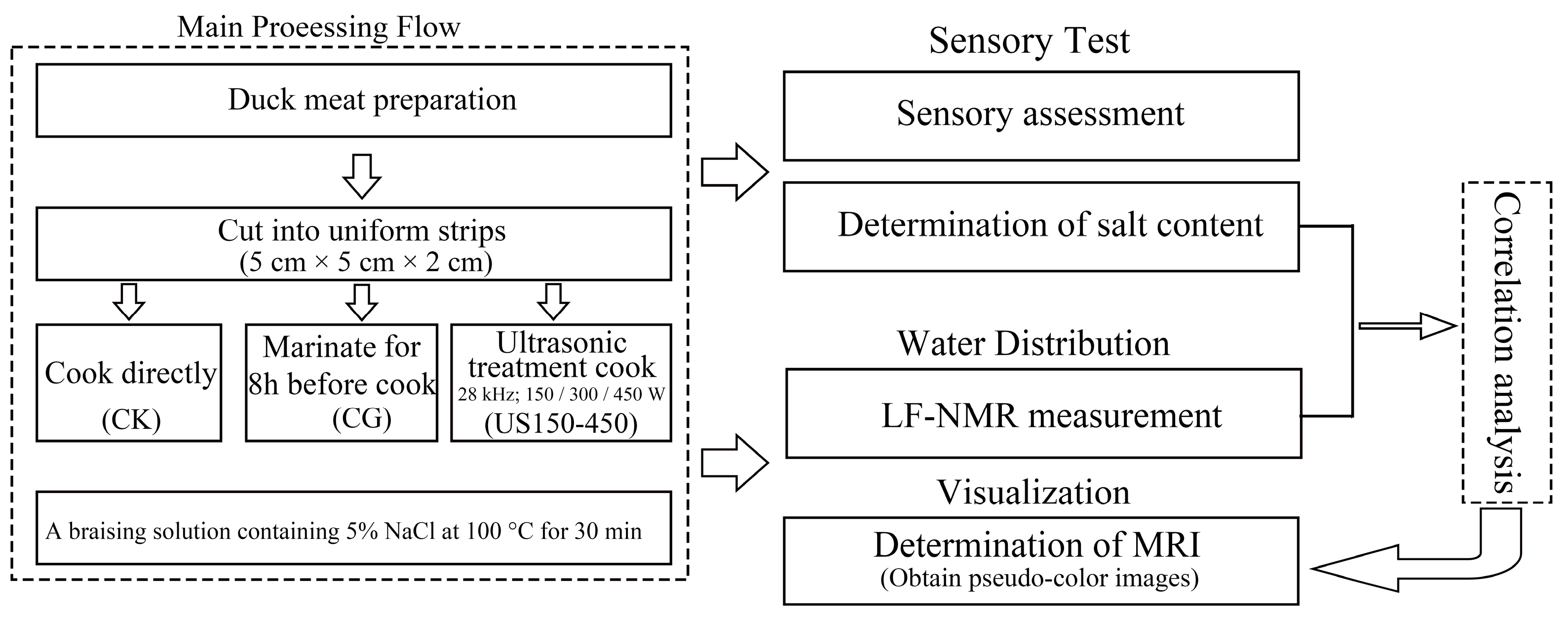
2. Materials and Methods
2.1. Preparation of Sauced Ducks
2.2. Sensory Assessment
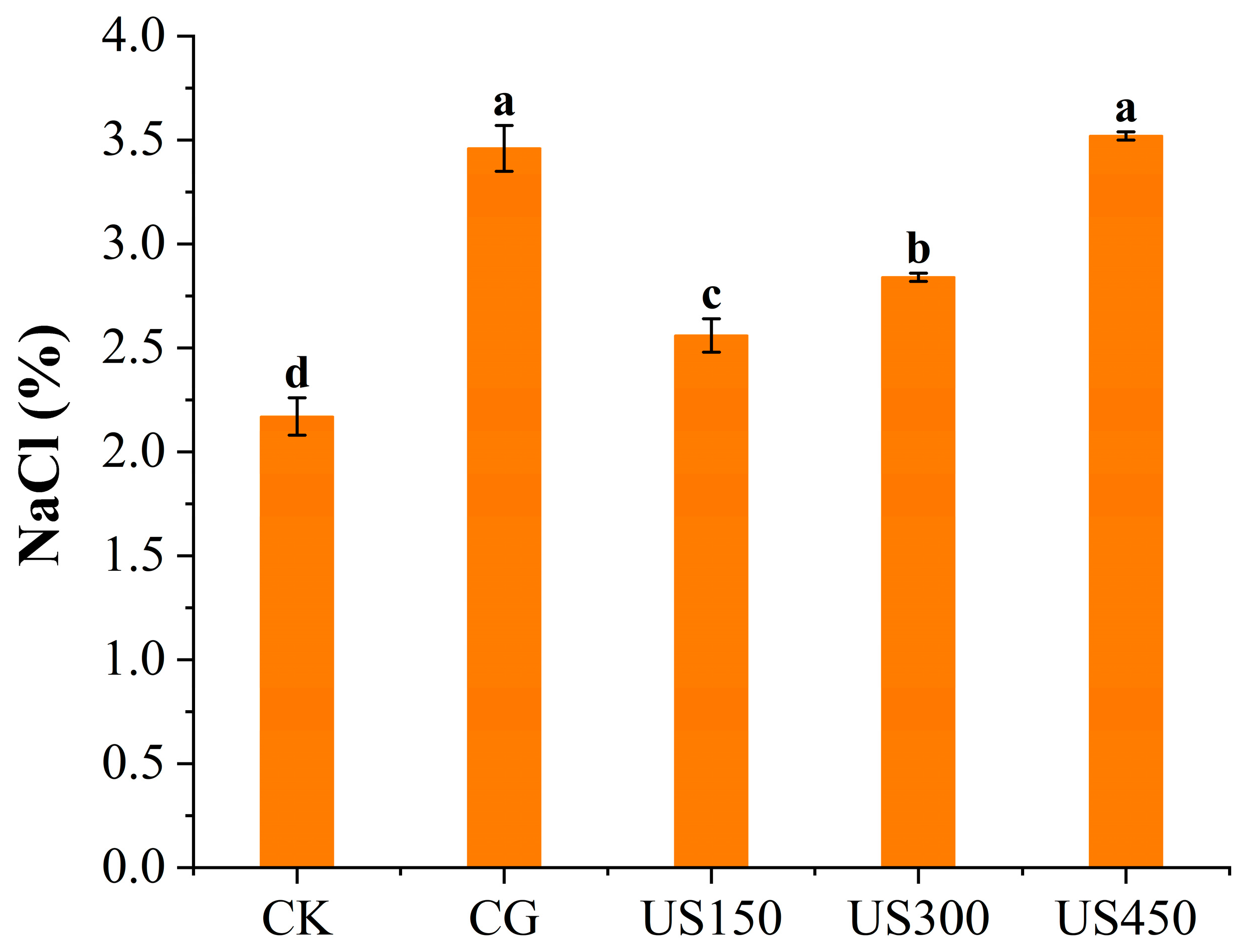
2.3. Determination of Salt Content
2.4. Determination of LF-NMR
2.5. Determination of MRI
2.6. Statistical Analysis
3. Results and Discussion
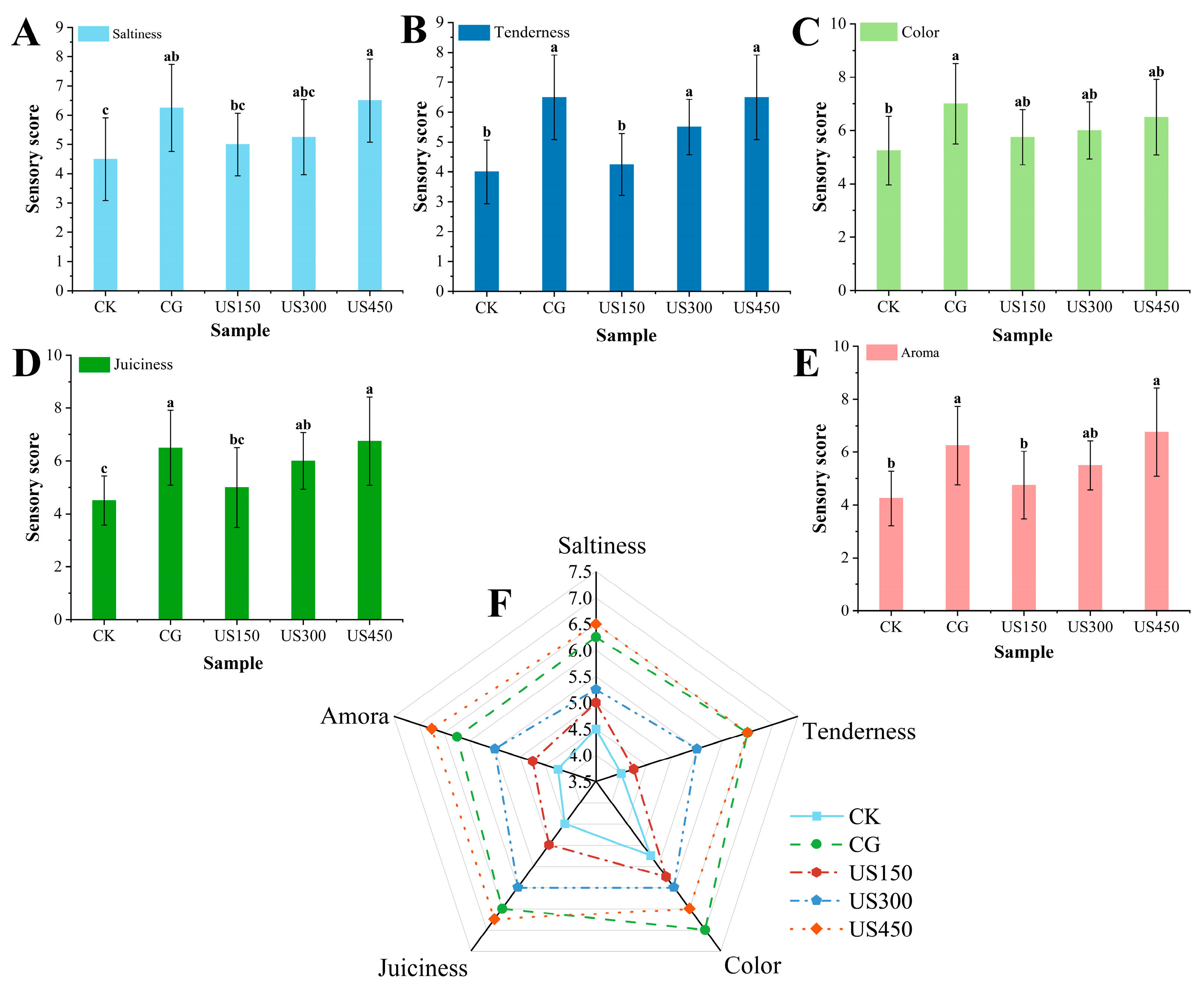
3.1. Sensory Attributes
3.2. Food Compositional Analysis by LF-NMR
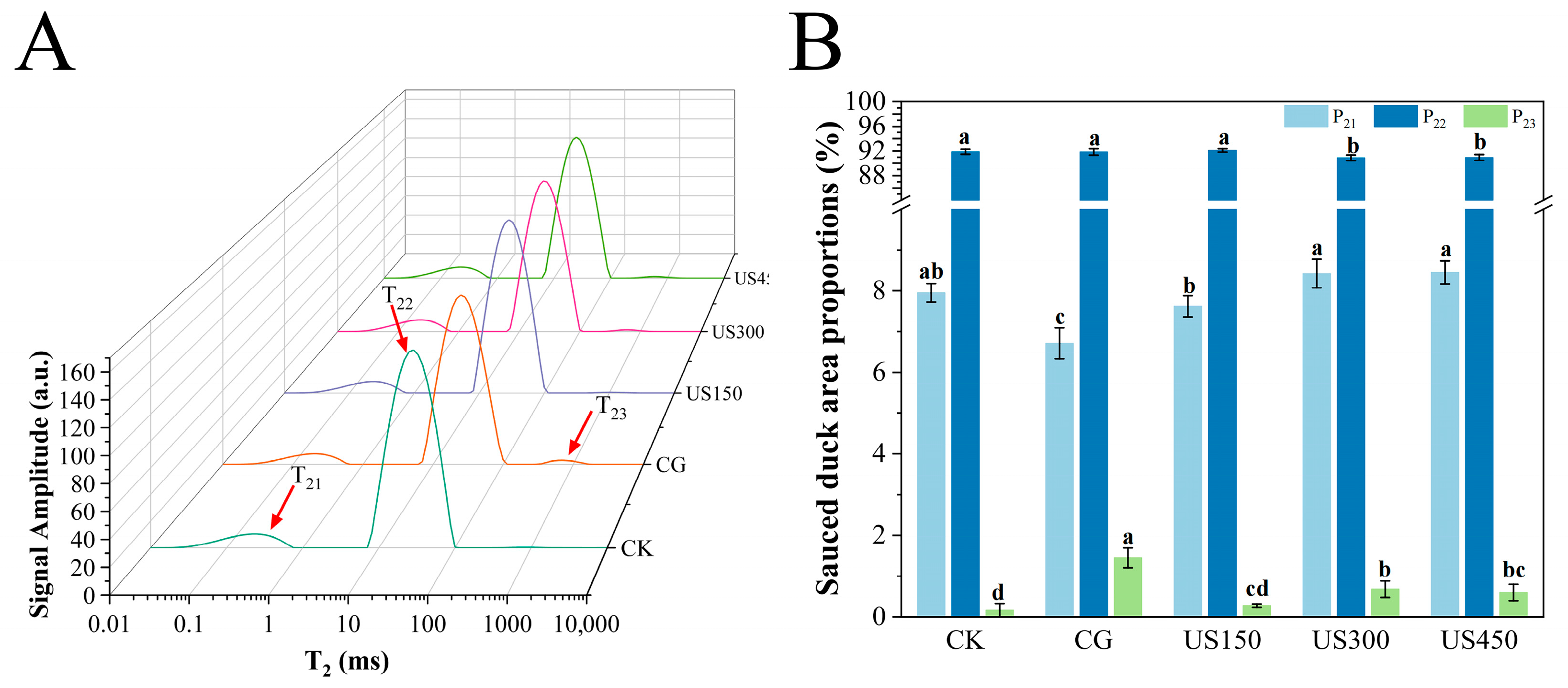
3.2.1. Distribution of Water
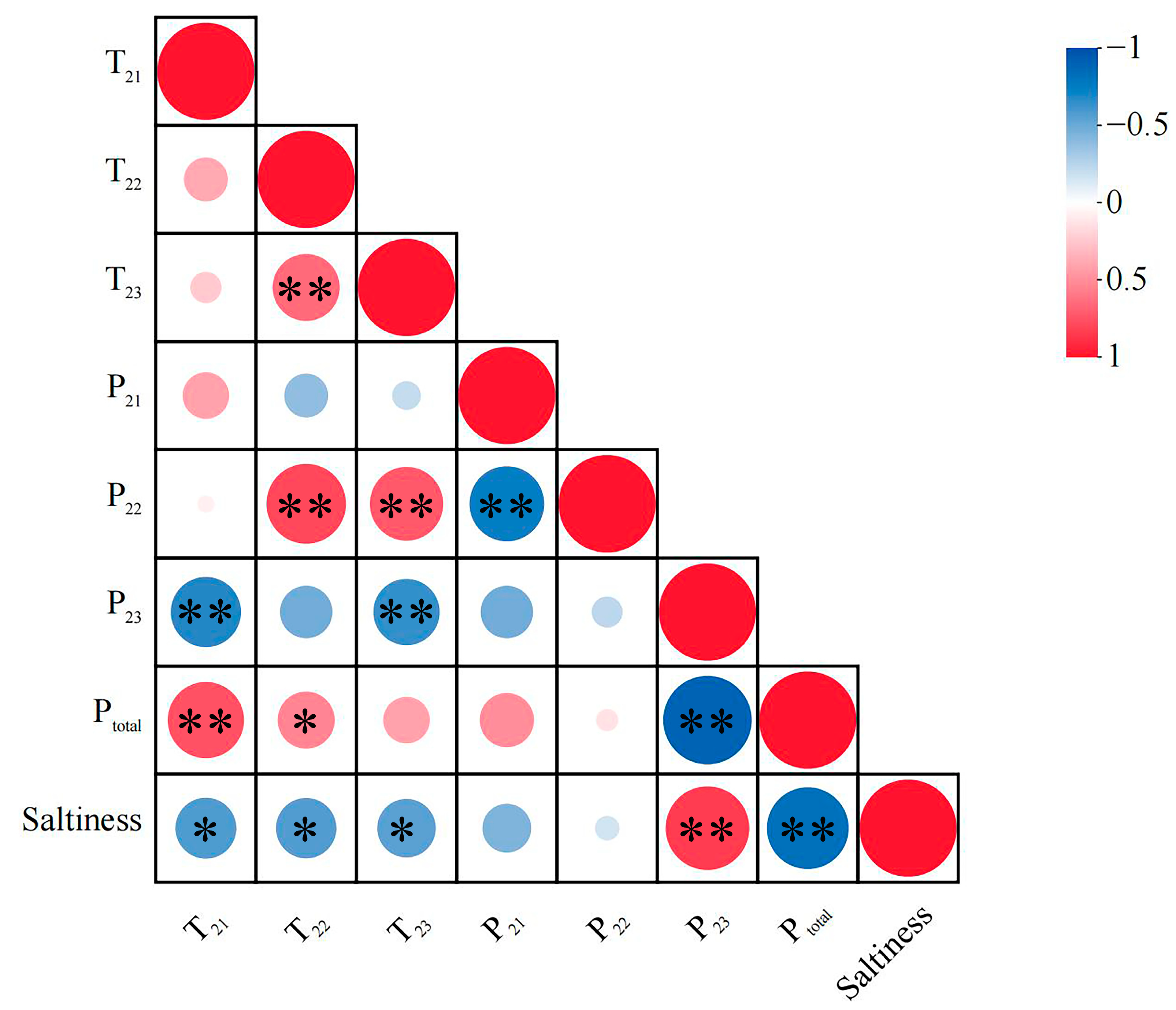
3.2.2. Correlation Analysis
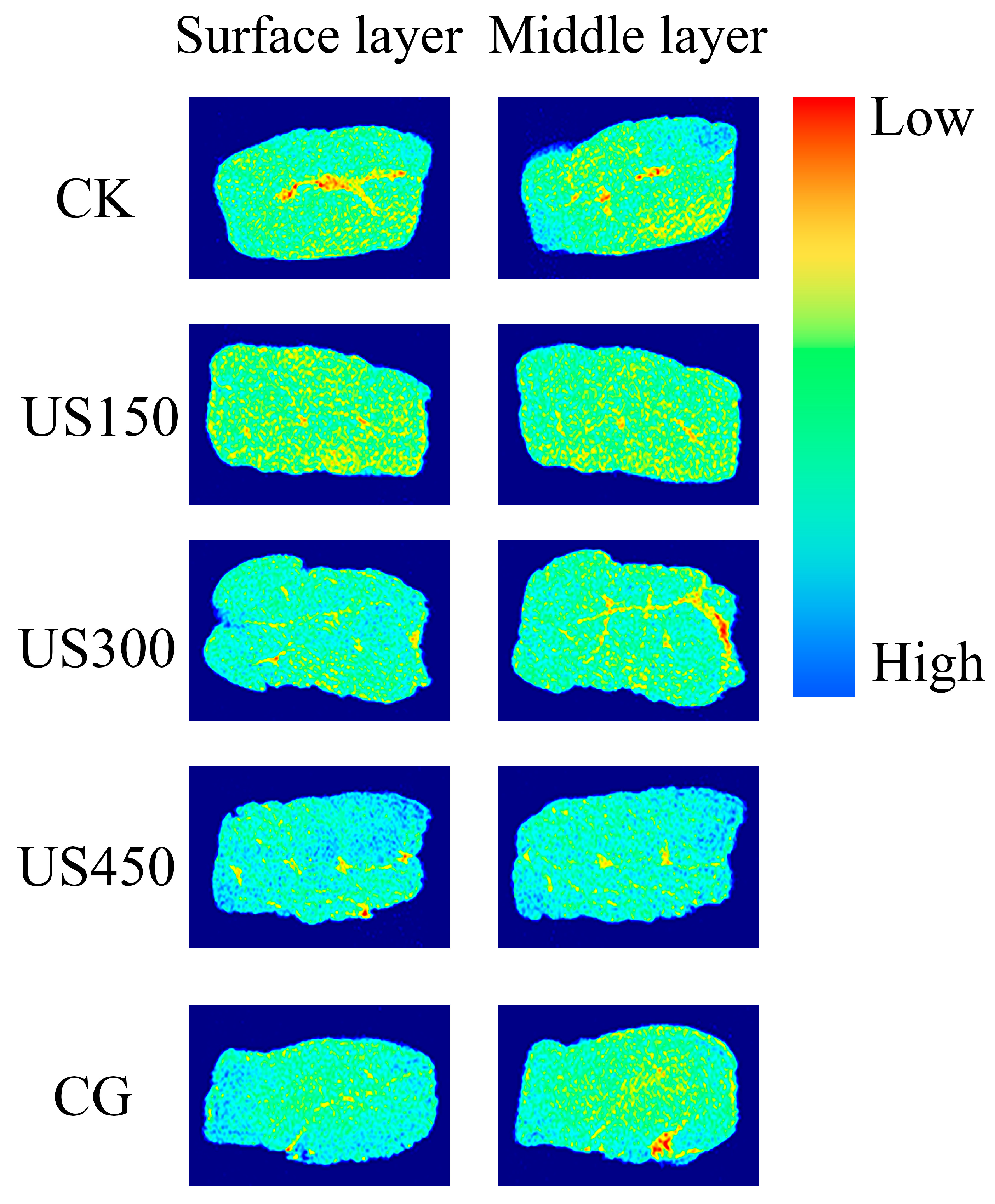
3.3. Visualization of Salt Diffusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, Y.S.; Chen, J.W.; Wu, Y.S.; Wang, S.Y.; Chen, Y.C. A possible systematic culinary approach for spent duck meat: Sous-vide cuisine and its optimal cooking condition. Poult. Sci. 2023, 102, 102636. [Google Scholar] [CrossRef] [PubMed]
- Inguglia, E.S.; Zhang, Z.; Burgess, C.; Kerry, J.P.; Tiwari, B.K. Influence of extrinsic operational parameters on salt diffusion during ultrasound assisted meat curing. Ultrasonics 2018, 83, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Boateng, E.F.; Nasiru, M.M. Applications of Ultrasound in Meat Processing Technology: A Review. Food Sci. Technol. 2019, 7, 11–15. [Google Scholar] [CrossRef]
