Dynamics of Physicochemical Properties, Flavor, and Bioactive Components in Lactobacillus-Fermented Pueraria lobata with Potential Hypolipidemic Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Sample Preparation of PL Fermentation Products
2.3. Physicochemical Properties Analysis
2.3.1. Microbial Viability Determination
2.3.2. Determination of pH and Titratable Acidity
2.3.3. Quantification of Total Flavonoids, Total Phenolics, and Reducing Sugars
2.3.4. Antioxidant Capacity Assessment
2.4. Flavor Profiling of PL Fermentation via Intelligent Sensory Technology
2.4.1. Flavor Discrimination Using Electronic Tongue and Electronic Nose Technologies
2.4.2. Analysis via Heracles NEO Ultra-Fast Gas Chromatography Electronic Nose
2.5. Analysis of Non-Volatile Components
2.6. Network Pharmacological Analysis
2.6.1. Screening of Active Components and Prediction of Their Putative Targets
2.6.2. Collection of Disease-Associated Targets and Screening of Intersection Targets
2.6.3. Protein–Protein Interaction (PPI) Network Construction and Hub Target Screening
2.6.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis with Construction of the “Active Components-Targets-Pathways-Disease” Network
2.7. Molecular Docking (MD) of Active Components with Hub Targets
2.8. Molecular Dynamics Simulation (MDS) of Active Components with Hub Targets
2.9. Statistical Analysis
3. Results
3.1. Microbial Viability and Physicochemical Dynamics During PL Fermentation
3.2. Dynamic Changes in Antioxidant Activity
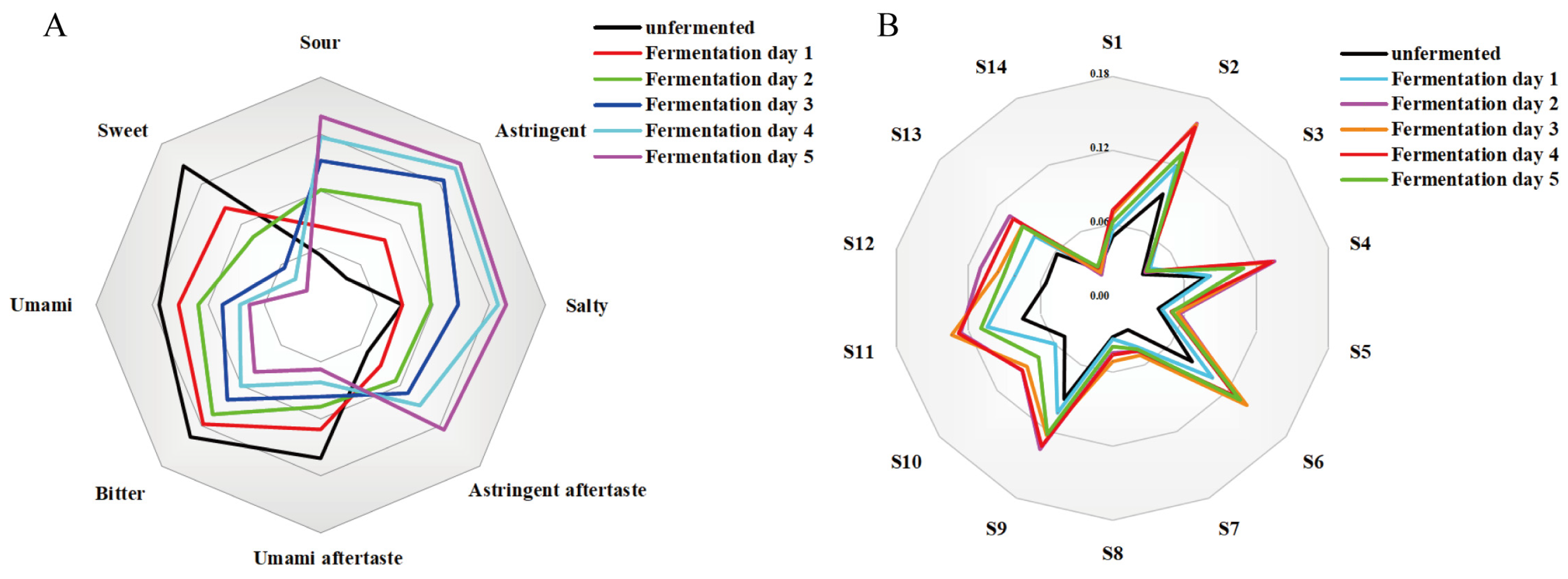
3.3. Flavor Dynamics Characterization Using Intelligent Sensory Technologies
3.3.1. Electronic Tongue and Electronic Nose Characterisation
3.3.2. Heracles NEO Ultra Rapid Gas Phase Electronic Nose
3.4. Non-Volatile Component Dynamics
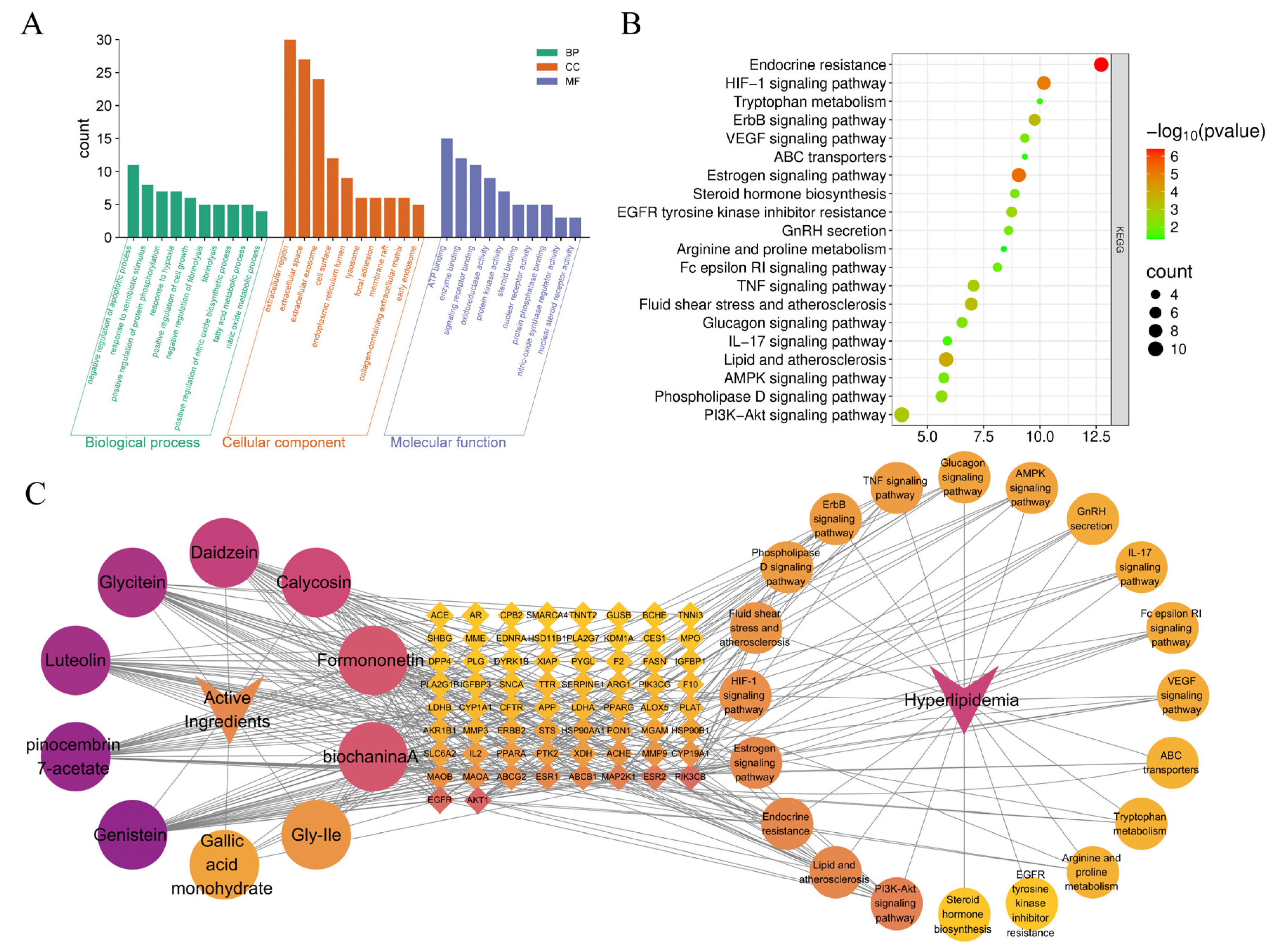
3.5. Network Pharmacological Analysis
3.5.1. Screening of Key Enriched Active Components, Target Prediction, and Disease-Associated Target Acquisition
3.5.2. PPI Network Construction and Hub Target Identification
3.5.3. Functional Enrichment of GO and KEGG Pathways with Construction of the “Component-Target-Pathway-Disease” Network
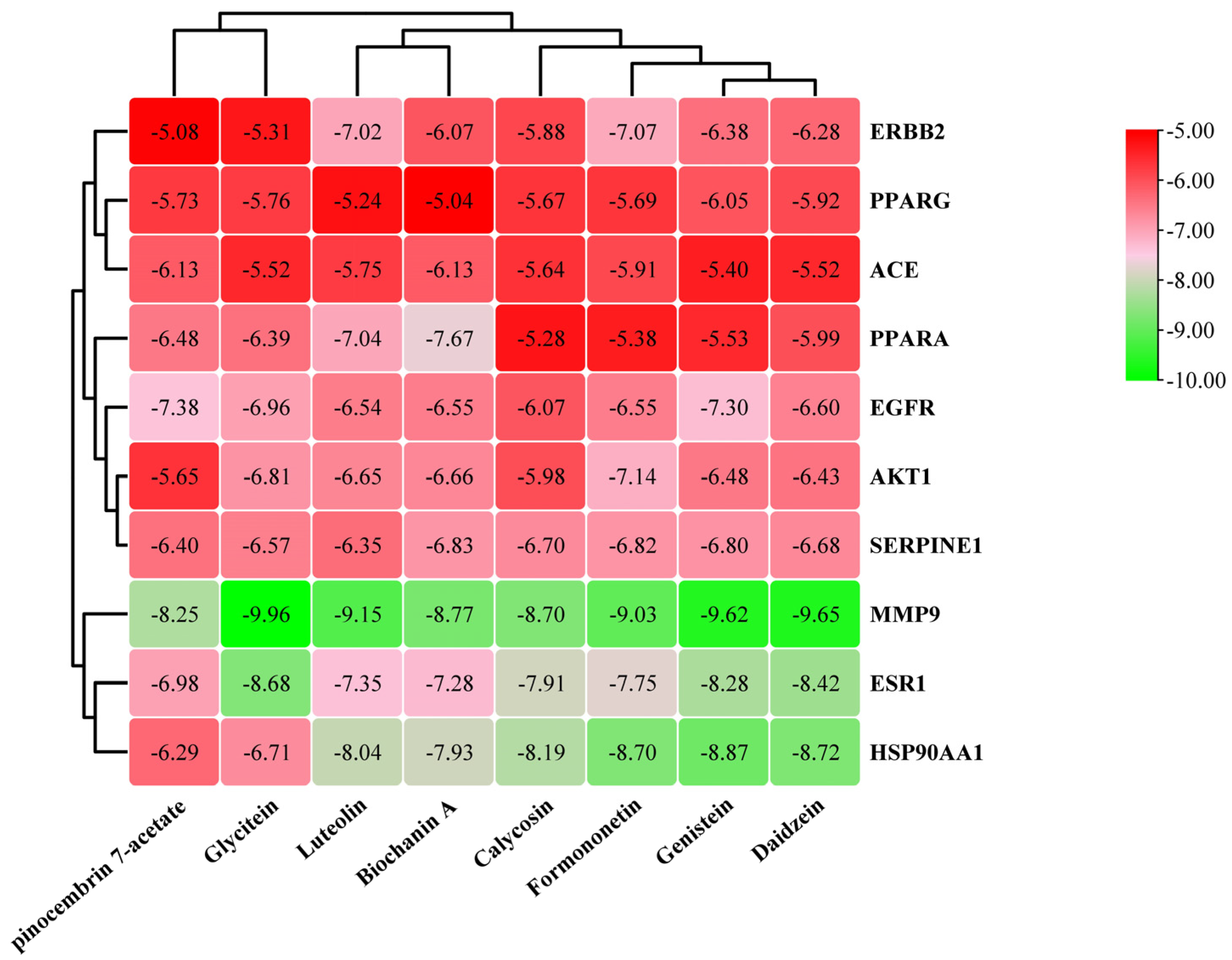
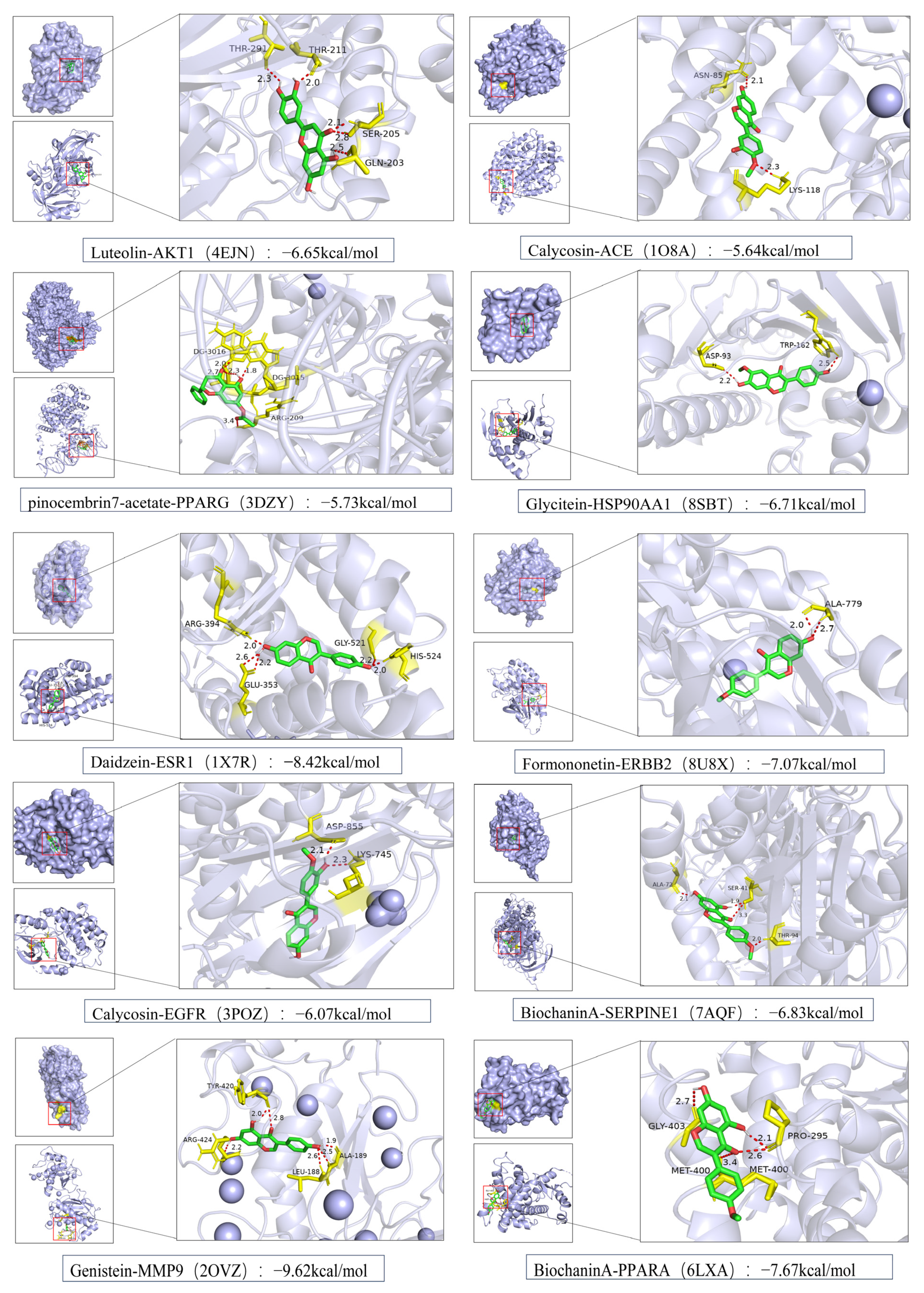
3.6. Molecular Docking
3.7. Molecular Dynamics Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Zhao, D.; Qi, Y. Global Trends in the Epidemiology and Management of Dyslipidemia. J. Clin. Med. 2022, 11, 6377. