Ultrasound-Assisted Extraction Followed by Inductively Coupled Plasma Mass Spectrometry and Multivariate Profiling of Rare Earth Elements in Coffee
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Samples Digestion and Instrument Analysis
2.3. Analytical Method Validation
2.4. Multivariate Statistics
2.5. Health Risks from REE in Coffee
3. Results and Discussion
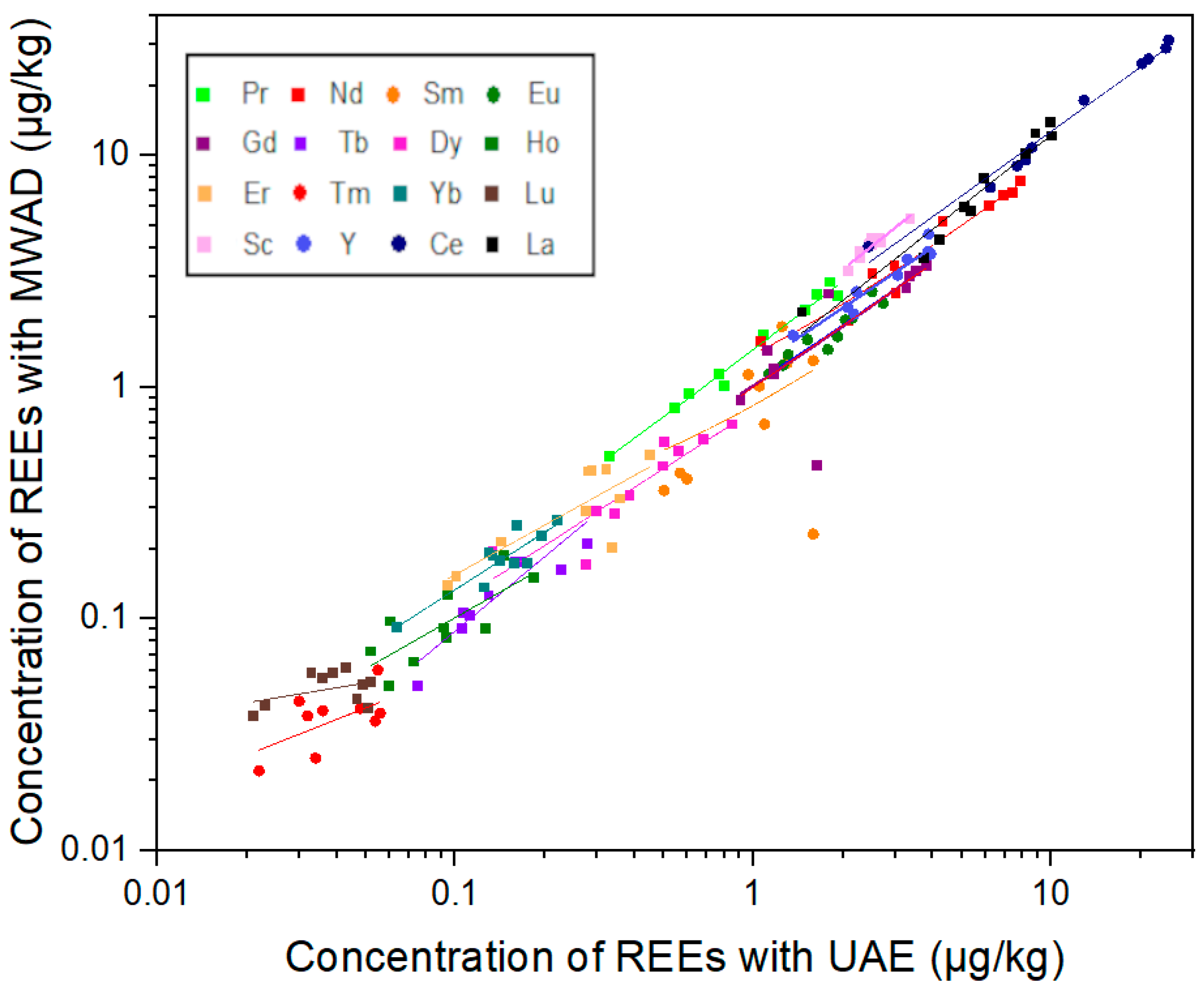
3.1. Method Development and Validation
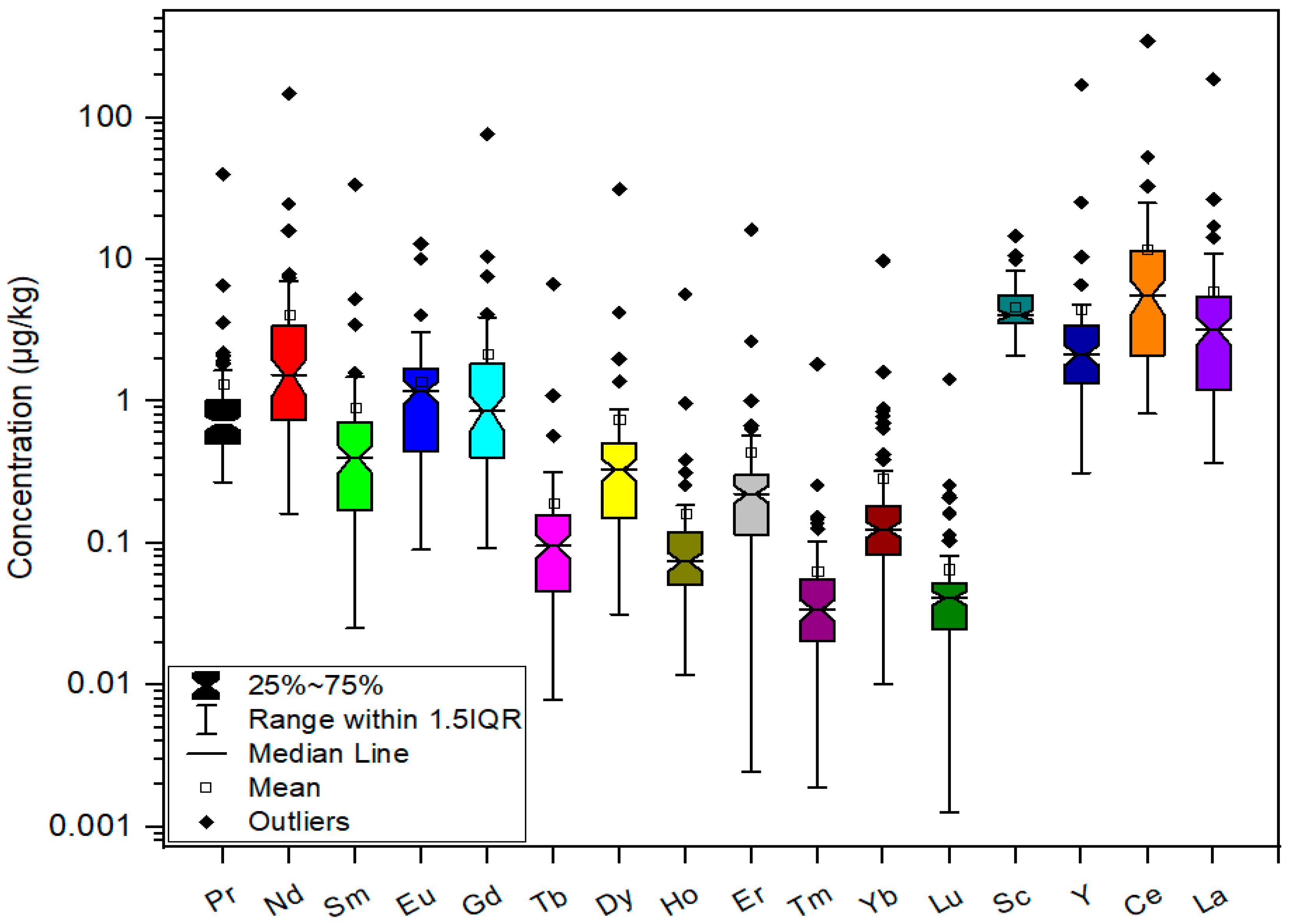
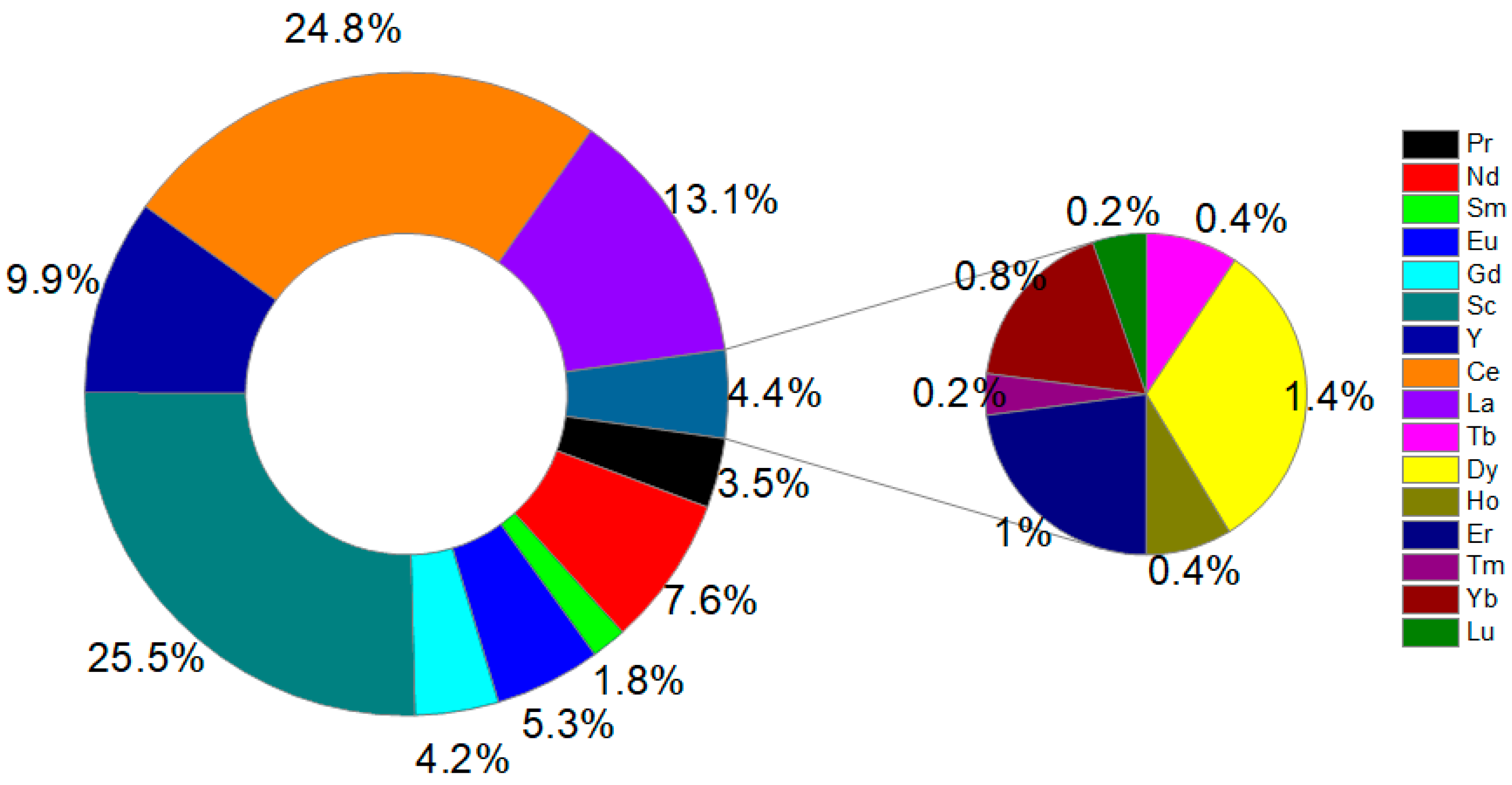
3.2. REE Content in Coffee
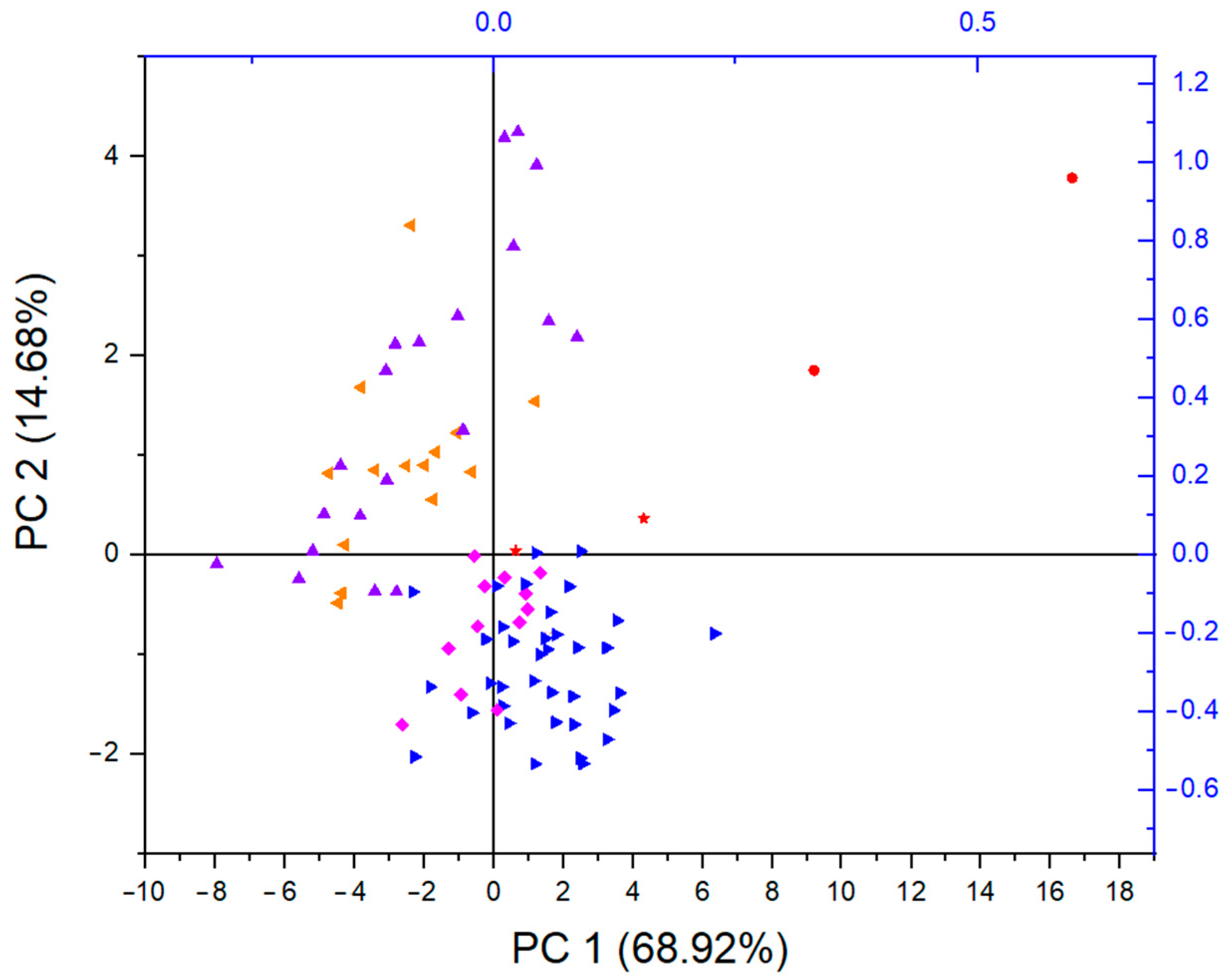
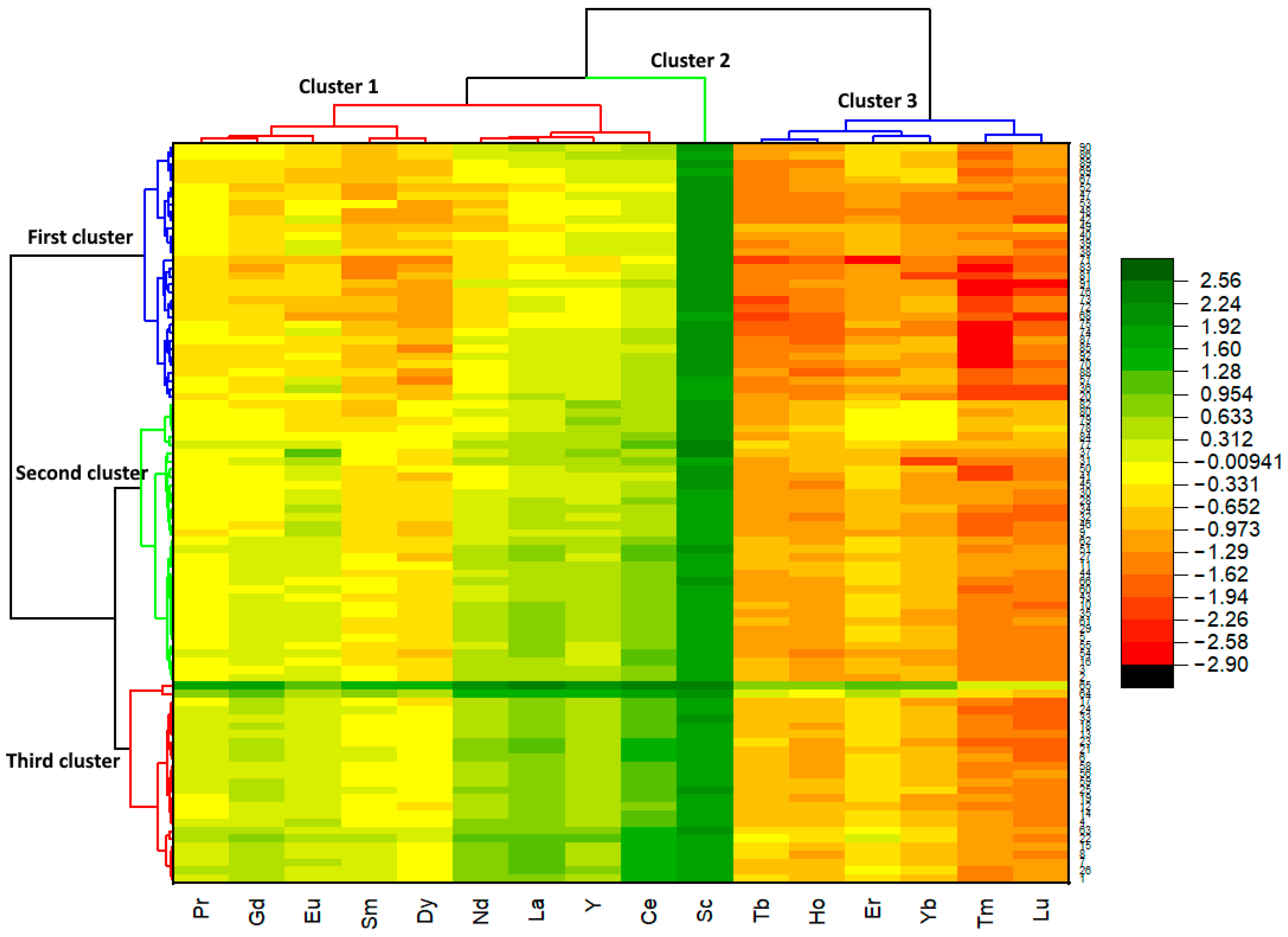
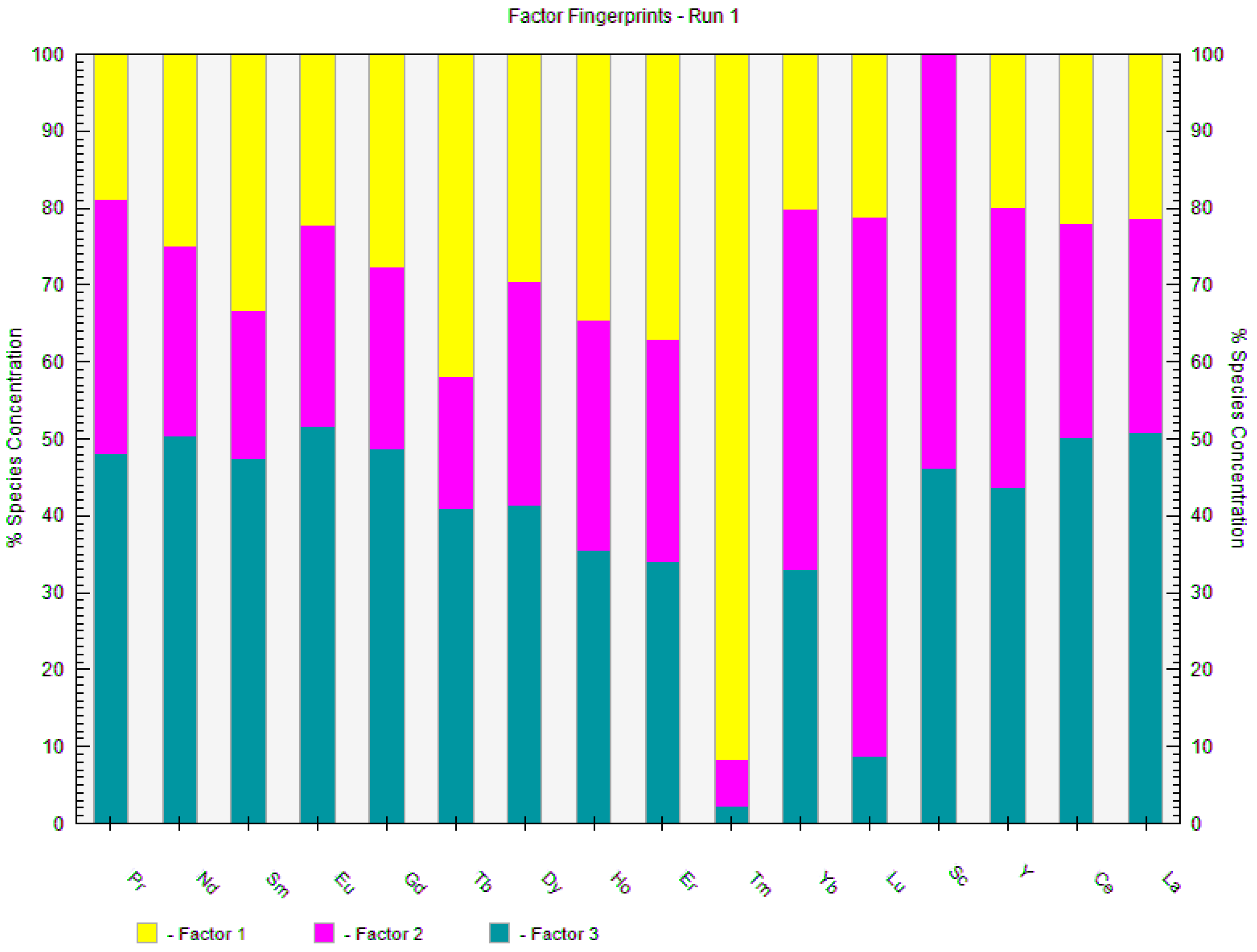
3.3. Multivariate Analyses
3.4. Potential REE Hazards to Human Health
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butt, M.S.; Sultan, M.T. Coffee and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef] [PubMed]
- George, S.E.; Ramalakshmi, K.; Rao, L.J.M. A Perception on Health Benefits of Coffee. Crit. Rev. Food Sci. Nutr. 2008, 48, 464–486. [Google Scholar] [CrossRef] [PubMed]
- Santato, A.; Bertoldi, D.; Perini, M.; Camin, F.; Larcher, R. Using Elemental Profiles and Stable Isotopes to Trace the Origin of Green Coffee Beans on the Global Market. J. Mass Spectrom. 2012, 47, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Samoggia, A.; Riedel, B. Consumers’ Perceptions of Coffee Health Benefits and Motives for Coffee Consumption and Purchasing. Nutrients 2019, 11, 653. [Google Scholar] [CrossRef]
- Markos, M.U.; Tola, Y.; Kebede, B.T.; Ogah, O. Metabolomics: A Suitable Foodomics Approach to the Geographical Origin Traceability of Ethiopian Arabica Specialty Coffees. Food Sci. Nutr. 2023, 11, 4419–4431. [Google Scholar] [CrossRef]
- Debastiani, R.; Iochims dos Santos, C.E.; Maciel Ramos, M.; Sobrosa Souza, V.; Amaral, L.; Yoneama, M.L.; Ferraz Dias, J. Elemental Analysis of Brazilian Coffee with Ion Beam Techniques: From Ground Coffee to the Final Beverage. Food Res. Int. 2019, 119, 297–304. [Google Scholar] [CrossRef]