- Tong, H.; Cao, C.; Du, Y.; Liu, Y.; Huang, W. Ultrasonic-assisted phosphate curing: A novel approach to improve curing rate and chicken meat quality. Int. J. Food Sci. Technol. 2022, 57, 2906–2917. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Wang, M.S.; Liu, Z.W.; Han, Z.; Zhang, Z.H.; Hong, J.; Jabbar, S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1144–1150. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Zhang, J.; Mora, L.; Toldrá, F.; Zhang, W.; Flores, M. Effects of ultrasound pretreatment on flavor characteristics and physicochemical properties of dry-cured ham slices during refrigerated vacuum storage. LWT 2024, 199, 116132. [Google Scholar] [CrossRef]
- Gómez-Salazar, J.A.; Ochoa-Montes, D.A.; Cerón-García, A.; Ozuna, C.; Sosa-Morales, M.E. Effect of Acid Marination Assisted by Power Ultrasound on the Quality of Rabbit Meat. J. Food Qual. 2018, 2018, 5754930. [Google Scholar] [CrossRef]
- Liu, L.; Niu, F.; Xiong, Y.; Wang, P.; Lyu, X.; Yang, Z. Ultrasound-assisted low-sodium salt curing to modify the quality characteristics of beef for aging. Ultrason. Sonochem. 2024, 111, 107134. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Xia, Q.; He, J.; Sun, Y.Y.; Dang, Y.L.; Zhou, G.H.; Geng, F.; Pan, D.D.; Cao, J.X. Insights into ultrasonic treatment on the mechanism of proteolysis and taste improvement of defective dry-cured ham. Food Chem. 2022, 388, 133059. [Google Scholar] [CrossRef]
