Comparative Study of Bulgarian Linden Honey (Tilia sp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples and Study Area
2.2. Methods
2.2.1. Pollen Analysis
2.2.2. Physicochemical Properties
- pH—dissolving 10 g sample in 75 mL of carbon dioxide-free water in a 250 mL beaker. Stirred with a magnetic stirrer, immersion of the pH electrodes in the solution and the pH recorded.
- Water content is determined using a refractometer. Directly after melting, if needed, and homogenization of the sample, the surface of the prism is covered evenly with the sample. After 2 min, the refractive index is ready to be recorded. The water content is the value determined from the refractive index of the honey by reference to a standard table.
- Electrical conductivity—the electrical conductivity of honey is defined as that of a 20% weight in volume solution in water at 20 °C, where the 20% refers to the honey dry matter. The result is expressed in milliSiemens per centimeter (mS.cm−1)/microsiemens per centimeter (µS.cm−1).
- Diastase activity—the unit of Diastase Activity, the Gothe unit, is defined as the amount of enzyme which will convert 0.01 g of starch to the prescribed end-point in one hour at 40 °C under the conditions of the test. The results are expressed as Gothe units (or Schade units) per gram of honey.
- HMF content—the determination of the hydroxymethylfurfural (HMF) content is based on the determination of UV absorbance of HMF at 284 nm. In order to avoid the interference of other components at this wavelength, the difference between the absorbances of a clear aqueous honey solution and the same solution after the addition of bisulfite is determined. The HMF content is calculated after subtraction of the background absorbance at 336 nm. This method is based on the original work of White.
2.2.3. Antioxidant Properties
- Total Phenolic Content (TPC)
- Phenolic compounds by the method of Glories
- 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical activity
- Radical scavenging capacity relative to ABTS•+
- Iron-reducing antioxidant capacity (FRAP) assay.
- CUPRAC (CUPric reducing antioxidant capacity)
- Oxygen Radical Absorbance Capacity (ORAC)
2.2.4. Sensory Analysis
- -
- Visual assessment (appearance, color, purity): 0–5 points;
- -
- Aroma (authenticity, matching the type): 0–4 points;
- -
- Taste (authenticity, matching the type): 0–8 points;
- -
- Texture (consistency, crystallization): 0–3 points.
- -
- Pollen analysis: Tiliaceae 54.1%, Asteraceae 26%, Rosaceae 12.1%, Fabaceae 4.4%, Lamiaceae 1.7%, and Brassicaceae 1.7%;
- -
- Visual assessment: light to medium yellow;
- -
- Physical characteristics: fine crystals;
- -
- Olfactory evaluation: strong smell, chemical, and fresh;
- -
- Taste assessment: medium sweet, chemical and minty, and long and persistent astringent aftertaste.
2.2.5. Statistical Analysis
- All assays were performed in triplicate. One-way ANOVA (p = 0.05) was used for descriptive statistics.
- Principal component analysis (PCA).
3. Results and Discussion
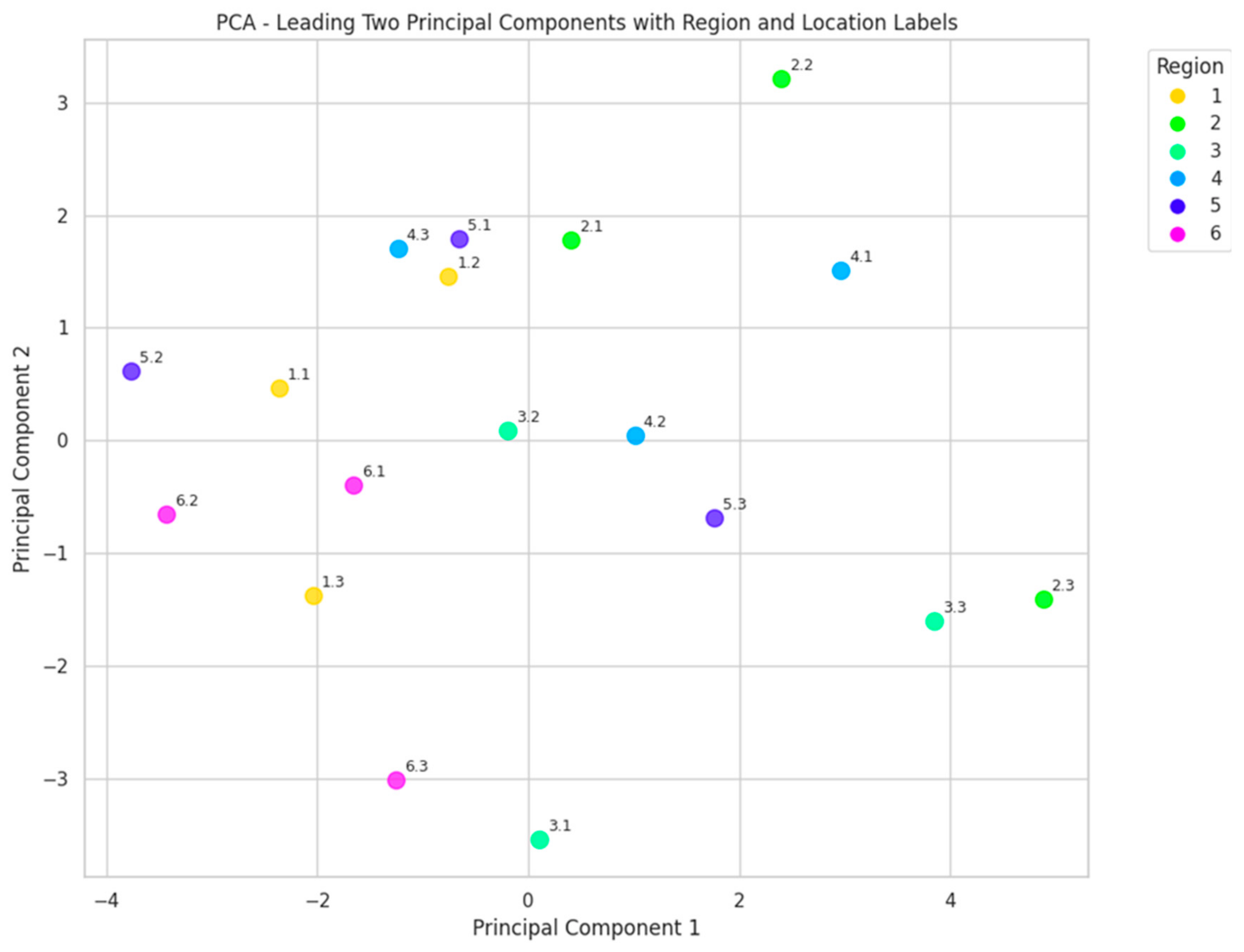
3.1. Pollen Analysis
3.2. Physicochemical Properties
3.3. Antioxidant Activity
