L-Theanine Extends the Lifespan of Caenorhabditis elegans by Reducing the End Products of Advanced Glycosylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Subjects and Treatments
2.2. Longevity and Phenotype Measurement
2.3. AGE Detection by Fluorescence and ELISA
2.4. Quantification of gst-4::GFP Expression
2.5. Statistical Analysis
3. Results
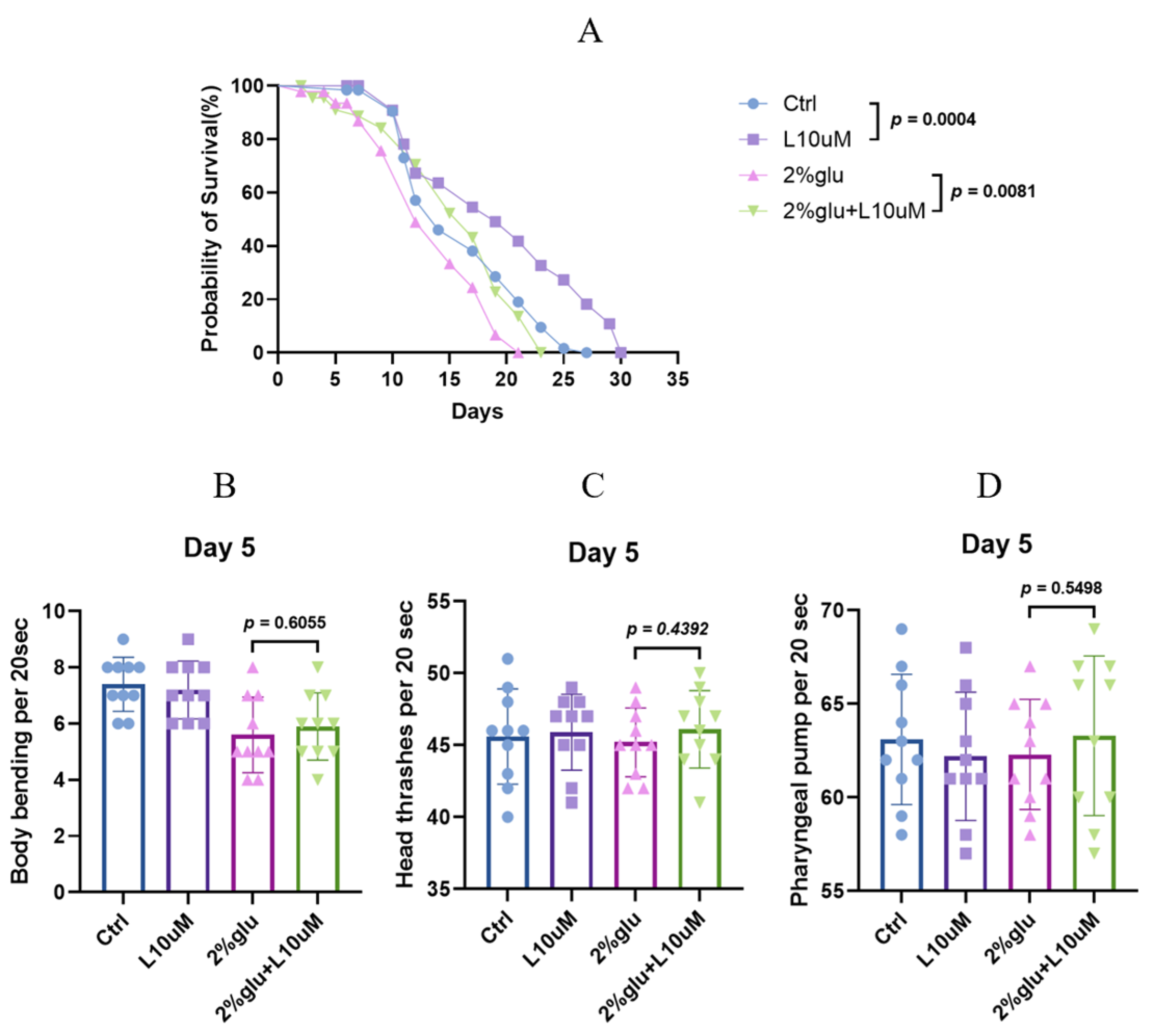
3.1. L-Theanine Prolongs Lifespan, Both With and Without Glucose Addition
3.2. L-Theanine Reduces Accumulation of AGEs Under High-Glucose Conditions
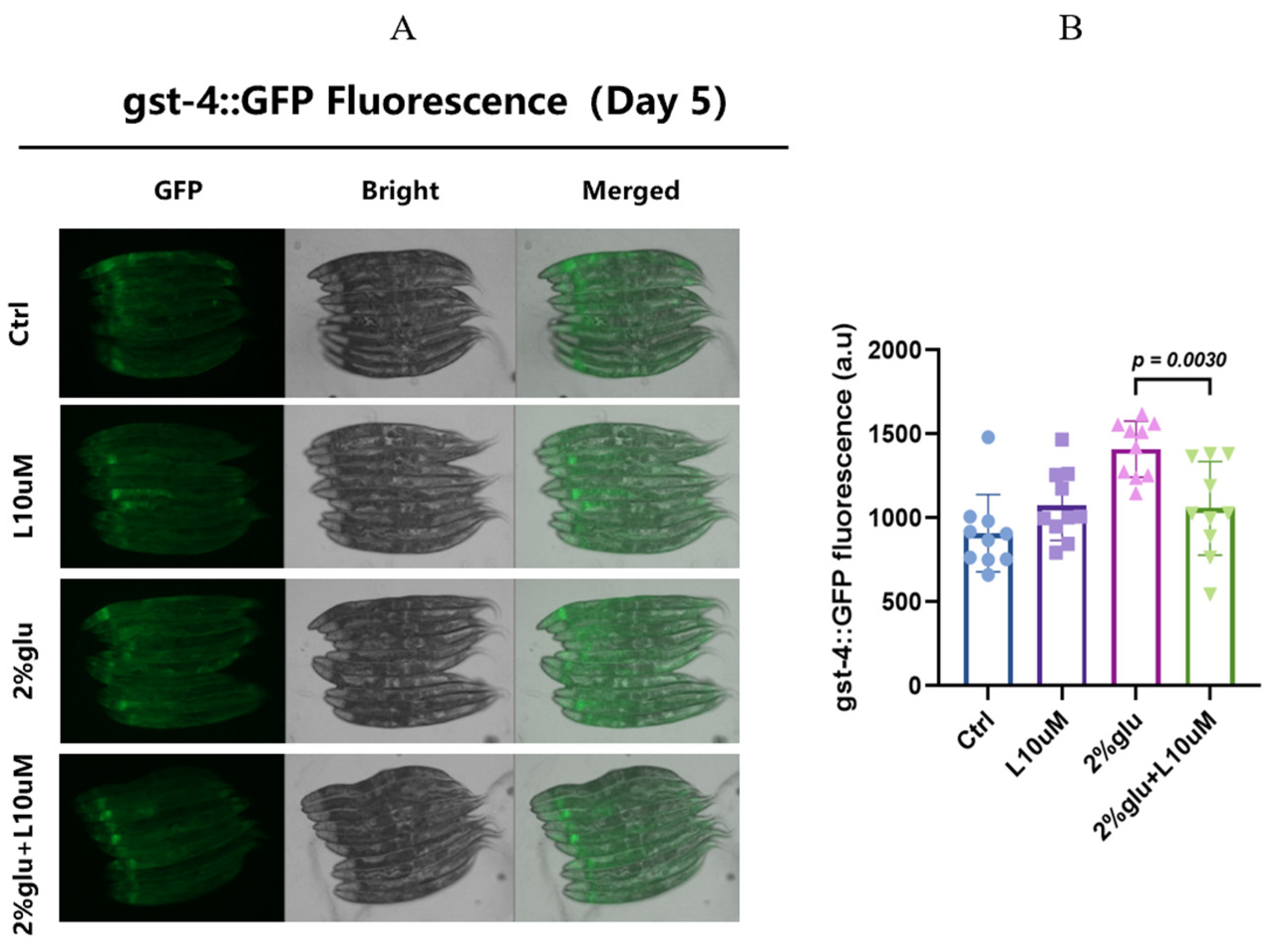
3.3. L-Theanine Alleviates Oxidative Stress Under High-Glucose Conditions by Regulating gst-4 Expression
3.4. L-Theanine Modulates Lifespan and AGE Accumulation in C. elegans via the daf-2/daf-16 Pathway
4. Discussion
4.1. Mechanisms of AGEs and RAGE
4.2. Mechanisms of gst-4 Gene Expression in Reducing Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Guan, L.; Feng, H.; Gong, D.; Zhao, X.; Cai, L.; Wu, Q.; Yuan, B.; Yang, M.; Zhao, J.; Zou, Y. Genipin ameliorates age-related insulin resistance through inhibiting hepatic oxidative stress and mitochondrial dysfunction. Exp. Gerontol. 2013, 48, 1387–1394. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lin, L.; Xiao, W.; Li, Y. L-theanine protects rat kidney from D-galactose-induced injury via inhibition of the AGEs/RAGE signaling pathway. Eur. J. Pharmacol. 2022, 927, 175072. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pasupulati, A.; Chitra, P.S.; Reddy, G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts 2016, 7, 293–309. [Google Scholar] [CrossRef]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Hollenbach, M. The Role of Glyoxalase-I (Glo-I), Advanced Glycation Endproducts (AGEs), and Their Receptor (RAGE) in Chronic Liver Disease and Hepatocellular Carcinoma (HCC). Int. J. Mol. Sci. 2017, 18, 2466. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Palanissami, G.; Paul, S.F.D. AGEs and RAGE: Metabolic and molecular signatures of the glycation-inflammation axis in malignant or metastatic cancers. Explor. Target Antitumor Ther. 2023, 4, 812–849. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Muthyalaiah, Y.S.; Jonnalagadda, B.; John, C.M.; Arockiasamy, S. Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression. Glycoconj. J. 2021, 38, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Drury, S.; Hudson, B.I.; Gleason, M.R.; Qu, W.; Lu, Y.; Lalla, E.; Chitnis, S.; Monteiro, J.; Stickland, M.H.; et al. RAGE and arthritis: The G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002, 3, 123–135. [Google Scholar] [CrossRef]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Lakowski, B.; Hekimi, S. The genetics of caloric restriction in C. elegans. Proc. Natl. Acad. Sci. USA 1998, 95, 13091–13096. [Google Scholar] [CrossRef]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Dorman, J.B.; Albinder, B.; Shroyer, T.; Kenyon, C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of C. elegans. Genetics 1995, 141, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Kim, S.S.; Park, S.; Kwon, H.C.; Ha, S.G.; Bae, Y.; Lee, G.Y.; Lee, S.V. Combinatorial transcriptomic and genetic dissection of insulin/IGF-1 signaling-regulated longevity in C. elegans. Aging Cell. 2024, 23, e14151. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef]
- Golegaonkar, S.; Tabrez, S.S.; Pandit, A.; Sethurathinam, S.; Jagadeeshaprasad, M.G.; Bansode, S.; Sampathkumar, S.G.; Kulkarni, M.J.; Mukhopadhyay, A. Rifampicin reduces advanced glycation end products and activates DAF-16 to increase lifespan in C. elegans. Aging Cell. 2015, 14, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Jabin, S.; Ristow, M. L-Theanine extends lifespan of adult C. elegans. Eur. J. Nutr. 2012, 51, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.C.; Rao, A.; Owen, G.N. L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac. J. Clin. Nutr. 2008, 17, 167–168. [Google Scholar]
- Du, Z.; Wu, G.; Cheng, H.; Han, T.; Li, D.; Xie, Z. L-Theanine Ameliorates Obesity-Related Complications Induced by High-Fat Diet in Mice: Insights from Transcriptomics and Metabolomics. Foods 2024, 13, 2977. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, S.; Sadamori, K.; Konishi, R.; Majima, T.; Mukai, A.; Uno, K.; Kinjo, T.; Komori, K.; Kuramoto, N.; Kawada, K. Pharmacokinetics of L-theanine and the effect on amino acid composition in mice administered with L-theanine. Amino Acids 2024, 56, 29. [Google Scholar] [CrossRef]
- Wang, F.; Huang, X.; Wang, W.; Li, X.; Hao, M.; Taylor, E.W.; Zhang, J. L-Theanine Effectively Protects Against Copper-Facilitated Dopamine Oxidation: Implication for Relieving Dopamine Overflow-Associated Neurotoxicities. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Borah, A. L-theanine, the unique constituent of tea, improves neuronal survivability by curtailing inflammatory responses in MPTP model of Parkinson’s disease. Neurochem. Int. 2024, 179, 105830. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Gray, M.A.; Oliver, C.; Liley, D.T.; Harrison, B.J.; Bartholomeusz, C.F.; Phan, K.L.; Nathan, P.J. The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum. Psychopharmacol. 