Soil Amendment with Poultry Manure, Biochar, and Coenzyme A Enhances Yield and Nutritional Composition of Moringa oleifera Lam.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Design, Climate, and Treatments
2.3. Extraction and Determination of Intact Glucosinolates and Phenolic Compounds
2.4. Analysis of Mineral Nutrients and Trace Elements
2.5. Statistical Analysis
3. Results
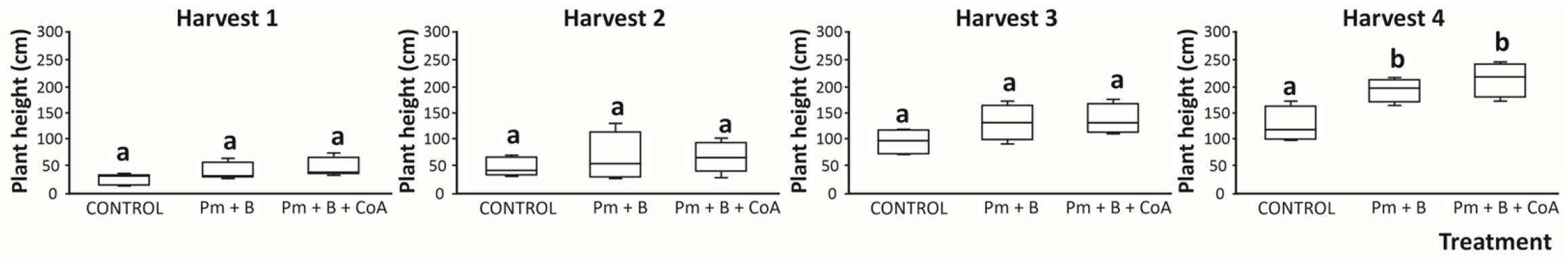
3.1. Plant Height of Moringa oleifera as Influenced by Soil Amendments
3.2. Number of Pinnae of Moringa oleifera as Influenced by Soil Amendments
3.3. Petiole Girth of Moringa oleifera Tree as Influenced by Soil Amendments
3.4. Number of Branches of Moringa oleifera as Influenced by Soil Amendments
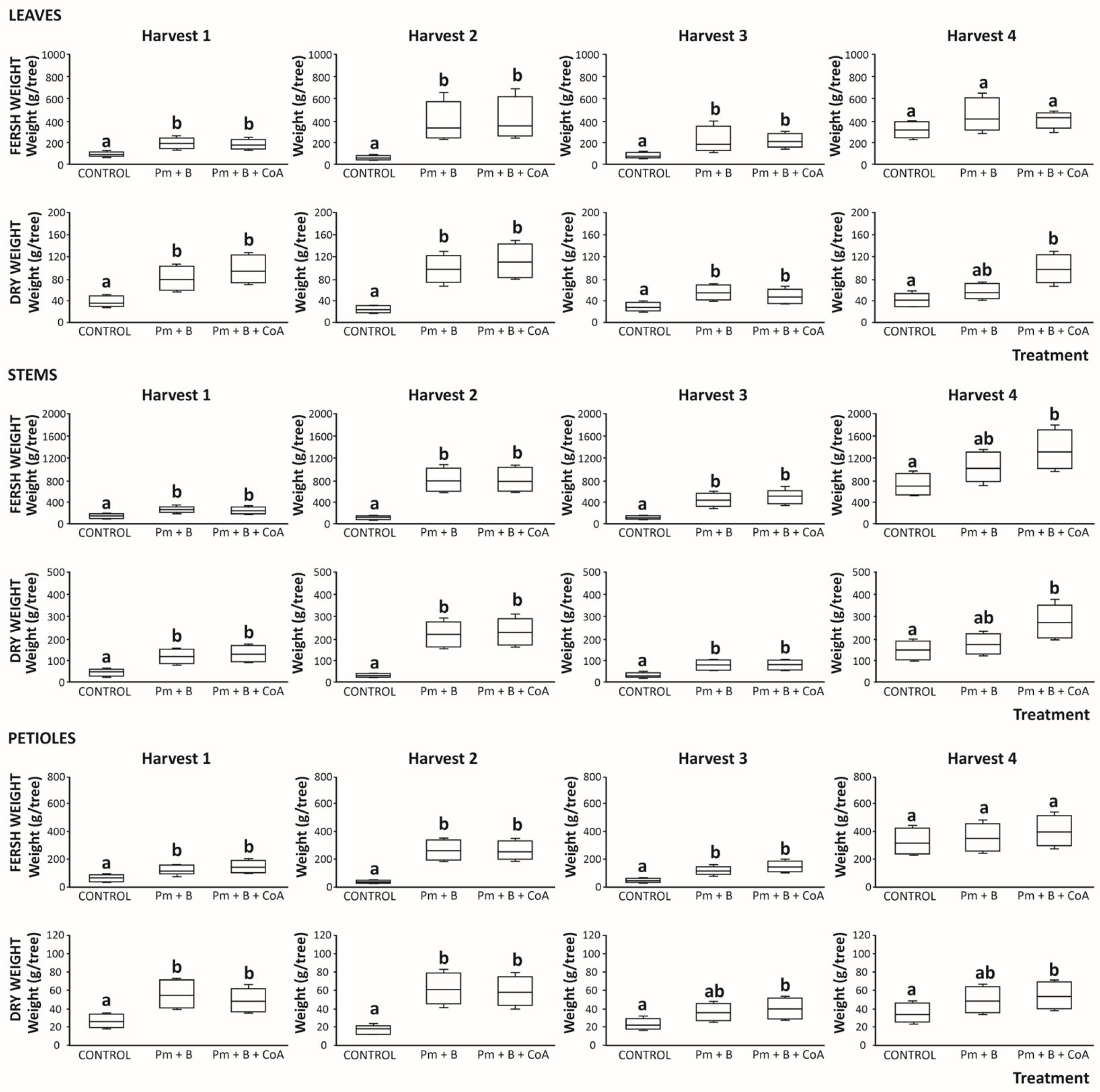
3.5. Leaf, Stems, and Petioles’ Yield of Moringa oleifera as Influenced by Soil Amendments: Fresh and Dry Matter Assessments
3.5.1. Fresh and Dried Leaves Yield
3.5.2. Fresh and Dried Stems Yield
3.5.3. Fresh and Dried Petioles Yield
3.6. Glucosinolate Content of the Tissues with the Soil Amendments
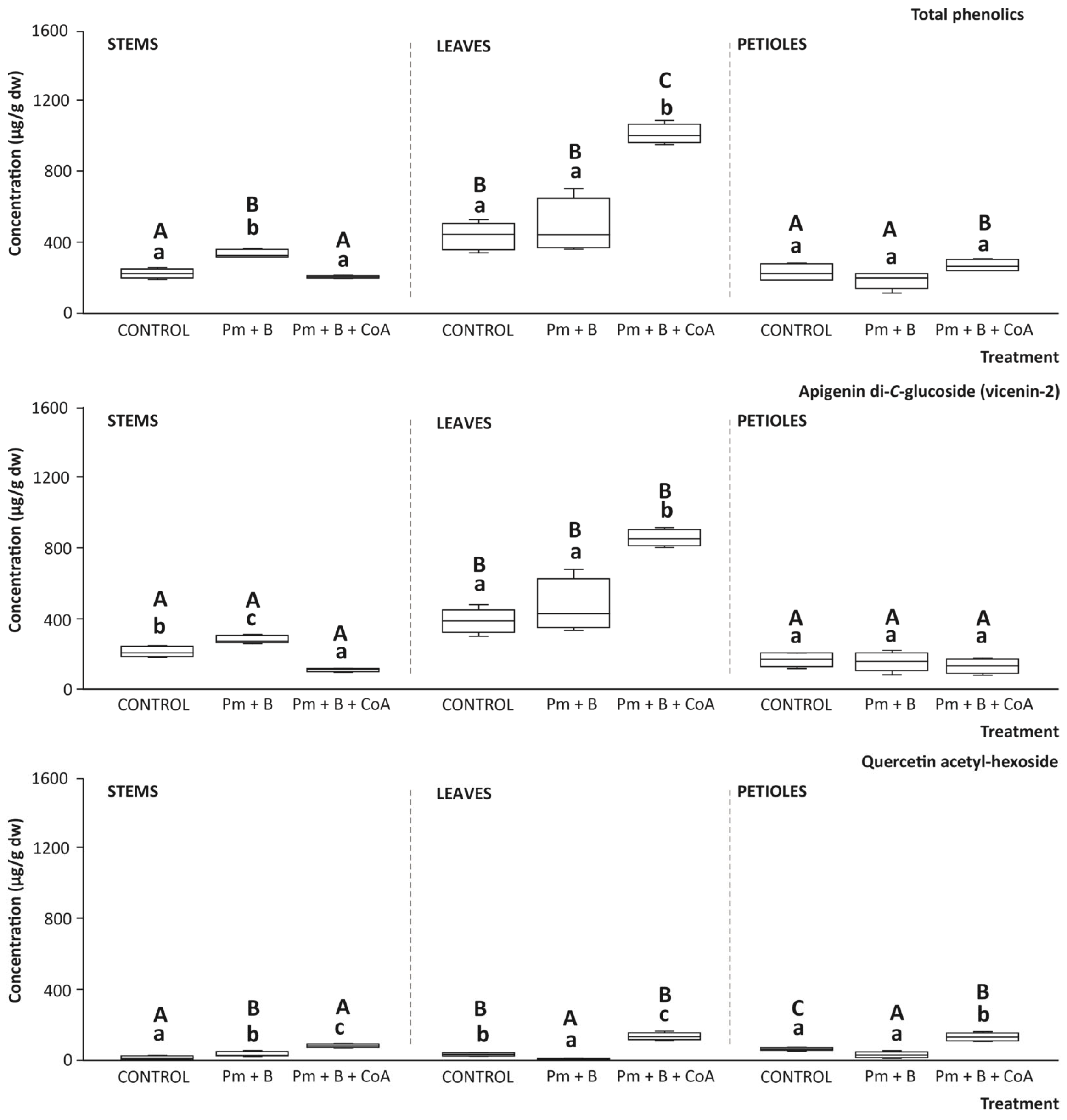
3.7. Phenolic Content of the Tissues with the Soil Amendments
3.8. Mineral Nutrients and Trace Elements
4. Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef] [PubMed]
- Gandji, K.; Chadare, F.J.; Idohou, R.; Salako, V.K.; Assogbadjo, A.E.; Kakaï, R.L.G. Status and Utilisation of Moringa oleifera Lam: A Review. Afr. Crop Sci. J. 2018, 26, 137–156. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M.R.; Karim, R. Moringa Oleifera Is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef]
- Siddique, F.; Ahmad, S.; Hussain, A.; Firdous, N.; Nisar, R.; Bilal, M.; Najam, A.; Salik, A.; Zia, M.; Elkhedir, A.E. Development, Physicochemical, and Sensory Analysis of Moringa oleifera L. Powder Added Buffalo Milk Yoghurt with Pharmacological Potential. Sci. Rep. 2025, 15, 31519. [Google Scholar] [CrossRef]
- Alavilli, H.; Poli, Y.; Verma, K.S.; Kumar, V.; Gupta, S.; Chaudhary, V.; Jyoti, A.; Sahi, S.V.; Kothari, S.L.; Jain, A. Miracle Tree Moringa oleifera: Status of the Genetic Diversity, Breeding, In vitro Propagation, and a Cogent Source of Commercial Functional Food and Non-Food Products. Plants 2022, 11, 3132. [Google Scholar] [CrossRef]