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Council Directive of the European Union. Council directive 2001/110/ec of 20 december 2001 relating to honey. Off. J. Eur. Communities 2002, 47–52.
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern Methods for Assessing the Quality of Bee Honey and Botanical Origin Identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Lazarieva, L.; Akymenko, L.; Postoienko, H.; Postoienko, V.; Nikitina, L.; Zasiekin, D.; Razanov, S.; Nedosekov, V.; Amons, S.; Razanova, A.; et al. Specific quality indicators of monofloral linden honey. IJEES 2023, 13, 161–168. [Google Scholar] [CrossRef]
- Tzonev, R. Silver linden (Tilia tomentosa) Forests. 2003. Available online: http://e-ecodb.bas.bg/rdb/bg/vol3/23G1.html (accessed on 29 November 2024).
- Regulation 9 from 22 June 2005 about the Terms and Conditions for Approval and Registration of Wax Processing Enterprises and Production of Wax Bases, as well as the Enterprises for Production and Trade of Honey and Bee Products, State Gazette, no. 54 dated 1 July 2005. Available online: https://bfsa.egov.bg/wps/wcm/connect/bfsa.egov.bg19113/b9db2e69-4027-4821-ac9c-47aebaacaa57/NAREDBA___9_ot_22062005_g_za_usloviqta_i_reda_za_odobrqvane_i_registraciq_na_predpriqtiqta_za_pre.pdf?MOD=AJPERES&CVID=obJPTht (accessed on 2 January 2025).
- BDS 3050:1980; Honey. Rules for Sampling and Testing Methods. Bulgarian Institute for Standardization: Sofia, Bulgaria, 1980. Available online: https://bds-bg.org/en/project/show/bds:proj:22905 (accessed on 2 January 2025).
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- International Honey Commission. World Network of Honey Science. Harmonized Methods of the International Honey Commission. 2009. Available online: http://ihc-platform.net/ihcmethods2009.pdf (accessed on 29 November 2024).
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and odor of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Shopska, V.; Teneva, D.; Denkova-Kostova, R.; Ivanova, K.; Denev, P.; Kostov, G. Modeling of malt mixture for the production of wort with increased biological value. Beverages 2022, 8, 44. [Google Scholar] [CrossRef]
- Nedyalkov, P.; Qnkova-Nikolova, A.; Kolev, N.; Vlahova-Vangelova, D. Sensory and antioxidant properties of mead with added beehive products. Food Sci. Appl. Biotechnol. 2024, 7, 231–238. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Dinkova, R.; Heffels, P.; Shikov, V.; Weber, F.; Schieber, A.; Mihalev, K. Effect of enzyme-assisted extraction on the chilled storage stability of bilberry (Vaccinium myrtillus L.) anthocyanins in skin extracts and freshly pressed juices. Int. Food Res. 2014, 65, 35–41. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescence probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidant properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Nakib, R.; Ghorab, A.; Harbane, S.; Saker, Y.; Ouelhadj, A.; Rodríguez-Flores, M.S.; Seijo, M.C.; Escuredo, O. Sensory Attributes and Chemical Composition: The Case of Three Monofloral Honey Types from Algeria. Foods 2024, 13, 2421. [Google Scholar] [CrossRef]
- Persano Oddo and Piro, International Honey Commission, Appendix I. Glossary for Unifloral Honeys Sensory Description. 2004. Available online: https://www.ihc-platform.net/appendix.pdf (accessed on 29 November 2024).
