Valorization of Cavia porcellus By-Products via Ultrasound-Assisted Collagen Extraction: Optimization and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Material
2.2. Extraction Method
2.3. Experimental Design
2.4. Collagen Extraction Yield
- R: collagen extraction yield (%)
- MCollagen: Collagen mass obtained (g)
- Msample: Initial guinea pig head and leg mass (g).
2.5. Quantification of Hydroxyproline
- Y: Hydroxyproline content (μg/mL)
- X: Absorbance (nm).
2.6. Determination of ζ-Potential by Dynamic Light Scattering (DLS)
2.7. Particle Morphology and Size Analysis by SEM
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Statistical Analysis
3. Results
3.1. Effect of Variables on Yield and Hydroxyproline
3.1.1. Model Fitting and Statistical Analysis by Response Surface Methodology (RSM)
3.1.2. Interaction Effects of Process Parameters on Yield and Hydroxyproline Content in Ultrasound-Assisted Extraction
3.1.3. Validation of the Predictive Model
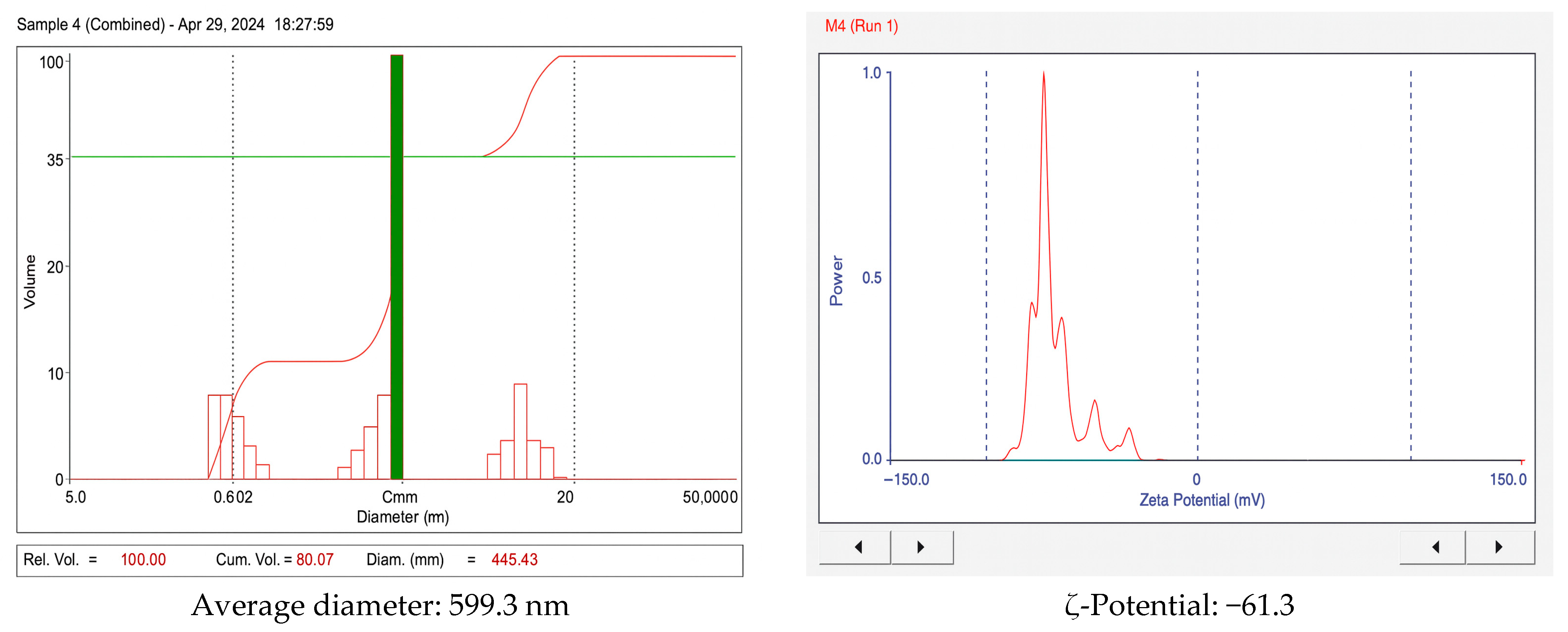
3.2. Analysis of Particle Size, ζ-Potential, and Polydispersity Index (PDI)
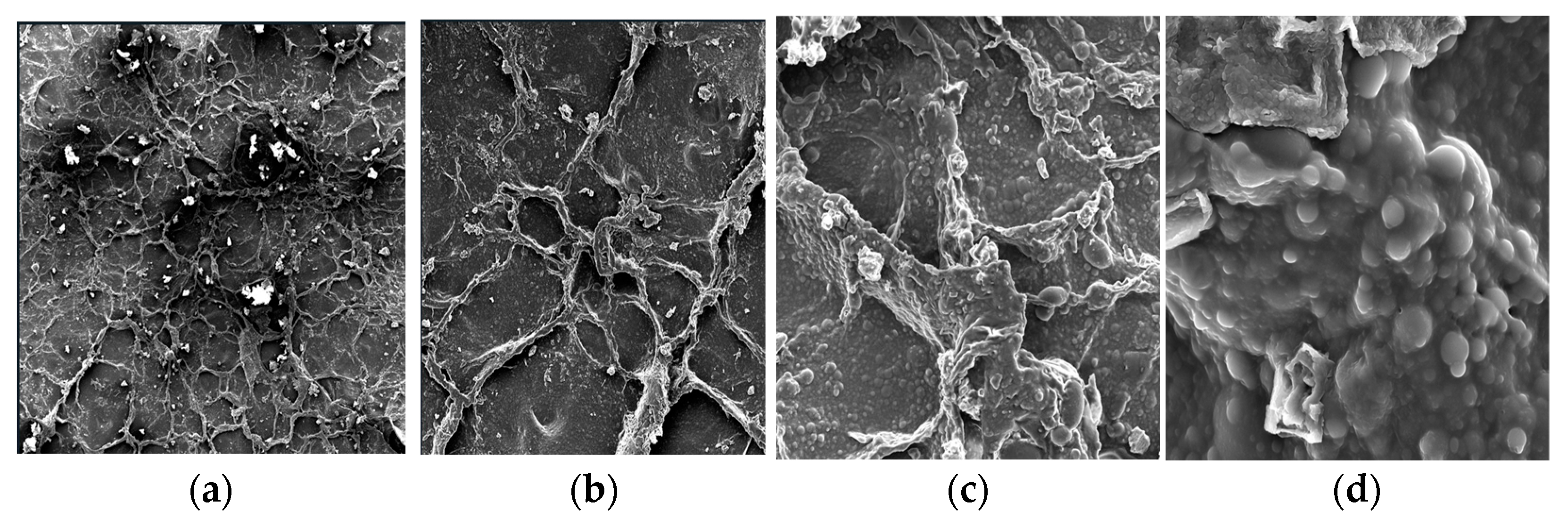
3.3. SEM Analysis of Surface Morphology
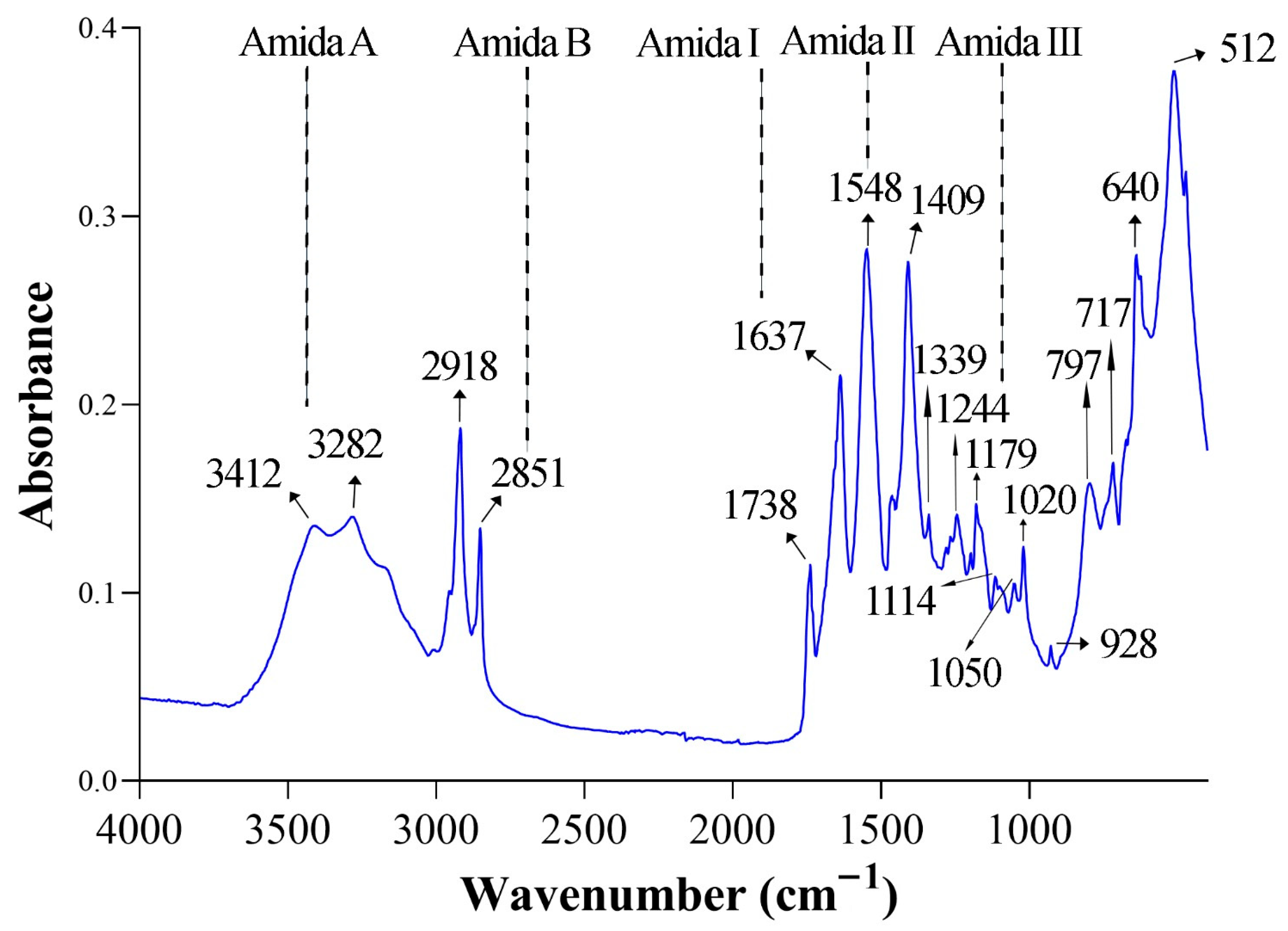
3.4. Fourier Transform Infrared (FTIR) Spectral Analysis
| Observed Band (cm−1) | Reference Band (cm−1) | Vibrational Mapping | Structural Meaning | References |
|---|---|---|---|---|
| 3412 | 3307–3428 | Stretching N–H (Amid A) | Hydrogen bridge network that stabilises the triple helix | [20] |
| 3282 | 3282 | Amid A–Streching N–H2 | Indicates extra reinforcement of H bonds with the peptide skeleton. | [21] |
| 2851 | 2924 | Symmetrical stretching C–H2 (Amid B) | Indicates the presence of prolines and glycines in collagen. | [22] |
| 1637 | 1638 | Stretching C=O (Amid I) | It reflects the α/β helix conformation of the polymer and the formation of H bonds between adjacent N–H and C=O. | [23,24] |
| 1548 | 1548 | Flexion N–H + stretching C–N (Amid II) | Reflects peptide packaging in the triple helix. | [25,26] |
| 1409 | 1410–1460 | Symmetrical stretching COO− | Indicates the presence of deprotonated acid residues (glutamate/aspartic acid) and calcium/inorganic salts. | [27] |
| 1339 | 1338 | CH2 balance (Amid III) | Triple propeller integrity indicator. | [28] |
| 1244 | 1243 | Stretching C–N + flexion N–H (Amid III) | Vibrational support of the native helix | [29] |
| 1179 | 1180 | Stretching C–O–C | It is often associated with ether bonds in collagen/hydroxyapatite mixtures. | [30] |
| 1020/1050 | 1023 | Vibration PO43− | Its presence suggests the infiltration of mineral salts or bone remains (apatite) in the sample. | [31] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–11. Available online: https://scispace.com/papers/type-i-collagen-3oyrkf3god (accessed on 15 July 2025).