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Kano, Y.; Yuan, D.; Qu, J. Oriental traditional herbal Medicine-Puerariae Flos: A systematic review. J. Ethnopharmacol. 2023, 306, 116089. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, J.; Wan, Q.; Wang, W.; Yu, X.; You, J.; Ouyang, H.; Chen, X.; Cong, Y.; Huang, S.; et al. Efficacy and safety of Pueraria lobata radix and Pueraria thomsonii radix for patients with mild dyslipidemia: A randomized, double-blind, placebo-controlled trial. J. Funct. Foods 2022, 98, 105284. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The Application of Fermentation Technology in Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Fonseca, H.C.; Melo, D.d.S.; Ramos, C.L.; Menezes, A.G.T.; Dias, D.R.; Schwan, R.F. Sensory and flavor-aroma profiles of passion fruit juice fermented by potentially probiotic Lactiplantibacillus plantarum CCMA 0743 strain. Food Res. Int. 2022, 152, 110710. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yin, X.; Fang, Y.; Xiong, T.; Peng, F. Effect of Limosilactobacillus fermentum NCU001464 fermentation on physicochemical properties, xanthine oxidase inhibitory activity and flavor profile of Pueraria Lobata. Food Chem. 2025, 476, 143490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Peng, D.; Li, W.; Chen, S.; Liu, B.; Huang, P.; Wu, J.; Du, B.; Li, P. Probiotic-fermented