- Savić, A.; Mutić, J.; Lučić, M.; Onjia, A. Dietary Intake of Minerals and Potential Human Exposure to Toxic Elements via Coffee Consumption. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Tian, L.; Guo, Y.; Zhang, A.; Zhong, H. Simultaneously Verifying the Original Region of Green and Roasted Coffee Beans by Stable Isotopes and Elements Combined with Random Forest. J. Food Qual. 2022, 2022, 1308645. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A. The Characteristics, Occurrence, and Geochemical Behavior of Rare Earth Elements in the Environment: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 429–471. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Musgrove, M.; White, S.J.O. Outlining Potential Biomarkers of Exposure and Effect to Critical Minerals: Nutritionally Essential Trace Elements and the Rare Earth Elements. Toxics 2023, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.G.; Yang, J.; Zhang, S.; Yang, D.J. A Survey of 16 Rare Earth Elements in the Major Foods in China. Biomed. Environ. Sci. 2012, 25, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of Rare Earth Elements (REEs) and Their Roles in Plant Growth: A Review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, Y.; Kamitani, M. Rare Earth Minerals and Resources in the World. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Turra, C. Sustainability of Rare Earth Elements Chain: From Production to Food—A Review. Int. J. Environ. Health Res. 2017, 28, 23–42. [Google Scholar] [CrossRef]
- Khan, A.M.; Bakar, N.K.A.; Bakar, A.F.A.; Ashraf, M.A. Chemical Speciation and Bioavailability of Rare Earth Elements (REEs) in the Ecosystem: A Review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789. [Google Scholar] [CrossRef]