- Siemiątkowski, R.; Haber, M.; Czachor, A.; Kula, P.; Juśkiewicz, A.; Grelewicz, O.; Kucy, N.; Servaas, E.; Kotula, A. Comparative Analysis of Collagen Supplementation Forms and Their Effects on Multiple Health Parameters. J. Educ. Health Sport 2024, 65, 55474. [Google Scholar] [CrossRef]
- VSandhu, S.; Gupta, S.; Bansal, H.; Singla, K. Collagen in Health and Disease. J. Orofac. Res. 2012, 2, 153–159. [Google Scholar] [CrossRef]
- Pillai, N.S.; Khan, S.A.; Mehrotra, N.; Jadhav, K. A Comprehensive Review on the Role of Collagen in Health and Disease. Biosci. Biotechnol. Res. Asia 2024, 21, 1329–1347. [Google Scholar] [CrossRef]
- Palamutoğlu, R.; Palamutoğlu, M.İ. Beneficial Health Effects of Collagen Hydrolysates. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2024; Volume 80, pp. 477–503. Available online: https://scispace.com/papers/beneficial-health-effects-of-collagen-hydrolysates-2s1madkh6c (accessed on 15 July 2025).
- Ye, T.; Xiang, Q.; Yang, Y.; Huang, Y. Research, development and application of collagen: A review. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2023, 39, 942–960. [Google Scholar] [CrossRef]
- Gulevsky, A.K. Collagen: Structure, Metabolism, Production and Industrial Application. Biotechnol. Acta 2020, 13, 42–61. [Google Scholar] [CrossRef]
- Rosenfeld, S.A. Delicious guinea pigs: Seasonality studies and the use of fat in the pre-Columbian Andean diet. Quat. Int. 2008, 180, 127–134. [Google Scholar] [CrossRef]
- Apaza-Ticona, J.; Arocutipa, V.A.; Cutipa-Añamuro, G.; Calderón-Torres, A.; Chambilla-Laquiticona, A.; Maquera-Maquera, Y. Diagnóstico con Cavia Porcellus Aymara, Rayos X y Tratamiento en Tiempos de COVID-19. Rev. Gestão—RGSA 2024, 18, e06618. [Google Scholar] [CrossRef]
- García-Coronado, J.M.; Martínez-Olvera, L.; Elizondo-Omaña, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Bilotto, F.; Harrison, M.T.; Vibart, R.; Mackay, A.; Christie-Whitehead, K.M.; Ferreira, C.S.; Cottrell, R.S.; Forster, D.; Chang, J. Towards resilient, inclusive, sustainable livestock farming systems. Trends Food Sci. Technol. 2024, 152, 104668. [Google Scholar] [CrossRef]
- Niu, J.; Wu, H.; Ma, S.; Yan, Z.; Wang, X.; Li, J.; Hu, R.; Qi, Q. Optimization of Ultrasonic Assisted Extraction of Tibetan Sheep Skin Collagen Peptide by Response Surface Methodology and Its Antioxidant Activity in Vivo. Sci. Technol. Food Ind. 2023, 44, 163–170. [Google Scholar] [CrossRef]
