1. Introduction
Plastic food packaging materials readily release microplastics (MPs) from packaging into foods, contributing to human exposure to MPs [
1]. MPs are of particular concern due to their long-term durability in the environment and great potential for releasing plastic oligomers, additives, and chemicals and their vector capacity for adsorbing or collecting other pollutants [
2]. Once introduced into aqueous or biological environments, MPs readily acquire surface-bound biomolecular layers, so-called “protein coronas” comprising loosely bound (soft) and tightly adsorbed (hard) proteins. These coronas may alter particle size, surface chemistry, and biological reactivity, complicating downstream analysis and influencing toxicological outcomes [
3]. A mechanistic understanding of protein–MP interactions is therefore essential for assessing MP behavior in complex biological matrices and for developing effective protocols to isolate or characterize MPs in digested food, serum, or tissue samples.
Recent studies have begun to explore how MPs behave in simulated digestive environments, with a particular focus on the intestinal phase. For instance, Stock et al. [
3] demonstrated that MPs incubated in simulated intestinal fluids formed stable bio-coronas composed of bile salts and digestive enzymes, significantly altering their colloidal stability and cellular uptake. Similarly, Schöpfer et al. [
4] found that protein adsorption onto MPs persisted during digestion, supporting the presence of resilient hard coronas even under harsh enzymatic conditions and influencing subsequent analytical outcomes, including particle detection and interaction profiling. In biological matrices, such as digested food or serum, MP coronas can interfere with size characterization, compositional analysis, and surface reactivity assessments [
3,
4]. Such protein coatings can persist even after exposure to digestive enzymes, forming so-called “hard coronas” that resist removal and may bias analytical detection and toxicological interpretation. These findings reinforce the importance of understanding corona stability under gastrointestinal conditions, especially when designing effective decontamination protocols for analytical or toxicological studies.
Among the various types of MPs, polyethylene terephthalate (PET) is frequently found in food packaging, bottled water, and environmental samples [
5]. Its relatively hydrophobic and chemically stable surface makes it prone to interacting with proteins such as bovine serum albumin (BSA), especially under physiologically relevant conditions like digestion. BSA is widely used as a model protein in studies of MP corona formation due to its well-characterized structure, amphiphilic properties, and relevance in mimicking biological fluid composition [
6]. BSA shares structural and surface interaction characteristics with human serum albumin and digestive enzymes, making it a representative surrogate for protein adsorption studies [
7]. Its ability to form stable hard coronas on hydrophobic surfaces allows for a controlled evaluation of adsorption mechanisms and removal strategies under physiologically relevant conditions. Furthermore, BSA’s widespread availability and stability under varied pHs and temperatures make it ideal for an in vitro simulation of gastrointestinal exposure [
7]. The PET backbone is composed of terephthalic acid and ethylene glycol moieties linked via an ester bond, making it susceptible to degradation by hydrolytic enzymes that have been discovered in both bacteria and eukaryotes. Conversely, no functional enzymes have been found to degrade other plastic materials containing a hydrocarbon backbone, such as polypropylene and polystyrene [
8]. Furthermore, these enzymes share a common mechanism of action, a catalytic triad consisting of serine, histidine, and a negatively charged amino acid [
9], which is also found in pancreatic lipase and serine proteases [
10], making PET an interesting candidate for studying the effects of gastrointestinal digestion.
The exposure of PET MPs to simulated digestive fluids can lead to measurable, although generally minor, spectral changes detectable by FTIR and Raman spectroscopy. These changes may include shifts or broadening in characteristic PET bands, such as the ester carbonyl stretch (~1715–1725 cm
−1) and benzene ring modes (~1408–1450 cm
−1), attributed to plastic–protein or plastic–lipid interactions in the intestinal environment [
3,
4]. Raman spectroscopy can also reveal reduced peak intensity or subtle shifts due to surface adsorption or mild oxidative changes [
11]. Nevertheless, the core polymeric structure of PET generally remains intact, suggesting that gastrointestinal digestion does not induce significant degradation.
Recent advances in the clean-up of protein coronas on microplastics under simulated gastrointestinal conditions have increasingly focused on combining chemical and biological strategies. Proteolytic enzymes such as trypsin and proteinase K have been reported to selectively degrade adsorbed proteins [
12], while oxidative approaches including hydrogen peroxide, Fenton reactions, and photocatalysis improve the removal of more persistent corona layers [
13]. Protocols based on surfactants remain widely used, but their efficiency can be enhanced when applied together with enzymatic or alkaline treatments, thus minimizing residual protein coverage while preserving polymer integrity [
14,
15]. Collectively, these developments highlight the need for tailored protocols that address the complexity of corona formation in simulated intestinal fluid and ensure the reliable downstream spectroscopic identification of polymers. In this study, we use fluorescently labeled BSA to quantify removal efficiency after corona formation on PET MPs in simulated intestinal fluid conditions. By comparing three clean-up protocols—ionic detergent and hydrogen peroxide, hydrogen peroxide oxidation, and hydrogen peroxide combined with an alkali—we assessed both removal efficiency and the mechanisms of protein–polymer interaction in conditions simulating intestinal fluid. Quantification by fluorescence, protein analysis by SDS-PAGE, and structural confirmation via FTIR provide an integrated view of corona stability and clean-up effectiveness. Furthermore, we confirmed that layers of hard bound protein corona do not interfere with downstream characterization nor affect polymer identification and characterization. Additionally, relevant conditions were applied to investigate the removal efficiency of trypsin, chymotrypsin, and lipase coronas, as well as the effect of these digestive enzymes on the spectral changes in PET.
2. Materials and Methods
2.1. Materials
Polyethylene terephthalate (PET) microplastics (MPs) (<80 µm) were prepared at the University of Vienna. Powders of food-grade PET (CAS:25038-59-9) were produced using a ZM 200 ultra-centrifugal mill (Retsch GmbH, Haan, Germany) with an 80 μm ring sieve with trapezoid holes (03.647.0465) and cyclone accessories. MP fractions <80 µm were collected and kept at 4 °C as a dry powder. The characteristics of the obtained PET MPs were described in detail by Lujic et al. [
16].