- Adegun, M.K.; Ayodele, O.J. Growth and Yield of Moringa Oleifera as Influenced by Spacing and Organic Manures in South-Western Nigeria. Int. J. Agron. Agric. Res. 2015, 6, 30–37. [Google Scholar]
- Amaglo, N.K.; Deng, J.; Foidl, N. The Agro-Forestry Uses and Benefits of Moringa Oleifera. J. Biopr. Engin. Bioref. 2015, 3, 182–189. [Google Scholar] [CrossRef]
- Pituya, P.; Sriburi, T.; Wijitkosum, S. Optimization of Biochar Preparation from Acacia Wood for Soil Amendment. Engin. J. 2017, 21, 99–105. [Google Scholar] [CrossRef]
- Mastrolonardo, G.; Calderaro, C.; Cocozza, C.; Hardy, B.; Dufey, J.; Cornelis, J.T. Long-Term Effect of Charcoal Accumulation in Hearth Soils on Tree Growth and Nutrient Cycling. Front. Environ. Sci. 2019, 7, 430967. [Google Scholar] [CrossRef]
- Steiner, C.; García, M.; Zech, W. Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A., Rebellato, L., Eds.; Chapter Effects of Charcoal as Slow Release Nutrient Carrier on N-P-K Dynamics and Soil Microbial Population: Pot Experiments with Ferralsol Substrate. In Amazonian Dark Earths: Wim Sombroek’s Vision; Springer Nature+Bussines Media B.V.: Berlin, Germany, 2009; pp. 325–338. ISBN 978-1-4020-9031-8. [Google Scholar]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Ndubuaku, U.M.; Ede, A.E.; Baiyeri, K.P.; Ezeaku, P.I. Application of Poultry Manure and Its Effect on Growth and Performance of Potted Moringa (Moringa oleifera Lam.) Plants Raised for Urban Dwellers’ Use. Am. J. Plant Nutr. Fertil. Technol. 2014, 5, 33–39. [Google Scholar] [CrossRef]
- Liao, Z.; Fan, J.; Lai, Z.; Bai, Z.; Wang, H.; Cheng, M.; Zhang, F.; Li, Z. Response Network and Regulatory Measures of Plant-Soil-Rhizosphere Environment to Drought Stress. Adv. Agron. 2023, 180, 93–196. [Google Scholar]
- Anjorin, T.S.; Ikokoh, P.; Okolo, S. Mineral Composition of Moringa Oleifera Leaves, Pods and Seeds from Two Regions in Abuja, Nigeria. Int. J. Agric. Biol. 2010, 12, 431–434. [Google Scholar]
- Salem, L.R.; Saleh, M.E.; El, A.; El-Henawy, M.A.; El-Absy, K.M.; El Gamal, E.H. Biochar as an Amendment to Sandy Soil Properties and Its Effect on Biochemical Composition and Growth of Moringa oleifera. Alex. Sci. Exh. J. 2025, 46, 613–635. [Google Scholar] [CrossRef]
- Oloyede, O. Effects of Cow Dung and N.P. K Fertilizer at Different Levels on the Growth Performance and Nutrient Composition of Moringa Oleifera. Ann. Exp. Biol. 2016, 4, 35–39. [Google Scholar]