2004, 19, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xiang, X.; Lin, L.; Gong, Z.H.; Xiao, W.J. L-Theanine delays d-galactose-induced senescence by regulating the cell cycle and inhibiting apoptosis in rat intestinal cells. J. Sci. Food Agric. 2024, 104, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T. Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol. Res. 2011, 64, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Su, L.; You, H.; Dong, Z.; Liu, M.; Zhou, C. L-Theanine Inhibits Chemoresistance of Lung Cancer Cells to Cisplatin by Regulating STAT3/NOTCH1-BMAL1 Signaling. Front. Biosci. Landmark Ed. 2024, 29, 226. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, Z.; Cai, H.; Li, Z.; Zhu, J.; Wu, D.; Xu, W.; Qiu, H.; Zhang, N.; Li, G.; et al. WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging. Int. J. Mol. Sci. 2024, 25, 9675. [Google Scholar] [CrossRef]
- Komura, T.; Yamanaka, M.; Nishimura, K.; Hara, K.; Nishikawa, Y. Autofluorescence as a noninvasive biomarker of senescence and advanced glycation end products in C. elegans. NPJ Aging Mech. Dis. 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- McElwee, J.J.; Schuster, E.; Blanc, E.; Thomas, J.H.; Gems, D. Shared transcriptional signature in C. elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004, 279, 44533–44543. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in C. elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Kaeberlein, M.; Powers, R.W., 3rd; Steffen, K.K.; Westman, E.A.; Hu, D.; Dang, N.; Kerr, E.O.; Kirkland, K.T.; Fields, S.; Kennedy, B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005, 310, 1193–1196. [Google Scholar] [CrossRef]
- Apfeld, J.; Kenyon, C. Regulation of lifespan by sensory perception in C. elegans. Nature 1999, 402, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.L. Aging and resistance to oxidative damage in C. elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 8905–8909. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Partridge, L. Stress-response hormesis and aging: “That which does not kill us makes us stronger”. Cell Metab. 2008, 7, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of C. elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 target genes that control C. elegans life-span and metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R.A. C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Liang, B.; Moussaif, M.; Kuan, C.J.; Gargus, J.J.; Sze, J.Y. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 2006, 4, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef]
