Optimization of Calibration Strategies for the Quantification of Volatile Compounds in Virgin Olive Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Quality Parameters and Sensory Assessment
2.4. Volatile Compound Analysis
2.4.1. External Matrix-Matched Standard Calibration (EC)
2.4.2. External Matrix-Matched Standard with Internal Standard Calibration (EC with IS)
2.4.3. Standard Addition Calibration (AC)
2.4.4. Standard Addition with Internal Standard Calibration (AC with IS)
2.5. Analytical–Statistical Approach
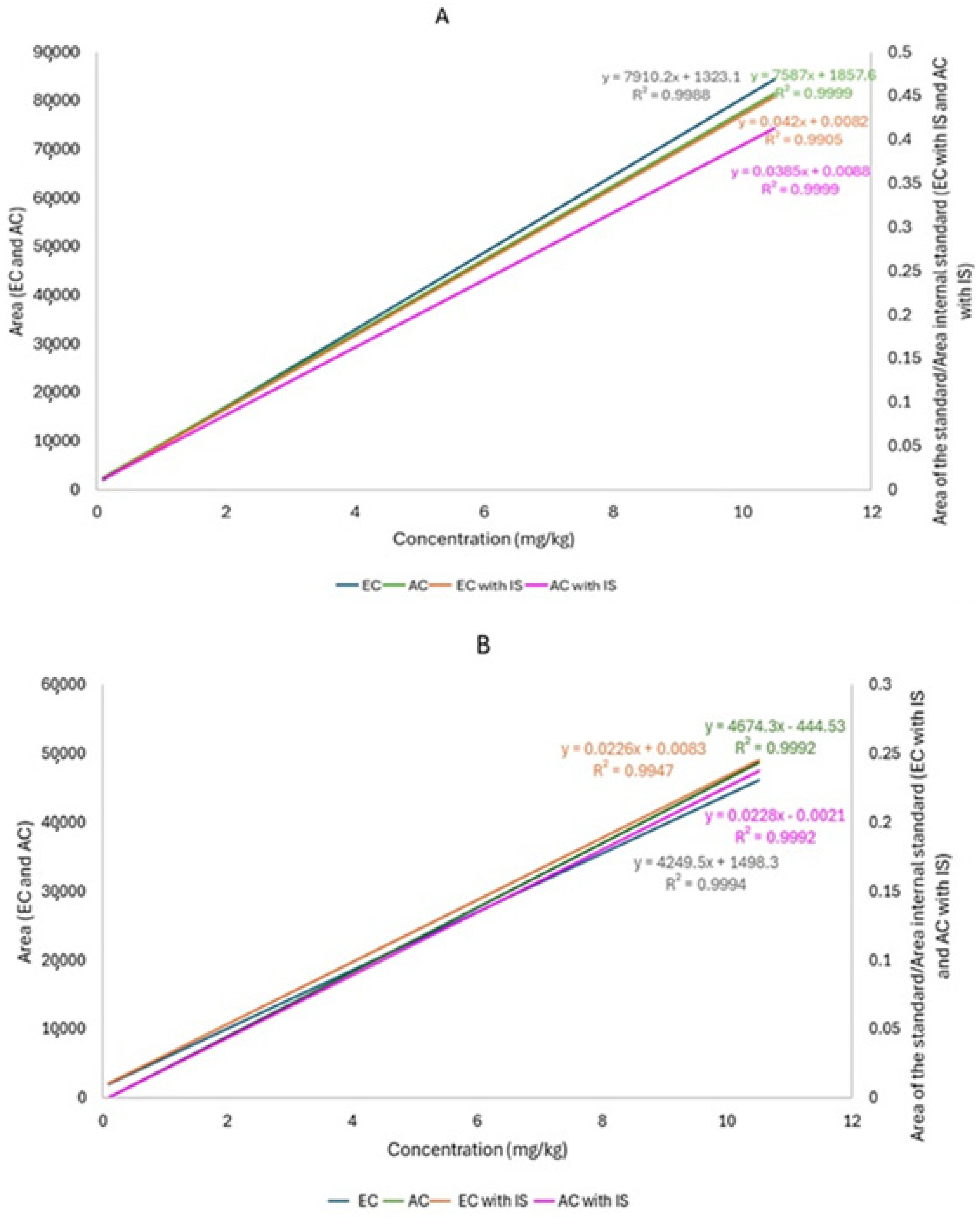
2.5.1. Linearity
2.5.2. Sensitivity
2.5.3. Accuracy
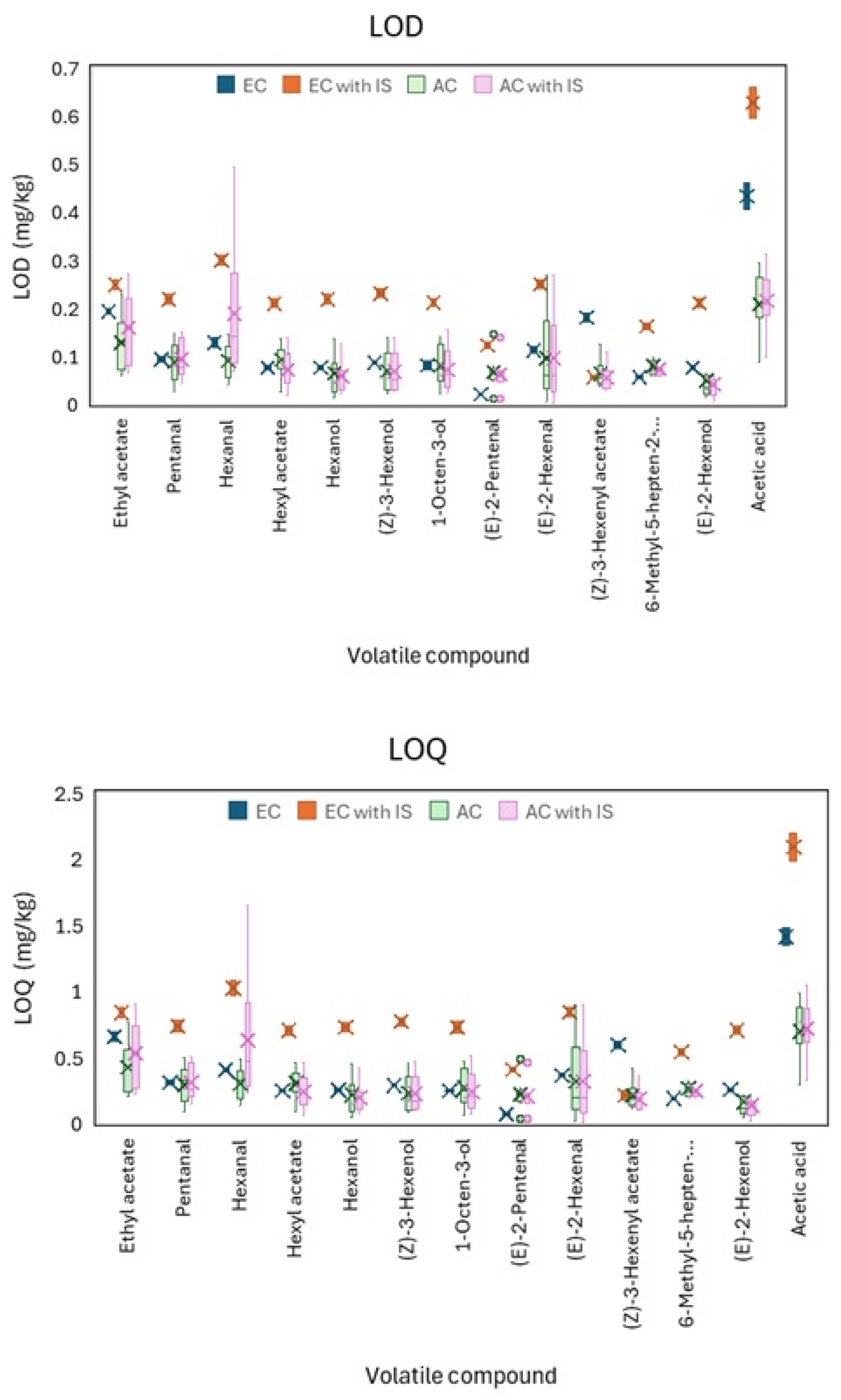