- Vesković, J.; Bulatović, S.; Ražić, S.; Lučić, M.; Miletić, A.; Nastasović, A.; Onjia, A. Arsenic-Contaminated Groundwater of the Western Banat (Pannonian Basin): Hydrogeochemical Appraisal, Pollution Source Apportionment, and Monte Carlo Simulation of Source-Specific Health Risks. Water Environ. Res. 2024, 96, e11087. [Google Scholar] [CrossRef]
- Yan, Y.; Chi, H.; Liu, J.; Hu, G.; Yu, R.; Huang, H.; Lin, C. Provenance and Bioaccessibility of Rare Earth Elements in Atmospheric Particles in Areas Impacted by the Optoelectronic Industry. Environ. Pollut. 2020, 263, 114349. [Google Scholar] [CrossRef]
- Miclean, M.; Levei, E.A.; Tanaselia, C.; Cadar, O. Rare Earth Elements Transfer from Soil to Vegetables and Health Risks Associated with Vegetable Consumption in a Former Mining Area. Agronomy 2023, 13, 1399. [Google Scholar] [CrossRef]
- Arciszewska, Z.; Gama, S.; Lesniewska, B.; Malejko, J.; Nalewajko-Sieliwoniuk, E.; Zambrzycka-Szelewa, E.; Godlewska-Zyłkiewicz, B. The Translocation Pathways of Rare Earth Elements from the Environment to the Food Chain and Their Impact on Human Health. Process Saf. Environ. Prot. 2022, 168, 205–223. [Google Scholar] [CrossRef]
- Shi, Z.; Yong, L.; Liu, Z.; Wang, Y.; Sui, H.; Mao, W.; Zhang, L.; Li, Y.; Liu, J.; Wei, S.; et al. Risk Assessment of Rare Earth Elements in Fruits and Vegetables from Mining Areas in China. Environ. Sci. Pollut. Res. 2022, 29, 48694–48703. [Google Scholar] [CrossRef]
- Kollander, B.; Rodushkin, I.; Sundström, B. Multi-Element Assessment of Potentially Toxic and Essential Elements in New and Traditional Food Varieties in Sweden. Foods 2023, 12, 1831. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, A.; Squillante, J.; Esposito, F.; Velotto, S.; Romano, R.; Aponte, M.; Giarra, A.; Toscanesi, M.; Montella, E.; Cirillo, T. Coffee Silverskin: Chemical and Biological Risk Assessment and Health Profile for Its Potential Use in Functional Foods. Foods 2022, 11, 2834. [Google Scholar] [CrossRef]
