Preservation of Beef with Limonene-Rich Citrus Peel Extracts: Antioxidant, Antimicrobial and Textural Benefits
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of the Composition and Activities of Citrus Peel
2.3. Processing and Storage of Citrus Peel Beef
2.4. Sensory Variations in the Preserved Beef
2.5. Hardiness and Springiness of the Preserved Beef
2.6. Bacterial Activity in the Preserved Beef
2.7. Protein Degradation in the Preserved Beef
2.8. Microstructure Integrity in the Preserved Beef
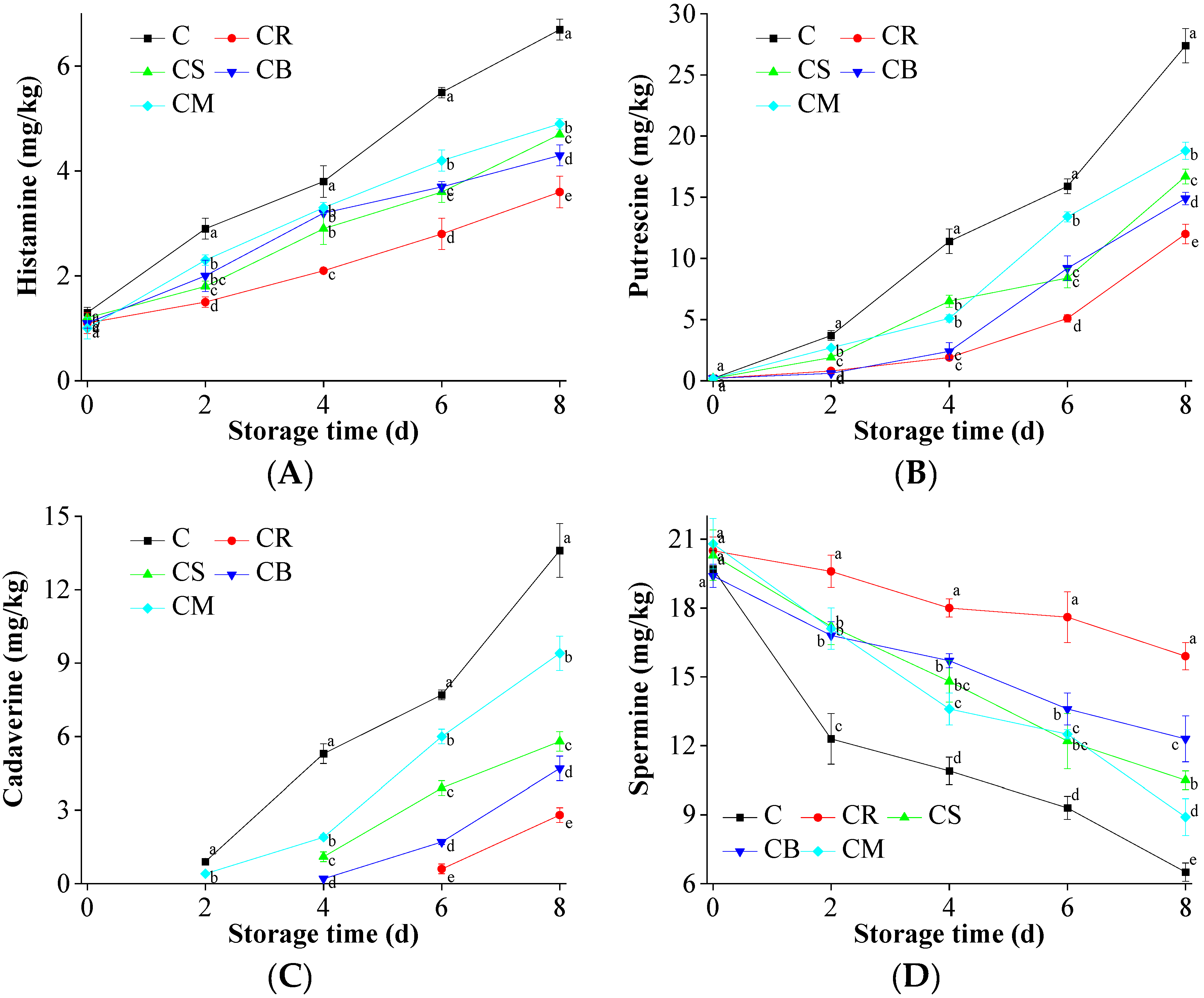
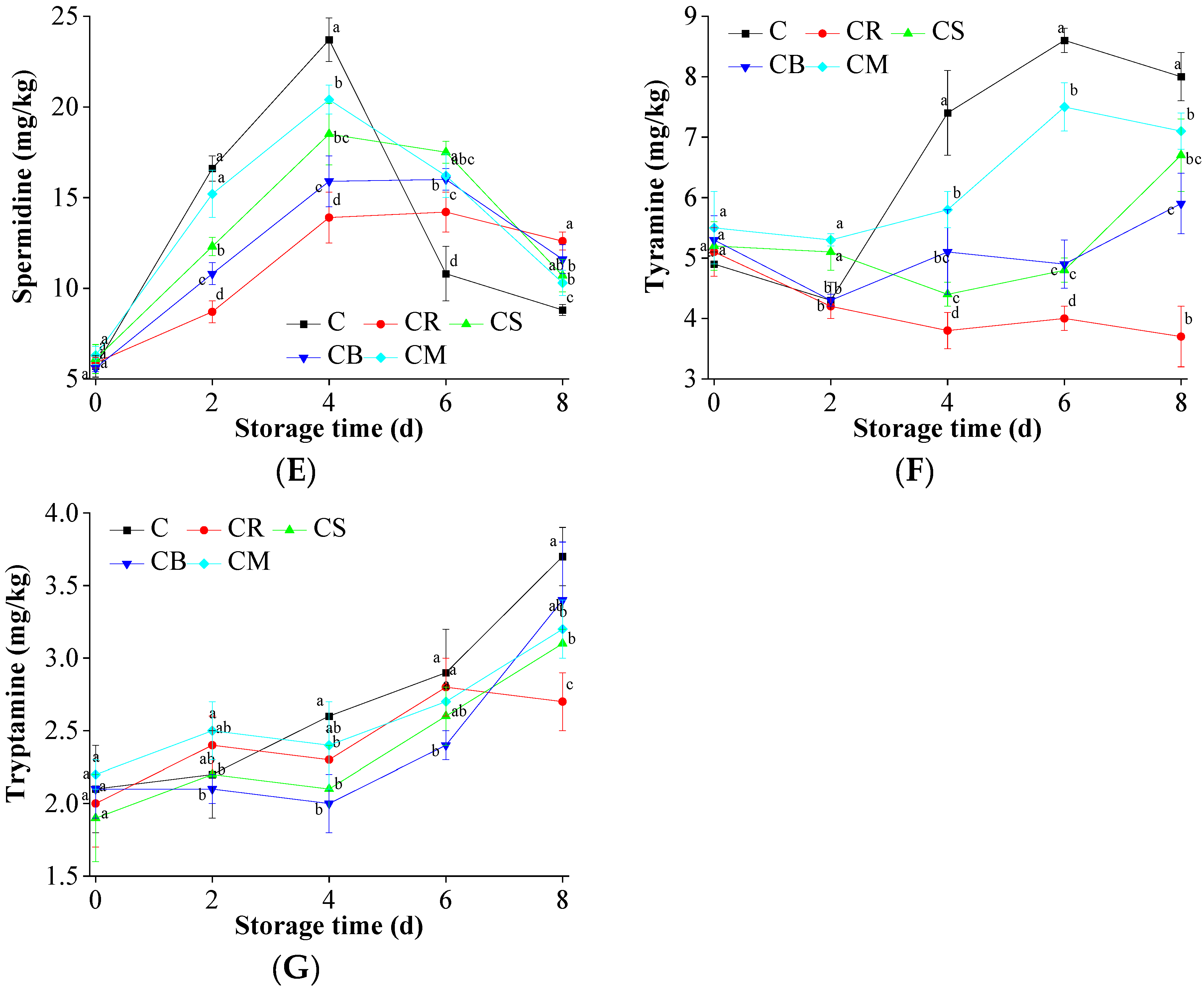
2.9. Biogenic Amines Accumulation in the Preserved Beef
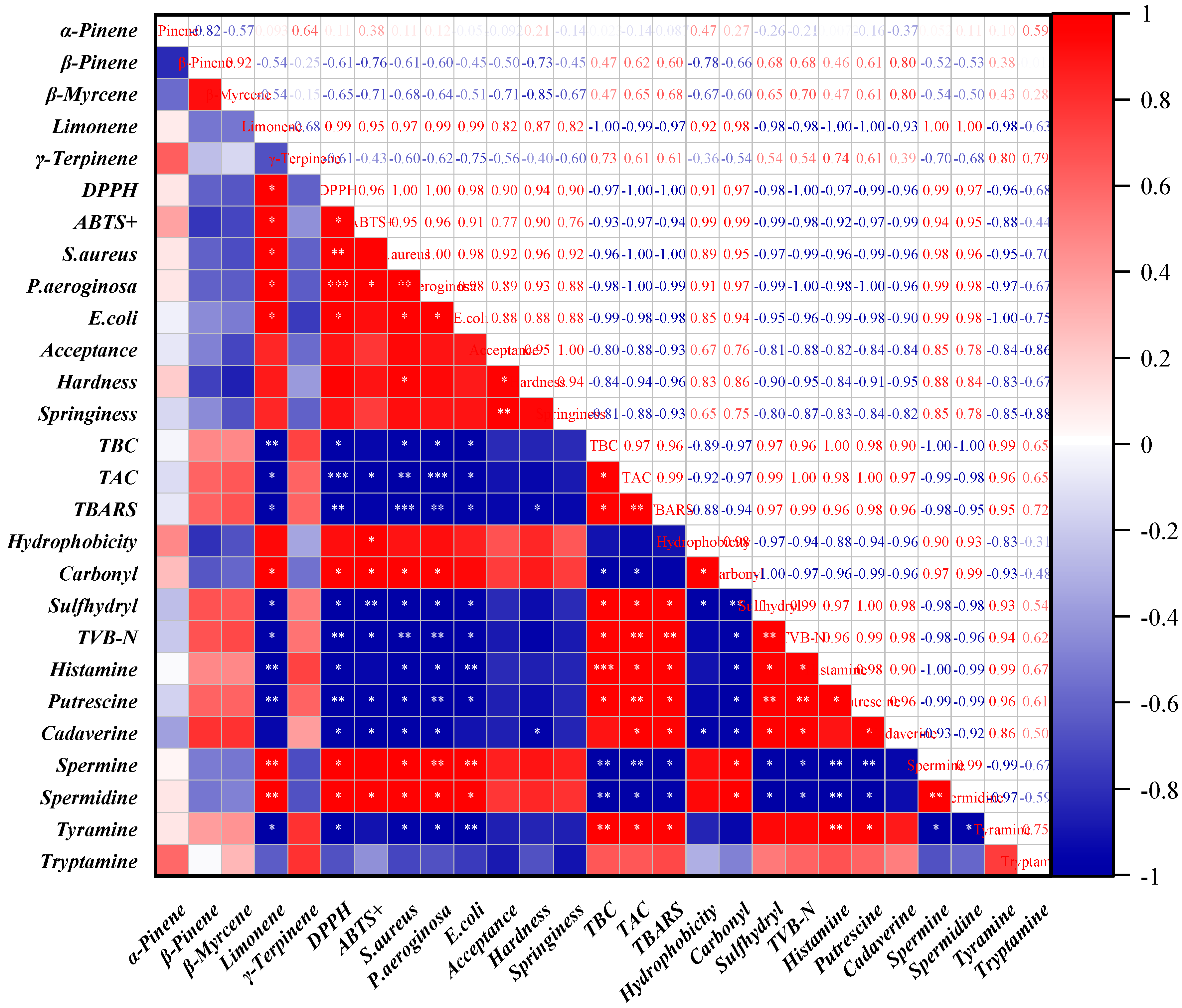
2.10. Correlation Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Component Extract and Characterization of Citrus Peel
3.2. Characterization of Citrus Peel Activities
3.3. Sensory and Texture Variations in the Beef
3.4. Microbial and Chemical Degradation in the Beef
3.5. Microstructure Variations in the Beef
3.6. Biogenic Amines Accumulation in the Beef
3.7. Correlation Analysis and Mechanistic Insights
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negrea, M.; Cocan, I.; Jianu, C.; Alexa, E.; Berbecea, A.; Poiana, M.A.; Silivasan, M. Valorization of citrus peel byproducts: A sustainable approach to nutrient-rich jam production. Foods 2025, 14, 1339. [Google Scholar] [CrossRef]
- Magalhaes, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional ingredients and additives from lemon by-products and their applications in food preservation: A review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mkadem, W.; Belguith, K.; Indio, V.; Oussaief, O.; Guluzade, G.; Elhatmi, H.; Serraino, A.; De Cesare, A.; Boudhrioua, N. Assessment of the anti-listeria effect of citrus limon peel extract in silico, in vitro, and in fermented cow milk during cold storage. Foods 2025, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J.; Blesa, J. Comprehensive analysis of polyphenols from hybrid Manda-rin peels by SPE and HPLC-UV. LWT 2022, 165, 113770. [Google Scholar] [CrossRef]
- Sanches, V.L.; Cunha, T.A.; Vigano, J.; Mesquita, L.M.D.; Faccioli, L.H.; Breitkreitz, M.C.; Rostagno, M.A. Comprehensive analysis of phenolics compounds in citrus fruits peels by UPLC-PDA and UPLC-Q/TOF MS using a fused-core column. Food Chem. X 2022, 14, 100262. [Google Scholar] [CrossRef]
