The Effects of Hot Air and Microwave Drying on the Structural and Physicochemical Properties of Soluble Dietary Fiber from Sugar Beet Pulp
Abstract
1. Introduction
2. Materials and Methods
2.1. Optimization of Drying Conditions and Extraction of Soluble Dietary Fiber
2.2. Compositional Analysis of SDF
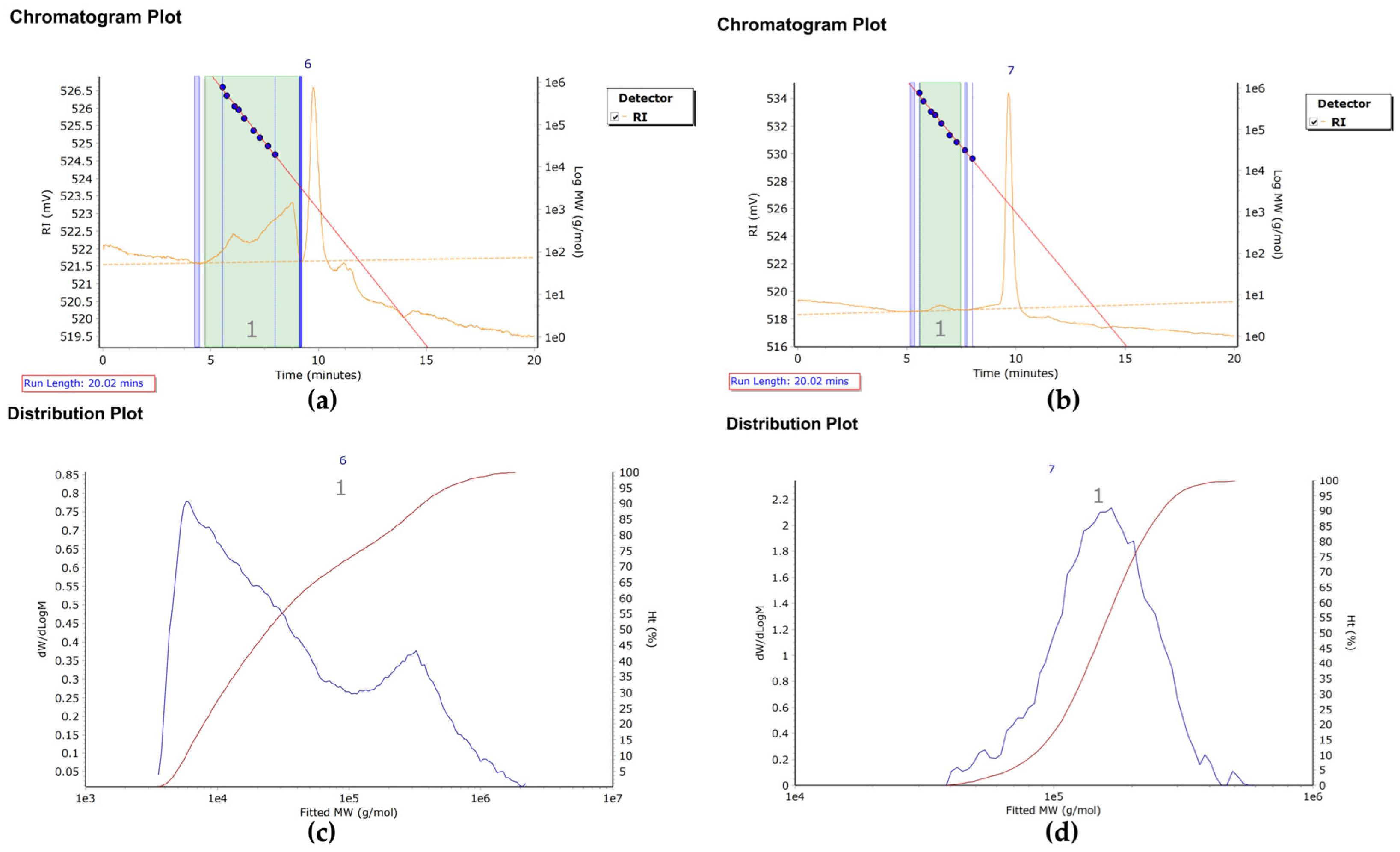
2.2.1. Molecular Weight Distribution
2.2.2. Monosaccharide Composition
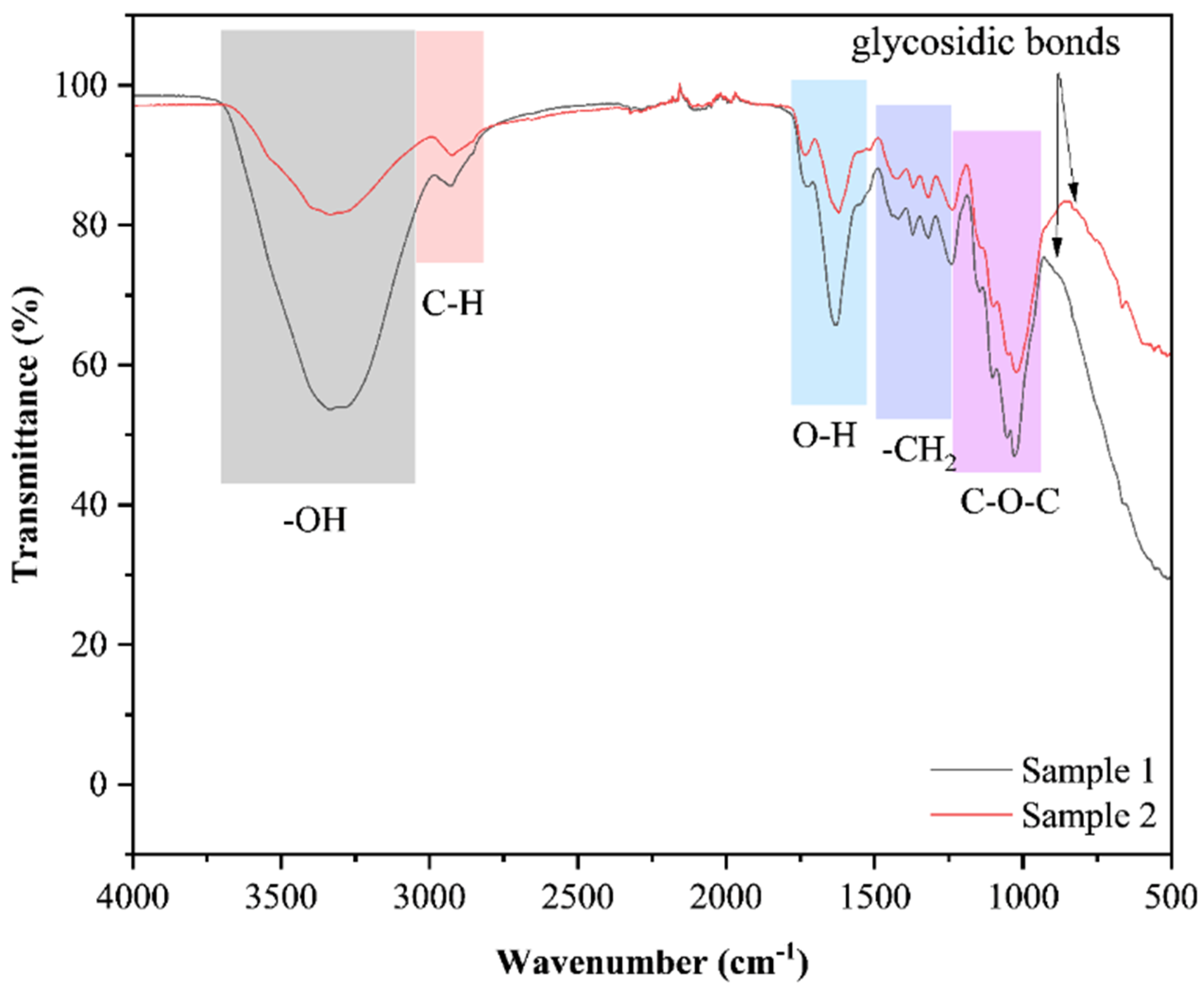
2.2.3. Fourier Transform Infrared Spectroscopy
2.3. Structural Analysis of SDF
2.3.1. Scanning Electron Microscopy
2.3.2. Zeta Potential
2.4. Physicochemical Properties of SDF
2.4.1. Water Holding Capacity and Swelling Capacity
2.4.2. Oil Holding Capacity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition of SDF
3.1.1. Molecular Weight Characteristics
3.1.2. Monosaccharide Composition Analysis
3.1.3. Fourier Transform Infrared Spectroscopy Analysis
3.2. Structural Characterization of SDF
3.2.1. Scanning Electron Microscopy Analysis
3.2.2. Zeta Potential Analysis
3.3. Physicochemical Functional Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habeeb, A.; Gad, A.; El-Tarabany, A.; Mustafa, M.; Atta, M. Using of sugar beet pulp by-product in farm animals feeding. Int. J. Sci. Res. Sci. Technol. 2017, 3, 107–120. [Google Scholar]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Jiao, A.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, Y.; Luo, J.; Mao, X.; Chen, D. Beet Pulp: An Alternative to Improve the Gut Health of Growing Pigs. Animals 2020, 10, 1860. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, H.; Zhang, H.; Yun, Y.; Chen, W.; Zhong, Q.; Chen, W.; Fu, X. Preparation and properties of ferulic acid-sugar beet pulp pectin ester and its application as a physical and antioxidative stabilizer in a fish oil-water emulsion. Int. J. Biol. Macromol. 2019, 139, 290–297. [Google Scholar] [CrossRef]
- Baryga, A.; Ziobro, R.; Gumul, D.; Rosicka-Kaczmarek, J.; Miśkiewicz, K. Physicochemical Properties and Evaluation of Antioxidant Potential of Sugar Beet Pulp—Preliminary Analysis for Further Use (Future Prospects). Agriculture 2023, 13, 1039. [Google Scholar] [CrossRef]