- Temerdashev, Z.; Bolshov, M.; Abakumov, A.; Khalafyan, A.; Kaunova, A.; Vasilyev, A.; Sheludko, O.; Ramazanov, A. Can Rare Earth Elements Be Considered as Markers of the Varietal and Geographical Origin of Wines? Molecules 2023, 28, 4319. [Google Scholar] [CrossRef]
- Lymperopoulou, T.; Balta-Brouma, K.; Tsakanika, L.A.; Tzia, C.; Tsantili-Kakoulidou, A.; Tsopelas, F. Identification of Lentils (Lens Culinaris Medik) from Eglouvi (Lefkada, Greece) Based on Rare Earth Elements Profile Combined with Chemometrics. Food Chem. 2024, 447, 138965. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical Origin and Botanical Type Honey Authentication through Elemental Metabolomics via Chemometrics. Food Chem. 2021, 338, 127936. [Google Scholar] [CrossRef]
- Tanaka, K.; Zhao, L.; Tazoe, H.; Iizuka, T.; Murakami-Sugihara, N.; Toyama, K.; Yamamoto, T.; Yorisue, T.; Shirai, K. Using Neodymium Isotope Ratio in Ruditapes Philippinarum Shells for Tracking the Geographical Origin. Food Chem. 2022, 382, 131914. [Google Scholar] [CrossRef]
- Barbosa, R.M.; Batista, B.L.; Varrique, R.M.; Coelho, V.A.; Campiglia, A.D.; Barbosa, F., Jr. The Use of Advanced Chemometric Techniques and Trace Element Levels for Controlling the Authenticity of Organic Coffee. Food Res. Int. 2014, 61, 246–251. [Google Scholar] [CrossRef]
- Vezzulli, F.; Fontanella, M.C.; Lambri, M.; Beone, G.M. Specialty and High-Quality Coffee: Discrimination through Elemental Characterization via ICP-OES, ICP-MS, and ICP-MS/MS of Origin, Species, and Variety. J. Sci. Food Agric. 2023, 103, 4303–4316. [Google Scholar] [CrossRef]
- Spalla, S.; Baffi, C.; Barbante, C.; Turretta, C.; Cozzi, G.; Beone, G.M.; Bettinelli, M. Determination of Rare Earth Elements in Tomato Plants by Inductively Coupled Plasma Mass Spectrometry Techniques. Rapid Commun. Mass Spectrom. 2009, 23, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Chiaudani, A.; Flamminii, F.; Consalvo, A.; Bellocci, M.; Pizzi, A.; Passamonti, C.; Cichelli, A. Rare Earth Element Variability in Italian Extra Virgin Olive Oils from Abruzzo Region. Foods 2024, 13, 141. [Google Scholar] [CrossRef]