- Agbede, T.M. Poultry Manure Improves Soil Properties and Grain Mineral Composition, Maize Productivity and Economic Profitability. Sci. Rep. 2025, 15, 16501. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, Limitations, Co-Benefits, and Trade-Offs of Biochar Applications to Soils for Climate Change Mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived by-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Kandil, H.; Gad, N. Effects of inorganic and organic fertilizers on growth and production of brocoli (Brassica oleracea Lam.). In Factori şi Procese Pedogenetice din Zona Temperată; Editura Universităţii Al. I. Cuza: Iași, Romania, 2009; Volume 8, pp. 61–69. [Google Scholar]
- Mohammed, T.; Shareef, E.; Zhao, B.; Shareef, T.M.E.; Zhao, B.W. Review Paper: The Fundamentals of Biochar as a Soil Amendment Tool and Management in Agriculture Scope: An Overview for Farmers and Gardeners. J. Agric. Chem. Environ. 2016, 6, 38–61. [Google Scholar]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The Role of Biochar and Biochar-Compost in Improving Soil Quality and Crop Performance: A Review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Barritt, S.A.; DuBois-Coyne, S.E.; Dibble, C.C. Coenzyme A Biosynthesis: Mechanisms of Regulation, Function and Disease. Nat. Metab. 2024, 6, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.G.; Akmtoye, H.A.; Olufolaji, A.O.; Aina, O.O.; Olatunji, M.T.; Shokalu, A.O. Assessment of Organic Amendments on Vegetative Development and Nutrient Uptake of Moringa oleifera Lam. in the Nursery. Asian J. Plant Sci. 2011, 10, 74–79. [Google Scholar] [CrossRef]
- Truong, H.T.H.; Tran, T.V.; Nguyen, T.T.T.; Nguyen, P.D.; Do, A.T. Germplasm Evaluation and Influence of Soil Type, Plant Density and Pruning Height on Biomass Yield of Moringa in Central Vietnam. Acta Hortic. 2017, 1158, 133–142. [Google Scholar] [CrossRef]
- Kauser, M.; Waraich, E.A.; Rehman, H.U.; Haq, M.A. Organic and Inorganic Amendments Improved the Morpho-Physiological and Nutritional Status of Moringa (Moringa Oleifera) Genotypes. Acta Physiol. Plant 2025, 47, 1959–1972. [Google Scholar] [CrossRef]