- Mella, C.; Vega-Gálvez, A.; Uribe, E.; Pasten, A.; Mejias, N.; Quispe-Fuentes, I. Impact of vacuum drying on drying characteristics and functional properties of beetroot (Beta vulgaris). Appl. Food Res. 2022, 2, 100120. [Google Scholar] [CrossRef]
- Kerr, W.L.; Varner, A. Chemical and physical properties of vacuum-dried red beetroot (Beta vulgaris) powders compared to other drying methods. Drying Technol. 2020, 38, 1165–1174. [Google Scholar] [CrossRef]
- TaŞOva, M.; Dursun, S.K. Comparison of microwave assisted foam drying processes to improve the physicochemical properties and to reduce GHG values of the melon powder processes. Biomass Convers. Biorefin. 2025, 15, 8419–8426. [Google Scholar] [CrossRef]
- Liu, Y.; Sabadash, S.; Duan, Z.; Deng, C. The Influence of Different Drying Methods on the Quality Attributes of Beetroots. East.-Eur. J. Enterp. Technol. 2022, 117, 60–68. [Google Scholar] [CrossRef]
- Liu, Y.; Sabadash, S.; Duan, Z.; Gao, D. Influence of different microwave-assisted drying methods on the physical properties, bioactive compounds and antioxidant activity of beetroots. East.-Eur. J. Enterp. Technol. 2022, 1, 15–25. [Google Scholar] [CrossRef]
- Tamova, M.; Barashkina, E.; Tretyakova, N.y.; Zhuravlev, R.; Penov, N. Beet pulp dietary fiber exposed to an extremely low-frequency electromagnetic field: Detoxification properties. Foods Raw Mater. 2020, 9, 2–9. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Chau, H.K.; Strahan, G.D.; Nuñez, A.; Simon, S.; White, A.K.; Dieng, S.; Heuberger, E.R.; Yadav, M.P.; Hirsch, J. Structural characterization of red beet fiber and pectin. Food Hydrocoll. 2022, 129, 107549. [Google Scholar] [CrossRef]
- Zhang, H.; Zu, Q.; Zhang, J.; Liu, S.; Zhang, G.; Chang, X.; Li, X. Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice. Foods 2024, 13, 2220. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Mironeasa, S. Effect of drying on the extraction and physicochemical properties of sugar beet pulp pectin. Food Chem. 2020, 318, 126–133. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Z.; Zhong, H.; Xiao, J.; Li, S.; Zhu, Z. Ultrasound pretreatment on soluble dietary fiber-polyphenol from lotus root: Preparation, interaction, and characterization. LWT 2025, 225, 117883. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C.; Li, J.; Hussain, S.; Yan, S.; Wang, Q. Effects of extrusion on structural and physicochemical properties of soluble dietary fiber from nodes of lotus root. LWT 2018, 93, 204–211. [Google Scholar] [CrossRef]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, Y.; Chen, J.; Hu, J.; Yang, C.; Chen, F.; Zhu, T.; Xin, Y.; Geng, X. Optimization of Solid-State Fermentation Process for Dietary Fiber in Flaxseed Meal and Analysis of Its Microstructure and Functional Properties. Foods 2025, 14, 1722. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhang, S.; Li, J.; Zheng, H.; Jin, H.; Xu, J. Comparison of Different Protein Emulsifiers on Physicochemical Properties of β-Carotene-Loaded Nanoemulsion: Effect on Formation, Stability, and In Vitro Digestion. Nanomaterials 2021, 11, 167. [Google Scholar] [CrossRef]
- Yang, C.; Si, J.; Chen, Y.; Xie, J.; Tian, S.; Cheng, Y.; Hu, X.; Yu, Q. Physicochemical structure and functional properties of soluble dietary fibers obtained by different modification methods from Mesona chinensis Benth. residue. Food Res. Int. 2022, 157, 111489. [Google Scholar] [CrossRef]
- Xiong, M.; Feng, M.; Chen, Y.; Li, S.; Fang, Z.; Wang, L.; Lin, D.; Zhang, Q.; Liu, Y.; Luo, Y.; et al. Comparison on structure, properties and functions of pomegranate peel soluble dietary fiber extracted by different methods. Food Chem. X 2023, 19, 100827. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, M.; Hu, H.; Tu, Y.; Gao, P.; Li, T.; Zhang, X.; Teng, J.; Wang, L. Comparative study on chemical composition, functional properties of dietary fibers prepared from four China cereal brans. Int. J. Biol. Macromol. 2024, 257, 128510. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Shen, M.; Chen, Y.; Yu, Q.; Xie, J. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Zheng, J.; Kan, J.; Chen, G.; Du, M. Physicochemical, structure properties and in vitro hypoglycemic activity of soluble dietary fiber from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran treated by steam explosion. Front. Nutr. 2023, 10, 1124012. [Google Scholar] [CrossRef]