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological Maps for the Main European Woody Species. Data Brief 2017, 12, 662–666. Available online: https://commons.wikimedia.org/wiki/File:Tilia_tomentosa_range.svg (accessed on 2 January 2025). [CrossRef]
- Ramly, N.S.; Sujanto, I.S.R.; Ghani, A.A.; Huat, J.T.Y.; Alias, N.; Ngah, N. The Impact of Processing Methods on the Quality of Honey: A Review. MyJAS 2021, 6, 99–110. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality assessment of raw honey issued from eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T.J. Review article: Methods of analysis of honey. Apic. Res. 2018, 57, 38–74. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Raweh, H.S.A.; Badjah-Hadj-Ahmed, A.Y.; Iqbal, J.; Alqarni, A.S. Physicochemical Composition of Local and Imported Honeys Associated with Quality Standards. Foods 2023, 12, 2181. [Google Scholar] [CrossRef]
- Raweh, H.S.A.; Badjah-Hadj-Ahmed, A.Y.; Iqbal, J.; Alqarni, A.S. Impact of Different Storage Regimes on the Levels of Physicochemical Characteristics, Especially Free Acidity in Talh (Acacia gerrardii Benth.) Honey. Molecules 2022, 27, 5959. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.N.; Hanková, M.; Kružík, V.; Grégrová, A.; Škorpilová, T.; Štarha, P.; Tran, V.N.; Čížková, H. Determination of volatile compound profiles and physico-chemical analysis of linden and acacia Czech honey. J. Apic. Res. 2023, 62, 374–382. [Google Scholar] [CrossRef]
- Zhuk, A.; Sytnikova, I.; Fylypchuk, T.; Bahlei, O.; Shkrobanets, O.; Danihlík, J.; Moskalyk, H.; Panchuk, I.; Burkut, V.; Angelstam, P.; et al. Physicochemical quality indicators of honey: An evaluation in a Ukrainian socioecological gradient. Regul. Mech. Biosyst. 2022, 13, 354–361. [Google Scholar] [CrossRef]
- Bodor, Z.; Benedek, C.; Urbin, A.; Szabó, D.; Sipos, L. Color of honey: Can we trust the Pfund scale?—An alternative graphical tool covering the whole visible spectra. LWT 2021, 149, 111859. [Google Scholar] [CrossRef]
- Zou, S.; Tao, H.; Chang, Y.-N. Characterization of antioxidant activity and analysis of phenolic acids and flavonoids in linden honey. Food Sci. Technol 2022, 42, e76621. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Petrova, P.; Mrmošanin, J.; Petrović, S.; Chochkova, M.; Jovanović, V. Evaluation of Antioxidant Properties and Phytochemical Composition in Varietal Honeys. JUCTM 2024, 59, 779–788. [Google Scholar] [CrossRef]
- Tkač, M.; Abdullah, F.A.A.; Vorlová, L.; Bartáková, K.; Bursová, Š.; Javůrková, Z. Quality Characterization of Honeys from Iraqi Kurdistan and Comparison with Central European Honeys. Foods 2024, 13, 2228. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Farkas, Á.; Balázs, V.L.; Kõszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked With Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef]