- Ezeanaka, M.C.; Nsor-Atindana, J.; Zhang, M. Online Low-field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI) for Food Quality Optimization in Food Processing. Food Bioprocess Technol. 2019, 12, 1435–1451. [Google Scholar] [CrossRef]
- Nordon, A.; McGill, C.A.; Littlejohn, D. Process NMR spectrometry. Analyst 2001, 126, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, X.; Lai, B.; Liu, R.; Wu, M.; Ge, Q.; Yu, H. Effects of ultrasound-assisted cooking on the physicochemical properties and microstructure of pork meatballs. Meat Sci. 2024, 208, 109382. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Tong, H.; Tao, X.; Gu, D.; Wu, Y.; Xu, Z.; Ge, C. Effect of ultrasound-assisted enzyme treatment on the quality of chicken breast meat. Food Bioprod. Process. 2021, 125, 193–203. [Google Scholar] [CrossRef]
- Li, D.-Y.; Huang, Y.; Wang, K.-X.; Dong, X.-P.; Yu, D.; Ge, L.-H.; Zhou, D.-Y.; Yu, C.-X. Microstructural characteristics of turbot (Scophthalmus maximus) muscle: Effect of salting and processing. Int. J. Food Prop. 2018, 21, 1291–1302. [Google Scholar] [CrossRef]
- Cho, W.H.; Choi, J.S. Sensory Quality Evaluation of Superheated Steam-Treated Chicken Leg and Breast Meats with a Combination of Marination and Hot Smoking. Foods 2021, 10, 1924. [Google Scholar] [CrossRef]
