Mealworm-Derived Protein Hydrolysates Enhance Adipogenic Differentiation via Mitotic Clonal Expansion in 3T3-L1 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Protein Hydrolysate from Mealworm
2.3. Differentiation of 3T3-L1 Preadipocytes
2.4. Cell Viability Assay
2.5. Oil Red O Staining
2.6. Bromodeoxyuridine (BrdU) Assay
2.7. Western Blot Analysis
2.8. Quantitative Real-Time PCR (qPCR) Analysis
2.9. Statistical Analysis
3. Results and Discussion
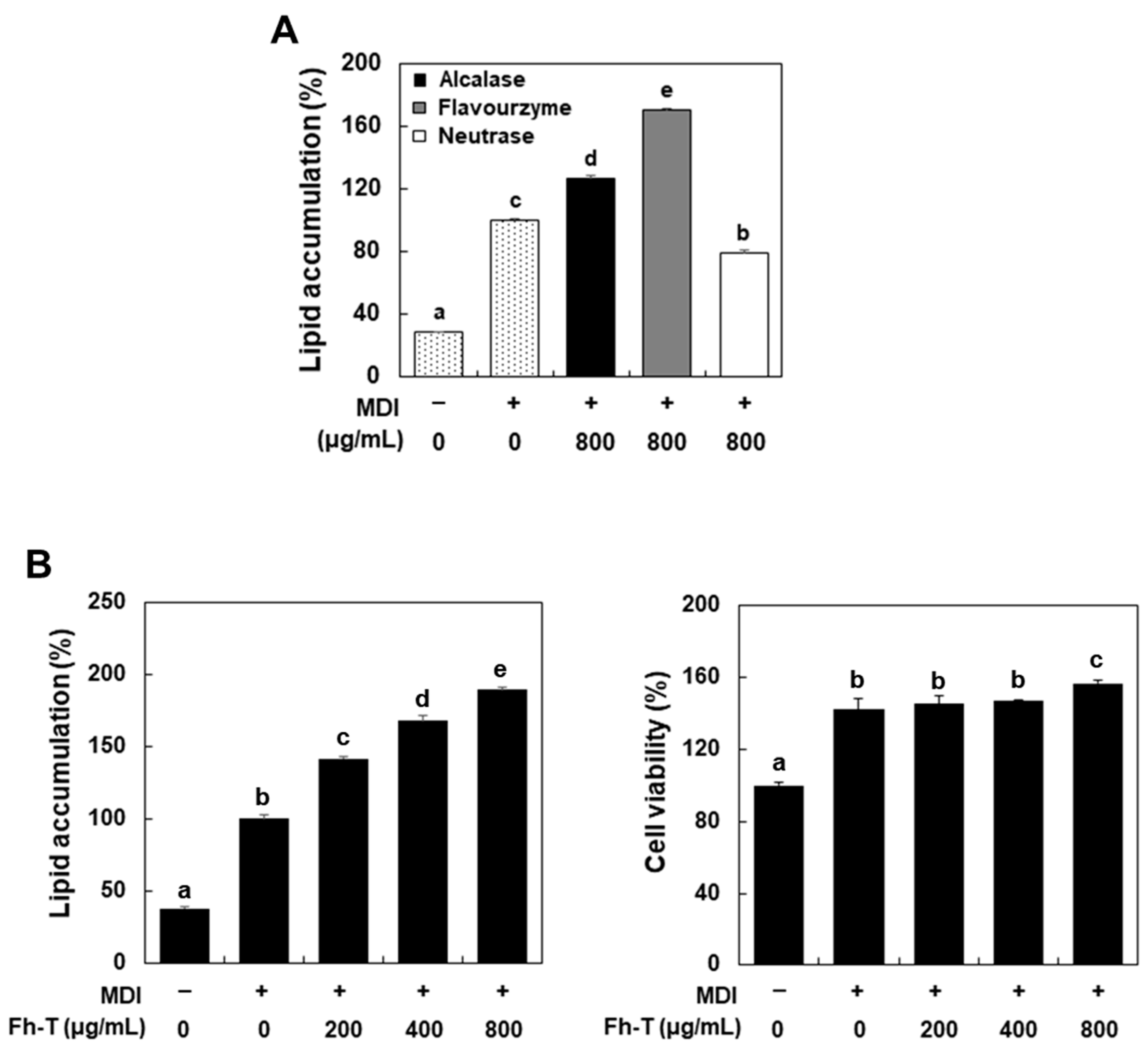
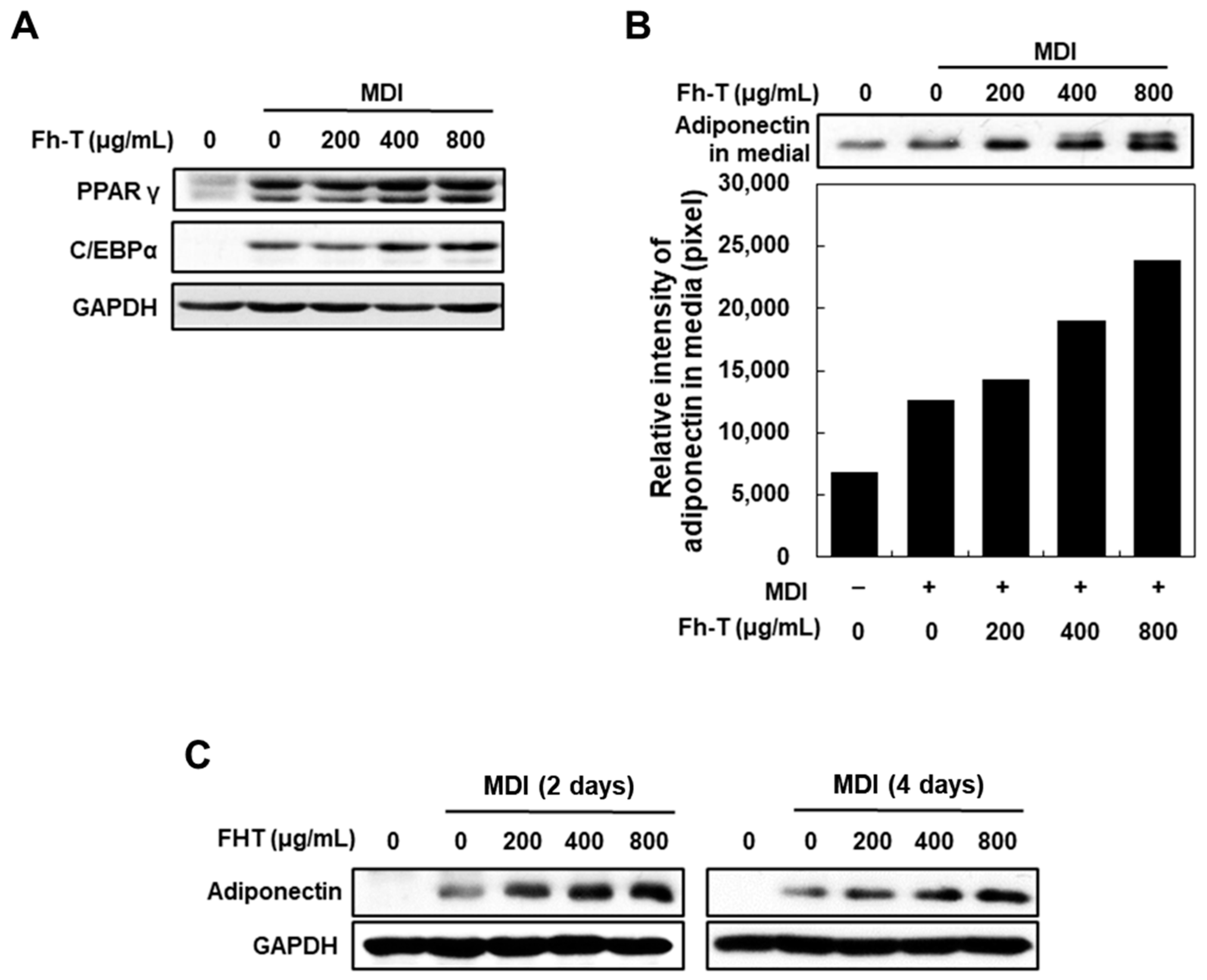
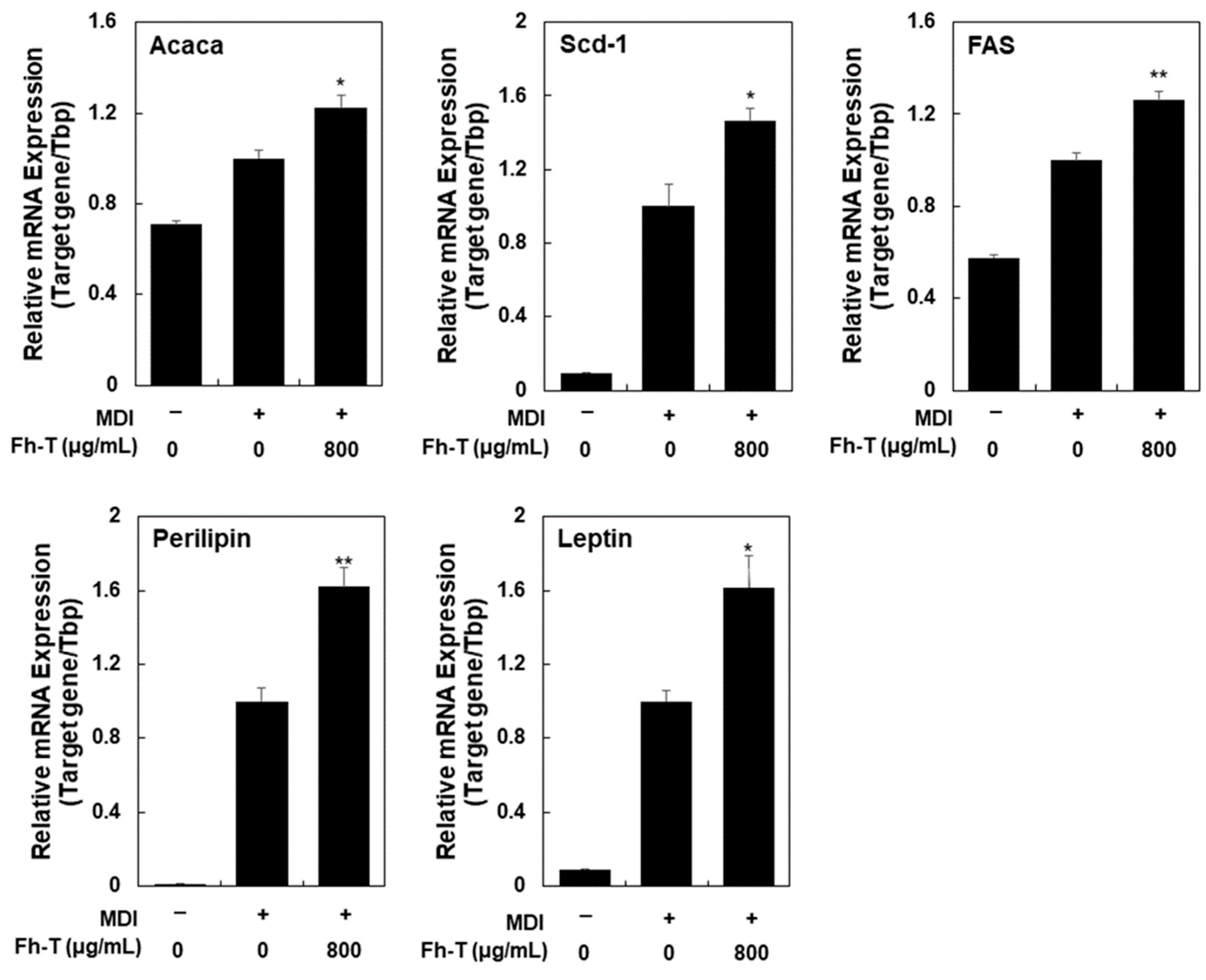
3.1. Fh-T Promotes Adipogenesis of MDI-Treated 3T3-L1 Cells
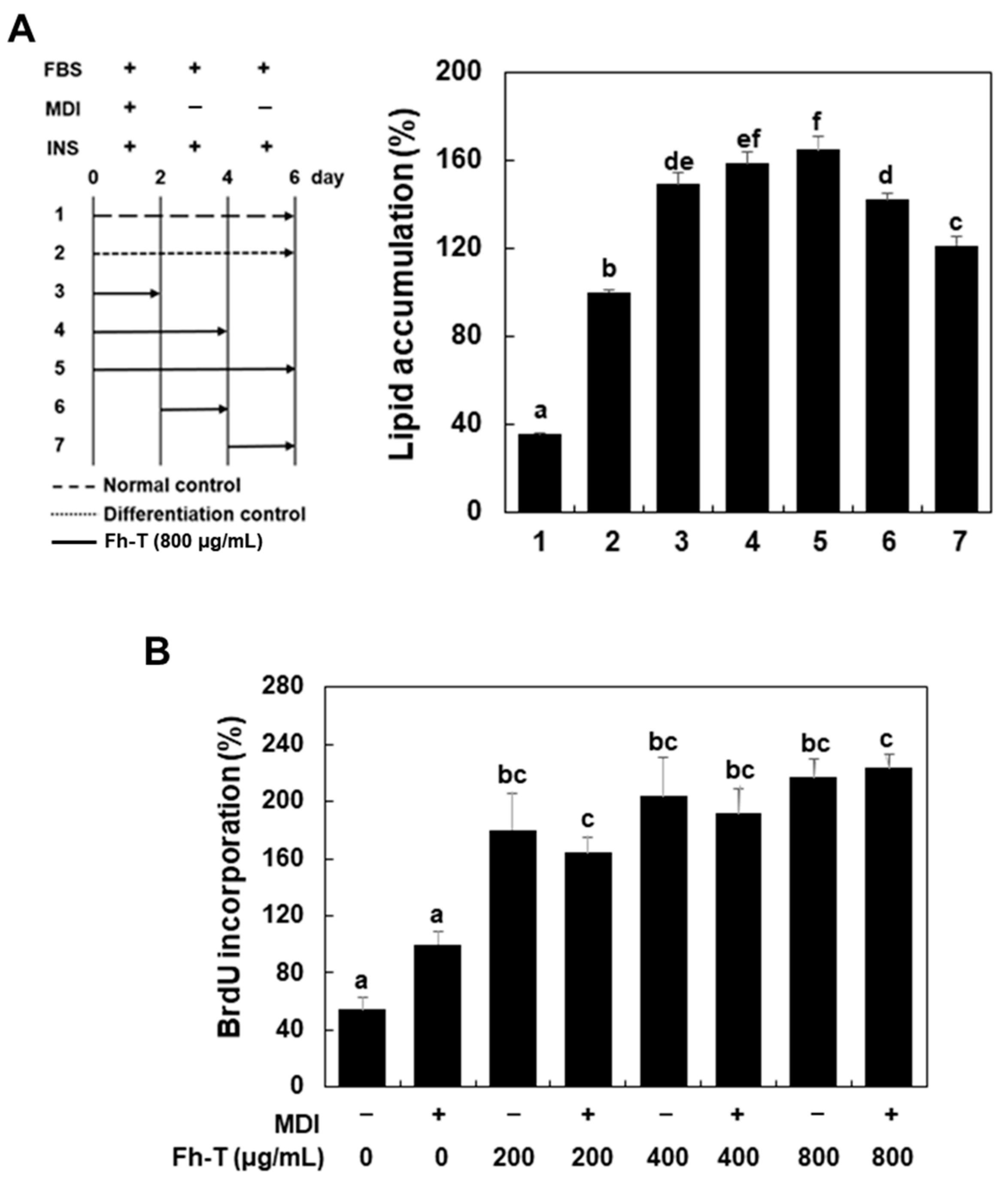
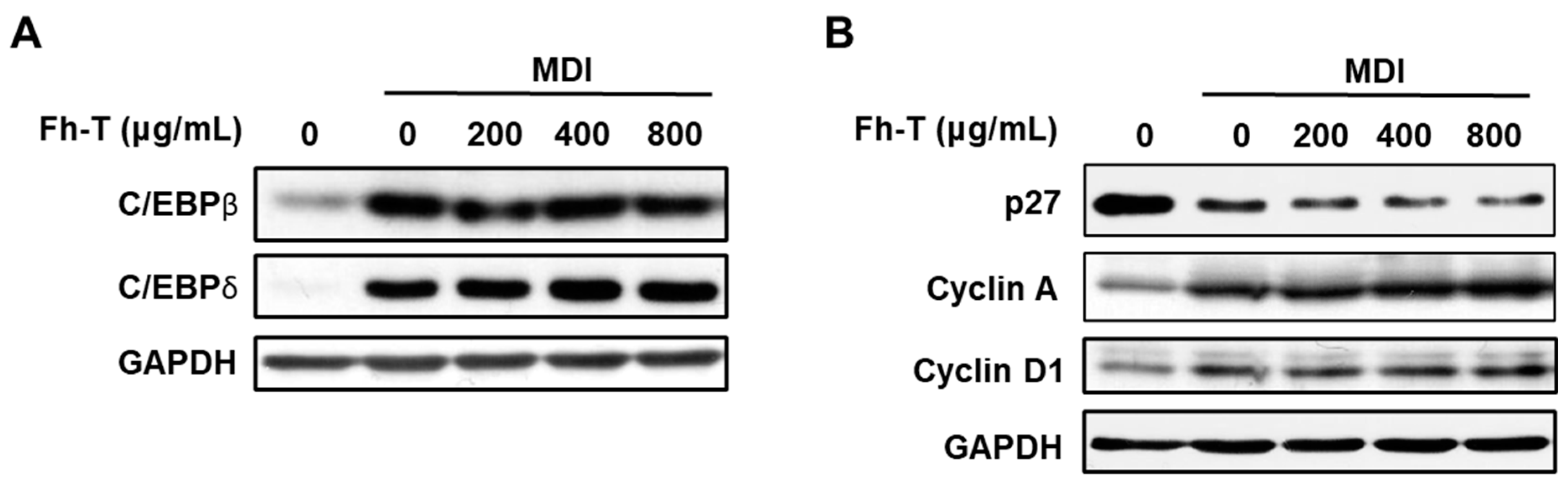
3.2. Fh-T Promotes Adipogenesis of 3T3-L1 Cells Partially Via Regulation of Mitotic Clonal Expansion (MCE)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The role of adipose tissue and nutrition in the regulation of adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, J.; Zhang, Z.; Yin, W. Insights into the unique roles of dermal white adipose tissue (dWAT) in wound healing. Front. Physiol. 2024, 15, 1346612. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, A.D.V.; Reis, A.F.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017, 6, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Zhao, S. Molecular mechanisms of obesity predisposes to atopic dermatitis. Front. Immunol. 2024, 15, 1473105. [Google Scholar] [CrossRef]
- Yuan, C.; Liao, J.; Zheng, L.; Ding, L.; Teng, X.; Lin, X.; Wang, L. Current knowledge of Leptin in wound healing: A collaborative review. Front. Pharmacol. 2022, 13, 968142. [Google Scholar] [CrossRef]
- Cho, H.R.; Lee, S.O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E.; Alkoaik, F. The yellow mealworm as a novel source of protein. Am. J. Agric. Biol. Sci. 2009, 4, 319–331. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jędras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar]
- Baek, N.H.; Seo, M.C.; Kim, M.A.; Yun, E.Y.; Hwang, J.S. The antioxidant activities and hair-growth promotion effects of Tenebrio molitor larvae extracts. J. Life Sci. 2017, 27, 1269–1275. [Google Scholar]
- Dai, C.; Ma, H.; Luo, L.; Yin, X. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 681–689. [Google Scholar] [CrossRef]
- Seo, M.C.; Baek, M.H.; Lee, J.H.; Lee, H.J.; Kim, I.W.; Kim, S.Y.; Kim, M. Osteoblastogenic activity of Tenebrio molitor larvae oil on the MG-63 osteoblastic cell. J. Life Sci. 2019, 29, 1027–1033. [Google Scholar]
- Yu, M.H.; Lee, H.S.; Cho, H.R.; Lee, S.O. Enzymatic preparation and antioxidant activities of protein hydrolysates from Tenebrio molitor larvae. J. Korean Soc. Food Sci. Nutr. 2017, 46, 435–441. [Google Scholar] [CrossRef]
- Park, G.H.; Lee, J.M.; Lim, N.Y.; Lee, S.O. Enzymatic preparation and antioxidant activities of protein hydrolysates derived from tuna byproducts. Korean J. Food Preserv. 2023, 30, 885–895. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lee, H.S.; Cho, H.R.; Kim, K.J.; Kim, J.H.; Safe, S.; Lee, S.O. Dual targeting of Nur77 and AMPKα by isoalantolactone inhibits adipogenesis in vitro and decreases body fat mass in vivo. Int. J. Obes. 2019, 43, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Liu, M.; Zhao, H.; Yaqoob, S.; Zheng, M.; Liu, J. Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. J. Nutr. 2018, 10, 830. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, J.; Park, S.H.; Lee, Y.G. Anti-obesity effects of Glycyrrhiza uralensis ethanol extract on the inhibition of 3T3-L1 adipocyte differentiation in high-fat diet-induced C57BL/6J mice. Korean J. Food Preserv. 2023, 30, 716–728. [Google Scholar] [CrossRef]