- Dumbrava, D.G.; Bordean, D.M.; Raba, D.N.; Druga, M.; Moldovan, C.; Popa, M.V. Antioxidant properties and other physicochemical characteristics of some honey varieties from the western Romanian area. In Proceedings of the International Multidisciplinary Scientific GeoConference SGEM 2013, Albena, Bulgaria, 16–22 June 2013; pp. 101–108. [Google Scholar]
- Aktaş, K.A.; Huseyin, S. Determination of enzyme inhibition and antioxidant activity in some chestnut honeys. Foods Raw Mater. 2018, 6, 210–221. [Google Scholar]
- Kaygusuz, H.; Tezcan, F.; Erim, F.B.; Yildiz, O.; Sahin, H.; Can, Z.; Kolayli, S. Characterization of Anatolian honeys based on minerals, bioactive components and principal compon. LWT—Food Sci. Technol. 2016, 68, 273–279. [Google Scholar] [CrossRef]
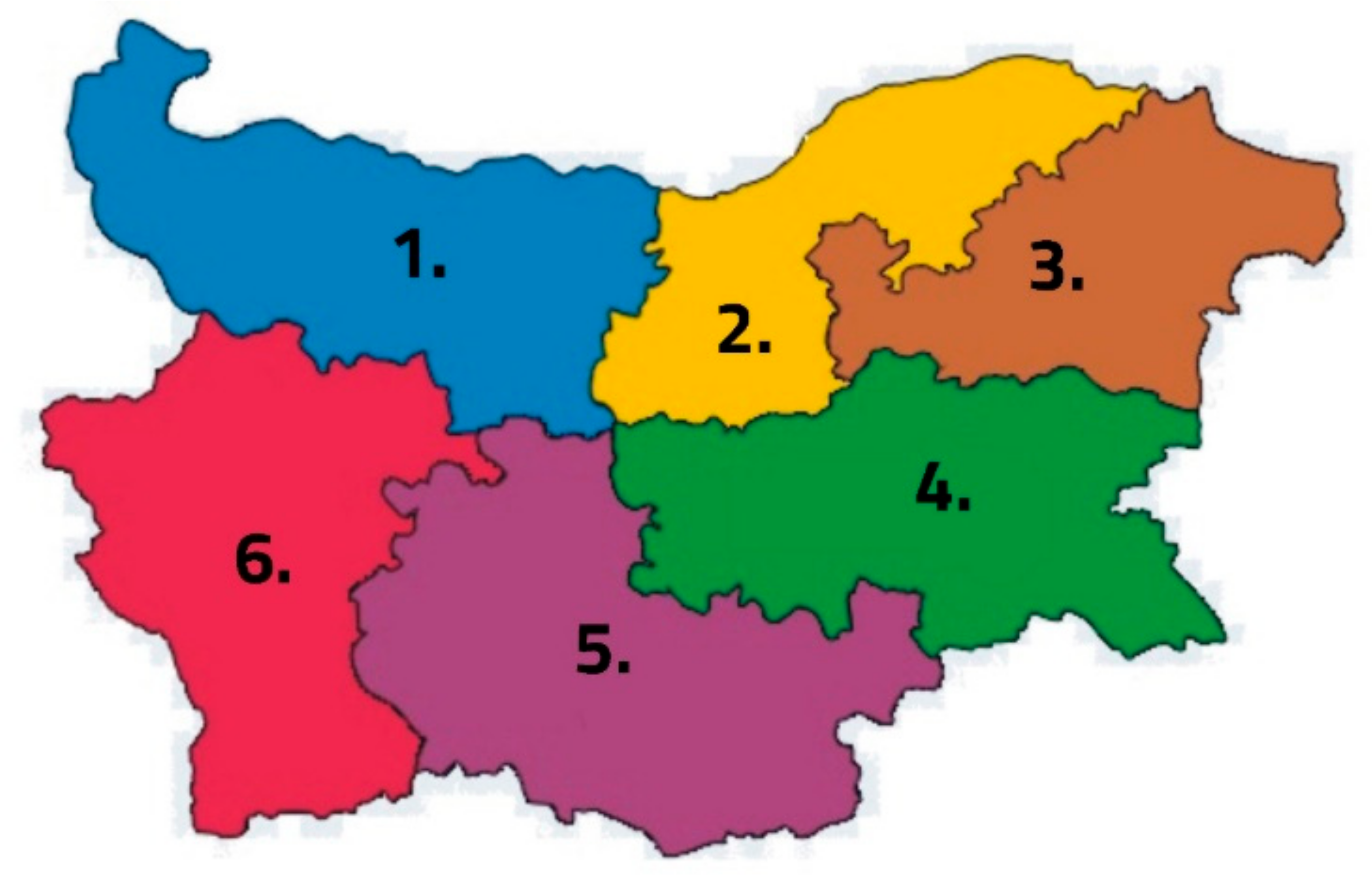

| District | Sample | Town/Village | GPS Coordinates of the Region | Bee Type | Municipality | Region |
|---|---|---|---|---|---|---|
| Northwest | 1.1 | Mramoren village | 43.298096°, 23.678137° | Apis mellifera rodopica | Vratza | Vratza |
| 1.2 | Gorno Pestene village | 43.277092°, 23.710482° | Apis mellifera rodopica | Vratza | Vratza | |
| 1.3 | Koynare village | 43.355699°, 24.143577° | Apis mellifera rodopica | Cherven bryag | Pleven | |
| North Central | 2.1 | Yuper village | 43.916667°, 26.4° | Apis mellifera rodopica | Kubrat | Razgrad |
| 2.2 | Dulovo village | 43.822946°, 27.141233° | Apis mellifera rodopica | Dulovo | Silistra | |
| 2.3 | The town of Silistra | 44.109238°, 27.265381° | Apis mellifera rodopica | Silistra | Silistra | |
| Northeast | 3.1 | Avren village | 43.110168°, 27.667995° | Apis mellifera rodopica | Avren | Varna |
| 3.2 | Prilep village | 42.849899°, 26.929908° | Apis mellifera rodopica | Dobrich | Dobrich | |
| 3.3 | Donchevo village | 43.516667°, 27.766667° | Apis mellifera rodopica | Dobrich | Dobrich | |
| Southeast | 4.1 | Srednogorovo village | 42.531195°, 25.330981° | Apis mellifera rodopica | Kazanlyk | Stara Zagora |
| 4.2 | The town of Aytos | 42.698961°, 27.248972° | Apis mellifera rodopica | Aytos | Sliven | |
| 4.3 | Omarchevo village | 42.45791°, 26.144843° | Apis mellifera rodopica | Nova Zagora | Sliven | |
| South Central | 5.1 | The town of Kardzhali | 41.644721°, 25.374966° | Apis mellifera rodopica | Kardzhali | Kardzhali |
| 5.2 | The town of Simeonovrgad | 42.03236°, 25.834458° | Apis mellifera rodopica | Simeonovgrad | Haskovo | |
| 5.3 | The town of Kalofer | 42.611157°, 24.974847° | Apis mellifera rodopica | Karlovo | Plovdiv | |
| Southwest | 6.1 | The town of Petrich | 41.398129°, 23.206857° | Apis mellifera rodopica | Petrich | Blagoevgrad |
| 6.2 | The town of Petrich | 41.398129°, 23.206857° | Apis mellifera rodopica | Petrich | Blagoevgrad | |
| 6.3 | Vaksevo village | 42.162079°, 22.857866° | Apis mellifera rodopica | Nevestino | Kyustendil |
| Basic Category | Sub-Category | Individual Aroma |
|---|---|---|
| Vegetal | green | raw bean, crumpled leave, vegetation, after the rain |
| dry | pale malt, straw, tea, dry hay | |
| Woody | dry | leafy wood, dust, walnut, hazelnut |
| resinous | cedar, pine resin, propolis | |
| spicy | clove, nutmeg, coffee | |