- Contreras-Lopez, G.; Carnero-Hernandez, A.; Huerta-Jimenez, M.; Alarcon-Rojo, A.D.; Garcia-Galicia, I.; Carrillo-Lopez, L.M. High-intensity ultrasound applied on cured pork: Sensory and physicochemical characteristics. Food Sci. Nutr. 2020, 8, 786–795. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Zhou, Y.; Wang, X.; Ma, J.; Xiong, G.; Wang, L.; Wang, S.; Sun, W. Gel properties of grass carp myofibrillar protein modified by low-frequency magnetic field during two-stage water bath heating. Food Hydrocoll. 2020, 107, 105920. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Yang, D.; Wu, G.; Li, P.; Qi, X.; Zhang, H.; Wang, X.; Jin, Q. The effect of fatty acid composition on the oil absorption behavior and surface morphology of fried potato sticks via LF-NMR, MRI, and SEM. Food Chem. X 2020, 7, 100095. [Google Scholar] [CrossRef]
- Ojha, K.S.; Keenan, D.F.; Bright, A.; Kerry, J.P.; Tiwari, B.K. Ultrasound assisted diffusion of sodium salt replacer and effect on physicochemical properties of pork meat. Int. J. Food Sci. Technol. 2015, 51, 37–45. [Google Scholar] [CrossRef]
- Goli, T.; Ricci, J.; Bohuon, P.; Marchesseau, S.; Collignan, A. Influence of sodium chloride and pH during acidic marination on water retention and mechanical properties of turkey breast meat. Meat Sci. 2014, 96, 1133–1140. [Google Scholar] [CrossRef]