- de Andrade Melo Junior, J.L.; Soares, L.B.F.; de Andrade Melo, L.D.F.; Gomes, L.C.A.; Neto, J.A.; Berto, T.D.S.; Santos, E.B.D.; de Mendonça Santos, K. Optimal Temperature and Energy Efficiency in the Germination of Moringa Oleifera Lam.: Implications for Use in Ecological Restoration Programs in Degraded Areas. S. Afr. J. Bot. 2025, 178, 33–38. [Google Scholar] [CrossRef]
- Siwach, P.; Gill, A.R. Micropropagation of Ficus Religiosa L. via Leaf Explants and Comparative Evaluation of Acetylcholinesterase Inhibitory Activity in the Micropropagated and Conventionally Grown Plants. 3 Biotech 2014, 4, 477–491. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of Nutrient Deficiency in Maize and Tomato Plants by in vivo Chlorophyll a Fluorescence Measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Akhtar, S.; Khalid, N.; Ahmed, I.; Shahzad, A.; Suleria, H.A.R. Physicochemical Characteristics, Functional Properties, and Nutritional Benefits of Peanut Oil: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1562–1575. [Google Scholar] [CrossRef]
- Srikrishnah, S.; Perns, S.E.; Sutharsan, S. Effect of Shade Levels on Leaf Area and Biomass Production of Three Varieties of Dracaena Sanderiana L. in the Dry Zone of Sri Lanka. Trop. Agric. Res. 2012, 23, 142–143. [Google Scholar] [CrossRef]
- Botanicae Horti Agrobotanici, N.; Sam Dalirie, M.; Seyed Sharifi, R.; Farzaneh, S. Evaluation of Yield, Dry Matter Accumulation and Leaf Area Index in Wheat Genotypes as Affected by Terminal Drought Stress. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2010, 38, 182–186. [Google Scholar]
- Çelikel, G. Effect of different substrates on yield and quality of tomato. Acta Hortic. 1999, 491, 353–356. [Google Scholar] [CrossRef]
- Maldini, M.; Maksoud, S.A.; Natella, F.; Montoro, P.; Petretto, G.L.; Foddai, M.; De Nicola, G.R.; Chessa, M.; Pintore, G. Moringa Oleifera: Study of Phenolics and Glucosinolates by Mass Spectrometry. J. Mass Spectrom. 2014, 49, 900–910. [Google Scholar] [CrossRef] [PubMed]
- López-Rodríguez, N.A.; Gaytán-Martínez, M.; de la Luz Reyes-Vega, M.; Loarca-Piña, G. Glucosinolates and Isothiocyanates from Moringa Oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Gil, J.I.; Ferreres, F.; García-Viguera, C.; Martínez-Sanz, J.M.; Gil-Izquierdo, A. A Ultra-Pressure Liquid Chromatography/Triple Quadrupole Tandem Mass Spectrometry Method for the Analysis of 13 Eicosanoids in Human Urine and Quantitative 24 Hour Values in Healthy Volunteers in a Controlled Constant Diet. Rapid Comm. Mass Spectrom. 