Potassium hydroxide (KOH), sodium dodecyl sulfate (SDS), hydrogen peroxide (H2O2, 30%), and absolute ethanol (EtOH, HPLC grade, Merck, Darmstadt, Germany) were of analytical grade and used in three clean-up protocols. Bovine serum albumin (BSA) (cat. no. A7906), lipase from porcine pancreas (cat. no. L3126-100G), and chymotrypsin (cat. no. C4129) were obtained from Sigma Aldrich (St. Louis, MI, USA) and used for the preparation of the PET MP protein’s corona. Before preparing the MP-BSA corona, BSA was conjugated with AlexaFluor488 (AF488) fluorescent dye (BSA-AF488) using an AF488 protein labeling kit (Invitrogen, Molecular Probes, Inc., Eugene, OR, USA) according to the manufacturer’s instructions. Briefly, 75 µL amino-reactive dye AF488 solution in dimethyl sulfoxide (10 mg/mL) was slowly added in 1 mL BSA solution (conc. 10 mg/mL in 0.1 M sodium bicarbonate buffer pH 8.3). After 1 h incubation at room temperature (RT), BSA-AF488 was separated from unbound AF488 by gel filtration using Sephadex G-25 (Sigma Aldrich, St. Louis, MI, USA). After chromatography, BSA-AF488 protein concentration was determined using Bicinchoninic acid (BCA) Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Enzyme solutions were freshly prepared following the procedures described below.
A PVDF membrane filter, 0.22 μm, (Millipore, Merck, Darmstadt, Germany) and stainless steel filter with a pore size of 10 μm (Xinmingde Machinery, Xinxiang, China) were used for filtration.
Ultrapure water (Barnstead Smart2Pure Water Purification System, Thermo Fisher Scientific, Waltham, MA, USA) was used for all experiments. Before use, all working solutions (distilled water, 10% KOH, and 50% ethanol) were filtered through a 0.22 μm PVDF membrane filter.
2.2. Preparation of PET MP BSA-AF488 Hard Corona
To evaluate the efficiency of three different clean-up protocols for removing hard corona from the surface of PET MPs by measuring residual fluorescence, a model system of the hard corona was prepared by incubating PET MPs with BSA-AF488. Briefly, 10 mg of PET MPs (˂80 µm) was mixed with 500 µL of BSA-AF488 working solution (1 mg/mL in simulated intestinal fluid, SIF) in a 2 mL glass vial (Thermo Fisher Scientific, Waltham, MA, USA). The working BSA-AF488 solution was prepared by diluting BSA-AF488 stock solution (5.7 mg/mL) with SIF, which was prepared according to Minekus et al. [
17]. The protein/MP ratio in the mixture was 1:20 (
w/
w). The reaction mixture was continuously mixed on a rotator (Multi Bio RS-24 Multi-rotator, Biosan, Riga, Latvia) at 37 °C for 4 h. After that, MPs were separated from the supernatant (bulk BSA-AF488 solution) by centrifugation at 1000 rpm for 5 min (Centrifuge 5804 R, Eppendorf, Hamburg, Germany). The pellet of MPs, from which the supernatant was removed, was washed three times with distilled water (1 mL each time) to remove any soft corona from the MPs. For each wash, the pellet was vortexed for 30 s, incubated for 5 min in water, and centrifuged for 2 min at 1000 rpm. After removing the water, the pellet of PET MPs with the formed BSA-AF488 hard corona was subjected to different clean-up protocols.
In this experiment, eight experimental replicates of PET MPs with the formed BSA-AF488 hard corona were prepared. Six replicates were used for three different clean-up protocols (each protocol was repeated in duplicate), and two replicates were used as positive controls (100% fluorescence). Additionally, six experimental replicates of PET MPs incubated with SIF without BSA-AF488 were prepared as negative controls (0% fluorescence) and further subjected to the three different clean-up protocols (each protocol was repeated in duplicate).
2.3. Clean-Up Protocols for Removing Adsorbed BSA-AF488 Hard Corona from PET MPs
Three different approaches to the removal of the hard corona of BSA-AF488 from PET MPs were evaluated: the combination of ionic detergent (SDS) with H
2O
2 oxidation (protocol I), only oxidation with H
2O
2 (protocol II), and the combination of H
2O
2 oxidation with an alkali (KOH) (protocol III). Each protocol was performed with two prepared experimental replicates of PET MPs with the formed BSA-AF488 hard corona and negative controls, described in
Section 2.2.
2.3.1. Clean-Up Protocol I: The Combination of IONIC Detergent (SDS) and H2O2 Oxidation
Pellets of PET MPs with the formed BSA-AF488 hard corona that remained in a glass vial were first washed twice with 10% SDS (1 mL each time). Each time, the mixture was continuously mixed on a rotator (Multi Bio RS-24 Multi-rotator, Biosan, Riga, Latvia) at RT for 20 min. After that, MPs were separated from the supernatant by centrifugation at 1000 rpm for 5 min (Centrifuge 5804 R, Eppendorf, Hamburg, Germany). In the next step, MP pellets were washed twice with 1 mL of water. Each time, the pellet was vortexed for 30 s and centrifuged for 2 min at 1000 rpm. After carefully removing water, PET MPs were transferred from the glass vials using a glass pipette onto a vacuum filtration system equipped with a stainless steel filter (mesh size 10 μm, 25 mm diameter) (Xinmingde Machinery, Xinxiang, China). For the transfer of MPs and their further SDS rinsing, 50 mL of water was used. The filter containing PET MPs was transferred into glass beakers, and 30 mL of 15% H2O2 solution was added. After 30 min of incubation at RT, the samples were sonicated for 1 min in an Elmasonic P30 H ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany). Finally, the PET MPs were transferred from the beaker onto a new stainless steel filter.