- Razali, U.H.M.; Juraimy, A.M.M.; Jusoh, Y.M.M.; Dailin, D.J.; Ya’Akob, H.; Zainool, N.; Zaidel, D.N.A. Effect of Ultrasonic Amplitude on the Yield and Properties of Barramundi (Lates calcarifer) Skin Collagen. J. Trop. Life Sci. 2023, 13, 247–256. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, X.; Ni, J.; Liang, Q.; Jiang, X.; Zhou, Z.; Shi, W. Effects of Ultrasonic Power on the Structure and Rheological Properties of Skin Collagen from Albacore (Thunnus alalunga). Mar. Drugs 2024, 22, 84. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Vidal, A.R.; Mello, R.O.; Mazutti, M.A.; Cansian, R.L.; Dornelles, R.C.P.; Demiate, I.M.; Kubota, E.H. Ultrasound as an alternative method to increase the extraction yield from chicken mecanically separated meatresidue collagen. J. Food Sci. Technol. 2021, 58, 2487–2496. [Google Scholar] [CrossRef]
- Pezeshk, S.; Rezaei, M.; Abdollahi, M. Impact of ultrasound on extractability of native collagen from tuna by-product and its ultrastructure and physicochemical attributes. Ultrason. Sonochem. 2022, 89, 106129. [Google Scholar] [CrossRef]
- Akram, A.N.; Zhang, C. Effect of ultrasonication on the yield, functional and physicochemical characteristics of collagen-II from chicken sternal cartilage. Food Chem. 2020, 307, 125544. [Google Scholar] [CrossRef]
- Metodología de Superficie de Respuesta (MSR) en el Diseño de Experimentos—SixSigma.us. Available online: https://www.6sigma.us/six-sigma-in-focus/response-surface-methodology-rsm/?utm_source=chatgpt.com (accessed on 20 August 2025).
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A. Could glutaric acid (GA) replace glutaraldehyde in the preparation of biocompatible biopolymers with high mechanical and thermal properties? J. Chem. Sci. 2014, 126, 127–140. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, H.S.; Lee, O.J.; Chao, J.R.; Park, H.J.; Lee, J.M.; Ju, H.W.; Moon, B.M.; Park, Y.R.; Song, J.E.; et al. Fabrication of duck’s feet collagen–silk hybrid biomaterial for tissue engineering. Int. J. Biol. Macromol. 2016, 85, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Déléris, G. Chemical mapping of tumor progression by FT-IR imaging: Towards molecular histopathology. Trends Biotechnol. 2006, 24, 455–462. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J.; Skorupska, M.; Michalska, M. Isolation and characterization of collagen from the skin of Brama australis. Int. J. Biol. Macromol. 2015, 80, 605–609. [Google Scholar] [CrossRef]