- McDonnell, C.K.; Allen, P.; Morin, C.; Lyng, J.G. The effect of ultrasonic salting on protein and water-protein interactions in meat. Food Chem. 2014, 147, 245–251. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Huang, C.; Liu, S.; Chen, X.; Wang, X.; Hu, P. Evaluation of ultrasound-assisted tomato sour soup marination on beef: Insights into physicochemical, sensory, microstructural, and flavour characteristics. Ultrason. Sonochem. 2024, 110, 107028. [Google Scholar] [CrossRef]
- Li, H.; Feng, J.; Shi, S.; Wang, X.; Xia, X. Evaluation of effects of ultrasound-assisted saucing on the quality of chicken gizzards. Ultrason. Sonochem. 2022, 86, 106038. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, B.; Liu, S.; Zheng, O.; Zhang, C. Ultrasound-assisted thawing accelerates the thawing of common carp (Cyprinus carpio) and improves its muscle quality. LWT 2021, 141, 111080. [Google Scholar] [CrossRef]
- Dinçer, C.; Topuz, A. Inactivation of Escherichia coli and Quality Changes in Black Mulberry Juice Under Pulsed Sonication and Continuous Thermosonication Treatments. J. Food Process. Preserv. 2015, 39, 1744–1753. [Google Scholar] [CrossRef]
- Fantazzini, P.; Gombia, M.; Schembri, P.; Simoncini, N.; Virgili, R. Use of Magnetic Resonance Imaging for monitoring Parma dry-cured ham processing. Meat Sci. 2009, 82, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, R.; Götz, J.; Noll, M.; Wolfschoon, A.; Eibel, H.; Weisser, H. Characterisation of the water-holding capacity of fresh cheese samples by means of low resolution nuclear magnetic resonance. Food Res. Int. 2004, 37, 667–676. [Google Scholar] [CrossRef]