| Chemical | petrochemical | styrene, paint, solvent |
| medicine | household soap, vitamin B1 | |
| Fresh | refreshing | mint, eucalyptus, aniseed |
| citrus fruit | lemon, orange, grapefruit | |
| Floral, fresh fruit | floral | orange blossom, violet, rose, hyacinth |
| fruit | pear, apple, red fruit, blackcurrant, coconut, apricot, exotic fruit | |
| Warm | burned | molasses, burned sugar |
| of cooked fruit | dates, plums, figs, raisins, candied fruits | |
| caramelized | toffee, caramel, brown sugar | |
| subtle | fresh butter, vanilla, beeswax, almond paste | |
| Spoiled | pungent | piquant cheese, vinegar |
| animal | cheese, perspiration, cowshed, cat’s urine | |
| moldy | damp floorcloth, humus, stuffy | |
| sulfur | globe artichoke, cabbage |
| Sample | Tilia | Fabaceae | Helianthus annuus | Paliurus spina christi | Apiaceae/Umbelliferae | Cruciferae/Brassica | Coriandrum | Asteraceae | Foeniculum | Rhamnaceae | Lamiaceae | Rosaceae | Trifolium | Amorpha | Vicki | Ericaceae/Vaccinium | Lotus | Liliaceae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 60% | 15% | 4% | 2% | 2% | |||||||||||||
| 1.2 | 78% | 10% | 2% | 3% | 1% | |||||||||||||
| 1.3 | 12% | 62% | 2% | 10% | 3% | |||||||||||||
| 2.1 | 78% | 2% | 10% | 1% | ||||||||||||||
| 2.2 | 77% | 2% | 1% | 1% | 12% | |||||||||||||
| 2.3 | 75% | 7% | 13% | 2% | ||||||||||||||
| 3.1 | 8% | 10% | 27% | 2% | 33% | 2% | 1% | |||||||||||
| 3.2 | 65% | 4% | 20% | 2% | ||||||||||||||
| 3.3 | 68% | 5% | 7% | 5% | 2% | |||||||||||||
| 4.1 | 60% | 15% | 3% | 1% | ||||||||||||||
| 4.2 | 42% | 10% | 26% | 2% | 2% | |||||||||||||
| 4.3 | 15% | 27% | 29% | 8% | 2% | 4% | 1% | 1% | ||||||||||
| 5.1 | 54% | 27% | 2% | 1% | 1% | |||||||||||||
| 5.2 | 11% | 9% | 3% | 1% | 3% | 32% | 9% | 3% | ||||||||||
| 5.3 | 35% | 40% | 4% | 2% | 1% | 1% | 4% | |||||||||||
| 6.1 | 16% | 31% | 3% | 39% | ||||||||||||||
| 6.2 | 39% | 2% | 8% | 41% | 1% | |||||||||||||
| 6.3 | 59% | 5% | 10% | 8% |
| Sample | pH | HMF, mg/kg | Diastase Activity, Goethe Units | Water Content, % | Electrical Conductivity, μS·cm−1 | mm Pfund | The Pfund Honey Color Grading System | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 4.44 f | 3.20 ± 1,13 e | 5.84 g | 17.0 d | 348 ± 1.41 m | 25 gf | white | 68.70 ± 1.40 cd | −1.78 ± 0.60 i | 34.49 ± 1.54 e |
| 1.2 | 4.93 b | 6.03 ± 1.49 d | 8.08 d | 17.8 c | 671 ± 2.12 d | 28 f | white | 75.12 ± 1.35 b | −3.11 ± 0.12 j | 31.27 ± 2.07 e |
| 1.3 | 4.00 h | 1.38 ± 0.53 f | 8.48 d | 18.1 c | 283 ± 2.83 o | 41 d | extra white amber | 64.22 ± 0.60 d | 1.29 ± 0.42 ef | 56.62 ± 2.46 a |
| 2.1 | 4.79 cd | 4.79 ± 1.64 d | 10.20 c | 18.9 ab | 627 ± 4.24 f | 38 e | extra white amber | 71.14 ± 1.64 c | −4.25 ± 0.40 k | 47.64 ± 1.88 b |
| 2.2 | 5.51 a | n.d | 5.52 g | 16.2 e | 670 ± 2.83 d | 39 de | extra white amber | 67.01 ± 1.06 d | −0.98 ± 0.57 g | 42.32 ± 1.01 d |
| 2.3 | 4.77 cd | 5.92 ± 0.96 d | 13,20 a | 16.2 e | 834 ± 2.83 b | 69 a | light amber | 59.93 ± 2.05 e | 6.87 ± 1.20 c | 45.49 ± 1.26 c |