- Safandowska, M.; Pietrucha, K. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, H.; Li, H.; Dai, K.; Wang, J.; Zhang, X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials 2009, 30, 4369–4376. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Xie, Q.; Hong, B.; Chen, J.; Hua, F.; Bai, K.; He, J.; Yi, R.; Wu, H. Rapid isolation of high purity pepsin-soluble type I collagen from scales of red drum fish (Sciaenops ocellatus). Food Hydrocoll. 2016, 52, 468–477. [Google Scholar] [CrossRef]
- Ramanathan, N.; Sundaram, J. Man and insects—Altruism and above. Curr. Sci. 2023, 125, 241–246. [Google Scholar] [CrossRef]
- Henchman, R.H.; McCammon, J.A. Structural and dynamic properties of water around acetylcholinesterase. Protein Sci. 2002, 11, 2080–2090. [Google Scholar] [CrossRef]
- Begam, H.; Nandi, S.K.; Chanda, A.; Kundu, B. Effect of bone morphogenetic protein on Zn-HAp and Zn-HAp/collagen composite: A systematic in vivo study. Res. Veter-Sci. 2017, 115, 1–9. [Google Scholar] [CrossRef]
- Kaewbangkerd, K.; Hamzeh, A.; Yongsawatdigul, J. Ultrasound-assisted extraction of collagen from broiler chicken trachea and its biochemical characterization. Ultrason. Sonochem. 2023, 95, 106372. [Google Scholar] [CrossRef]
- Ramli, R.A.; Mohamad Razali, U.H.; Izzreen Mohd Noor, N.Q. Optimization of extraction conditions of gelatin from buffalo (Bubalus bubalis) skins using response surface methodology. Heliyon 2023, 9, e14367. [Google Scholar] [CrossRef] [PubMed]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Starek-Wójcicka, A.; Sagan, A. Effect of the duty cycle of the ultrasonic processor on the efficiency of extraction of phenolic compounds from Sorbus intermedia. Sci. Rep. 2022, 12, 8311. [Google Scholar] [CrossRef]
- Woessner, J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type i affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Jazani, R.S.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
 ) they indicate that the design points are below the predicted value, the red dots (
) they indicate that the design points are below the predicted value, the red dots (  ) indicate that the design points are above the predicted value and the change from blue to red (