- Shao, J.H.; Deng, Y.M.; Jia, N.; Li, R.R.; Cao, J.X.; Liu, D.Y.; Li, J.R. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chem. 2016, 200, 308–314. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.K.; Allen, P.; Duggan, E.; Arimi, J.M.; Casey, E.; Duane, G.; Lyng, J.G. The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: A low field NMR study. Meat Sci. 2013, 95, 51–58. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Kang, D.C.; Gao, X.Q.; Ge, Q.F.; Zhou, G.H.; Zhang, W.G. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017, 38, 317–325. [Google Scholar] [CrossRef]
- Xiong, G.; Fu, X.; Pan, D.; Qi, J.; Xu, X.; Jiang, X. Influence of ultrasound-assisted sodium bicarbonate marination on the curing efficiency of chicken breast meat. Ultrason. Sonochem. 2020, 60, 104808. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, P.; Wu, H.; Zhang, M.; Sun, Z.; Sun, C.; Wang, D.; Cao, J.; Xu, W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018, 113, 640–647. [Google Scholar] [CrossRef]
- Bal, A.; Pati, S.G.; Panda, F.; Mohanty, L.; Paital, B. Low salinity induced challenges in the hardy fish Heteropneustes fossilis; future prospective of aquaculture in near coastal zones. Aquaculture 2021, 543, 737007. [Google Scholar] [CrossRef]