- Rajendran, K.; Ganesan, S.; Manikandan, V.; Sivaselvam, S.; AlSalhi, M.S.; Asemi, N.N.; Angayarkanni, J.; Rajendiran, N.; Lo, H.M. Facile synthesis of carbon/titanium oxide quantum dots from lignocellulose-rich mandarin orange peel extract via microwave irradiation: Synthesis, characterization and bio-imaging application. Int. J. Biol. Macromol. 2023, 241, 124546. [Google Scholar] [CrossRef]
- Benmebarek, I.E.; Gonzalez-Serrano, D.J.; Aghababaei, F.; Ziogkas, D.; Garcia-Cruz, R.; Boukhari, A.; Moreno, A.; Hadidi, M. Optimizing the microwave-assisted hydrothermal extraction of pectin from tangerine by-product and its physicochemical, structural, and functional properties. Food Chem. X 2024, 23, 101615. [Google Scholar] [CrossRef]
- Gomez-Mejia, E.; Dias, M.I.; Pereira, C.; Pires, T.C.S.P.; Pala-Paul, J.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Calhelha, R.; Roriz, C.L. A biorefinery approach for the simultaneous obtention of essential oils, organic acids and polyphenols from citrus peels: Phytochemical characterization and bioactive potential. Food Chem. 2025, 486, 144641. [Google Scholar] [CrossRef]
- Lima, M.D.; Monteiro, L.I.D.; Carvvalho, A.J.D.A.; Bastos, D.C.; Pimentel, T.C.; Magnani, M. A robust method for quantifying 42 phenolic compounds by RP-HPLC/DAD: Columns performance and characterization of Brazilian Citrus peels. Food Chem. 2024, 460, 140807. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K.J. The effects of tangerine peel (Citri reticulatae pericarpium) essential oils as glazing layer on freshness preserva-tion of bream (Megalobrama amblycephala) during superchilling storage. Food Control 2016, 69, 339–345. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus waste as source of bioactive compounds: Extraction and utilization in health and food industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Ping, C.Y.; Zhao, X.D.; He, C.C.; Zheng, Y.Y.; Zhang, H.B. Comparing effects of tangerine-peel (Citrus reticulata Blanco) age and concentration on deep-fried rabbit meat: Impact on heterocyclic aromatic amines, amino acids, and flavor compound formation. Food Chem. X 2024, 24, 101902. [Google Scholar] [CrossRef]