- Wang, L.; Fan, R.; Yan, Y.; Yang, S.; Wang, X.; Zheng, B. Characterization of the structural, physicochemical, and functional properties of soluble dietary fibers obtained from the peanut shell using different extraction methods. Front. Nutr. 2023, 9, 1103673. [Google Scholar] [CrossRef]
- Lorenzo-Santiago, M.A.; Rodríguez-Campos, J.; Rendón-Villalobos, R.; García-Hernández, E.; Vallejo-Cardona, A.A.; Contreras-Ramos, S.M. Thermal Treatment to Obtain 5-Hydroxymethyl Furfural (5-HMF), Furfural and Phenolic Compounds from Vinasse Waste from Agave. Molecules 2023, 28, 1063. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Malaika, A.; Ptaszynska, K.; Pereira, M.F.R.; Figueiredo, J.L. Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials 2020, 10, 1685. [Google Scholar] [CrossRef]
- Kryeziu, A.; Slovák, V.; Parchaňská, A. Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials. Polymers 2022, 14, 1621. [Google Scholar] [CrossRef]
- Fawzy, R.M.; Abdel-Aziz, A.A.; Bassiouny, K.; Fayed, A.M. Phytocompounds-based therapeutic approach: Investigating curcumin and green tea extracts on MCF-7 breast cancer cell line. J. Genet. Eng. Biotechnol. 2024, 22, 100339. [Google Scholar] [CrossRef]
- Srikanlaya, C.; Therdthai, N. Characterization of Plant-Based Meat Treated with Hot Air and Microwave Heating. Foods 2024, 13, 2697. [Google Scholar] [CrossRef]
- Khurshida, S.; Deka, S.C. Application of microwave and hydrothermal treatments for modification of cassava starch of Manipur region, India and development of cookies. J Food Sci Technol 2022, 59, 344–354. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef]
- Noui, Y.; Lekbir, A.; Moussa, H.; Haffas, M.; Bousselma, A.; Hameurlaine, S.; Tahraoui, H.; Zhang, J.; Boufahja, F.; Bendif, H.; et al. Effects of microwave drying on physicochemical characteristics, microstructure and antioxidant activities of date fruit powders. J. Food Meas. Charact. 2025, 19, 5554–5571. [Google Scholar] [CrossRef]
- Pattarapanawan, M.; Phuengjayaem, S.; Akrimajirachoote, N.; Kotatha, D. Partial enhancement of soluble fiber through pyrodextrinization of the residual starch in cassava pulp: Developing a novel dietary fiber with modified functional and improved prebiotic properties. Food Res. Int. 2025, 217, 116747. [Google Scholar] [CrossRef]