2.5.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
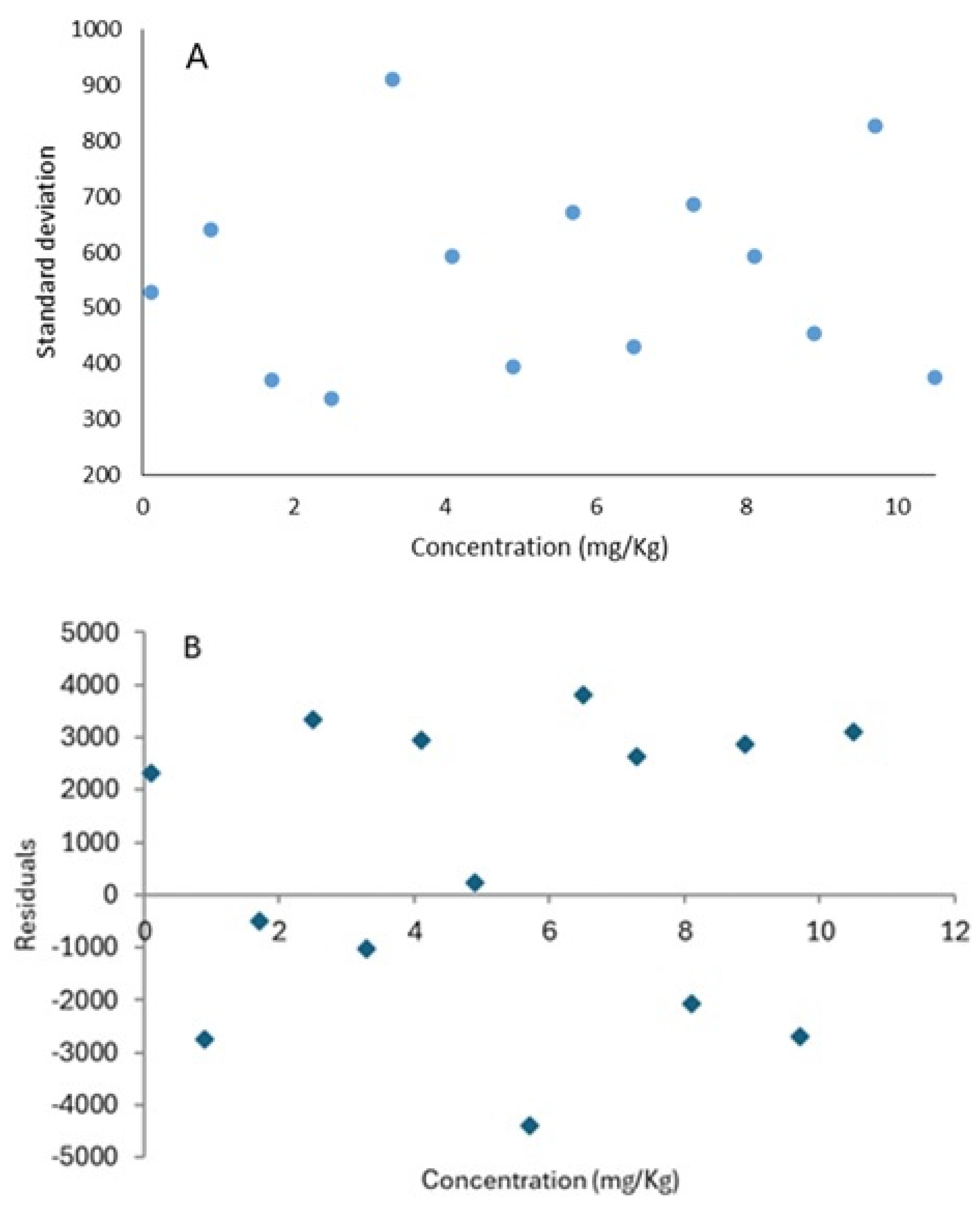
2.5.5. Precision
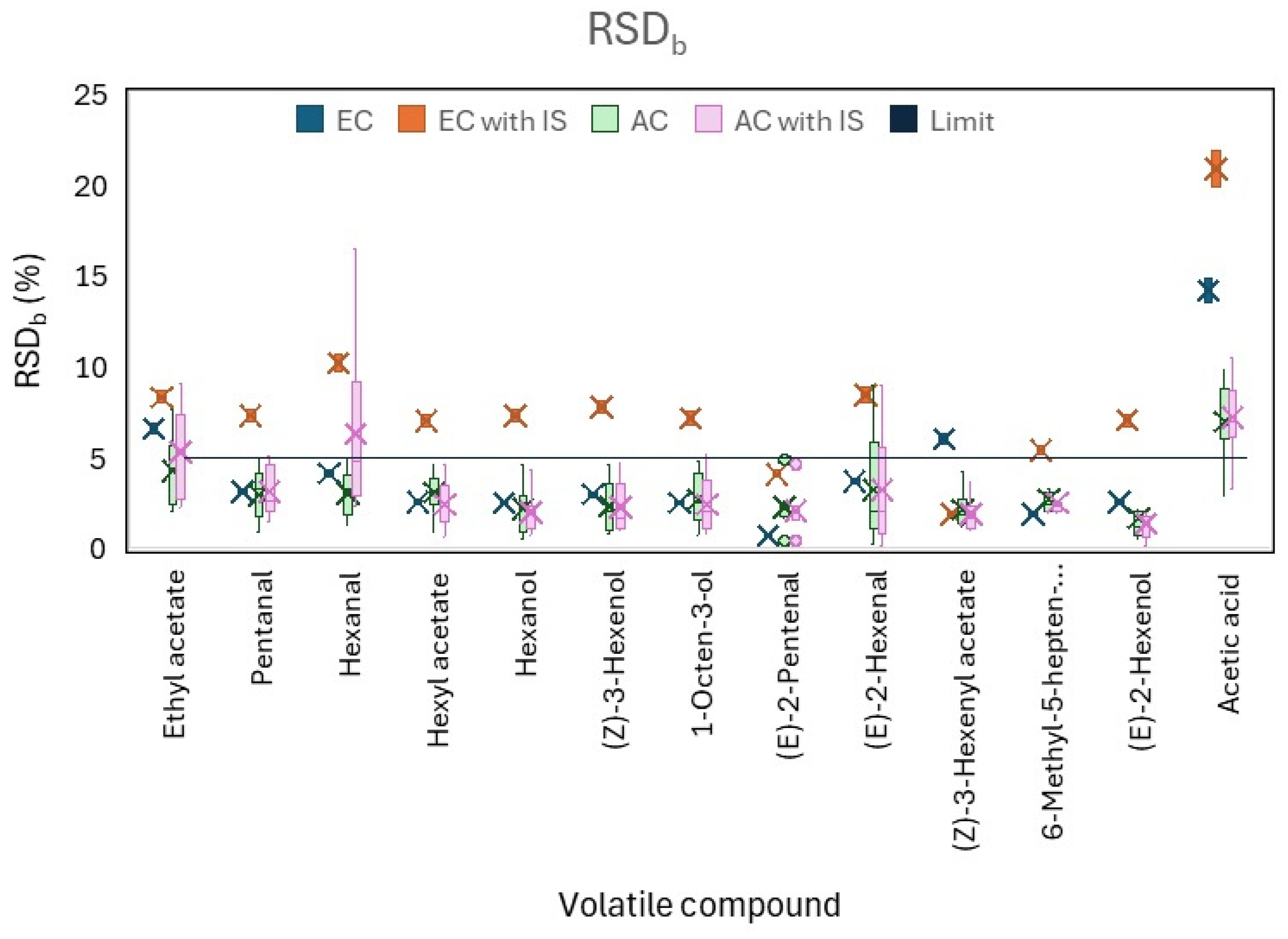
2.5.6. Matrix Effect
2.6. Statistical Analysis
3. Result and Discussion
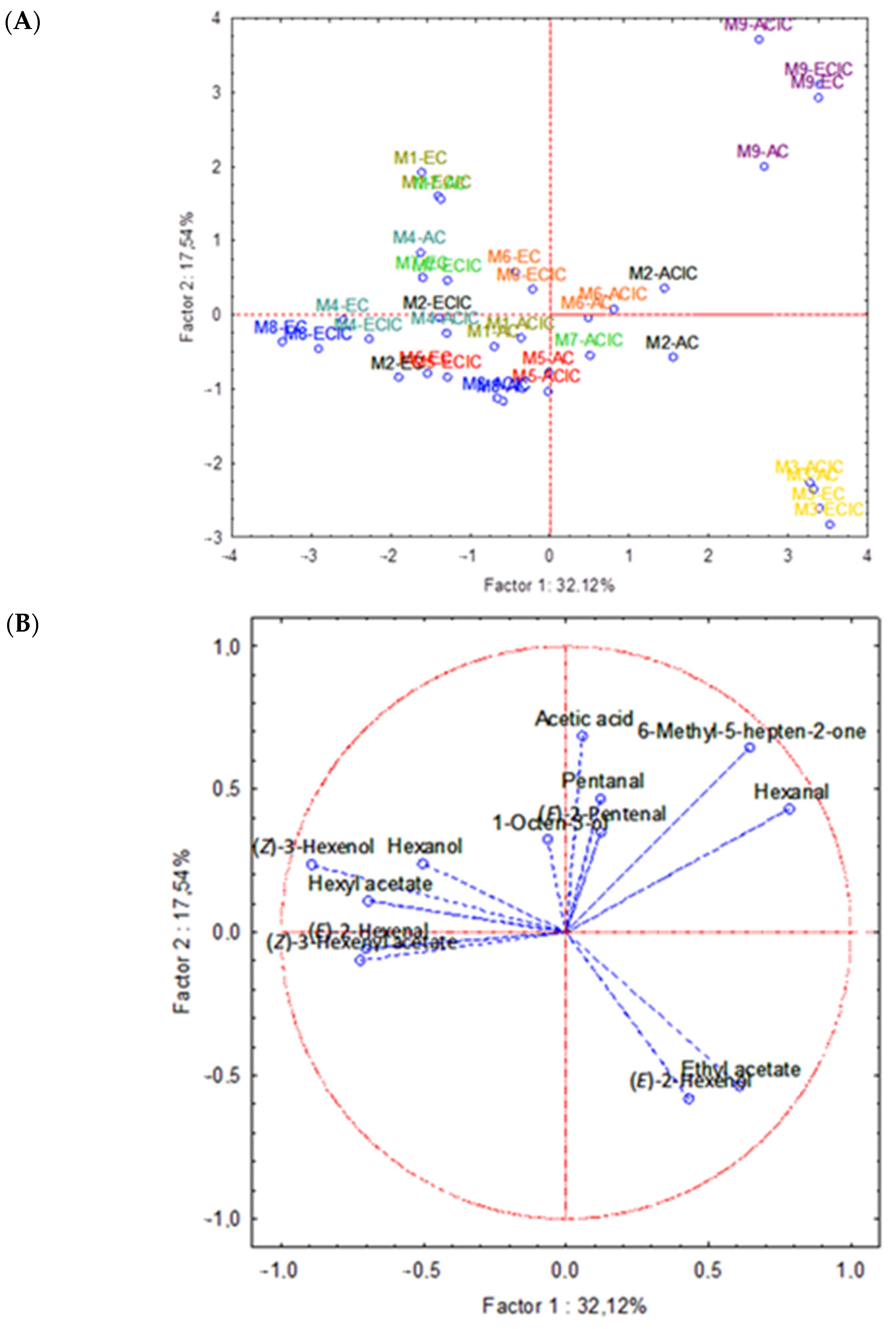
3.1. Samples Characterization
3.2. Analytical–Statistical Method Validation
3.2.1. Linearity
3.2.2. Sensitivity
3.2.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.2.4. Accuracy
3.2.5. Precision
3.2.6. Matrix Effect
3.3. Analytical–Statistical Method Application in VOO Volatile Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Chamorro, F.; Gonzalez Pereira, A.; Carrera-Casais, A.; Fraga-Corral, M.; Carpena, M.; Simal-Gandara, J.; Prieto, M.A. Evolution