Pueraria lobata (Willd.) Ohwi alleviates alcoholic liver injury by enhancing antioxidant defense and modulating gut microbiota. J. Sci. Food Agr. 2022, 102, 6877–6888. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Deng, L.; Zhao, M.; Tang, J.; Liu, T.; Zhang, H.; Feng, F. Probiotic-fermented blueberry juice prevents obesity and hyperglycemia in high fat diet-fed mice in association with modulating the gut microbiota. Food Funct. 2020, 11, 9192–9207. [Google Scholar] [CrossRef]
- Yang, J.; Shi, H.; Cai, L.; Zhang, S.; Zhao, Y.; Su, X.; Sun, H.; Cao, Y.; Li, Y. Effects of different lactic acid bacteria fermentation on active substances and functional characteristics of honeysuckle liquid: Analysis of metabolites of honeysuckle liquid based on metabolomics. Front Microbiol. 2025, 16, 1595351. [Google Scholar] [CrossRef]
- Guan, Y.; Tang, Y.; Li, L.; Dong, H.; Pan, H.; Zhang, H.; Zhu, W.; Zang, Z. Screening of Lactic Acid Bacteria Strains for Pueraria lobata Fermentation and Optimization of Synergistic Fermentation Process. Chin. J. Tradit. Chin. Med. Pharm. 2025, 1–18. Available online: https://link.cnki.net/urlid/21.1546.r.20250822.1038.002 (accessed on 22 August 2025).