- Huang, Z.M.; Xu, Y.; Jin, M.; Jiang, Z.X.; Mo, L.; Li, M.Q.; Lou, A.H.; Liu, Y.; Xue, C.Y.; Luo, J. Synergistic effects of polymethoxyflavonoids from citrus peel extracts on harmful compound formation and flavor quality in grilled beef patties. Food Chem. 2025, 481, 144089. [Google Scholar] [CrossRef]
- Kosker, A.R. The effects of nanoemulsions based on citrus essential oils on the formation of biogenic amines in trout fillets stored at 4 ± 2°C. J. Food Saf. 2020, 40, 12762. [Google Scholar] [CrossRef]
- Wang, W.X.; Liu, K.; Dong, H.; Liao, W.Z.; Yang, X.F.; He, Q. A frontier exploration of ancient craftsmanship: Effects of various tea products in traditional Chinese cuisine “tea flavored beef”. Food Chem. 2024, 454, 139834. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Zhang, Y.L.; Zhao, Y.H.; Dong, H.; Peng, J.; He, Q. Preparation of an antimicrobial and antioxidant bio-polymer film and its application as glazing shell for postharvest quality of fresh-cut apple. Foods 2022, 11, 985. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, Y.H.; Feng, J.; Dong, H.; Liao, W.Z.; Yang, X.F.; Chen, S.; He, Q. Development of equilibrium modified atmosphere packaging (EMAP) for postharvest strawberries based on material modification of permeability and selectivity: Theoretical design, model validation, and application effects. Postharvest Biol. Technol. 2024, 211, 112799. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Yang, B.; Huang, H.H.; Dong, H.; Liao, W.Z.; Yang, X.F.; He, Q. Does the plant growth stage affect its function-ality? impact of a distinctive plant-derived marinade on the edible quality and biogenic amine safety of sashimi. Food Med. Homol. 2025, 2, 9420062. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Yang, B.; Xu, S.Y.; Liu, K.; Dong, H.; Pan, J.L.; Liao, W.Z.; Yang, X.F.; He, Q. Emulsion gels based on soy protein–pectin complexes as fat replacers: Enhancing pork sausage quality through structural and molecular insights. Int. J. Biol. Macromol. 2025, 312, 146331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Lu, J.K.; Liu, L.Z.; Sui, Z.N.; Peng, Y.Y.; Zhang, Y.; Qu, H.; Yi, J.J. Fortification effect of mixed fermentation product of Russula vinosa Lindblad supplementation on physicochemical, sensory and antioxidant properties of wheat bread. Food Med. Homol. 2025, 2, 9420071. [Google Scholar] [CrossRef]