- Simić, M.; Nikolić, V.; Sarić, B.; Milovanović, D.; Kostadinović, M.; Žilić, S. Changes in Nutritional and Techno-Functional Properties of Whole Grain Maize Flours Induced by Dry-Heat Treatment. Foods 2024, 13, 3314. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, R.; Wang, L.; Ge, Y.; Fang, H.; Chen, G. Comparative study on the effects of different drying technologies on the structural characteristics and biological activities of polysaccharides from Idesia polycarpa maxim cake meal. Food Chem. X 2025, 26, 102348. [Google Scholar] [CrossRef]
- Khashaba, R.A.; Lou, H.; Gu, Z.; Omer, S.H.S.; Wang, X.; Zhao, R. Enhancing wheat bran quality: Comparing the effects of conventional thermal processing and microwave-assisted hydrolysis. Food Chem. 2025, 489, 144984. [Google Scholar] [CrossRef]
- Vargas, L.; Kapoor, R.; Nemzer, B.; Feng, H. Application of different drying methods for evaluation of phytochemical content and physical properties of broccoli, kale, and spinach. LWT 2022, 155, 112892. [Google Scholar] [CrossRef]
- Ozdemir, M.A.O.; Karagoz, S.A.O. Effects of microwave drying on physicochemical characteristics, microstructure, and antioxidant properties of propolis extract. J. Sci. Food Agric. 2025, 104, 2189–2197. [Google Scholar] [CrossRef]
- Dong, Y.A.O.X.; Shu, Z.; Wang, S.; Wang, J.; Wu, N. Effects of microwave treatment on structural and functional properties of germinated corn starch. J. Sci. Food Agric. 2025, 105, 2246–2254. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, Z.; Gao, X.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Functional protection of different structure soluble dietary fibers from Lentinus edodes as effective delivery substrate for Lactobacillus plantarum LP90. LWT 2021, 136, 110339. [Google Scholar] [CrossRef]
- Stahl, M.A.; Lüdtke, F.L.; Grimaldi, R.; Gigante, M.L.; Ribeiro, A.P.B. Characterization and stability of solid lipid nanoparticles produced from different fully hydrogenated oils. Food Res. Int. 2024, 176, 113821. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Y.; Yang, Y.; Wang, G.; Zhou, S. Establishment of a stable complex formed from whey protein isolate and chitosan and its stability under environmental stresses. Int. J. Biol. Macromol. 2020, 165, 2823–2833. [Google Scholar] [CrossRef]