of Flavors in Extra Virgin Olive Oil Shelf-Life. Antioxidants 2021, 10, 368. [Google Scholar] [CrossRef]
- Tucci, R.; Cevoli, C.; Bendini, A.; Barbieri, S.; Casadei, E.; Valli, E.; Gallina-Toschi, T. Changes in the Volatile Profile, Fruity Flavor, and Rancidity in Virgin Olive Oils During Storage by Targeted and Untargeted Analytical Approaches. Foods 2025, 14, 1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Zhou, M.; Wang, Y.; Shehzad, Q.; Wei, W.; Jin, J.; Zhang, H.; Jin, Q.; Wang, X. Comparative analysis of olive oil from laboratory and industrial production: Quality characteristics and chemical composition of Chinese olive oils. Food Meas. 2025, 19, 6069–6081. [Google Scholar] [CrossRef]
- Psathas, D.; Lioupi, A.; Rebholz, A.M.; Zinoviadou, K.; Tsaftaris, A.; Theodoridis, G.; Papoti, V.T. Volatile profile and quality characteristics of the Greek “Chondrolia Chalkidikis” virgin olive oils: Effect of ripening stage. Eur. Food Res. Technol. 2022, 248, 1977–1990. [Google Scholar] [CrossRef]
- European Commission Regulation (EC). Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union 2022, L 284, 1–22.
- Olive Oil Council (COI). Sensory Analysis of Olive Oil—Method for the Organoleptic Assessment of Virgin Olive Oil. 2024, COI/T.20/Doc. No 15. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 30 November 2022).