- Hui, X.D.; Wan, Y.; Dong, H.; Peng, J.; Wu, W.L.; Yang, X.F.; He, Q. A promising insight into the inhibition of lipid oxidation, protein degradation and biogenic amine accumulation in postmortem fish: Functional glazing layers of modified bio-polymer. LWT 2023, 177, 114575. [Google Scholar] [CrossRef]
- Çelebi, Y.; Kavrut, E.; Bulut, M.; Çetintas, Y.; Tekin, A.; Hayaloglu, A.A.; Alwazeer, D. Incorporation of hydrogen-producing magnesium into minced beef meat protects the quality attributes and safety of the product during cold storage. Food Chem. 2024, 448, 139185. [Google Scholar] [CrossRef]
- Guo, H.; Bai, J.R.; Jin, X.C.; Liu, H.Y.; Wu, D.T.; Gan, R.Y.; Gao, H. Innovative edible films for food preservation: Combining pectin and flavonoids from citrus peels with soy protein isolates. LWT 2024, 214, 117102. [Google Scholar] [CrossRef]
- Gawat, M.; Boland, M.; Chen, J.M.; Singh, J.; Kaur, L. Effects of microwave processing in comparison to sous vide cooking on meat quality, protein structural changes, and in vitro digestibility. Food Chem. 2024, 434, 137442. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Agerelated-changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [PubMed]
- Chmiel, M.; Cegielka, A.; Swider, O.; Roszko, M.; Hac-Szymanczuk, E.; Adamczak, L.; Pietrzak, D.; Florowski, T.; Bryla, M.; Florowska, A. Effect of high pressure processing on biogenic amines content in skin-packed beef during storage. LWT 2023, 175, 114483. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C.L.; Huang, Q.Y.; Wang, W.X.; Dong, H.; Liao, W.Z.; Yang, X.F.; He, Q. “Chilling-induced hardening” or “chilling-induced softening”? A scientific insight on cold shock effects on strawberry phenotypic quality and pectin metabolism. Food Chem. 2025, 487, 144831. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Chen, Q.L.; Liu, Y.J.; Zhou, X.Y.; Wang, X.C. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Mahdavi, S.; Sadeghi, R.; Esfanjani, A.F.; Hedayati, S.; Shaddel, R.; Dima, C.; Malekjani, N.; Boostani, S.; Jafari, S.M. Nanodelivery systems for d-limonene, techniques and applications. Food Chem. 2022, 384, 132479. [Google Scholar] [CrossRef]