- Greco, R.; Amantea, D.; Mangione, A.S.; Petrelli, F.; Gentile, R.; Nappi, G.; Blandini, F.; Corasaniti, M.T.; Tassorelli, C. Modulation of RAGE isoforms expression in the brain and plasma of rats exposed to transient focal cerebral ischemia. Neurochem. Res. 2012, 37, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Faruqui, T.; Khan, M.S.; Akhter, Y.; Khan, S.; Rafi, Z.; Saeed, M.; Han, I.; Choi, E.H.; Yadav, D.K. RAGE Inhibitors for Targeted Therapy of Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2022, 24, 266. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells. 2022, 11, 1312. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Tuo, M.; Ma, J.; Xie, A. RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci. Lett. 2018, 672, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Tóbon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef]
- Li, J.S.; Ji, T.; Su, S.L.; Zhu, Y.; Chen, X.L.; Shang, E.X.; Guo, S.; Qian, D.W.; Duan, J.A. Mulberry leaves ameliorate diabetes via regulating metabolic profiling and AGEs/RAGE and p38 MAPK/NF-κB pathway. J. Ethnopharmacol. 2022, 283, 114713. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Cheng, W.; Jia, X.; Bai, X.; Zhao, Y.; Lu, Y.; Zhu, L.; Zhu, Y.; Wang, L.; Shu, Y.; et al. AGEs promote atherosclerosis by increasing LDL transcytosis across endothelial cells via RAGE/NF-κB/Caveolin-1 pathway. Mol. Med. 2023, 29, 113. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.S.; Manoharan, R.; Karthikeyan, S.; Alhosaini, K.; Odeibat, H.A.M.; Ahmad, M.D.I.; Al-Okail, M.; Al-Twaijry, N. Inhibitory Potential of Carnosine and Aminoguanidine Towards Glycation and Fibrillation of Albumin: In-vitro and Simulation Studies. J. Fluoresc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.; Nasu, R.; Furukawa, A.; Takeuchi, M.; Koriyama, Y. Pyridoxamine and Aminoguanidine Attenuate the Abnormal Aggregation of β-Tubulin and Suppression of Neurite Outgrowth by Glyceraldehyde-Derived Toxic Advanced Glycation End-Products. Front. Pharmacol. 2022, 13, 921611. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Sang, S. Dietary Quercetin Reduces Plasma and Tissue Methylglyoxal and Advanced Glycation End Products in Healthy Mice Treated with Methylglyoxal. J. Nutr. 2021, 151, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tang, F.; Liu, Q.; Xiao, J.; Cao, H.; Chen, X. Inhibition of resveratrol glucosides (REs) on advanced glycation endproducts (AGEs) formation: Inhibitory mechanism and structure-activity relationship. Nat. Prod. Res. 2020, 34, 2490–2494. [Google Scholar] [CrossRef]