) indicate that the design points are above the predicted value and the change from blue to red (  ) indicates the minimum and maximum value of the variables yield and hydroxyproline content respectively.
) indicates the minimum and maximum value of the variables yield and hydroxyproline content respectively.
 ) they indicate that the design points are below the predicted value, the red dots (
) they indicate that the design points are below the predicted value, the red dots (  ) indicate that the design points are above the predicted value and the change from blue to red (
) indicate that the design points are above the predicted value and the change from blue to red (  ) indicates the minimum and maximum value of the variables yield and hydroxyproline content respectively.
) indicates the minimum and maximum value of the variables yield and hydroxyproline content respectively.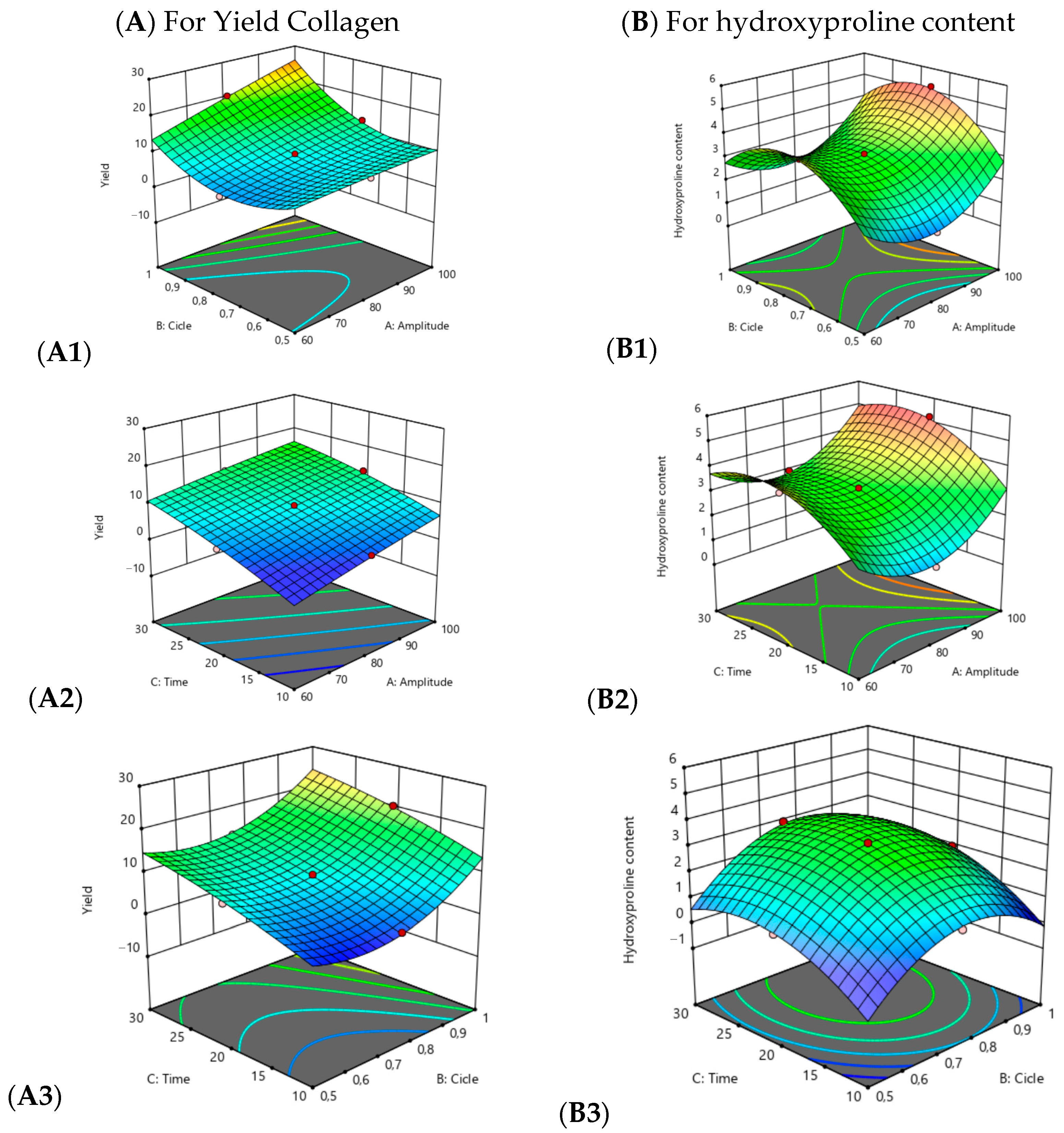
| Factor | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Amplitude (%) | X1 | 60 | 80 | 100 |
| Cycle | X2 | 0.5 | 0.75 | 1 |
| Time (min) | X3 | 10 | 20 | 30 |
| Run Numbers | Amplitude (X1) | Cycle (X2) | Time (X3) | Yield (%) | Hydroxyproline Content (%) |
|---|---|---|---|---|---|
| 1 | 60 | 1 | 30 | 19.03 | 2.531 |
| 2 | 60 | 0.5 | 30 | 14.61 | 1.552 |
| 3 | 100 | 1 | 30 | 29.11 | 3.971 |
| 4 | 60 | 0.75 | 20 | 4.81 | 3.921 |
| 5 | 60 | 1 | 10 | 6.11 | 0.831 |
| 6 | 80 | 0.75 | 20 | 9.23 | 3.111 |
| 7 | 100 | 1 | 10 | 20.12 | 1.891 |
| 8 | 80 | 0.75 | 20 | 9.23 | 3.161 |
| 9 | 80 | 0.5 | 20 | 9.71 | 0.891 |
| 10 | 80 | 0.75 | 20 | 9.63 | 3.152 |
| 11 | 80 | 1 | 20 | 20.22 | 1.951 |
| 12 | 100 | 0.5 | 30 | 14.82 | 2.421 |
| 13 | 100 | 0.5 | 10 | 4.51 | 0.974 |
| 14 | 100 | 0.75 | 20 | 13.12 | 5.211 |
| 15 | 60 | 0.5 | 10 | 2.02 | 0.261 |
| 16 | 80 | 0.75 | 10 | 3.21 | 1.061 |
| 17 | 80 | 0.75 | 30 | 13.21 | 2.951 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| For Yiel Collagen | ||||||
| Model | 836.18 | 9 | 92.91 | 266.20 | <0.0001 | **** |
| X1-Amplitude | 123.20 | 1 | 123.20 | 352.99 | <0.0001 | |
| X2-Cycle | 239.32 | 1 | 239.32 | 685.68 | <0.0001 | |
| X3-Time | 300.41 | 1 | 300.41 | 860.73 | <0.0001 | |
| X1X2 | 57.19 | 1 | 57.19 | 163.86 | <0.0001 | |
| X1X3 | 4.82 | 1 | 4.82 | 13.81 | 0.0075 | |
| X2X3 | 0.1225 | 1 | 0.1225 | 0.3510 | 0.5722 | |
| X12 | 0.1665 | 1 | 0.1665 | 0.4771 | 0.5120 | |
| X22 | 88.60 | 1 | 88.60 | 253.87 | <0.0001 | |
| X32 | 2.70 | 1 | 2.70 | 7.74 | 0.0272 | |
| Residual | 2.44 | 7 | 0.3490 | |||