| 3.1 | 4.10 g | 16.52 ± 2.09 a | 11.56 b | 17.0 d | 479 ± 0.71 k | 57 c | light amber | 55.86 ± 1.21 f | 9.45 ± 0.62 b | 45.22 ± 1.83 c |
| 3.2 | 4.42 f | 7.94 ± 1.39 d | 8.24 d | 17.2 d | 545 ± 4.24 i | 39 de | extra white amber | 64.50 ± 1.95 d | −0.45 ± 0.55 g | 55.32 ± 2.74 a |
| 3.3 | 4.78 cd | n.d | 6.84 f | 18.2 c | 858 ± 7.07 a | 61 bc | light amber | 51.70 ± 0.67 g | 13.73 ± 0.84 a | 37.70 ± 2.07 e |
| 4.1 | 4.97 a b | n.d | 4.92 h | 16.9 d | 566 ± 4.95 h | 43 d | extra white amber | 59.93 ± 0.54 e | 4.57 ± 0.59 d | 45.25 ± 0.37 c |
| 4.2 | 4.41 f | 8.60 ± 3.82 d | 7.28 e | 16.6 de | 494 ± 1.41 j | 58 c | light amber | 65.58 ± 2.55 d | 1.65 ± 1.76 ip | 56.96 ± 1.22 a |
| 4.3 | 4.50 e | 22.12 ± 4.00 a | 3.24 i | 16.0 ef | 475 ± 1.41 l | 44 d | extra white amber | 74.88 ± 1.35 b | −4.81 ± 0.52 k | 51.87 ± 2.58 b |
| 5.1 | 4.82 c | 11.27 ± 2.16 b | 8.56 d | 17.4 cd | 577 ± 0.71 g | 32 f | white | 78.39 ± 1.20 a | −3.26 ± 0.04 j | 25.95 ± 1.63 f |
| 5.2 | 4.85 c | 18.48 ± 3.45 a | 5.16 g | 19.4 a | 307 ± 0.71 n | 23 g | white | 74.40 ± 1.34 b | −1.63 ± 0.05 i | 21.87 ± 1.64 g |
| 5.3 | 4.44 f | n.d | 9.80 c | 15.4 g | 647 ± 4.24 e | 42 d | extra white amber | 66.90 ± 0.51 d | 1.42 ± 0.62 ip | 41.05 ± 1.43 d |
| 6.1 | 4.91 bc | 6.54 ± 1.15 d | 10.08 c | 18.0 c | 721 ± 2.83 c | 36 ef | extra white amber | 70.32 ± 1.96 c | −1.65 ± 0.42 i | 33.95 ± 2.78 e |
| 6.2 | 3.88 i | 11.90 ± 0.89 c | 6.44 f | 17.8 c | 550 ± 0.71 i | 17 h | extra white | 70.08 ± 0.51 c | −1.36 ± 0.16 h | 25.88 ± 0.54 f |
| 6.3 | 4.00 h | 14.35 ± 1.49 b | 10.00 c | 16.8 e | 309 ± 2.12 n | 43 d | extra white amber | 66.45 ± 1.23 d | 2.54 ± 0.56 ip | 36.35 ± 0.90 e |
| Glories Method | Glories Method | Glories Method | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | TPC (mg GA·kg−1) | Total Phenols (mg GAE·L−1) | Phenolic Acids (mg CAE·L−1) | Flavonoids (mg QE·L−1) | DPPH (µmol·L−1) | ABTS (µmol TE·L−1) | FRAP (µmol·L−1) | CUPRAC (µmol TE·L−1) | ORAC (µmol TE·100−1) |
| 1.1 | 3456.28 ± 7.01 m | 751.33 ± 2.78 k | 583.09 ± 1.64 j | NF | 962.37 ± 6.21 k | 1090.37 ± 7.33 q | 1776.75 ± 7.30 n | 899.90 ± 4.32 o | 475.3 ± 7.4 h |
| 1.2 | 3682.22 ± 6.49 j | 1458.00 ± 3.07 g | 1128.62 ± 2.58 g | NF | 578.20 ± 1.07 p | 1881.33 ± 4.10 h | 2671.84 ± 4.86 k | 1280.99 ± 3.74 m | 436.3 ± 4.6 hj |
| 1.3 | 3781.63 ± 6.88 i | 328.00 ± 3.76 q | 182.28 ± 2.70 q | NF | 903.71 ± 6.48 l | 1233.20 ± 3.37 o | 2618.96 ± 4.85 l | 1380.24 ± 2.93 l | 595.2 ± 21.5 g |
| 2.1 | 3376.91 ± 5.02 n | 1688.00 ± 3.29 c | 1325.37 ± 2.52 b | NF | 1070.38 ± 3.26 j | 1815.07 ± 4.28 j | 2898.83 ± 5.91 i | 1462.14 ± 4.92 j | 913.2 ± 1.2 e |
| 2.2 | 4028.40 ± 4.64 f | 1648.00 ± 4.53 d | 1243.25 ± 3.66 d | NF | 542.52 ± 2.28 q | 2067.49 ± 4.01 g | 2614.29 ± 3.15 l | 7273.68 ± 3.37 a | 1177.1 ± 13.6 c |
| 2.3 | 4554.54 ± 7.61 c | 1833.00 ± 3.84 a | 1387.97 ± 2.88 a | NF | 2064.52 ± 5.05 c | 4766.60 ± 26.80 a | 5220.40 ± 2.13 a | 2204.77 ± 3.52 d | 1019.2 ± 6.3 d |
| 3.1 | 4733.72 ± 7.28 b | 684.67 ± 2.64 l | 446.50 ± 2.45 m | NF | 1115.35 ± 5.67 i | 1729.10 ± 7.03 k | 4218.10 ± 6.30 e | 2061.02 ± 4.07 f | 405.7 ± 15.3 h |