- Shanmugam, G.; Mohankumar, A.; Kalaiselvi, D.; Nivitha, S.; Murugesh, E.; Shanmughavel, P.; Sundararaj, P. Diosgenin a phytosterol substitute for cholesterol, prolongs the lifespan and mitigates glucose toxicity via DAF-16/FOXO and GST-4 in C. elegans. Biomed. Pharmacother. 2017, 95, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, J.; Bu, L.L.; Liao, D.F.; Cheng, S.W.; Zheng, X.L. Curcumin Acetylsalicylate Extends the Lifespan of C. elegans. Molecules 2021, 26, 6609. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain. Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yi, J.; Lu, J.; Nie, M.; Huang, M.; Rong, J.; Zhu, Z.; Chen, J.; Zhou, X.; Li, B.; et al. N-Acetylcysteine Reduces ROS-Mediated Oxidative DNA Damage and PI3K/Akt Pathway Activation Induced by Helicobacter pylori Infection. Oxid. Med. Cell Longev. 2018, 2018, 1874985. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Shakir, H.A.; Mughal, T.A.; Mumtaz, S.; Summer, M.; Farooq, M.A. Aging and its treatment with vitamin C: A comprehensive mechanistic review. Mol. Biol. Rep. 2021, 48, 8141–8153. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, E.; Hassan, H.M.; Abdallah, Z.; Magdy, A.; Farrag, E.A. Renoprotective effect of N-acetylcystein and vitamin E in bisphenol A-induced rat nephrotoxicity; Modulators of Nrf2/ NF-κB and ROS signaling pathway. Acta Biomed. 2022, 93, e2022301. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Jing, H.; Pan, Y.; Cai, H.; Zhang, W.; Zhu, J.; Zhang, N.; Wu, D.; Xu, W.; Qiu, H.; et al. L-Theanine Extends the Lifespan of Caenorhabditis elegans by Reducing the End Products of Advanced Glycosylation. Foods 2025, 14, 221. https://doi.org/10.3390/foods14020221
Huang Z, Jing H, Pan Y, Cai H, Zhang W, Zhu J, Zhang N, Wu D, Xu W, Qiu H, et al. L-Theanine Extends the Lifespan of Caenorhabditis elegans by Reducing the End Products of Advanced Glycosylation. Foods. 2025; 14(2):221. https://doi.org/10.3390/foods14020221
Chicago/Turabian StyleHuang, Zhihang, Haiming Jing, Yan Pan, Hongxia Cai, Wenjing Zhang, Jingyuan Zhu, Nan Zhang, Dan Wu, Wentao Xu, Hexiang Qiu, and et al. 2025. "L-Theanine Extends the Lifespan of Caenorhabditis elegans by Reducing the End Products of Advanced Glycosylation" Foods 14, no. 2: 221. https://doi.org/10.3390/foods14020221
APA StyleHuang, Z., Jing, H., Pan, Y., Cai, H., Zhang, W., Zhu, J., Zhang, N., Wu, D., Xu, W., Qiu, H., Bao, H., Li, G., Ning, J., Xian, B., & Gao, S. (2025). L-Theanine Extends the Lifespan of Caenorhabditis elegans by Reducing the End Products of Advanced Glycosylation. Foods, 14(2), 221. https://doi.org/10.3390/foods14020221