- Mariotti, R.; Núñez-Carmona, E.; Genzardi, D.; Pandolfi, S.; Sberveglieri, V.; Mousavi, S. Volatile Olfactory Profiles of Umbrian Extra Virgin Olive Oils and Their Discrimination through MOX Chemical Sensors. Sensors 2022, 22, 7164. [Google Scholar] [CrossRef] [PubMed]
- Breschi, C.; Cecchi, L.; Mattagli, F.; Zanoni, B.; Ugolini, T.; Ieri, F.; Calamai, L.; Bellumori, M.; Mulinacci, N.; Boncinelli, F.; et al. Chemical, Sensory, and Nutraceutical Profiling, and Shelf-Life Assessment of High-Quality Extra Virgin Olive Oil Produced in a Local Area near Florence (Italy). Molecules 2025, 30, 2811. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Rossetti, A.; Cane, A.; Mulinacci, N. New Volatile Molecular Markers of Rancidity in Virgin Olive Oils under Nonaccelerated Oxidative Storage Conditions. J. Agric. Food Chem. 2019, 67, 13150–13163. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, I.; Krapac, M.; Koprivnjak, O. Exploring the Connection between the Occurrence and Intensity of “Grubby” Defect and Volatile Composition of Olive Oil. Foods 2023, 12, 4473. [Google Scholar] [CrossRef]
- García-González, D.L.; Casadei, E.; Aparicio-Ruiz, R.; Ortiz Romero, C.; Valli, E.; Brereton, P.; Koidis, A.; Korytkowska, M.; Servilli, M.; Selvaggini, R.; et al. Multianalyte analysis of volatile compounds in virgin olive oils using SPME-GC with FID or MS detection: Results of an international interlaboratory validation. Eur. J. Lipid Sci. Technol. 2023, 126, 2300079. [Google Scholar] [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. GC-IMS data on the discrimination between geographic origins of olive oils. Data Brief 2022, 45, 108730. [Google Scholar] [CrossRef]
- Fella, P.; Stylianou, M.; Agapiou, A. A green sorptive extraction method (HiSorb-TD-GC-MS) for determining the extra virgin olive oil (EVOO) aroma profile. Pure App. Chem. 2023, 95, 595–610. [Google Scholar] [CrossRef]
- Díaz-Montaña, E.J.; Aparicio-Ruiz, R.; Morales, M.T. Effect of Flavorization on Virgin Olive Oil Oxidation and Volatile Profile. Antioxidants 2023, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Díez-Betriu, A.; Romero, A.; Ninot, A.; Tres, A.; Vichi, S.; Guardiola, F. Subzero Temperature Storage to Preserve the Quality Attributes of Veiled Virgin Olive Oil. Foods 2023, 12, 1228. [Google Scholar] [CrossRef] [PubMed]
- Freitas Lima, A.; da Silva Oliveira, W.; de Oliveira Garcia, A.; Vicente, E.; Teixeira Godoy, H. Identifying markers volatiles in Brazilian virgin oil by multiple headspace solid-phase microextraction, and chemometrics tools. Food Res. Int. 2023, 167, 112697. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Rodríguez, L.; Gámiz-Gracia, L.; Almansa-López, E.M.; Bosque-Sendra, J.M. Calibration in chemical measurement processes. II. A methodological approach. Trends Anal. Chem. 2001, 20, 620–636. [Google Scholar] [CrossRef]
- Carter, J.A.; Barros, A.I.; Nóbrega, J.A.; Donati, G.L. Traditional Calibration Methods in Atomic Spectrometry and New Calibration Strategies for Inductively Coupled Plasma Mass Spectrometry. Front. Chem. 2018, 6, 504. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Bagur-González, M.G.; Sánchez-Viñas, M.; González-Casado, A.; Gómez-Sáez, A.M. Principles of analytical calibration/quantification for the separation sciences. J. Chromatogr. A 2007, 1158, 33–46. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Thompson, M. Standard additions: Myth and reality. Analyst 2008, 133, 992–997. [Google Scholar] [CrossRef]
- Compañó, R.; Ríos, A. Garantía de la Calidad en los Laboratorios Analíticos, 1st ed.; Síntesis: Madrid, Spain, 2002. [Google Scholar]
- Anagnostopoulos, C.; Miliadis, G.E.; Aplada-Sarlis, P.; Ziogas, B.N. Comparison of external and internal standard methods in pesticide residue determinations. Int. J. Environ. Anal. Chem. 2006, 86, 77–82. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Danzer, K.; Currie, L.A. Guidelines for calibration in analytical chemistry. Part I. Fundamentals and single component calibration (IUPAC Recommendations 1998). Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- SOP 12. SOP 12: Validation of Bioanalytical Methods. Onkologie 2003, 26, 52–55. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Digiglio, I.; Ugolini, T.; Trapani, S.; Zanoni, B.; Mulinacci, N.; Melani, F. Robust application of a chemometric model based on the relationships between 10 volatile compounds and sensory attributes to support the panel test in virgin olive oil quality classification in olive oil companies. J. Food Compos. Anal. 2025, 141, 107362. [Google Scholar] [CrossRef]
- Díaz-Montaña, E.J.; Brignot, H.; Aparicio-Ruiz, R.; Thomas-Danguin, T.; Morales, M.T. Phenols and saliva effect on virgin olive oil aroma release: A chemical and sensory approach. Food Chem. 2024, 437, 137855. [Google Scholar] [CrossRef]
- Gomes, H.O.; Da, S.; Cardoso, R.; da Costa, J.G.M.; Andrade da Silva, V.P.; de A. Nobre, C.; Pereira Teixeira, R.N.; do Nascimento, R.F. Statistical evaluation of analytical curves for quantification of pesticides in bananas. Food Chem. 2021, 345, 128768. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Prentice Hall: Saddle River, NJ, USA, 2010. [Google Scholar]
- Guedes, J.A.C.; Silva, R.D.O.; Lima, C.G.; Milhome, M.A.L.; do Nascimento, R.F. Matrix effect in guava multiresidue analysis by QuEChERS method and gas chromatography coupled to quadrupole mass spectrometry. Food Chem. 2016, 199, 380–386. [Google Scholar] [CrossRef]
- ISO/IEC Guide 98–3:2008; Uncertainty of Measurement—Part 3: Guide to the Expression of Uncertainty in Measurement (GUM: 1995). International Organization for Standardization (ISO): Geneva, Switzerland, 2008.