- Jung, Y.S.; Jeong, Y.J.; Kim, J.H.; Jeon, C.H.; Lee, S.O. Elecampane (Inula helenium) root extract and its major sesquiterpene lactone, alantolactone, inhibit adipogenesis of 3T3-L1 preadipocytes. Molecules 2022, 27, 4765. [Google Scholar] [CrossRef] [PubMed]
- Caldas, B.V.; Guimarães, V.H.D.; Ribeiro, G.H.M.; dos Santos, T.A.X.; Nobre, D.A.; de Castro, R.J.S.; da Costa, D.V.; de Paula, A.M.B.; Guimarães, A.L.S.; Mafra, V.; et al. Effect of dietary supplementation with Tenebrio molitor wholemeal and fermented flour modulating adipose lipogenesis gene expression in obese mice. J. Insects Food Feed. 2023, 9, 625–635. [Google Scholar] [CrossRef]
- Chilakala, R.; Moon, H.J.; Jung, M.S.; Han, J.W.; Ko, K.H.; Lee, D.S.; Cheong, S.H. Bioactive peptides from Meretrix lusoria enzymatic hydrolysate as a potential treatment for obesity in db/db Mice. Nutrients 2024, 16, 1913. [Google Scholar] [CrossRef]
- Kuptawach, K.; Noitung, S.; Buakeaw, A.; Puthong, S.; Sawangkeaw, R.; Sangtanoo, P.; Srimongkol, P.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Lemon basil seed-derived peptide: Hydrolysis, purification, and its role as a pancreatic lipase inhibitor that reduces adipogenesis by downregulating SREBP-1c and PPAR-γ in 3T3-L1 adipocytes. PLoS ONE 2024, 19, e0301966. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Mojica, L.; González de Mejía, E.; Camacho Ruiz, R.M.; Luna-Vital, D.A. Combinations of legume protein hydrolysates synergistically inhibit biological markers associated with adipogenesis. Foods 2020, 9, 1678. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.B.; Je, J.Y. Low molecular weight blue mussel hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through upregulating HO-1/Nrf2 pathway. Food Res. Int. 2020, 136, 109603. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hao, Y.; Teng, C.; Yao, Y.; Ren, G. Functional properties and adipogenesis inhibitory activity of protein hydrolysates from quinoa (Chenopodium quinoa Willd.). Food Sci. Nutr. 2019, 7, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, M.; Wu, T.; Fang, L.; Liu, C.; Min, W. Novel anti-obesity peptide (RLLPH) derived from hazelnut (Corylus heterophylla Fisch) protein hydrolysates inhibits adipogenesis in 3T3-L1 adipocytes by regulating adipogenic transcription factors and AMPK activation. J. Biosci. Bioeng. 