- Bandoniene, D.; Walkner, C.; Ringdorfer, F.; Meisel, T. Authentication of Meat and Dairy Products Using Rare Earth Element Labeling and Detection by Solution Based and Laser Ablation ICP-MS. Food Res. Int. 2020, 132, 109106. [Google Scholar] [CrossRef] [PubMed]
- Kalogiouri, N.P.; Manousi, N.; Klaoudatos, D.; Spanos, T.; Topi, V.; Zachariadis, G.A. Rare Earths as Authenticity Markers for the Discrimination of Greek and Turkish Pistachios Using Elemental Metabolomics and Chemometrics. Foods 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Costa, T.; Rangel Botelho, J.; Cassago Nascimento, M.H.; Krause, M.; Weitzel Dias Carneiro, M.T.; Coelho Ferreira, D.; Filgueiras, P.R.; de Oliveira Souza, M. A One-Class Classification Approach for Authentication of Specialty Coffees by Inductively Coupled Plasma Mass Spectroscopy (ICP-MS). Food Chem. 2024, 442, 138268. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Zotos, A.; Thomatou, A.A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), a Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11, 3705. [Google Scholar] [CrossRef]
- Gamela, R.R.; Costa, V.C.; Pereira-Filho, E.R. Multivariate Optimization of Ultrasound-Assisted Extraction Procedure for the Determination of Ca, Fe, K, Mg, Mn, P, and Zn in Pepper Samples by ICP OES. Food Anal. Methods 2020, 13, 69–77. [Google Scholar] [CrossRef]
- Saleem, M.; Durani, A.I.; Alharthy, R.D.; Ahmed, M.; Shafiq, M.I.; Hameed, A.; Mubasher Hussain, S.; Bashir, W. Ultrasound-Assisted Extraction of Micro- and Macroelements in Fruit Peel Powder Mineral Supplement for Osteoporosis Patients and Their Determination by Flame Atomic Absorption Spectrometry. J. Chem. 2021, 2021, 5151560. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Ahmed, E.; Sharif, A.; Ahmed, M.; Ahmad, W. Optimization of Ultrasound-Assisted Extraction of Essential and Non-Essential/Toxic Trace Metals in Vegetables and Their Determination by FAAS and ICP-OES: An Evaluation of Human Health Risk. Food Anal. Methods 2021, 14, 2262–2275. [Google Scholar] [CrossRef]
- Lima, E.C.; Barbosa, O.F.; Krug, F.J.; Silva, M.M.; Vale, M.G.R. Comparison of Ultrasound-Assisted Extraction, Slurry Sampling and Microwave-Assisted Digestion for Cadmium, Copper and Lead Determination in Biological and Sediment Samples by Electrothermal Atomic Absorption Spectrometry. J. Anal. At. Spectrom. 2000, 15, 995–1000. [Google Scholar] [CrossRef]
- Mansour, F.R.; Płotka-Wasylka, J.; Locatelli, M. Modified GAPI (MoGAPI) Tool and Software for the Assessment of Method Greenness: Case Studies and Applications. Analytica 2024, 5, 451–457. [Google Scholar] [CrossRef]
- Gohlke, G.; Cauduro, V.H.; Frozi, E.; Rocha, L.F.; Machado, G.R.; Henn, A.S.; Tao, Y.; Mesko, M.F.; Flores, E.M.M. Low Cost Sample Preparation Method Using Ultrasound for the Determination of Environmentally Critical Elements in Seaweed. Ultrason. Sonochem. 2024, 103, 106788. [Google Scholar] [CrossRef] [PubMed]
- ISO 11294:1994; ISO International Standard—Roasted Ground Coffee—Determination of Moisture Content—Method by Determination of Loss in Mass at 103 °C (Routine Method). ISO: Geneva, Switzerland, 1994.
- EN 17851:2023; Comite Europeen de Normalisation—Foodstuffs—Determination of Elements and Their Chemical Species—Determination of Ag, As, Cd, Co, Cr, Cu, Mn, Mo, Ni, Pb, Se, Tl, U and Zn in Foodstuffs by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) after Pressure Digestion. Comite Europeen de Normalisation: Brussels, Belgium, 2023.
- USEPA Human Health Risk Assessment|US EPA. Available online: https://www.epa.gov/risk/human-health-risk-assessment (accessed on 15 October 2024).