- Eduardo, L.D.; Farias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial activity and time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef]
- Jin, K.S.; Bak, M.J.; Jun, M.; Lim, H.J.; Jo, W.K.; Jeong, W.S. Alpha-pinene triggers oxidative stress and related signaling pathways in A549 and HepG2 cells. Food Sci. Biotechnol. 2010, 19, 1325–1332. [Google Scholar] [CrossRef]
- Vimal, A.; Pal, D.; Tripathi, T.; Kumar, A. Eucalyptol, sabinene and cinnamaldehyde: Potent inhibitors of salmonella target protein L-asparaginase. 3 Biotech 2017, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of themonoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Q.; Guo, Q.; Cai, Y.J.; Wang, T.; Mao, L.K.; Gao, Y.X.; Yuan, F.; Van der Meeren, P. Effect of Ultra-high temperature pro-cessing on the physicochemical properties and antibacterial activity of D-limonene emulsions stabilized by beta-lactoglobulin/Gum arabic bilayer membranes. Food Chem. 2020, 332, 127391. [Google Scholar] [CrossRef]
- de Castro, J.A.M.; Monteiro, O.S.; Coutinho, D.F.; Rodrigues, A.A.C.; da Silva, J.K.R.; Maia, J.G.S. Seasonal and circadian study of a thymol/gamma-terpinene/p-cymene type oil of Ocimum gratissimum L. and its antioxidant and antifungal effects. J. Brazil. Chem. Soc. 2019, 30, 930–938. [Google Scholar] [CrossRef]
- Wróblewska, A.; Retajczyk, M.; Kadziolka, D.; Markowska-Szczupak, A. Microbiological tests of natural limonene and the compounds obtained after isomerization of limonene in the presence of Ti-SBA-15 catalyst-α-terpinene, γ-terpinene, terpinolene, and p-cymene. J. Cosmet. Sci. 2019, 70, 137–147. [Google Scholar]
- Nupur, A.H.; Mazumder, M.A.R.; Karmokar, P.; Alim, M.A. Effectiveness of orange peel extract on the quality of minced beef during frozen storage. J. Food Process. Preserv. 2022, 46, 16659. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Tang, X.X.; Li, Z.; Wei, L.F.; Wu, X.P.; Zhao, H. Effects of lactic acid bacteria fermentation on antioxidant activity and sensory quality of Rosa sterilis S D Shi. Food Med. Homol. 2025, 2, 9420026. [Google Scholar] [CrossRef]