- Liu, Z.F.; Gao, X.Q.; Yu, J.X.; Qian, X.M.; Xue, G.P.; Zhang, Q.Y.; Liu, B.L.; Hong, L. Effects of different salinities on growth performance, survival, digestive enzyme activity, immune response, and muscle fatty acid composition in juvenile American shad (Alosa sapidissima). Fish Physiol. Biochem. 2017, 43, 761–773. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Yang, H.; Lin, R.; Wang, H.; Tan, M. Characterization of moisture migration of beef during refrigeration storage by low-field NMR and its relationship to beef quality. J. Sci. Food Agric. 2020, 100, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Cheng, J.-H.; Ma, J.; Sun, D.-W. LF-NMR and MRI analyses of water status and distribution in pork patties during combined roasting with steam cooking. Food Biosci. 2023, 56, 103325. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.; Wang, Y.; Wang, B.; Wang, S.; Chang, J.; Liu, S.; Wang, H. Proanthocyanidin B2 and transglutaminase synergistically improves gel properties of oxidized myofibrillar proteins. Food Chem. 2022, 391, 133262. [Google Scholar] [CrossRef]
- Fantazzini, P.; Bortolotti, V.; Garavaglia, C.; Gombia, M.; Riccardi, S.; Schembri, P.; Virgili, R.; Soresi Bordini, C. Magnetic resonance imaging and relaxation analysis to predict noninvasively and nondestructively salt-to-moisture ratios in dry-cured meat. Magn. Reson. Imaging 2005, 23, 359–361. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Ma, C.; Cai, J. Evaluation of ultrasound-assisted process as an approach for improving the overall quality of unsmoked bacon. Ultrason. Sonochem. 2023, 98, 106490. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Anese, M.; Marzona, S.; Innocente, N.; Lagazio, C.; Nicoli, M.C. Monitoring dry-curing of S. Daniele ham by magnetic resonance imaging. Food Chem. 2013, 141, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xia, C.; Li, H.; Sun, Y.; Pan, D.; He, J. Ultrasound-Assisted Salt Penetration in Sauced Duck: Insights from LF-NMR and MRI Combined Analysis. Foods 2025, 14, 3553. https://doi.org/10.3390/foods14203553
Wang X, Xia C, Li H, Sun Y, Pan D, He J. Ultrasound-Assisted Salt Penetration in Sauced Duck: Insights from LF-NMR and MRI Combined Analysis. Foods. 2025; 14(20):3553. https://doi.org/10.3390/foods14203553
Chicago/Turabian StyleWang, Xiangyu, Chenlan Xia, Huimin Li, Yangying Sun, Daodong Pan, and Jun He. 2025. "Ultrasound-Assisted Salt Penetration in Sauced Duck: Insights from LF-NMR and MRI Combined Analysis" Foods 14, no. 20: 3553. https://doi.org/10.3390/foods14203553
APA StyleWang, X., Xia, C., Li, H., Sun, Y., Pan, D., & He, J. (2025). Ultrasound-Assisted Salt Penetration in Sauced Duck: Insights from LF-NMR and MRI Combined Analysis. Foods, 14(20), 3553. https://doi.org/10.3390/foods14203553