- Joint Committee for Guides in Metrology (JCGM). International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM), 3rd ed.; JCGM: Paris, France, 2012. [Google Scholar]
- Sayago, A.; Asuero, A.G. Fitting Straight Lines with Replicated Observations by Linear Regression: Part II. Testing for Homogeneity of Variances. Crit. Rev. Anal. Chem. 2004, 34, 133–146. [Google Scholar] [CrossRef]
- Katajamaa, M.; Oresic, M. Data processing for mass spectrometry-based metabolomics. J. Chromatogr. A 2007, 1158, 318–328. [Google Scholar] [CrossRef]
| Labeled | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 |
|---|---|---|---|---|---|---|---|---|---|
| Variety | Picual | Arbequina | Coratina | Hojiblanca | Coratina | Mixture (not specified) | Picuda | Arbequina | Cornicabra |
| Harvesting | 2022/23 | 2022/23 | 2022/23 | 2022/23 | 2022/23 | 2022/23 | 2016/17 | 2016/17 | 2016/17 |
| Category | EVOO | EVOO | EVOO | VOO | VOO | VOO | Lampante | Lampante | Lampante |
| Origin | Andalusia (Spain) | ||||||||
| Sensory analysis | |||||||||
| Md a | 0 | 0 | 0 | 0.5 | 1.0 | 0.5 | 3.0 | 2.8 | 4.2 |
| Mf b | 2.0 | 2.5 | 4.5 | 1.0 | 0.5 | 0.5 | 1.0 | 1.5 | 0.5 |
| Physico-chemical parameters | |||||||||
| Acidity index c | 0.61 | 0.54 | 0.23 | 1.34 | 1.01 | 1.64 | 3.16 | 2.34 | 3.01 |
| Peroxide value d | 8.22 | 7.63 | 12.57 | 14.13 | 10.94 | 9.86 | 22.61 | 23.85 | 19.21 |
| K232 | 1.25 | 1.46 | 0.98 | 2.14 | 2.47 | 2.56 | 2.49 | 2.86 | 3.01 |
| K270 | 0.09 | 0.14 | 0.19 | 0.21 | 0.17 | 0.25 | 0.18 | 0.22 | 0.26 |
| ΔK | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | - | - | - |
| Volatile Compounds | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Ethyl acetate | 9.65 | 5.17 | 15.99 | 39.19 | 1.13 |
| Pentanal | 6.97 | 6.14 | 33.76 | 82.71 | 0.94 |
| Hexanal | 7.81 | 7.27 | 25.48 | 62.41 | 2.01 |
| Hexyl acetate | 2.53 | 5.29 | 41.38 | 101.35 | 1.67 |
| Hexanol | 3.26 | 4.11 | 41.64 | 101.99 | 0.68 |
| (Z)-3-hexenol | 7.27 | 2.75 | 35.53 | 87.07 | 1.21 |
| 1-Octen-3-ol | 9.84 | 3.25 | 42.08 | 103.07 | 1.98 |
| (E)-2-Pentenal | 10.14 | 3.03 | 150.18 | 367.85 | 1.46 |
| (E)-2-Hexenal | 11.94 | 6.94 | 28.40 | 69.56 | 0.85 |
| (Z)-3-Hexenyl acetate | 2.87 | 4.08 | 17.51 | 42.88 | 1.12 |
| 6-Methyl-5-hepten-2-one | 4.51 | 3.11 | 55.49 | 135.92 | 1.06 |
| (E)-2-Hexenol | 5.02 | 3.76 | 41.41 | 101.43 | 0.79 |
| Acetic acid | 11.05 | 2.18 | 7.40 | 18.14 | 2.11 |
| Chemical Compound | Concentration Level (mg/kg) | EC Relative Recovery ± SD (%) | EC with IS Relative Recovery ± SD (%) | AC Relative Recovery ± SD (%) | AC with IS Relative Recovery ± SD (%) |
|---|---|---|---|---|---|
| Ethyl acetate | 0.1 | 101.60 ±0.16 a | 70.48 ± 0.18 b | 101.28 ± 0.44 a | 95.02 ± 0.41 c |
| 1 | 100.47 ± 0.18 a | 71.39 ± 0.15 b | 98.92 ± 0.60 a,c | 97.14 ± 0.52 c | |
| 10 | 98.55 ± 0.11 a,c | 74.06 ± 0.08 b | 101.96 ± 1.01 a | 96.05 ± 0.87 c | |
| (Z)-3-hexenyl acetate | 0.1 | 100.18 ± 0.09 a | 86.86 ± 0.12 b | 98.36 ± 0.54 a | 100.01 ± 0.63 a |
| 1 | 99.60 ± 0.10 a | 79.11 ± 0.27 b | 101.65 ± 0.98 a | 101.90 ± 0.98 a | |
| 10 | 101.02 ± 0.09 a,c | 83.57 ± 0.11 b | 98.37 ± 0.32 a | 101.91 ± 0.59 c | |
| 1-octen-3-ol | 0.1 | 101.04 ± 0.12 a | 71.77 ± 0.24 b | 100.29 ± 0.51 a | 102.23 ± 0.97 a |
| 1 | 100.26 ± 0.13 a | 79.07 ± 0.22 b | 98.40 ± 0.81 a | 103.18 ± 0.37 c | |
| 10 | 98.94 ± 0.08 a | 75.2 ± 0.02 b | 98.56 ± 0.34 a | 101.97 ± 0.19 c | |