| 3.2 | 3652.75 ± 2.84 k | 1166.33 ± 4.29 i | 911.54 ± 3.14 h | NF | 876.34 ± 4.74 m | 1629.60 ± 4.86 m | 2744.29 ± 6.29 j | 1509.80 ± 4.56 i | 799.4 ± 8.6 e |
| 3.3 | 5191.89 ± 6.50 a | 1619.67 ± 4.71 e | 1231.87 ± 2.79 e | NF | 1608.50 ± 7.71 d | 2535.19 ± 4.40 d | 4486.86 ± 6.31 d | 2261.73 ± 4.82 d | 742.3 ± 6.9 f |
| 4.1 | 4267.31 ± 7.26 e | 1738.00 ± 3.79 b | 1313.983.21 c | NF | 1358.75 ± 7.49 f | 2742.44 ± 5.89 b | 4132.50 ± 6.18 f | 5794.19 ± 4.25 b | 1267.1 ± 22.4 b |
| 4.2 | 4254.34 ± 6.68 e | 781.33 ± 3.38 j | 538.37 ± 2.55 k | NF | 1534.70 ± 2.55 e | 2243.79 ± 4.81 e | 4796.52 ± 5.14 c | 4640.16 ± 2.98 c | 1041.4 ± 16.7 d |
| 4.3 | 3785.96 ± 6.54 j | 668.00 ± 3.60 m | 496.10 ± 2.74 l | NF | 2074.29 ± 7.30 c | 1656.65 ± 4.04 l | 2935.64 ± 8.54 h | 1516.23 ± 4.45 i | 1423.9 ± 5.7 a |
| 5.1 | 3886.16 ± 7.05 g | 1248.00 ± 1.90 h | 918.05 ± 2.14 i | NF | 691.10 ± 5.65 o | 1871.24 ± 5.54 i | 5101.50 ± 4.62 b | 1539.62 ± 4.09 h | 794.3 ± 63.0 e |
| 5.2 | 3798.14 ± 5.70 h | 628.00 ± 10.25 n | 307.48 ± 2.48 n | NF | 1335.78 ± 7.84 g | 1182.31 ± 3.35 p | 3598.19 ± 6.59 g | 895.29 ± 4.46 o | 448.4 ± 35.1 h |
| 5.3 | 4386.37 ± 3.35 d | 1511.33 ± 3.73 f | 1193.66 ± 2.74 f | NF | 2582.60 ± 4.81 b | 2609.24 ± 3.63 c | 1486.37 ± 4.61 p | 2121.91 ± 4.85 e | 617.8 ± 22.8 g |
| 6.1 | 3780.06 ± 6.79 i | 466.33 ± 3.09 o | 289.59 ± 2.13 o | NF | 2630.99 ± 4.72 a | 1596.81 ± 4.99 n | 1987.96 ± 4.84 m | 1420.52 ± 5.32 k | 435.4 ± 0.7 hj |
| 6.2 | 3513.26 ± 7.93 l | 196.33 ± 3.32 r | 110.73 ± 2.16 r | NF | 775.66 ± 9.25 n | 959.69 ± 4.12 r | 1726.50 ± 8.67 o | 1156.04 ± 4.07 n | 412.6 ± 9.5 hk |
| 6.3 | 5203.68 ± 6.08 a | 433.00 ± 3.53 p | 187.97 ± 2.23 p | NF | 1203.32 ± 3.66 h | 2220.18 ± 4.05 f | 4481.89 ± 7.57 d | 1910.65 ± 5.37 g | 455.7 ± 4.7 Hi |
| Sample | I. Visual Evaluation—Appearance, Color, Cleanliness (0–5 Points) | II. Aroma (0–4 Points) | III. Taste (0–8 Points) | IV. Texture (0–3 Points) | Overall Rating | Notes |
|---|---|---|---|---|---|---|
| 1.1 | 4.0 ± 0.63 | 3.3 ± 0.82 | 5.71.37 ± | 2.8 ± 1.33 | 15.8 ± 0.98 | Light white/beige color, large crystals, fresh, not so strong linden taste, very sweet |
| 1.2 | 4.4 ± 1.19 | 3.9 ± 0.35 | 7.1 ± 1.13 | 2.8 ± 0.46 | 18.1 ± 2.64 | Light yellow color, medium and fine crystal, herbal freshness, intense taste of linden, light pleasant aroma, typical astringent aftertaste |
| 1.3 | 3.9 ± 1.36 | 3.3 ± 0.89 | 5.9 ± 1.46 | 2.3 ± 0.71 | 15.3 ± 3.58 | Light yellow color, small, fine crystals, very smooth structure, fruity taste and aroma, does not meet the characteristics of the linden type; short aftertaste |
| 2.1 | 4.0 ± 1.00 | 3.3 ± 1.15 | 5.7 ± 2.52 | 2.3 ± 1.15 | 15.3 ± 5.69 | Intense yellow color, small and round crystals, perfume aroma and fruity taste, atypical for linden, very sweet and slightly sour taste |
| 2.2 | 4.5 ± 0.58 | 3.5 ± 1.00 | 4.8 ± 2.50 | 3.0 ± 0.00 | 15.8 ± 3.20 | Light white/beige color, medium size crystals, weak aroma, fresh herbal, quite astringent taste. |