- Niu, Y.; Li, N.; Xia, Q.; Hou, Y.; Xu, G. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT 2018, 88, 56–63. [Google Scholar] [CrossRef]
- Gu, M.; Fang, H.; Gao, Y.; Su, T.; Niu, Y.; Yu, L. Characterization of enzymatic modified soluble dietary fiber from tomato peels with high release of lycopene. Food Hydrocoll. 2020, 99, 105321. [Google Scholar] [CrossRef]
- Baskaya-Sezer, D. The characteristics of microwave-treated insoluble and soluble dietary fibers from grape and their effects on bread quality. Food Sci. Nutr. 2023, 11, 7877–7886. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Naseem, Z. Effect of microwave-assisted vacuum and hot air oven drying methods on quality characteristics of apple pomace powder. Food Prod. Process. Nutr. 2023, 5, 26. [Google Scholar] [CrossRef]
- Sui, W.; Wang, S.; Chen, Y.; Li, X.; Zhuang, X.; Yan, X.; Song, Y. Insights into the Structural and Nutritional Variations in Soluble Dietary Fibers in Fruits and Vegetables Influenced by Food Processing Techniques. Foods 2025, 14, 1861. [Google Scholar] [CrossRef]
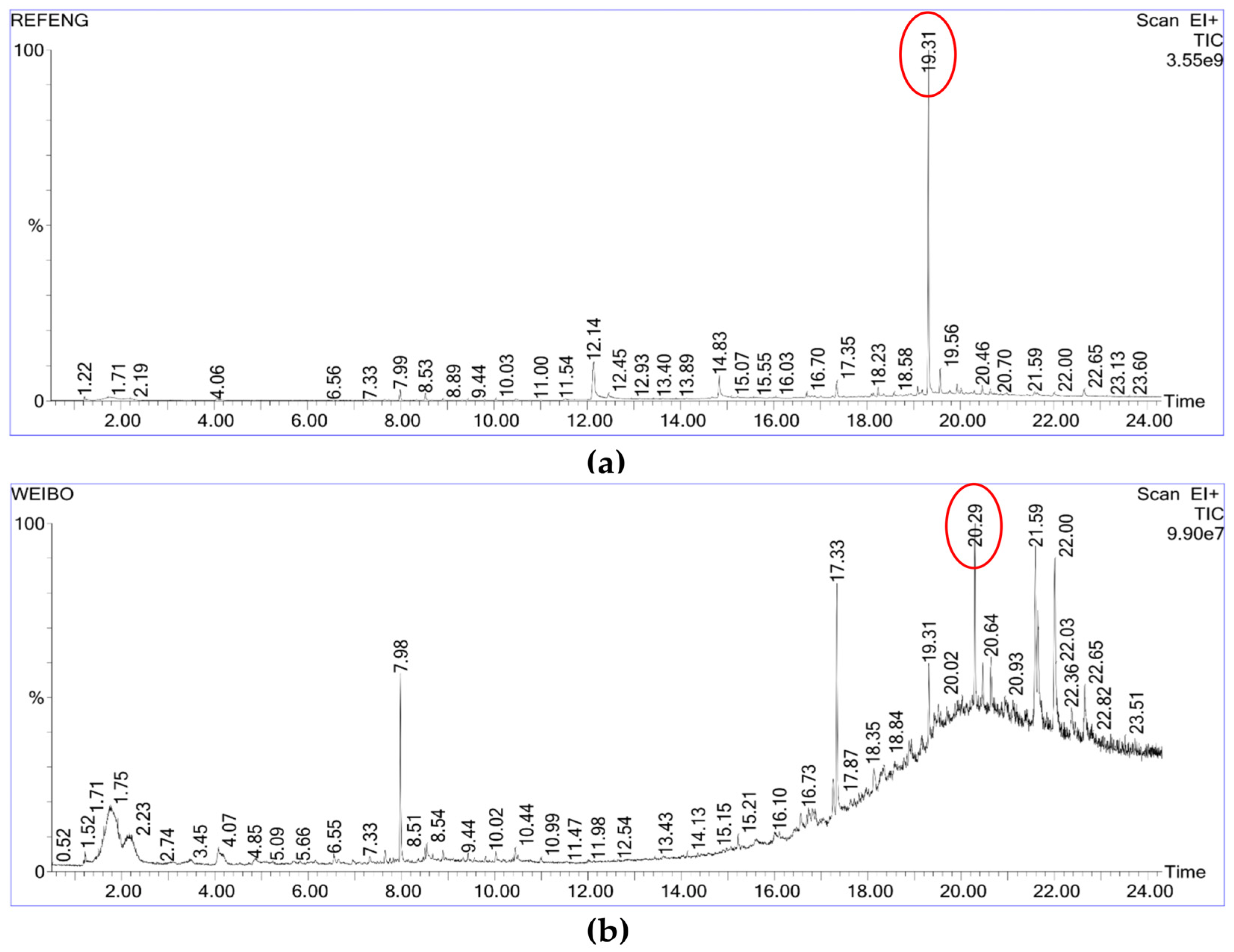

| Method | RT (min) | Compound Name 1 | Match/R Match | Area (%) | CAS No. | Identification Confidence 2 |
|---|---|---|---|---|---|---|
| Hot air Drying | 1.775 3 | Ethyne, fluoro- | 913/930 | 3.914 | 2971-09-9 | High confidence |
| 2.154 | Aceticacid | 912/927 | 1.686 | 64-19-7 | High confidence | |
| 4.073 | 3-Furaldehyde | 916/924 | 0.417 | 498-60-2 | High confidence | |
| 8.526 | 5-Hydroxymethylfurfural | 915/918 | 0.784 | 67-47-0 | High confidence | |
| 12.137 | Benzoicacid,2-(1-oxopropyl)- | 813/822 | 12.429 | 2360-45-4 | Tentative | |
| 14.831 | Biphenyl-4-carboxylicacid | 897/908 | 5.936 | 92-92-2 | Tentative | |
| 17.349 | 1,8-Diazacyclotetradecane-2,9-dione | 870/873 | 2.254 | 5776-79-4 | High confidence | |
| 19.313 | 2H-pyrrol-2-one,1,5-dihydro-4-hydroxy-3-[(4-methylphenyl)thio]-1-phenyl- | 619/644 | 33.279 | / | Unidentified | |
| Microwave Drying | 1.775 3 | Ethyne, fluoro- | 920/949 | 4.977 | 2713-09-9 | High confidence |