| (E)-2-pentenal | 0.1 | 100.91 ± 0.03 a | 84.55 ± 0.15 b | 98.28 ± 1.03 a,c | 96.38 ± 0.86 c |
| 1 | 101.78 ± 0.16 a | 80.88 ± 0.21 b | 100.53 ± 0.67 a | 95.83 ± 0.77 c | |
| 10 | 101.11 ± 0.12 a | 78.90 ± 0.04 b | 99.53 ± 0.76 a | 98.80 ± 0.45 a | |
| (E)-2-hexenol | 0.1 | 99.33 ± 0.09 a | 68.62 ± 0.05 b | 101.23 ± 0.48 a | 104.67 ± 0.31 c |
| 1 | 101.48 ± 0.08 a,c | 72.58 ± 0.11 b | 100.56 ± 0.52 a | 102.99 ± 0.40 c | |
| 10 | 99.05 ± 0.17 a | 68.89 ± 0.18 b | 100.53 ± 0.99 a | 104.50 ± 0.64 c | |
| 6-methyl-5-hepten-2-one | 0.1 | 98.37 ± 0.23 a | 68.65 ± 0.12 b | 99.38 ± 0.49 a | 96.97 ± 0.31 c |
| 1 | 100.68 ± 0.31 a | 77.07 ± 0.15 b | 98.18 ± 0.88 a | 98.31 ± 0.51 a | |
| 10 | 99.92 ± 0.28 a | 75.98 ± 0.19 b | 98.79 ± 1.07 a,c | 96.69 ± 0.84 c | |
| Pentanal | 0.1 | 98.91 ± 0.09 a | 77.32 ± 0.11 b | 101.14 ± 0.89 a | 101.93 ± 0.46 a |
| 1 | 100.21 ± 0.24 a | 78.66 ± 0.10 b | 101.09 ± 0.76 a | 100.45 ± 0.72 a | |
| 10 | 99.16 ± 0.30 a | 76.00 ± 0.08 b | 98.12 ± 0.92 a | 100.56 ± 0.91 a | |
| Hexanal | 0.1 | 98.59 ± 0.08 a | 74.48 ± 0.21 b | 100.31 ± 1.00 a | 101.03 ± 1.04 a |
| 1 | 99.50 ± 0.14 a,c | 71.13 ± 0.16 b | 98.51 ± 0.84 a | 102.53 ± 0.32 c | |
| 10 | 99.47 ± 0.23 a | 74.06 ± 0.17 b | 98.95 ± 0.77 a | 104.12 ± 0.61 c | |
| Hexyl acetate | 0.1 | 98.51 ± 0.08 a | 75.92 ± 0.22 b | 99.70 ± 0.43 a | 98.15 ± 0.53 a |
| 1 | 98.02 ± 0.12 a | 77.38 ± 0.31 b | 100.58 ± 0.86 a | 101.43 ± 1.02 a | |
| 10 | 100.44 ± 0.31 a | 75.62 ± 0.28 b | 98.38 ± 0.74 a | 98.40 ± 0.79 a | |
| Hexan-1-ol | 0.1 | 98.37 ± 0.07 a | 83.49 ± 0.14 b | 99.17 ± 0.91 a | 97.27 ± 0.64 a |
| 1 | 99.35 ± 0.16 a | 84.12 ± 0.25 b | 100.80 ± 0.66 a | 97.80 ± 0.35 c | |
| 10 | 99.94 ± 0.31 a | 79.35 ± 0.16 b | 98.70 ± 0.81 a | 101.00 ± 0.76 a | |
| (Z)-3-hexenol | 0.1 | 100.46 ± 0.19 a | 66.46 ± 0.23 b | 101.66 ± 0.63 a | 99.86 ± 0.92 a |
| 1 | 101.83 ± 0.08 a | 68.00 ± 0.18 b | 100.62 ± 0.91 a | 101.48 ± 0.84 a | |
| 10 | 98.92 ± 0.17 a | 71.89 ± 0.27 b | 100.18 ± 0.84 a | 99.74 ± 0.62 a | |
| (E)-2-hexenal | 0.1 | 98.11 ± 0.04 a | 73.40 ± 0.16 b | 99.61 ± 0.37 a | 103.50 ± 0.49 c |
| 1 | 99.73 ± 0.18 a | 68.51 ± 0.31 b | 101.76 ± 0.96 a | 101.52 ± 1.00 a | |
| 10 | 101.58 ± 0.09 a | 72.53 ± 0.28 b | 99.21 ± 0.75 a | 102.46 ± 1.02 a | |
| Acetic acid | 0.1 | 84.63 ± 0.84 a | 51.58 ± 1.01 b | 83.21 ± 1.49 a | 78.42 ± 1.06 c |
| 1 | 82.87 ± 0.92 a | 56.34 ± 0.96 b | 86.71 ± 1.67 a | 76.99 ± 0.99 c | |
| 10 | 81.83 ± 0.87 a | 50.30 ± 0.97 b | 81.88 ± 1.52 a | 76.08 ± 1.14 c |
| Chemical Compound | Concentration Level (mg/kg) | EC | EC with IS | AC | AC with IS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Repeatability CV (%) | Intermediate Precision CV (%) | Repeatability CV (%) | Intermediate Precision CV (%) | Repeatability CV (%) | Intermediate Precision CV (%) | Repeatability CV (%) | Intermediate Precision CV (%) | ||
| Ethyl acetate | 0.1 | 2.07 | 2.78 | 2.30 | 3.19 | 5.29 | 8.68 | 4.95 | 8.05 |
| 1 | 2.28 | 3.24 | 1.89 | 2.58 | 7.13 | 12.05 | 6.21 | 10.36 | |
| 10 | 1.50 | 1.74 | 1.69 | 2.01 | 11.85 | 20.68 | 10.24 | 17.73 | |
| (Z)-3-hexenyl acetate | 0.1 | 1.27 | 1.29 | 1.61 | 1.93 | 6.44 | 10.79 | 7.13 | 12.05 |
| 1 | 1.38 | 1.53 | 3.34 | 5.10 | 11.50 | 20.05 | 12.50 | 21.05 | |
| 10 | 1.26 | 1.24 | 1.52 | 1.72 | 3.91 | 6.16 | 7.02 | 11.84 | |
| 1-octen-3-ol | 0.1 | 1.61 | 1.94 | 2.99 | 4.47 | 6.10 | 10.15 | 11.39 | 19.83 |
| 1 | 1.73 | 2.16 | 2.75 | 4.05 | 9.55 | 16.43 | 4.49 | 7.21 | |