2.3.2. Clean-Up Protocol II: Oxidation with H2O2
Pellets of PET MPs with the formed BSA-AF488 hard corona remaining in the glass vials were carefully transferred into beakers using a glass pipette and 15 mL of water. After that, 15 mL of 30% H2O2 was added (the final concentration of H2O2 was 15%), and the reaction mixture was first incubated for 1 h at RT and then sonicated for 1 min in an Elmasonic P30 H ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany). PET MPs from the beakers were transferred onto 10 µm stainless steel filters by filtration using a vacuum filtration system. The filters with the PET MPs were returned to the same beakers and covered with 30 mL of 15% H2O2 and incubated for 1 h at RT. After the sonication of the samples for 1 min in the ultrasonic bath, PET MPs were finally transferred from the beakers onto new stainless steel filters.
2.3.3. Clean-Up Protocol III: The Combination of H2O2 Oxidation with KOH Digestion
Pellets of PET MPs with the formed BSA-AF488 hard corona remaining in the glass vials were first treated with H2O2 using the same starting steps described in protocol II. When PET MPs were transferred onto 10 µm stainless steel filters after incubation in the presence of 15% H2O2 for 1 h at RT and sonication for 1 min, they were returned to the same beaker and covered with 30 mL of 10% KOH. After sonication for 5 min in the ultrasonic bath, the reaction mixture was incubated for 24 h at RT and transferred from the beaker onto new stainless steel filters.
The final step in all three protocols, as well as in the positive control, consisted of rinsing PET MPs three times with 30 mL of water, followed by a single rinse with 30 mL of 50% ethanol. Before subsequent analyses, the filters containing the MPs were placed in a Petri dish and dried at room temperature (RT) in the dark.
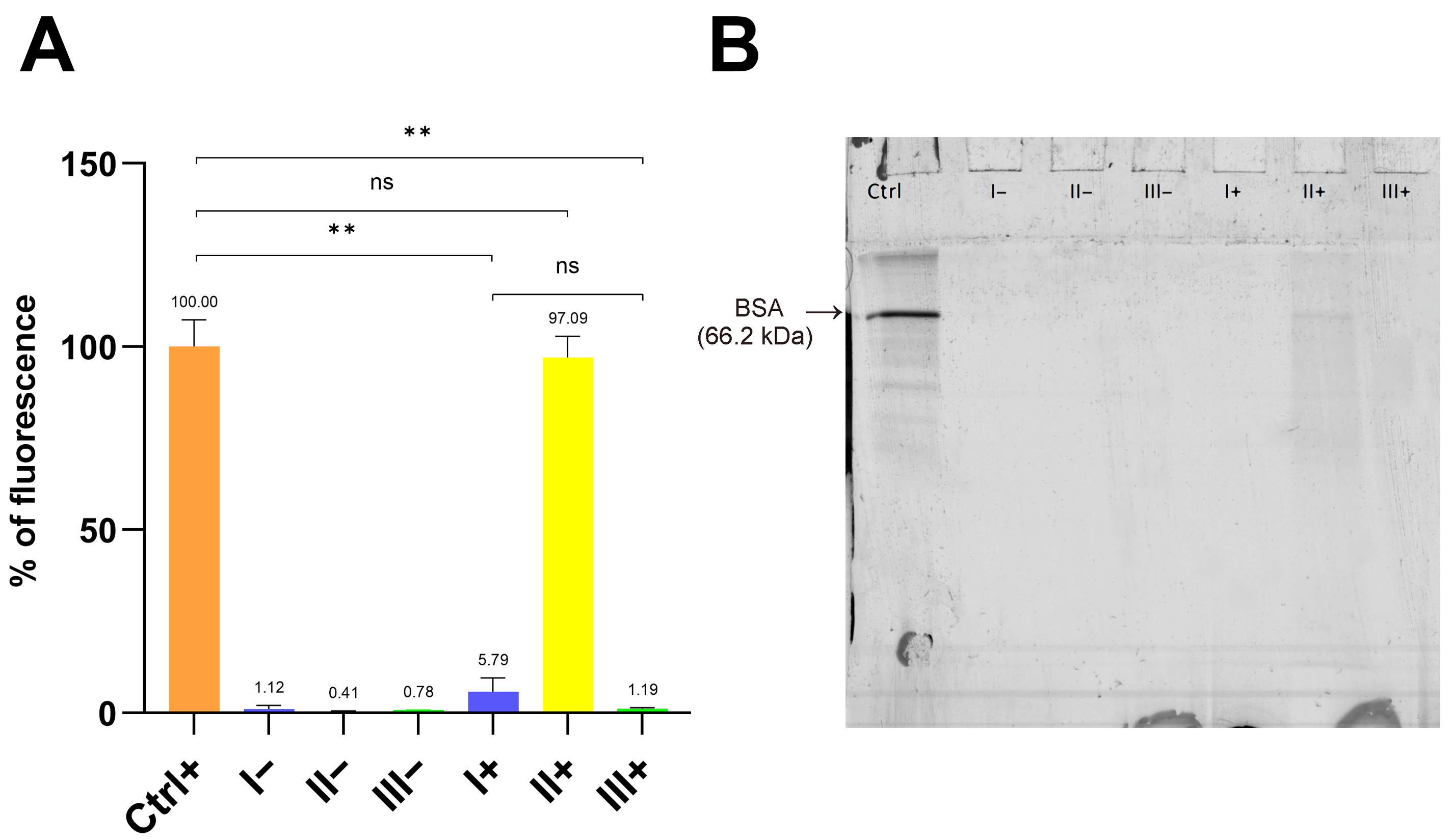
2.4. Quantification of Residual Fluorescence on PET MPs After Clean-Up Protocols
The clean-up efficiency of each protocol was assessed by the measurement of residual fluorescence on PET MPs using the Synergy LX multi-mode reader (BioTek Instruments; Winooski, VT, USA). Briefly, 2–3 mg of dried PET MP particles from each stainless steel filter was first weighed on a piece of aluminum foil using an analytical balance. In the next step, MP particles were quantitatively transferred using a spatula into the wells of a microtiter plate for fluorescence measurement (Sarstedt, Germany) and suspended in 150 µL of water. Residual fluorescence in the prepared samples and positive and negative controls was measured using a green filter (excitation: 485/20; emission: 528/20) on the Synergy LX multi-mode reader. The fluorescence intensity was first normalized to the mass of the PET MP particles weighed into microtiter wells. Residual fluorescence was calculated according to the following equation: Residual fluorescence of sample (%) = fluorescence intensity of sample × 100/fluorescence intensity of positive control. The positive control (PET MPs with BSA-AF488 hard corona), not subject to clean-up protocols, was considered as the maximal fluorescence (100% intensity). The results were expressed as the mean value ± SD of two measurements for all samples and controls (positive and negative).