| 2.176 | Aceticacid | 813/907 | 2.668 | 64-19-7 | High confidence | |
| 17.332 | 1,8-Diazacyclotetradecane-2,9-dione | 862/867 | 1.941 | 5776-79-4 | High confidence | |
| 20.293 | Squalene | 783/833 | 6.620 | 111-02-4 | High confidence | |
| 20.639 | (R)-9-[(R)-2-(Hydroxymethyl)pyrrolidin-1-yl]-3-isobutyl-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepine1,1-dioxide | 569/629 | 5.362 | 1334427-03-0 | Tentative | |
| 21.59 | Furanic/aromatic pyrolysis product | 540/580 | 3.29 | / | Tentative | |
| 22.003 | 2,2,4-Trimethyl-4-(4-hydroxyphenyl)chroman,tert-butyldimethylsilylether | 517/533 | 3.295 | / | Tentative | |
| 18–21 | Complex unknown product group 4 | 400–550 | ≈25 | / | Unidentified |
| Method | Water Holding Capacity (g/g) | Swelling Capacity (mL/g) | Oil Holding Capacity (g/g) |
|---|---|---|---|
| Hot air Drying | 4.71 ± 0.08 a | 5.18 ± 0.05 a | 2.59 ± 0.07 a |
| Microwave Drying | 5.01 ± 0.05 b | 4.88 ± 0.14 b | 2.61 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhang, Z.; Li, Y.; Yang, Y.; Mulati, A.; Shataer, D.; Wang, J. The Effects of Hot Air and Microwave Drying on the Structural and Physicochemical Properties of Soluble Dietary Fiber from Sugar Beet Pulp. Foods 2025, 14, 3435. https://doi.org/10.3390/foods14193435
Huang X, Zhang Z, Li Y, Yang Y, Mulati A, Shataer D, Wang J. The Effects of Hot Air and Microwave Drying on the Structural and Physicochemical Properties of Soluble Dietary Fiber from Sugar Beet Pulp. Foods. 2025; 14(19):3435. https://doi.org/10.3390/foods14193435
Chicago/Turabian StyleHuang, Xinmeng, Zunqi Zhang, Yuanpeng Li, Yuting Yang, Ailikemu Mulati, Dilireba Shataer, and Jiayi Wang. 2025. "The Effects of Hot Air and Microwave Drying on the Structural and Physicochemical Properties of Soluble Dietary Fiber from Sugar Beet Pulp" Foods 14, no. 19: 3435. https://doi.org/10.3390/foods14193435
APA StyleHuang, X., Zhang, Z., Li, Y., Yang, Y., Mulati, A., Shataer, D., & Wang, J. (2025). The Effects of Hot Air and Microwave Drying on the Structural and Physicochemical Properties of Soluble Dietary Fiber from Sugar Beet Pulp. Foods, 14(19), 3435. https://doi.org/10.3390/foods14193435