- Cheng, H.; Tang, Y.; Li, Z.; Guo, Z.; Heath, J.R.; Xue, M.; Wei, W. Non-mass spectrometric targeted single-cell metabolomics. Trends Anal. Chem. 2023, 168, 117300. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, B.; Chen, S.; Lin, M.; Chen, Y.; Jin, S.; Chen, W.; Zhang, Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid-based Compl Alt. 2020, 2020, 1646905. [Google Scholar] [CrossRef]
- GB 4789.2-2022; National Health Commission of the People’s Republic of China. National Food Safety Standard—Food Microbiological Examination—Aerobic Plate Count. Standards Press of China: Beijing, China, 2022. Available online: https://wjw.nmg.gov.cn/zzb/hybz/202212/t20221205_2183178.html (accessed on 20 March 2025).
- GB 5009.237-2016; National Health Commission of the People’s Republic of China. National Food Safety Standard—Determination of pH Value in Foods. Standards Press of China: Beijing, China, 2016. Available online: http://down.foodmate.net/wap/index.php?itemid=49406&moduleid=23 (accessed on 20 March 2025).
- GB 12456-2021; National Health Commission of the People’s Republic of China. National Food Safety Standard—Determination of Total Acid in Foods. Standards Press of China: Beijing, China, 2021. Available online: http://down.foodmate.net/wap/index.php?moduleid=23&itemid=97830 (accessed on 20 March 2025).
- Su, H.; Jiang, H.; Li, Y. Effects of PAL and ICS on the production of total flavonoids, daidzein and puerarin in Pueraria thomsonii Benth. suspension cultures under low light stress. J. Plant Biochem. Biot. 2015, 24, 34–41. [Google Scholar] [CrossRef]
- Rasheed, H.; Deng, B.; Ahmad, D.; Bao, J. Genetic Diversity and Genome-Wide Association Study of Total Phenolics, Fla-vonoids, and Antioxidant Properties in Potatoes (Solanum tuberosum L.). Int. J. Mol. Sci. 2024, 25, 12795. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Z.; Wen, M.; Wu, Z.; Zha, M.; Xu, W.; Zhang, L. Chemical Variation of Chenpi (Citrus Peels) and Corresponding Correlated Bioactive Compounds by LC-MS Metabolomics and Multibioassay Analysis. Front. Nutr. 2022, 9, 825381. [Google Scholar] [CrossRef]
- An, X.; Li, T.; Hu, J.; Li, Y.; Liu, H.; Fang, H.; Wei, X. Evaluation of physicochemical characteristics, bioactivity, flavor profile and key metabolites in the fermentation of goji juice by Lacticaseibacillus rhamnosus. Food Chem. X 2024, 23, 101755. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zheng, Z.; Wang, Z.; Gao, C.; Liang, Y.; Zeng, W.; Sun, W. Profiling of Potential Anti-Diabetic Active Compounds in White Tea: An Integrated Study of Polyphenol-Targeted Metabolomics, Network Pharmacology, and Computer Simulation. Foods 2024, 13, 3354. [Google Scholar] [CrossRef]
- Xu, N.; Zeng, X.; Wang, P.; Chen, X.; Xu, X.; Han, M. Investigation on taste characteristics and sensory perception of soft-boiled chicken during oral processing based on electronic tongue and electronic nose. Food Sci. Hum. Wellness 2024, 13, 313–326. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Z.; Dang, J.; Li, D.; Yu, D.; Qu, C.; Wu, Q. GC-MS Combined with Fast GC E-Nose for the Analysis of Volatile Components of Chamomile (Matricaria chamomilla L.). Foods 2024, 13, 1865. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, C.; Zang, Z.; Wan, X.; Naeem, A.; Zhang, R.; Zhu, W. Chitosan/xanthan gum-based (Hydroxypropyl methylcellulose-co-2-Acrylamido-2-methylpropane sulfonic acid) interpenetrating hydrogels for controlled release of amorphous solid dispersion of bioactive constituents of Pueraria lobatae. Int. J. Biol. Macromol. 2023, 224, 380–395. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, R.; Li, X.; Yang, D.; Ji, S.; Peng, L.; Sheng, J.; Wang, J. Integrating Strategy of Network Pharmacology, Molecular Dynamics Simulation, and Experimental Verification to Investigate the Potential Mechanism of Gastrodia elata Against Alcoholic Liver Injury. Foods 2025, 14, 2008. [Google Scholar] [CrossRef]