- Wu, N.; Pan, Y.; Liu, Q.; Shahidi, F.; Li, H.Y.; Chen, F.; Deng, Z.Y.; Zhang, Z.H. Protective benefits and mechanisms of Phyllanthus emblica Linn. on aging induced by oxidative stress: A system review. Food Med. Homol. 2025, 2, 9420029. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Elshebrawy, H.A.; Imre, K.; Morar, A.; Herman, V.; Pascalau, R.; Sallam, K.I. Antioxidant and antibacterial effect of fruit peel powders in chicken patties. Foods 2022, 11, 301. [Google Scholar] [CrossRef]
- Hui, L.C.; Zhang, C.; Yu, J.J.; Liu, M.; Yang, K.L.; Shen, L.; Hu, B.Q.; Tian, J.; Li, Y.X. Structural characterization, gelling properties, and beef preservation applications of pectin extracted from sweetpotato residue using a hydrothermal method. Int. J. Biol. Macromol. 2025, 314, 144348. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Mariño-Cortegoso, S.; Barbosa-Pereira, L.; Sendon, R.; Buonocore, G.G.; Stanzione, M.; Coelho, A.; Correia, C.B.; Saraiva, M. LDPE and PLA active food packaging incorporated with lemon by-products extract: Preparation, characterization and effectiveness to delay lipid oxidation in almonds and beef meat. Foods 2023, 12, 2450. [Google Scholar] [CrossRef]
- Bae, S.M.; Yoo, Y.; Park, J.; Kim, M.; Jeong, J.Y. Citrus peel extract powders as reducing agents for naturally cured pork sausages: Effects on cured color development. Foods 2025, 14, 1397. [Google Scholar] [CrossRef]
- Wang, W.X.; Liu, K.; Liu, C.L.; Yang, B.; Dong, H.; Liao, W.Z.; Yang, X.F.; He, Q. A modern scientific perspective on the flavor and functional properties of diverse teas in traditional cuisine “tea-flavored fish”: From macroscopic quality to microscopic variations. Food Chem. X 2024, 10, 102122. [Google Scholar] [CrossRef]
- Medeleanu, M.L.; Fa, A.C.; Coman, C.; Leopold, L.; Diaconeasa, Z.; Socaci, S.A. Citrus essential oils-Based nano-emulsions: Functional properties and potential applications. Food Chem. X 2023, 20, 100960. [Google Scholar] [CrossRef]
- He, Q.; Yang, Z.; Gong, B.; Wang, J.J.; Xiao, K.J.; Yang, S.T. Quality evaluation focusing on tissue fractal dimension and chemical changes for frozen tilapia with treatment by tangerine peel extract. Sci. Rep. 2017, 7, 42202. [Google Scholar] [CrossRef] [PubMed]