| 2.3 | 2.3 ± 0.58 | 2.0 ± 0.00 | 3.3 ± 2.89 | 2.3 ± 1.15 | 10.0 ± 4.36 | Fine crystals, atypical aroma and taste, bitter taste |
| 3.1 | 3.0 ± 1.63 | 2.7 ± 1.53 | 3.7 ± 3.06 | 1.3 ± 0.58 | 8.8 ± 6.55 | Light caramel color, atypical aroma and taste of caramel, too sweet, no aftertaste |
| 3.2 | 4.5 ± 0.58 | 3.5 ± 0.58 | 6.0 ± 1.63 | 2.5 ± 1.00 | 16.5 ± 2.52 | Creamy, fine crystals, fresh herbal aroma of linden, hint of mint, astringent aftertaste |
| 3.3 | 2.0 ± 1.41 | 3.0 ± 0.00 | 7.0 ± 0.00 | 2.0 ± 0.00 | 14.0 ± 1.41 | Large, coarse crystals, typical chemical plant aroma, sweet, no aftertaste |
| 4.1 | 3.8 ± 0.84 | 2.8 ± 1.10 | 6.8 ± 1.10 | 2.0 ± 0.71 | 15.4 ± 3.36 | Creamy, sticky, vegetal taste with a mint note, moderately sweet with a bitter aftertaste |
| 4.2 | 3.0 ± 0.00 | 2.0 ± 0.00 | 5.0 ± 0.00 | 3.0 ± 0.00 | 13.0 ± 0.00 | Darker than the control, less sweet |
| 4.3 | 3.3 ± 1.71 | 3.0 ± 1.15 | 6.3 ± 1.50 | 2.0 ± 0.00 | 14.5 ± 4.12 | Yellow color, fine crystals, pale fruity aroma, sour taste, no aftertaste. |
| 5.1 | 4.4 ± 0.55 | 3.4 ± 0.55 | 6.6 ± 0.89 | 2.4 ± 0.55 | 16.8 ± 1.64 | Compact and hard lumps of crystals, floral aroma, slightly sour taste of fermentation |
| 5.2 | 4.0 ± 1.00 | 1.7 ± 1.15 | 4.3 ± 0.58 | 1.7 ± 1.15 | 11.7 ± 3.79 | Creamy texture, sweet aroma and sour taste, fermented |
| 5.3 | 2.5 ± 2.12 | 3.0 ± 0.00 | 6.0 ± 0.00 | 2.0 ± 0.00 | 13.5 ± 2.12 | Fine crystal, weaker linden taste and aroma compared to the control |
| 6.1 | 2.2 ± 1.64 | 1.4 ± 1.67 | 3.2 ± 3.27 | 0.8 ± 0.84 | 7.6 ± 7.09 | Liquid without crystals, chestnut aroma, very aromatic sweet beginning, long aftertaste |
| 6.2 | 1.0 ± 0.00 | 1.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 2.0 ± 0.00 | Lightly fresh and sour, lemon-like in taste only, fruity aroma, taste and aroma not matching linden |
| 6.3 | 1.3 ± 0.58 | 1.0 ± 1.73 | 1.0 ± 1.73 | 1.3 ± 0.58 | 4.7 ± 4.62 | Coarse crystal, association with liquid soap and medicine, taste and aroma not corresponding to linden |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yankova-Nikolova, A.; Vlahova-Vangelova, D.; Balev, D.; Kolev, N.; Dragoev, S.; Lowndes-Nikolova, B. Comparative Study of Bulgarian Linden Honey (Tilia sp.). Foods 2025, 14, 175. https://doi.org/10.3390/foods14020175
Yankova-Nikolova A, Vlahova-Vangelova D, Balev D, Kolev N, Dragoev S, Lowndes-Nikolova B. Comparative Study of Bulgarian Linden Honey (Tilia sp.). Foods. 2025; 14(2):175. https://doi.org/10.3390/foods14020175
Chicago/Turabian StyleYankova-Nikolova, Anastasiya, Desislava Vlahova-Vangelova, Desislav Balev, Nikolay Kolev, Stefan Dragoev, and Biljana Lowndes-Nikolova. 2025. "Comparative Study of Bulgarian Linden Honey (Tilia sp.)" Foods 14, no. 2: 175. https://doi.org/10.3390/foods14020175
APA StyleYankova-Nikolova, A., Vlahova-Vangelova, D., Balev, D., Kolev, N., Dragoev, S., & Lowndes-Nikolova, B. (2025). Comparative Study of Bulgarian Linden Honey (Tilia sp.). Foods, 14(2), 175. https://doi.org/10.3390/foods14020175