- Wu, J.; Ge, F.; Zhu, L.; Liu, N. Potential Toxic Mechanisms of Neonicotinoid Insecticides in Rice: Inhibiting Auxin-Mediated Signal Transduction. Environ. Sci. Technol. 2023, 57, 4852–4862. [Google Scholar] [CrossRef]
- Carretero-González, R.; Kevrekidis, P.G.; Kevrekidis, I.G.; Maroudas, D.; Frantzeskakis, D.J. A Parrinello-Rahman approach to vortex lattices. Phys. Lett. A 2005, 341, 128–134. [Google Scholar] [CrossRef]
- Wang, J.; Dai, G.; Shang, M.; Wang, Y.; Xia, C.; Duan, B.; Xu, L. Extraction, structural-activity relationships, bioactivities, and application prospects of Pueraria lobata polysaccharides as ingredients for functional products: A review. Int. J. Biol. Macromol. 2023, 243, 125210. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Singh, A.; Eriksson, K.E. Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Adv Biochem. Eng. Biot. 1997, 57, 45–125. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agr. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Zhang, F.; Foo, H.; Cao, Z.; Lin, Q. Dynamics Changes in Physicochemical Properties, Antioxidant Activity, and Non-Volatile Metabolites During Bulang Pickled Tea Fermentation. Foods 2025, 14, 878. [Google Scholar] [CrossRef]
- Cho, S.; Moazzem, M.S. Recent Applications of Potentiometric Electronic Tongue and Electronic Nose in Sensory Evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef]
- Cai, W.; Zhou, W.; Liu, J.; Wang, J.; Kuang, D.; Wang, J.; Long, Q.; Huang, D. An Exploratory Study on the Rapid Detection of Volatile Organic Compounds in Gardenia Fruit Using the Heracles NEO Ultra-Fast Gas Phase Electronic Nose. Metabolites 2024, 14, 445. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Zhao, X.; Li, M.; Wang, Y.; Tian, M.; Zhou, J. Classification and characterization on sorghums based on HS-GC-IMS combined with OPLS-DA and GA-PLS. Curr. Res. Food Sci. 2024, 8, 100692. [Google Scholar] [CrossRef]
- Wang, J.; Xioa, Q.; Huang, H.; Wu, D.; Zeng, G.; Chen, W.; Tao, Y.; Ding, B. Non-target screening and identification of the significant quality markers in the wild and cultivated Cordyceps sinensis using OPLS-DA and feature-based molecular networking. Chin. J. Anal. Chem. 2023, 51, 100302. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Belen Florez, A.; Vazquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Z.; Cheng, B.; Liu, M.; Tao, S.; Guan, S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp. Ther. Med. 2019, 18, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, S.; Liu, C.; Wu, Y.; Liu, Y.; Cai, Y. Inhibitory Effect of Liquiritigenin on Migration Via Downregulation ProMMP-2 and PI3K/Akt Signaling Pathway in Human Lung Adenocarcinoma A549 cells. Nutr. Cancer 2012, 64, 627–634. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Ahn, J.; Jung, C.H.; Ha, T. Coumestrol modulates Akt and Wnt/β-catenin signaling during the attenuation of adipogenesis. Food Funct. 2016, 7, 4984–4991. [Google Scholar] [CrossRef]