2012, 26, 1249–1257. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Jiang, L.; Li, J.; Yu, B.; Ren, C.; Yan, T.; Jia, Y.; He, B. LC-MS/MS-Based Chemical Profiling of Water Extracts of Moringa Oleifera Leaves and Pharmacokinetics of Their Major Constituents in Rat Plasma. Food Chem. X 2024, 23, 101585. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the Role of Phenylpropanoids in Plant Defense against UV-B Stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- González-Coria, J.; Lozano-Castellón, J.; Jaime-Rodríguez, C.; Olmo-Cunillera, A.; Laveriano-Santos, E.P.; Pérez, M.; Lamuela-Raventós, R.M.; Puig, J.; Vallverdú-Queralt, A.; Romanyà, J. The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties. Antioxidants 2022, 11, 2127. [Google Scholar] [CrossRef]
- Zeru, A.E.; Hassen, A.; Apostolides, Z.; Tjelele, J. Relationships between Agronomic Traits of Moringa Accessions and In vitro Gas Production Characteristics of a Test Feed Incubated with or without Moringa Plant Leaf Extracts. Plants 2022, 11, 2901. [Google Scholar] [CrossRef]
- Parola-Contreras, I.; Guzmán-Rodríguez, L.F.; Tovar-Pérez, E.G.; Guerrero-Aguilar, B.Z.; Amaro-González, B.A.; Rojas-Molina, A.; Torres-Pacheco, I.; Pons-Hernández, J.L.; González-Chavira, M.M.; Guevara-González, R.G. Controlled Elicitation and Greenhouse Acclimation Time Effects on Morphological and Biochemical Variables in Collections of Heliopsis Longipes from Central México. J. Plant Growth Regul. 2024, 43, 889–902. [Google Scholar] [CrossRef]
- González-Coloma, A.; Andrés, M.F.; Francisco Quílez Del Moral, J.; Diaz, C.E.; Rusli, L.S.; Abdullah, R.; Yaacob, J.S.; Osman, N. Organic Amendments Effects on Nutrient Uptake, Secondary Metabolites, and Antioxidant Properties of Melastoma Malabathricum L. Plants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Leaseburg, E.E.; Lei, L.; Fink, L.S. Effects of Organic Amendments on Phenol Oxidase, Peroxidase, Urease, and Nitrogen Mineralization: A Laboratory Incubation Study. Agrochemicals 2022, 1, 3–16. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Pongrac, P.; Kreft, I.; Pelicon, P.; Vavpetič, P.; Jenčič, B.; van Elteren, J.T.; Kump, P.; Singh, S.P.; Regvar, M. Distribution of Nutritional and Mineral Components in Important Crop Plants. In Genome Engineering for Crop Improvement; WILEY: Hoboken, NJ, USA, 2021; pp. 22–42. [Google Scholar]