- Slavković-Beškoski, L.; Ignjatović, L.; Ćujić, M.; Vesković, J.; Trivunac, K.; Stojaković, J.; Perić-Grujić, A.; Onjia, A. Ecological and Health Risks Attributed to Rare Earth Elements in Coal Fly Ash. Toxics 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, Y.S.; Lee, H.J.; Koo, Y.E. Microwave-Assisted Digestion Method Using Diluted Nitric Acid and Hydrogen Peroxide for the Determination of Major and Minor Elements in Milk Samples by ICP-OES and ICP-MS. Food Chem. 2022, 373, 131483. [Google Scholar] [CrossRef]
- Telloli, C.; Cicconi, F.; Manzi, E.; Borgognoni, F.; Salvi, S.; Iapalucci, M.C.; Rizzo, A. Multi-Elemental Analysis of Commercial Wheat Flours by ICP-MS Triple Quadrupole in Function of the Milling Degree. Food Chem. 2024, 450, 139370. [Google Scholar] [CrossRef]
- Škrbić, B.; Živančev, J.; Mrmoš, N. Concentrations of Arsenic, Cadmium and Lead in Selected Foodstuffs from Serbian Market Basket: Estimated Intake by the Population from the Serbia. Food Chem. Toxicol. 2013, 58, 440–448. [Google Scholar] [CrossRef]
- Naicker, K.; Mahlambi, P.; Mahlambi, M. Comparison of Ultrasonic and Microwave Assisted Digestion Methods for the Determination of Heavy Metals in Soil and Sediment: The Effect of Seasonal Variations on Metal Concentrations and Risk Assessment. Soil Sediment Contam. Int. J. 2023, 32, 320–336. [Google Scholar] [CrossRef]
- Alves, V.; de Andrade, J.K.; Felsner, M.L. Green and Fast Ultrasound-Assisted Extraction Procedures for Fe, Mn, Mg and Ca Analysis in Cane Syrups by FAAS. J. Food Compos. Anal. 2023, 123, 105495. [Google Scholar] [CrossRef]
- Biondić Fučkar, V.; Nutrizio, M.; Grudenić, A.; Djekić, I.; Režek Jambrak, A. Sustainable Ultrasound Assisted Extractions and Valorization of Coffee Silver Skin (CS). Sustainability 2023, 15, 8198. [Google Scholar] [CrossRef]
- Messaoudi, M.; Begaa, S.; Hamidatou, L.; Salhi, M.; Ouakouak, H.; Mouzai, M.; Hassani, A. Neutron Activation Analysis of Major and Trace Elements in Arabica and Robusta Coffee Beans Samples Consumed in Algeria. Radiochim. Acta 2018, 106, 525–533. [Google Scholar] [CrossRef]
- Miletić, A.; Vesković, J.; Lučić, M.; Onjia, A. Monte Carlo Simulation of Source-Specific Risks of Soil at an Abandoned Lead-Acid Battery Recycling Site. Stoch. Environ. Res. Risk Assess. 2024, 38, 3313–3329. [Google Scholar] [CrossRef]
- Yazman, M.M.; Yüksel, B.; Ustaoğlu, F.; Şen, N.; Tepe, Y.; Tokatlı, C. Investigation of Groundwater Quality in the Southern Coast of the Black Sea: Application of Computational Health Risk Assessment in Giresun, Türkiye. Environ. Sci. Pollut. Res. 2024, 31, 52306–52325. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Brizio, P.; Stella, C.; Mantia, M.; Battuello, M.; Nurra, N.; Sartor, R.M.; Orusa, R.; Robetto, S.; Brusa, F.; et al. Rare Earth Elements in Marine and Terrestrial Matrices of Northwestern Italy: Implications for Food Safety and Human Health. Sci. Total Environ. 2019, 660, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Bau, M.; Merschel, G.; Tepe, N. Anthropogenic Gadolinium in Tap Water and in Tap Water-Based Beverages from Fast-Food Franchises in Six Major Cities in Germany. Sci. Total Environ. 2019, 687, 1401–1408. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Forward power | 1550 W |
| Nebulizer gas (Ar) | 0.99 L/min |
| Auxiliary gas (Ar) | 0.8 L/min |
| Cool gas flow | 14 L/min |
| Collision cell gas (He) | 4.5 mL/min |
| Sample uptake/wash time | 45 s each |
| Dwell time | 0.01 s |
| Repeats per sample | 3 |
| Pump speed | 40 rpm |
| m/z | Element | Polyatomic Interferences | LOD, ng/L | R2 | Linear Equation |
|---|---|---|---|---|---|
| 141 | Pr | / | 0.19 | 0.9962 | f(x) = 48.6 × 103x + 1.67 |
| 146 | Nd | BaO, RuO3 | 1.38 | 0.9970 | f(x) = 13.6 × 103x + 1.33 |
| 147 | Sm | RuO3 | 0.52 | 0.9999 | f(x) = 68.8 × 103x + 1.45 |
| 153 | Eu | BaO | 0.19 | 0.9986 | f(x) = 33.8 × 103x + 2.19 |
| 157 | Gd | CeO, PrO, LaO, BF | 0.50 | 0.9989 | f(x) = 69.1 × 103x + 0.70 |
| 159 | Tb | NdO, PrO | 0.14 | 0.9981 | f(x) = 45.9 × 103x+ 3.27 |
| 163 | Dy | NdO, SmO | 0.62 | 0.9998 | f(x) = 11.3 × 103x + 0.33 |
| 165 | Ho | SmO | 0.16 | 0.9964 | f(x) = 44.0 × 103x + 1.45 |
| 166 | Er | SmO, NdO | 0.48 | 0.9955 | f(x) = 14.9 × 103x + 1.01 |
| 169 | Tm | EuO | 0.04 | 0.9977 | f(x) = 41.0 × 103x + 0.45 |