- Qiu, J.; Fu, L.; Xue, Y.; Yang, Y.; Qiao, F.; Zhu, W.; Gao, Y.; Fang, M.; Liu, Y.; Gao, Z.; et al. Gallic acid mitigates high-fat and high-carbohydrate diet-induced steatohepatitis by modulating the IRF6/PPARγ signaling pathway. Front. Pharmacol. 2025, 16, 1563561. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Ni, W.; Ye, H.; Liu, Y.; Chen, L.; Wang, L.; Liu, C.; Chen, J.; Wang, X.; et al. A small molecule esculetin accelerates postprandial lipid clearance involving activation of C/EBPβ and CD36-mediated phagocytosis by adipose tissue macrophages. Theranostics 2025, 15, 5910–5930. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Ziqubu, K.; Dludla, P.V.; Tiano, L.; Silvestri, S.; Orlando, P.; Nyawo, T.A.; Louw, J.; Kappo, A.P.; Muller, C.J.F. Isoorientin ameliorates lipid accumulation by regulating fat browning in palmitate-exposed 3T3-L1 adipocytes. Metab. Open 2020, 6, 100037. [Google Scholar] [CrossRef]
- Luo, W.; Deng, J.; He, J.; Yin, L.; You, R.; Zhang, L.; Shen, J.; Han, Z.; Xie, F.; He, J.; et al. Integration of molecular docking, molecular dynamics and network pharmacology to explore the multi-target pharmacology of fenugreek against diabetes. J. Cell Mol. Med. 2023, 27, 1959–1974. [Google Scholar] [CrossRef]
- Fu, J.; Chen, D.; Zhao, B.; Zhao, Z.; Zhou, J.; Xu, Y.; Xin, Y.; Liu, C.; Luo, L.; Yin, Z. Luteolin Induces Carcinoma Cell Apoptosis through Binding Hsp90 to Suppress Constitutive Activation of STAT3. PLoS ONE 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Liu, C.; Yang, C.; Yao, S.; Qiu, H.; Yang, J.; Wu, K.; Liao, H.; Jiang, Q. Daidzein protects endothelial cells against high glucose-induced injury through the dual-activation of PPARα and PPARγ. Gen. Physiol. Biophys. 2024, 43, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, Z.-H.; Liu, C.Y.; Zhang, Y. Effect of Chinese Herbal Monomer Hairy Calycosin on Nonalcoholic Fatty Liver Rats and its Mechanism. Comb. Chem. High T Scr. 2019, 22, 194–200. [Google Scholar] [CrossRef]
- Xing, N.; Wang, Y.; Wang, W.; Zhong, R.; Xia, T.; Ding, Z.; Yang, Y.; Zhong, Y.; Shu, Z. Cardioprotective effect exerted by Timosaponin BIT through the regulation of endoplasmic stress-induced apoptosis. Phytomedicine 2020, 78, 153288. [Google Scholar] [CrossRef]
- Chai, S.; Yang, Y.; Wei, L.; Cao, Y.; Ma, J.; Zheng, X.; Teng, J.; Qin, N. Luteolin rescues postmenopausal osteoporosis elicited by OVX through alleviating osteoblast pyroptosis via activating PI3K-AKT signaling. Phytomedicine 2024, 128, 155516. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Rathor, L.S.; Dwivedi, S.D.; Shah, K.; Chauhan, N.S.; Singh, M.R.; Singh, D. A Review on Molecular Docking As an Interpretative Tool for Molecular Targets in Disease Management. Assay Drug Dev. Techn. 2024, 22, 40–50. [Google Scholar] [CrossRef]
- Perola, E.; Charifson, P.S. Conformational analysis of drug-like molecules bound to proteins: An extensive study of ligand reorganization upon binding. J. Med. Chem. 2004, 47, 2499–2510. [Google Scholar] [CrossRef]
- Wang, C.; Greene, D.A.; Xiao, L.; Qi, R.; Luo, R. Recent Developments and Applications of the MMPBSA Method. Front. Mol. Biosci. 2018, 4, 87. [Google Scholar] [CrossRef]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanislawczyk, R. Electronic Sensing Technologies in Food Quality Assessment: A Comprehensive Literature Review. Appl. Sci. 2025, 15, 1530. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Z.; Zou, L.; Liu, X.; Chai, C.; Zhao, H.; Yan, Y.; Wang, C. Quality Evaluation of Apocyni Veneti Folium from Different Habitats and Commercial Herbs Based on Simultaneous Determination of Multiple Bioactive Constituents Combined with Multivariate Statistical Analysis. Molecules 2018, 23, 573. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Chen, W.; Liu, C.; Zhang, T.; Shi, R.; Wang, X.; Xu, H.; Li, D. Soy protein compared with whey protein ameliorates insulin resistance by regulating lipid metabolism, AMPK/mTOR pathway and gut microbiota in high-fat diet-fed mice. Food Funct. 2023, 14, 5752–5767. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Chen, J.-J.; Deng, W.-Y.; Xu, X.-D.; Liu, Q.-X.; Shi, M.-W.; Ren, K. Biochanin A Mitigates Atherosclerosis by Inhibiting Lipid Accumulation and Inflammatory Response. Oxid. Med. Cell. Longev. 2020, 2020, 8965047. [Google Scholar] [CrossRef] [PubMed]