- Catia, A.S.; Marta, R.; Francisca, V.; Manuel, S. Phenazines: Natural products for microbial growth control. hLife 2024, 2, 100–112. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Tang, R.; Liu, S.; Han, S.T.; Feng, J.; Chi, K.X.; Yang, G.; Hou, X.Y.; Fang, Y.W. Effects of urolithin A producing Streptococcus thermophilus FUA329 fermentation on the composition and antioxidant bioactivities of black tea. Food Med. Homol. 2025, 2, 9420041. [Google Scholar] [CrossRef]
- Gomez-Urious, C.; Viñas-Ospino, A.; Puchades-Colera, P.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Choline chloride-based natural deep eutectic solvents for the extraction and stability of phenolic compounds, ascorbic acid, and antioxidant capacity from Citrus sinensis peel. LWT 2023, 177, 114595. [Google Scholar] [CrossRef]
- He, Q.; Gong, B.; He, J.P.; Yang, X.C.; Xiao, K.J.; Zhu, L. A novel superchilling storage - ice glazing (SS-IG) approach using biopolymer-based composite hydrogel to delay microbiological spoilage and organic oxidation of preserved tilapia. J. Sci. Food Agric. 2018, 98, 5045–5051. [Google Scholar] [CrossRef] [PubMed]




| RI | Components | %RA | Reported Bio-Activity | |||
|---|---|---|---|---|---|---|
| CR | CS | CB | CM | |||
| 937 | α-Pinene | 3.9 ± 0.2 a | 4.7 ± 1.2 a | 5.4 ± 0.7 a | 3.6 ± 1.1 a | Antimicrobial [32], antioxidant [33] |
| 974 | Sabinene | 1.6 ± 0.3 b | 3.5 ± 0.6 a | 1.1 ± 0.3 b | 0.9 ± 0.5 b | Antimicrobial [34], antioxidant [35] |
| 979 | β-Pinene | 2.4 ± 0.7 b | 1.4 ± 0.4 c | 0.6 ± 0.1 d | 7.4 ± 1.3 a | Antimicrobial [22], antioxidant [36] |
| 987 | β-Myrcene | 4.2 ± 1.1 a | 3.8 ± 1.1 a | 4.4 ± 0.6 a | 5.9 ± 0.7 a | Antioxidant [10] |
| 1030 | Limonene | 77.1 ± 2.6 a | 63.5 ± 4.1 b | 68.9 ± 4.5 b | 59.6 ± 5.2 b | Antimicrobial [37], Antioxidant [31] |
| 1059 | γ-Terpinene | 4.7 ± 0.3 c | 9.5 ± 0.6 a | 8.7 ± 0.8 ab | 7.4 ± 1.0 b | Antimicrobial, antioxidant [38] |
| 1185 | α-Terpinolene | 0.9 ± 0.6 a | 0.8 ± 0.2 b | 1.4 ± 0.3 a | 1.9 ± 0.7 a | Antimicrobial [39] |
| 1094 | Linalool | 1.4 ± 0.5 ab | 2.8 ± 1.2 a | 2.1 ± 0.7 a | 0.9 ± 0.1 b | Antimicrobial, antioxidant [10] |
| 1005 | Octanal | 1.1 ± 0.7 b | 3.0 ± 0.8 a | 3.6 ± 0.3 a | 1.8 ± 0.6 ab | Antimicrobial, antioxidant [10] |