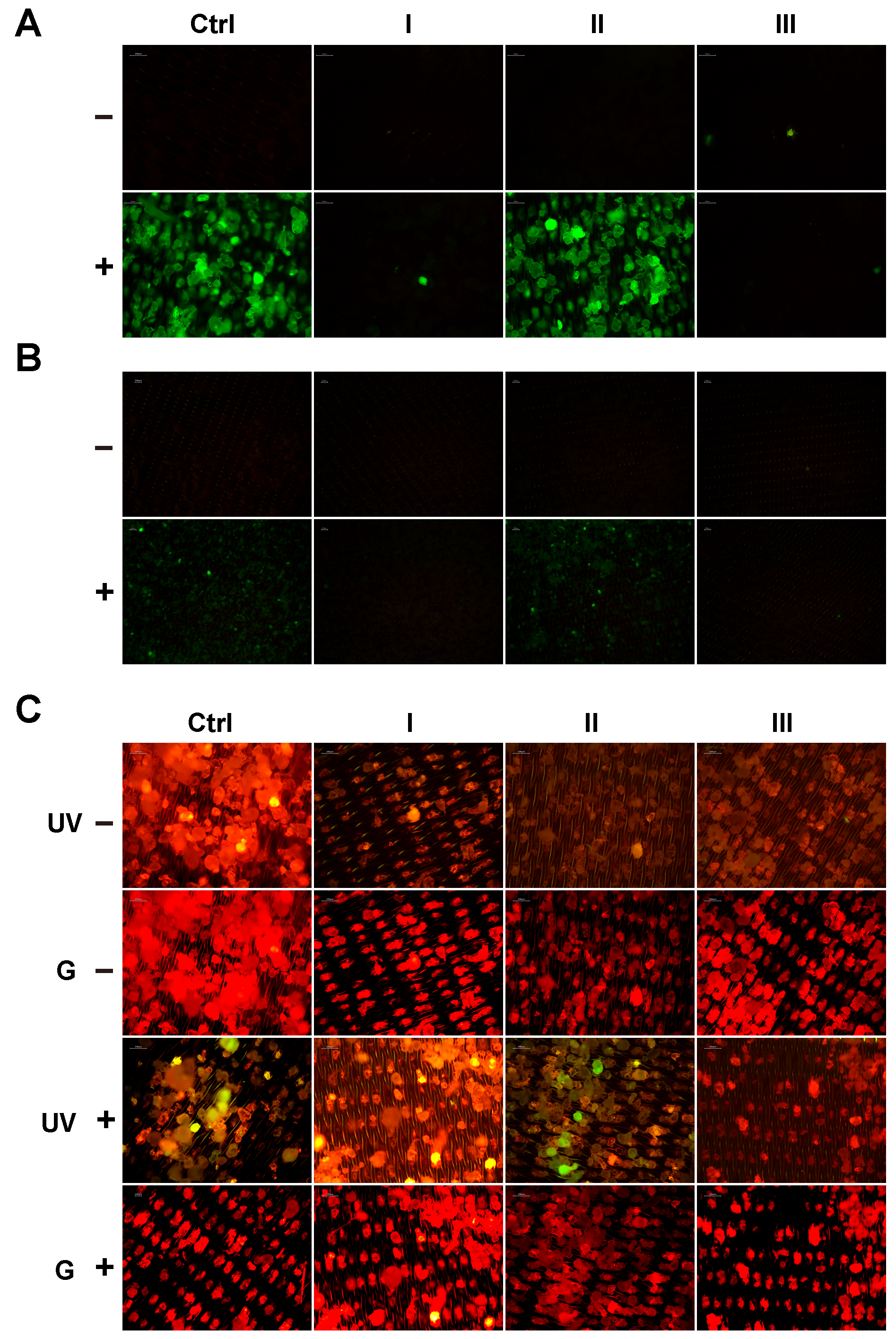
2.5. Qualitative Analysis of Residual Fluorescence on PET MPs After Clean-Up Protocols
The remaining fluorescence on the PET MPs was also checked qualitatively using reducing SDS polyacrylamide gel electrophoresis (PAGE) followed by fluorescence gel imaging using a Typhoon FLA 7000 imager (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), and images of PET MP particles were taken using an A16.2701 fluorescent microscope (Opto-Edu, Beijing, China).
2.5.1. SDS PAGE and Fluorescence Gel Imaging
After the measurement of residual fluorescence, the suspensions of PET MP particles in the microtiter plate wells were used for the preparation of samples for reducing SDS PAGE. Briefly, the PET MP suspension was quantitatively transferred into 1.5 mL plastic tubes (Eppendorf, Hamburg, Germany) using an automatic pipette, and 37 µL of 5× concentrated reducing buffer for samples was added. The samples were heated at 95 °C for 5 min. A total of 30 µL of each sample (vortexed and centrifugated 5 min 13,000× g, Eppendorf, Hamburg, Germany) was loaded into the wells of a 14% gel. After electrophoresis using the Mini-PROTEAN® system (Bio-Rad, Hercules, CA, USA), the gel was scanned using a Typhoon FLA 7000 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) imager.