- Caruso, G.; Formisano, L.; Cozzolino, E.; Pannico, A.; El-Nakhel, C.; Rouphael, Y.; Tallarita, A.; Cenvinzo, V.; De Pascale, S. Shading Affects Yield, Elemental Composition and Antioxidants of Perennial Wall Rocket Crops Grown from Spring to Summer in Southern Italy. Plants 2020, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced Growth of Halophyte Plants in Biochar-Amended Coastal Soil: Roles of Nutrient Availability and Rhizosphere Microbial Modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Guo, W.N.; Lin, Q.M.; Li, G.T.; Zhao, X.R. Improving Salt Leaching in a Simulated Saline Soil Column by Three Biochars Derived from Rice Straw (Oryza Sativa L.), Sunfower Straw (Helianthus Annuus), and Cow Manure. J. Soil Water Conserv. 2016, 71, 467–475. [Google Scholar] [CrossRef]
- Jin, F.; Ran, C.; Anwari, Q.; Geng, Y.; Guo, L.; Li, J.; Han, D.; Zhang, X.; Liu, X.; Shao, X. Effects of Biochar on Sodium Ion Accumulation, Yield and Quality of Rice in Saline-Sodic Soil of the West of Songnen Plain, Northeast China. Plant Soil Environ. 2018, 64, 612–618. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- de Freitas, S.T.; Amarante, C.V.T.; Dandekar, A.M.; Mitcham, E.J. Shading Affects Flesh Calcium Uptake and Concentration, Bitter Pit Incidence and Other Fruit Traits in “Greensleeves” Apple. Sci. Hortic. 2013, 161, 266–272. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Chemistry and Biochemistry of Magnesium. Mol. Aspects Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Marere, A.P.; Kimbi, G.G.; Nonga, D.L.M. Comparative Effectiveness of Animal Manures on Soil Chemical Properties, Yield and Root Growth of Amaranthus (Amaranthus Cruentus L.). AJST 2001, 1, 14–21. [Google Scholar] [CrossRef]
- Akande, M.O.; Adedira, J.A.; Oluwatoyinbo, F.I. Effects of Rock Phosphate Amended with Poultry Manure on Soil Available P and Yield of Maize and Cowpea. Afr. J. Biotechnol. 2005, 4, 444–448. [Google Scholar]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The Beneficial Roles of Trace and Ultratrace Elements in Plants. Plant Growth Regul. 2022, 100, 219–236. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, I.; Anjum, N.A.; Diva, I.; Abdin, M.Z.; Iqbal, M. Effect of Timing of Sulfur Fertilizer Application on Growth and Yield of Rapeseed. J. Plant Nutr. 2005, 28, 1049–1059. [Google Scholar] [CrossRef]