2020, 129, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lim, C.M.; Park, H.M.; Kim, J.; Pham, T.H.; Yang, Y.; Lee, H.P.; Hong, J.T.; Yoon, D.Y. MMPP promotes adipogenesis and glucose uptake via binding to the PPARγ ligand binding domain in 3T3-L1 MBX cells. Front. Pharmacol. 2022, 13, 994584. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Kumar, S.; Rawat, B.; Singh, R.; Purohit, R.; Kumar, D.; Padwad, Y. Kutkin, iridoid glycosides enriched fraction of Picrorrhiza kurroa promotes insulin sensitivity and enhances glucose uptake by activating PI3K/Akt signaling in 3T3-L1 adipocytes. Phytomedicine 2022, 103, 154204. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer Sequence | |
|---|---|---|
| Tbp | Sense | 5′-GTGAAGGGTACAAGGGGGTG-3′ |
| Antisense | 5′-ACATCTCAGCAACCCACACA-3′ | |
| Fas | Sense | 5′-AGCACTGCCTTCGGTTCAGTC-3′ |
| Antisense | 5′-AAGAGCTGTGGAGGCCACTTG-3′ | |
| Leptin | Sense | 5′-GAACCTGTCTACTCATGCCA-3′ |
| Antisense | 5′-CTGGTCCTGCAGCCTGTTTG-3′ | |