2.5.2. Fluorescent Microscopy
After the measurement of dried PET MP particles for the quantification of residual fluorescence, the remaining samples on the filters were used for fluorescence microscopy. One set of MP samples was used for additional staining with Nile red. Briefly, a working solution of Nile red (0.02 mg/mL) was prepared by diluting the stock solution (0.4 mg/mL in acetone) with 50% ethanol. Stainless steel filters containing PET MPs were placed in Petri dishes, and 120 µL of the Nile red working solution was added to cover the samples. After 10 min at RT, an additional 120 µL of the Nile red working solution was added and left for another 10 min. After that, the filters were transferred to a vacuum filtration system, rinsed with 8 mL of 50% ethanol, and left to dry in Petri dishes. Entire filters containing the PET MPs with or without additional staining with Nile red were placed between two microscope slides. To ensure stability during imaging, and the potential loss of MPs, the prepared sandwich was secured with tape on two sides. Each filter was first observed with 4× magnification using a fluorescence microscope (A16.2701, OptoEdu, Beijing, China) and later with 10× magnification. Several microscopic fields of view were captured per sample for each magnification using Image View version 3.7 microscope camera software (Beijing, China) after excitation using UV (340–380 nm) and green (527.5–552.5 nm) excitation filters. All obtained images were not further processed.
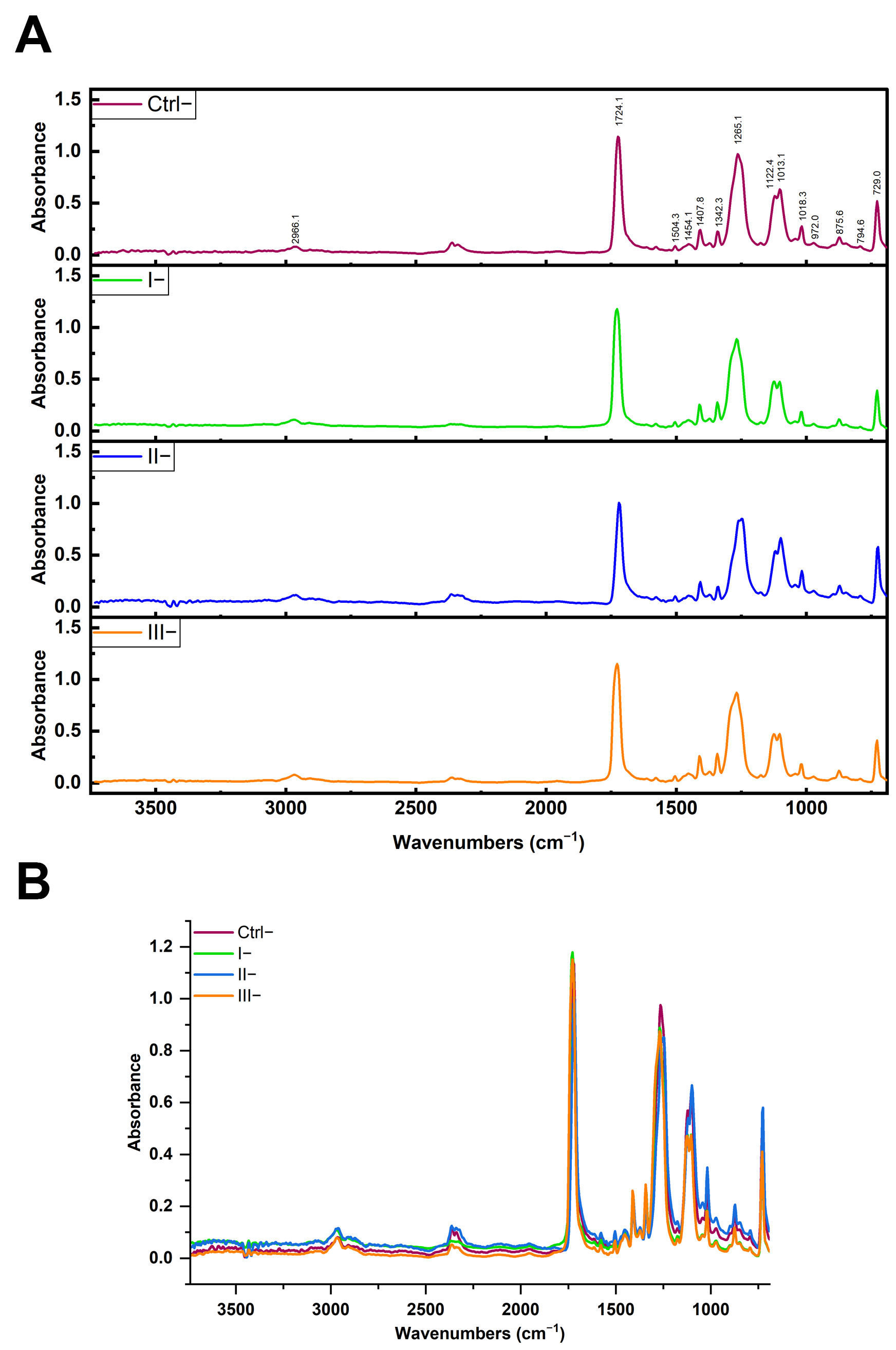
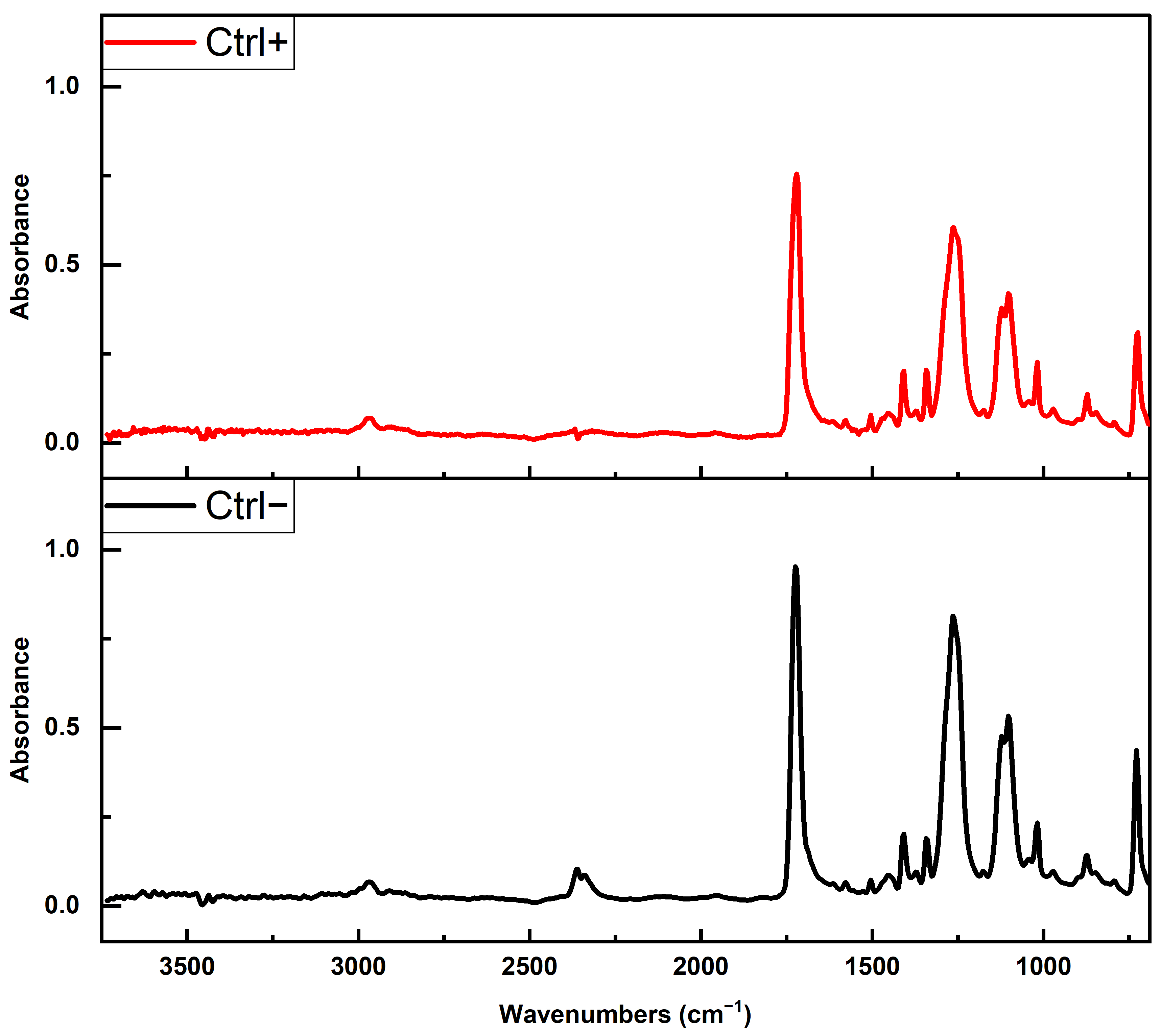
2.6. ATR FTIR Spectroscopy Analysis of Chemical and Morphological Characteristics of PET MPs
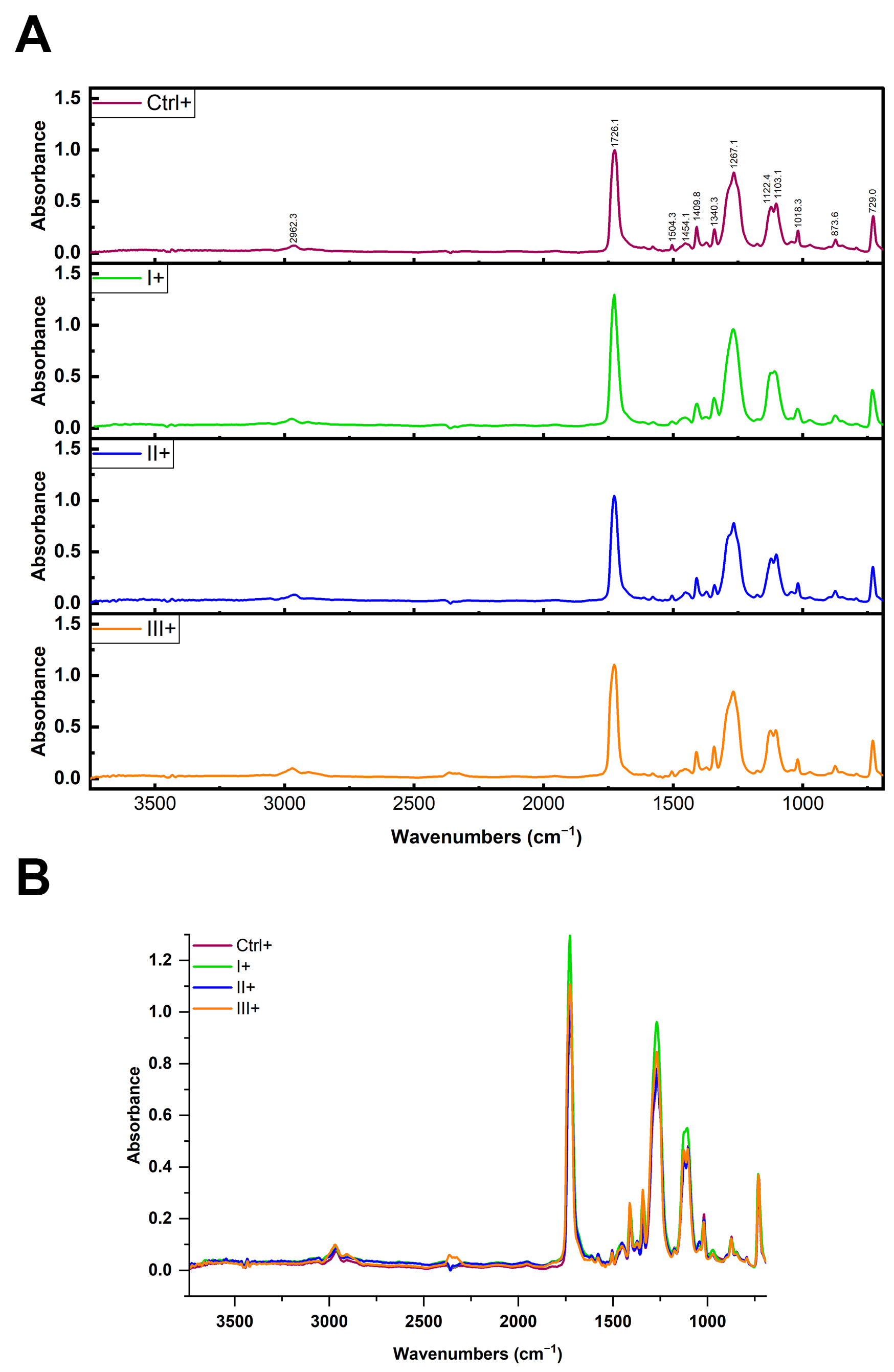
To investigate whether the reagents used in clean-up protocols or formed protein coronas altered the chemical composition and morphological characteristics of MPs and influenced the accurate identification of MP type, the FTIR spectra of PET MPs incubated with or without BSA-AF488 or digestive enzymes after clean-up protocols were compared with naïve PET spectra or with the corresponding spectrum of the control. FTIR spectroscopy was performed using two instruments. Clean-up protocol evaluation was conducted on a Thermo Scientific Nicolet iN10 instrument (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an ATR accessory (Ge crystal) and a liquid nitrogen-cooled MCT detector. Spectra were collected in the 4000–650 cm−1 range at 4 cm−1 resolution. PET MPs incubated with digestive