| 10 | 1.14 | 1.29 | 1.46 | 2.07 | 4.14 | 6.58 | 2.42 | 3.42 | |
| (E)-2-pentenal | 0.1 | 0.58 | 1.13 | 1.98 | 2.56 | 12.08 | 21.10 | 10.12 | 17.52 |
| 1 | 2.05 | 2.66 | 2.64 | 3.82 | 7.94 | 13.57 | 9.09 | 15.63 | |
| 10 | 1.60 | 1.95 | 1.70 | 2.31 | 8.96 | 15.15 | 5.41 | 8.89 | |
| (E)-2-hexenol | 0.1 | 1.32 | 1.18 | 1.81 | 2.47 | 5.75 | 9.52 | 3.80 | 5.94 |
| 1 | 1.17 | 1.46 | 1.50 | 1.75 | 6.21 | 10.36 | 4.83 | 7.84 | |
| 10 | 2.18 | 3.01 | 2.28 | 3.15 | 11.62 | 20.26 | 7.54 | 12.98 | |
| 6-methyl-5-hepten-2-one | 0.1 | 2.86 | 4.25 | 1.62 | 1.95 | 5.87 | 9.73 | 3.72 | 5.34 |
| 1 | 3.79 | 5.91 | 1.96 | 2.55 | 10.35 | 17.94 | 6.10 | 10.15 | |
| 10 | 3.45 | 5.31 | 2.42 | 3.42 | 12.54 | 21.94 | 9.59 | 17.04 | |
| Pentanal | 0.1 | 1.17 | 1.31 | 1.54 | 1.74 | 10.47 | 18.15 | 5.52 | 9.10 |
| 1 | 2.99 | 4.47 | 1.38 | 1.53 | 8.97 | 15.42 | 8.51 | 14.57 | |
| 10 | 3.68 | 5.73 | 1.91 | 2.62 | 10.81 | 18.78 | 10.70 | 18.57 | |
| Hexanal | 0.1 | 1.51 | 1.92 | 2.65 | 3.84 | 11.73 | 20.47 | 12.19 | 21.31 |
| 1 | 1.84 | 2.37 | 2.09 | 2.77 | 9.82 | 17.10 | 3.91 | 6.16 | |
| 10 | 2.88 | 4.26 | 2.19 | 3.02 | 9.09 | 15.63 | 7.25 | 12.26 | |
| Hexyl acetate | 0.1 | 1.43 | 1.84 | 2.76 | 4.01 | 5.18 | 8.47 | 6.33 | 10.57 |
| 1 | 1.54 | 1.87 | 3.80 | 5.94 | 10.12 | 17.52 | 11.47 | 20.61 | |
| 10 | 3.80 | 5.82 | 3.45 | 5.31 | 8.74 | 14.99 | 9.32 | 16.05 | |
| Hexan-1-ol | 0.1 | 1.01 | 1.89 | 1.84 | 2.37 | 10.71 | 18.54 | 7.59 | 12.89 |
| 1 | 2.18 | 2.79 | 3.11 | 4.68 | 7.82 | 13.31 | 4.26 | 6.79 | |
| 10 | 3.67 | 5.83 | 2.02 | 2.71 | 9.54 | 16.47 | 8.97 | 15.42 | |
| (Z)-3-hexenol | 0.1 | 2.42 | 3.42 | 2.88 | 4.26 | 7.48 | 12.68 | 10.81 | 18.78 |
| 1 | 1.15 | 1.99 | 2.31 | 3.21 | 10.64 | 18.57 | 9.89 | 17.10 | |
| 10 | 2.19 | 3.00 | 3.31 | 5.12 | 9.89 | 17.09 | 7.36 | 12.47 | |
| (E)-2-hexenal | 0.1 | 0.69 | 1.28 | 2.07 | 2.79 | 4.49 | 7.21 | 5.87 | 9.73 |
| 1 | 2.30 | 3.21 | 3.81 | 5.93 | 11.27 | 19.62 | 11.73 | 20.47 | |
| 10 | 1.09 | 1.39 | 3.46 | 5.28 | 8.86 | 15.20 | 11.96 | 20.89 | |
| Acetic acid | 0.1 | 9.89 | 17.10 | 11.85 | 20.68 | 17.37 | 30.78 | 12.42 | 21.73 |
| 1 | 10.81 | 18.78 | 11.27 | 19.62 | 19.44 | 34.57 | 11.62 | 20.26 | |
| 10 | 10.24 | 17.73 | 11.39 | 19.83 | 17.71 | 31.41 | 13.34 | 23.41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Montaña, E.J.; Barbero-López, M.; Aparicio-Ruiz, R.; García-González, D.L.; Morales, M.T. Optimization of Calibration Strategies for the Quantification of Volatile Compounds in Virgin Olive Oil. Foods 2025, 14, 3439. https://doi.org/10.3390/foods14193439
Díaz-Montaña EJ, Barbero-López M, Aparicio-Ruiz R, García-González DL, Morales MT. Optimization of Calibration Strategies for the Quantification of Volatile Compounds in Virgin Olive Oil. Foods. 2025; 14(19):3439. https://doi.org/10.3390/foods14193439
Chicago/Turabian StyleDíaz-Montaña, Enrique J., María Barbero-López, Ramón Aparicio-Ruiz, Diego L. García-González, and María T. Morales. 2025. "Optimization of Calibration Strategies for the Quantification of Volatile Compounds in Virgin Olive Oil" Foods 14, no. 19: 3439. https://doi.org/10.3390/foods14193439
APA StyleDíaz-Montaña, E. J., Barbero-López, M., Aparicio-Ruiz, R., García-González, D. L., & Morales, M. T. (2025). Optimization of Calibration Strategies for the Quantification of Volatile Compounds in Virgin Olive Oil. Foods, 14(19), 3439. https://doi.org/10.3390/foods14193439