- Abreu, I.A.; Cabelli, D.E. Superoxide Dismutases—A Review of the Metal-Associated Mechanistic Variations. Biochim. Biophys. Acta BBA Proteins Proteom. 2010, 1804, 263–274. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar]
- Vinodkumar, T.; Dinesh, T.; Ghosh, K.; Ilavarasan, R. A Comprehensive Analysis of Minerals in Moringa Oleifera Leaves, Seeds, Stem Bark, and Ash Using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). Discov. Chem. 2025, 2, 36. [Google Scholar] [CrossRef]
- Eswaramoorthy, D.; Krubanithy, K.; Mani, A. International Journal of Research Publication and Reviews Moringa Oleifera: An Overall Review on Nutritive Importance and Its Medicinal Application. Int. J. Res. Publ. Rev. 2023, 4, 3015–3023. [Google Scholar]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The Potential of Moringa oleifera in Food Formulation: A Promising Source of Functional Compounds with Health-Promoting Properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamudu, B.; García-Viguera, C.; Moreno, D.A.; Gaveh, E.; Appiah, F.; Idun, I.; Medina, S.; Domínguez-Perles, R. Soil Amendment with Poultry Manure, Biochar, and Coenzyme A Enhances Yield and Nutritional Composition of Moringa oleifera Lam. Foods 2025, 14, 3527. https://doi.org/10.3390/foods14203527
Mamudu B, García-Viguera C, Moreno DA, Gaveh E, Appiah F, Idun I, Medina S, Domínguez-Perles R. Soil Amendment with Poultry Manure, Biochar, and Coenzyme A Enhances Yield and Nutritional Composition of Moringa oleifera Lam. Foods. 2025; 14(20):3527. https://doi.org/10.3390/foods14203527
Chicago/Turabian StyleMamudu, Baba, Cristina García-Viguera, Diego A. Moreno, Eli Gaveh, Francis Appiah, Irene Idun, Sonia Medina, and Raúl Domínguez-Perles. 2025. "Soil Amendment with Poultry Manure, Biochar, and Coenzyme A Enhances Yield and Nutritional Composition of Moringa oleifera Lam." Foods 14, no. 20: 3527. https://doi.org/10.3390/foods14203527
APA StyleMamudu, B., García-Viguera, C., Moreno, D. A., Gaveh, E., Appiah, F., Idun, I., Medina, S., & Domínguez-Perles, R. (2025). Soil Amendment with Poultry Manure, Biochar, and Coenzyme A Enhances Yield and Nutritional Composition of Moringa oleifera Lam. Foods, 14(20), 3527. https://doi.org/10.3390/foods14203527