enzymes were analyzed on a Nicolet Summit with an Everest Diamond ATR, collecting spectra in the 4000–600 cm−1 range at 2 cm−1 resolution. Data acquisition and processing (normalization and subtraction of spectra) were performed using OMNIC Specta software version 2.2.155 (Thermo Fisher Scientific, Waltham, MA, USA). All spectra were averaged and normalized to a peak at 1408 cm−1 prior to spectral overlay or subtraction. The average matching rates (%) between the FTIR spectra obtained for untreated PET particles (native PET MPs) and after their exposure to different clean-up protocols were determined. The chemical identities (% matching) of the FTIR spectra of PET MP samples treated with different clean-up protocols and positive and negative controls with spectra libraries were determined.
2.7. Corona Formation on PET MPs with Intestinal Enzymes
To check the influence of intestinal enzymes (chymotrypsin and lipase) on PET MP integrity during the prolonged exposure of plastic to these hydrolytic enzymes, as well as the efficiency of the developed clean-up protocol for the removal of the protein corona, PET MPs were incubated with different concentrations/activities of enzymes in SIF for 24 or 72 h at 37 °C. For this experiment, SIF was prepared according to Minekus et al. [
17] with slight modification. NaHCO
3 was replaced with an equimolar concentration of NaCl to keep the pH more stable during incubation. Physiological enzyme activity was also evaluated based on the same paper [
17]. Furthermore, to prevent microbial growth, 0.01% of NaN
3 was added to SIF for the incubation of lipase for 72 h.
PET MPs were treated with chymotrypsin mimicking a physiological enzyme activity level of 25 IU/mL (one unit hydrolyzes 1 µmole of N-benzoyl-L-tyrosine ethyl ester per minute at pH 7.8 at 25 °C) and incubated for 24 h. To stop digestion after incubation, PMSF to a final concentration of 4.76 mM was added to the samples. In another experiment, PET MPs were exposed to different activity levels of intestinal lipase. MPs were treated with an activity level of lipase of 930 IU/mL (one unit releases 1 µmol of butyric acid per minute at 37 °C and pH 8) for 24 h and a much lower activity level of 75.6 IU/mL for 72 h. This is lower than the proposed physiological lipase activity level for adults, which is 2000 IU/mL [
17], but is close to the lipase activity level in infants [