| Lack of Fit | 2.34 | 5 | 0.4673 | 8.76 | 0.1056 | not significant |
| Pure Error | 0.1067 | 2 | 0.0533 | |||
| Cor Total | 838.62 | 16 | ||||
| For hydroxyproline content | ||||||
| Model | 28.85 | 9 | 3.21 | 329.49 | <0.0001 | **** |
| X1-Amplitude | 2.89 | 1 | 2.89 | 296.60 | <0.0001 | |
| X2-Cycle | 2.58 | 1 | 2.58 | 264.81 | <0.0001 | |
| X3-Time | 7.07 | 1 | 7.07 | 726.57 | <0.0001 | |
| X1X2 | 0.1053 | 1 | 0.1053 | 10.83 | 0.0133 | |
| X1X3 | 0.0359 | 1 | 0.0359 | 3.69 | 0.0962 | |
| X2X3 | 0.1357 | 1 | 0.1357 | 13.95 | 0.0073 | |
| X12 | 5.72 | 1 | 5.72 | 588.26 | <0.0001 | |
| X22 | 7.59 | 1 | 7.59 | 780.33 | <0.0001 | |
| X32 | 3.23 | 1 | 3.23 | 332.22 | <0.0001 | |
| Residual | 0.0681 | 7 | 0.0097 | |||
| Lack of Fit | 0.0667 | 5 | 0.0133 | 18.78 | 0.0513 | not significant |
| Pure Error | 0.0014 | 2 | 0.0007 | |||
| Cor Total | 28.92 | 16 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, G.L.; Valenzuela, M.E.; Hinostroza-Quiñonez, G.; Ramos, O.F.; López, E.A.; Saavedra, R.T.; Zacarias, E.R.; Bonilla, H.; Álvarez, E.I.; Espinoza Silva, C. Valorization of Cavia porcellus By-Products via Ultrasound-Assisted Collagen Extraction: Optimization and Characterization. Foods 2025, 14, 3542. https://doi.org/10.3390/foods14203542
Santos GL, Valenzuela ME, Hinostroza-Quiñonez G, Ramos OF, López EA, Saavedra RT, Zacarias ER, Bonilla H, Álvarez EI, Espinoza Silva C. Valorization of Cavia porcellus By-Products via Ultrasound-Assisted Collagen Extraction: Optimization and Characterization. Foods. 2025; 14(20):3542. https://doi.org/10.3390/foods14203542
Chicago/Turabian StyleSantos, Gussieff Lino, Milady Esteban Valenzuela, Greta Hinostroza-Quiñonez, Omar Flores Ramos, Edgar Acosta López, Rodolfo Tello Saavedra, Edgar Rojas Zacarias, Humberto Bonilla, Ever Ingaruca Álvarez, and Clara Espinoza Silva. 2025. "Valorization of Cavia porcellus By-Products via Ultrasound-Assisted Collagen Extraction: Optimization and Characterization" Foods 14, no. 20: 3542. https://doi.org/10.3390/foods14203542
APA StyleSantos, G. L., Valenzuela, M. E., Hinostroza-Quiñonez, G., Ramos, O. F., López, E. A., Saavedra, R. T., Zacarias, E. R., Bonilla, H., Álvarez, E. I., & Espinoza Silva, C. (2025). Valorization of Cavia porcellus By-Products via Ultrasound-Assisted Collagen Extraction: Optimization and Characterization. Foods, 14(20), 3542. https://doi.org/10.3390/foods14203542