| 172 | Yb | DyO, SmO, GdO | 0.02 | 0.9997 | f(x) = 19.6 × 103x + 0.79 |
| 175 | Lu | GdO, TbO | 0.12 | 0.9982 | f(x) = 40.7 × 103x + 3.40 |
| 45 | Sc | CO2, SiO, BO2, AlO, | 4.09 | 0.9982 | f(x) = 16.4 × 103x + 1.02 |
| 89 | Y | CaH, CHO2, SiOH, N2OH | 0.91 | 0.9985 | f(x) = 38.5 × 103x + 4.70 |
| 140 | Ce | / | 0.44 | 0.9987 | f(x) = 38.0 × 103x + 20.7 |
| 139 | La | / | 0.30 | 0.9988 | f(x) = 44.1 × 103x + 37.3 |
| REEs | Vezzulli et al. (2023) [30] | de Oliveira Costa et al. (2024) [35] 1 | Barbosa et al. (2014) [29] | Messaoudi et al. (2018) [53] | Santato et al. (2012) [3] | Present Study $ | Present Study # |
|---|---|---|---|---|---|---|---|
| Pr | <2.12–2.74 2; 8.96 3; 2.71 5; 13.61 6 | 3–200 | - | - | 0.16–3.38 | 0.27–3.58 | 0.89–39.7 |
| Nd | <3.12–11.9 1; 38.2 3; 3.5 4; 13.2 5; 76.8 6; 3.9 7 | 10–700 | - | - | 0.38–12.1 | <LOD–15.9 | 2.13–148 |
| Sm | <3.99–9.26 3; 8.8 6 | 3–400 | 3.5–62 | 7.8–8.3 a; 4.0–4.3 b | 0.20–2.36 | <LOD–3.46 | 0.62–33.8 |
| Eu | <3.93–4.3 6 | 1–10 | 1.3–15.1 | nd a; nd–11.7 b | 0.13–1.02 | 0.090–12.9 | 0.83–10.1 |
| Gd | <3.71–7.57 3; 6.9 6 | 3–60 | 0.9–24.9 | - | 0.18–2.39 | 0.092–7.59 | 1.18–76.2 |
| Tb | <3.25 | 1–10 | 0.07–10.6 | - | - | <LOD–0.57 | 0.096–6.68 |
| Dy | <3.94–7.4 3; 9.9 6 | 2–30 | 0.4–39.3 | 0.17–1.84 | <LOD–1.98 | 0.42–31.3 | |
| Ho | <2.79 | 1–10 | 0.11–6.5 | - | - | <LOD–0.38 | 0.072–5.69 |
| Er | <2.66–4.0 3; 3.4 6 | 2–20 | 0.3–11.1 | - | 0.21–1.07 | <LOD–1.00 | 0.25–16.1 |
| Tm | <2.23 | 1–8 | - | - | <LOD–0.21 | <LOD–0.15 | 0.043–1.83 |
| Yb | <3.82 | 2–10 | 0.1–9.2 | - | <LOD–0.94 | 0.010–0.89 | 0.17–9.74 |
| Lu | <2.46 | 3–20 | 0.13–0.72 | - | - | <LOD–0.26 | 0.040–1.42 |
| Ce | <2.55–23.2 1; 4.0 2; 64.2 3; 6.8 4; 28.5 5; 114.5 6; 9.4 7 | 10–2100 | 9.5–361 | 74–89 a; 490–510 b | 0.73–36.2 | 0.81–32.9 | 7.58–347 |
| La | <2.40–23.1 1; 4.9 2; 34.8 3; 5.3 4; 15.2 5; 85.9 6; 7.1 7 | 10–1000 | 3.2–122 | 35–36 a; 85–91 b | 0.59–15.5 | 0.37–17.1 | 3.45–186 |
| Sc | - | 10–40 | - | 37–38 a; 66–68 b | - | 2.09–10.6 | 3.81–14.5 |
| Y | - | 3–100 | - | - | - | 0.31–10.4 | 2.33–169 |
| Health Risk Index | REE | Mean | Min | Max |
|---|---|---|---|---|
| HQ | Ce | 6.77 × 10−8 | 4.69 × 10−9 | 2.00 × 10−6 |
| La | 3.43 × 10−8 | 2.11 × 10−9 | 1.07 × 10−6 | |
| Sc | 2.70 × 10−8 | 1.20 × 10−8 | 8.38 × 10−8 | |
| Y | 2.55 × 10−8 | 1.80 × 10−9 | 9.74 × 10−7 | |
| Nd | 2.37 × 10−8 | 9.29 × 10−10 | 8.50 × 10−7 | |
| Gd | 1.25 × 10−8 | 5.31 × 10−10 | 4.39 × 10−7 | |
| Eu | 8.03 × 10−9 | 5.17 × 10−10 | 7.42 × 10−8 | |
| Pr | 7.67 × 10−9 | 1.56 × 10−9 | 2.29 × 10−7 | |
| Sm | 5.26 × 10−9 | 1.45 × 10−10 | 1.95 × 10−7 | |
| Dy | 4.26 × 10−9 | 1.82 × 10−10 | 1.80 × 10−7 | |
| Er | 2.52 × 10−9 | 1.42 × 10−11 | 9.30 × 10−8 | |
| Yb | 1.65 × 10−9 | 5.90 × 10−11 | 5.61 × 10−8 | |
| Tb | 1.10 × 10−9 | 4.52 × 10−11 | 3.85 × 10−8 | |
| Ho | 9.25 × 10−10 | 6.79 × 10−11 | 3.27 × 10−8 | |
| Lu | 3.78 × 10−10 | 7.30 × 10−11 | 8.20 × 10−9 | |
| Tm | 3.64 × 10−10 | 1.09 × 10−11 | 1.06 × 10−8 | |
| HI | ΣREEs | 2.23 × 10−7 | 4.70 × 10−8 | 6.32 × 10−6 |
| TCR | ΣREEs | 1.24 × 10−13 | 2.61 × 10−14 | 3.51 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savić, A.; Mutić, J.; Lučić, M.; Vesković, J.; Miletić, A.; Onjia, A. Ultrasound-Assisted Extraction Followed by Inductively Coupled Plasma Mass Spectrometry and Multivariate Profiling of Rare Earth Elements in Coffee. Foods 2025, 14, 275. https://doi.org/10.3390/foods14020275
Savić A, Mutić J, Lučić M, Vesković J, Miletić A, Onjia A. Ultrasound-Assisted Extraction Followed by Inductively Coupled Plasma Mass Spectrometry and Multivariate Profiling of Rare Earth Elements in Coffee. Foods. 2025; 14(2):275. https://doi.org/10.3390/foods14020275
Chicago/Turabian StyleSavić, Aleksandra, Jelena Mutić, Milica Lučić, Jelena Vesković, Andrijana Miletić, and Antonije Onjia. 2025. "Ultrasound-Assisted Extraction Followed by Inductively Coupled Plasma Mass Spectrometry and Multivariate Profiling of Rare Earth Elements in Coffee" Foods 14, no. 2: 275. https://doi.org/10.3390/foods14020275
APA StyleSavić, A., Mutić, J., Lučić, M., Vesković, J., Miletić, A., & Onjia, A. (2025). Ultrasound-Assisted Extraction Followed by Inductively Coupled Plasma Mass Spectrometry and Multivariate Profiling of Rare Earth Elements in Coffee. Foods, 14(2), 275. https://doi.org/10.3390/foods14020275