| 1203 | Decanal | 0.9 ± 0.1 b | 1.2 ± 0.3 b | 0.7 ± 0.2 b | 3.4 ± 1.4 a | Antimicrobial, antioxidant [40] |
| Total | 98.2 ± 1.4 | 94.2 ± 4.3 | 96.9 ± 2.4 | 92.8 ± 5.0 | ||
| Activities | CR | CS | CB | CM | |
|---|---|---|---|---|---|
| Antioxidant activity (%) | DPPH | 60.8 ± 3.1 a | 49.6 ± 1.8 b | 52.1 ± 2.0 b | 43.2 ± 2.7 b |
| ABTS+ | 66.0 ± 3.6 a | 54.9 ± 2.2 b | 61.5 ± 4.9 ab | 46.4 ± 1.8 c | |
| Antimicrobial zone (mm) | S. aureus | 11.6 ± 0.8 a | 9.5 ± 0.3 b | 9.8 ± 0.5 b | 8.1 ± 0.4 c |
| L. monocytogenes | 13.4 ± 0.7 a | 10.8 ± 0.3 b | 9.9 ± 0.8 bc | 8.7 ± 0.6 c | |
| P. aeroginosa | 8.6 ± 0.6 a | 6.7 ± 0.2 b | 7.2 ± 0.6 b | 5.7 ± 0.5 c | |
| E. coli | 11.0 ± 0.2 a | 7.9 ± 0.5 bc | 8.6 ± 0.3 b | 7.1 ± 0.4 c | |
| S. typhimurium | 9.4 ± 0.5 a | 7.1 ± 0.5 bc | 7.6 ± 0.4 b | 6.5 ± 0.3 c | |
| MIC (mg/mL) | S. aureus | 4.8 ± 0.2 c | 5.7 ± 0.2 b | 5.9 ± 0.4 b | 6.9 ± 0.3 a |
| L. monocytogenes | 4.1 ± 0.3 d | 4.9 ± 0.4 c | 5.7 ± 0.1 b | 6.3 ± 0.3 a | |
| P. aeroginosa | 7.9 ± 0.5 c | 9.5 ± 0.5 b | 8.9 ± 0.6 bc | 12.1 ± 0.8 a | |
| E. coli | 5.1 ± 0.4 c | 5.5 ± 0.2 c | 6.4 ± 0.3 b | 8.6 ± 0.6 a | |
| S. typhimurium | 8.3 ± 0.6 c | 9.7 ± 0.5 b | 9.0 ± 0.5 bc | 11.4 ± 0.8 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Xu, S.; Liu, X.; Wang, W.; Liao, W.; Yang, X.; He, Q. Preservation of Beef with Limonene-Rich Citrus Peel Extracts: Antioxidant, Antimicrobial and Textural Benefits. Foods 2025, 14, 3506. https://doi.org/10.3390/foods14203506
Liu C, Xu S, Liu X, Wang W, Liao W, Yang X, He Q. Preservation of Beef with Limonene-Rich Citrus Peel Extracts: Antioxidant, Antimicrobial and Textural Benefits. Foods. 2025; 14(20):3506. https://doi.org/10.3390/foods14203506
Chicago/Turabian StyleLiu, Chunlong, Shiyang Xu, Xiuping Liu, Wenxia Wang, Wenzhen Liao, Xingfen Yang, and Qi He. 2025. "Preservation of Beef with Limonene-Rich Citrus Peel Extracts: Antioxidant, Antimicrobial and Textural Benefits" Foods 14, no. 20: 3506. https://doi.org/10.3390/foods14203506
APA StyleLiu, C., Xu, S., Liu, X., Wang, W., Liao, W., Yang, X., & He, Q. (2025). Preservation of Beef with Limonene-Rich Citrus Peel Extracts: Antioxidant, Antimicrobial and Textural Benefits. Foods, 14(20), 3506. https://doi.org/10.3390/foods14203506