18]. Digestion was stopped by either adjusting the pH to 5 when the sample is incubated for 24 h or with the addition of Orlistat to a final concentration of 50 µM. As a control for all experiments, PET MPs were incubated in SIF for the appropriate amount of time. In all experiments, the ratio of MPs to liquid was 1:50.
2.8. Statistic
Unpaired t-tests were used to compare residual fluorescence intensities after the clean-up protocols, using GraphPad Prism 10.4.2 (GraphPad Software, Boston, MA, USA). Clean-up experiments were performed in duplicate, and data are presented as the mean ± standard deviation (SD). A p value of less than 0.05 was considered statistically significant.
4. Discussion
The results of this study provide insights into the mechanistic underpinnings of protein–microplastic (MP) interactions and the stability of the hard protein coronas formed in simulated intestinal fluid conditions. Using BSA as a model protein and PET MPs under intestinally simulated conditions, we demonstrated that corona formation is not only robust but also resistant to disruption by oxidative treatment alone. These findings underscore the complexity of protein adsorption mechanisms on MP surfaces and the importance of selecting appropriate decontamination strategies in analytical workflows.
Our previous study showed that oxidative treatment with 15% H
2O
2 was less effective than digestion with 10% KOH for removing organic matter during the isolation of MPs from seafood samples [
34]. The development of new protocols that enable the investigation of eco- and bio-coronas of various types of MPs is essential for a better understanding of the interactions between coronas and MPs. Because the SDS/H
2O
2 protocol is less time-consuming and uses less aggressive reagents, it may be suitable for the rapid removal of hard coronas from various types of MPs. Given that environmental plastic pollution is one of the most critical environmental and toxicological issues [
37,
38], identifying natural resources capable of degrading plastics and elucidating the toxicity of MPs coronas are still major challenges for scientists. Currently, the methods used for the isolation of MPs from environmental and biological samples often involve prolonged exposure to aggressive reagents such as an acid or alkali, which can lead to the loss of eco- and bio-coronas but also the plastic itself [
39]. This may lead to a misinterpretation of the results concerning plastic integrity preservation, particularly when the primary aim of the investigation is to assess the influence of eco- and bio-coronas on plastic integrity.