| Scd-1 | Sense | 5′-CAGCCGAGCCTTGTAAGTTC-3′ |
| Antisense | 5′-GCTCTACACCTGCCTCTTCG-3′ | |
| Perilipin | Sense | 5′-GATGAGAGCCATGACGACCAGA-3′ |
| Antisense | 5′-TGTGTACCACACCACCCAGGA-3′ | |
| Acaca | Sense | 5′-GAAGCCACAGTGAAATCTCG-3′ |
| Antisense | 5′-GATGGTTTGGCCTTTCACAT-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-J.; Lee, S.-O. Mealworm-Derived Protein Hydrolysates Enhance Adipogenic Differentiation via Mitotic Clonal Expansion in 3T3-L1 Cells. Foods 2025, 14, 217. https://doi.org/10.3390/foods14020217
Ryu H-J, Lee S-O. Mealworm-Derived Protein Hydrolysates Enhance Adipogenic Differentiation via Mitotic Clonal Expansion in 3T3-L1 Cells. Foods. 2025; 14(2):217. https://doi.org/10.3390/foods14020217
Chicago/Turabian StyleRyu, Hee-Jeong, and Syng-Ook Lee. 2025. "Mealworm-Derived Protein Hydrolysates Enhance Adipogenic Differentiation via Mitotic Clonal Expansion in 3T3-L1 Cells" Foods 14, no. 2: 217. https://doi.org/10.3390/foods14020217
APA StyleRyu, H.-J., & Lee, S.-O. (2025). Mealworm-Derived Protein Hydrolysates Enhance Adipogenic Differentiation via Mitotic Clonal Expansion in 3T3-L1 Cells. Foods, 14(2), 217. https://doi.org/10.3390/foods14020217