In the current study, we observed no measurable changes to PET’s key FTIR spectral markers following treatment, indicating that none of the protocols, oxidative, alkaline, or surfactant-based, compromised polymer integrity. In our spectra, all PET samples maintained these distinct peaks regardless of treatment, indicating that no significant polymer chain cleavage or oxidation occurred. This confirms that none of the clean-up protocols, including the SDS/H2O2 or H2O2/KOH combination, adversely affected the chemical structure of PET MPs.
Among the tested protocols, the combination of SDS/H2O2 or H2O2/KOH achieved the most effective removal of the protein corona. This observation suggests that corona stability is maintained by a combination of hydrophobic and ionic interactions that require both surfactant-mediated disruption and alkaline hydrolysis for complete dissociation. The poor performance of hydrogen peroxide treatment implies that oxidative cleavage, valuable for general organic digestion, may not sufficiently disrupt the cohesive forces anchoring the hard corona to the PET surface.
SDS-PAGE and fluorescence quantification both supported the conclusion that significant fractions of BSA remained bound following only oxidative treatment. Moreover, FTIR analysis confirmed that none of the clean-up protocols visibly degraded the PET polymer, validating their use for subsequent spectroscopic analyses. Several recent studies have addressed the challenge of organic matter removal from MP surfaces prior to FTIR spectroscopy, particularly in cases of studying fine structural changes during the biodegradation of plastic [
30]. Furthermore, residual proteins remaining on the plastic may obscure or mimic spectral shifts occurring during biotransformation.
Therefore, it was particularly relevant to observe that even the partial removal of the protein corona (such as using only hydrogen peroxide) did not cause significant changes in the FTIR spectra of PET. Even though a layer of hard corona protein remained bound to the MP, it did not contribute to the signal recording by FTIR.
A growing body of work has also examined the behavior of MPs in digestive environments, particularly within intestinal fluids. Recent studies show that MPs incubated in simulated intestinal fluids form stable bio-coronas composed of bile salts and digestive enzymes, altering their colloidal stability and cellular uptake [
3,
40]. Similarly, Brower et al. reported that MPs exposed to intestinal environments exhibit protein adsorption that persists despite digestion, supporting the notion of resilient hard corona formation [
41].
Similarly, protein corona formation on polystyrene nanoparticles has been investigated under gastrointestinal conditions, revealing that digestive proteins can form resilient complexes that modulate particle bioactivity [
42]. Other authors used molecular modeling to show that plastic–protein interactions are dominated by hydrophobic forces, supporting our interpretation of hydrogen peroxide’s partial efficacy and the enhanced disruption achieved by SDS + H
2O
2 or H
2O
2 + KOH [
43].
Therefore, our data contributes not only to methodological refinement but also to a broader understanding of corona dynamics in real-world matrices, where hard protein coronas are known to form irreversible complexes that modulate surface properties and bioactivity. In the context of MPs, such persistent coronas may lead to the underestimation of particle numbers, misidentification of polymer types, or misinterpretation of surface properties in environmental and toxicological studies.
These results emphasize that the effective clean-up of MP-associated biomolecules requires a mechanistic understanding of corona formation and persistence. The SDS/H2O2 or H2O2/KOH protocol offers a practical, polymer-compatible strategy for improving MP analysis, particularly in biological matrices where protein adsorption can obscure detection and characterization. This work also contributes to broader efforts in standardizing MP isolation methods and deepens our understanding of MP–biomolecule interactions in realistic exposure scenarios.
The use of a single protein for protocol efficacy assessment gave us valuable information on the limitations of our detection method, e.g., the potential interference of the residual protein corona on the detection of subtle peak shifts. However, complex mixtures require further evaluation, as multiple proteins exhibit different affinities towards MPs and compete for a place in the hard corona [
20]. This is further complicated when all components of the digestive tract are taken into account, such as bile salts and the microbiome.
Prior studies on the gastrointestinal transformation of PET assessed by micro-Raman have investigated pancreatin digestion (a mixture of hydrolytic enzymes and bile acids) and also looked into microbiome transformations. The conditions tested were considered physiological (2 h incubation in the intestinal fluid) [
11]. Subtle morphological changes were observed with intestinal conditions (pancreatin), and they were more dramatic with microbial (colon) transformation. Our study did not apply physiological conditions but applied longer incubation times with individual enzymes to pinpoint the hydrolytic enzymes that may be responsible for mild PET MP surface modifications among pancreatin components observed by Tamargo et al. [
11].
Overall, the FTIR spectral analysis reveals enzyme-specific interactions with PET MPs, with lipase exhibiting the most pronounced effects. Together with cutinase and hydrolase, lipase is one of the common enzymes associated with plastic degradation. Lipases have been produced in many bacterial and fungal strains. Lipase is one of the best biocatalysts for PET degradation. Lipase B (CALB) from the yeast
Candida antarctica is known for its high selectivity and catalytic activity [
44]. CALB demonstrated high-efficiency hydrolysis steps and polymer scission that led to the accumulation of terephthalic acid [
45].
Intestinal lipase, a component of pancreatin, appears to induce the local hydrolysis of ester bonds, creating structural irregularities and disrupting crystalline regions, hence amorphization. This process increases polymer surface area and accessibility, reduces physical stability, and may precede or accompany biodegradation by the microbiome in the colon. The increased amorphization of PET may be a consequence of the plasticizing effect of polar solvent penetration into PET MP, which breaks hydrogen bonds and disrupts crystallinity. Any changes in crystallinity occurring because of MP exposure to enzymatic action (esterase, i.e., cutinase, lipase, PETase) occurring internally may be considered as internal aging or the weathering of the MP. This process is subtle but can facilitate the further action of the microbiome on the MP in the colon by providing more anchoring points for biofilm formation.