- Savva, C.; Korach-Andre, M. Estrogen Receptor beta (ERβ) Regulation of Lipid Homeostasis-Does Sex Matter? Metabolites 2020, 10, 116. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]






| Bacterial Viability and Physical and Chemical Indicators | Fermentation Time | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Bacterial Count/lgCFU·mL−1 | 7.23 ± 0.05 c | 8.89 ± 0.04 a | 8.97 ± 0.06 a | 8.98 ± 0.04 a | 8.89 ± 0.02 a | 8.74 ± 0.04 b |
| Fermentation Solution pH | 5.95 ± 0.05 a | 5.14 ± 0.06 b | 4.56 ± 0.07 c | 4.28 ± 0.03 d | 4.16 ± 0.03 de | 4.09 ± 0.03 e |
| Total Acid Content/g·L−1 | 1.02 ± 0.03 f | 1.43 ± 0.02 e | 2.32 ± 0.05 d | 2.99 ± 0.02 c | 3.32 ± 0.03 b | 3.48 ± 0.03 a |
| Total Flavonoid Content/mg·g−1 | 124.73 ± 1.24 b | 129.76± 1.15 a | 118.72 ± 1.50 c | 129.00 ± 1.68 a | 116.32 ± 0.68 cd | 113.91 ± 1.86 d |
| Total Phenol Content/mg·g−1 | 48.81 ± 0.70 a | 45.77 ± 1.87 c | 47.72 ± 1.34 ab | 45.53 ± 1.27 c | 47.02 ± 0.97 bc | 48.39 ± 1.88 ab |
| Reducing Sugar Content/mg·g−1 | 98.11 ± 1.71 a | 74.09 ± 0.78 b | 62.16 ± 0.87 c | 45.15 ± 0.87 e | 35.32 ± 0.99 f | 50.01 ± 1.53 d |
| DPPH Radical Scavenging Capacity/μmol TE·g−1 PL | 113.71 ± 3.68 a | 103.49 ± 1.91 b | 101.44 ± 3.95 b | 101.99 ± 3.03 b | 101.84 ± 2.29 b | 95.13 ± 4.66 c |
| ABTS·+ Radical Scavenging Capacity/μmol TE·g−1 PL | 736.29 ± 39.28 a | 663.86 ± 8.19 b | 599.51 ± 4.47 c | 642.39 ± 28.09 b | 585.19 ± 14.67 c | 550.08 ± 17.29 d |
| OH·Radical Scavenging Capacity/μmol VC·g−1 PL | 89.55 ± 1.04 a | 47.61 ± 1.10 b | 47.71 ± 2.52 b | 48.03 ± 1.31 b | 42.05 ± 1.19 c | 37.96 ± 2.21 d |
| Fe3+ Reducing Capability/μmol FeSO4·g−1 PL | 438.95 ± 9.05 a | 440.69 ± 4.62 a | 417.58 ± 3.89 c | 383.41 ± 8.19 d | 378.66 ± 7.90 d | 427.08 ± 4.51 b |
| Sensors | Compound Type | Sensors | Compound Type |
|---|---|---|---|
| S1 | Aromatic compounds | S8 | Amines |
| S2 | Nitrogen oxides, low molecular amines | S9 | Hydrogen |
| S3 | Sulphide | S10 | Furans |
| S4 | Organic acid esters and terpenes | S11 | Volatile organic compound |
| S5 | Terpenes, Esters | S12 | Sulfide |
| S6 | Sterols, triterpenes | S13 | Vinyl |
| S7 | Oxygenated derivatives of aliphatic hydrocarbons | S14 | Lactones, pyrazines |
| NO. | Possible Compounds | CAS | Formula | Fermentation Time (Day) | Odour Characteristics | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||||
| 1 | Ethanol | 64-17-5 | C2H6O | + | ↓ | ↑ | ↑ | ↑ | ↑ | Alcoholic; Spicy; Strong; Sweet |
| 2 | Methanol | 67-56-1 | CH4O | − | − | − | − | − | + | Alcohol; Spicy; Strong |
| 3 | β-Pinene | 127-91-3 | C10H16 | + | ↓ | ↑ | − | − | − | Dry; Freshly cut grass; Pine; Resin; Sweet |
| 4 | Propanal | 123-38-6 | C3H6O | − | + | ↑ | ↑ | ↑ | − | Acetaldehyde; Cocoa; Nuts; Plastic; Spicy |
| 5 | 2-Methylnonane | 871-83-0 | C10H22 | − | − | + | − | − | − | / |
| 6 | 2-Methyl-2-propanol | 75-65-0 | C4H10O | − | − | − | + | ↑ | ↑ | Camphor |
| 7 | (E)-2-Hexen-1-ol, butanoate | 53398-83-7 | C10H18O2 | − | − | − | + | − | − | Apple; Apricot; Banana (ripe); Cheese; Fermented; Freshly cut grass |
| 8 | 5-ethyl-3-hydroxy-4-methyl-2(5H)-furanone | 698-10-2 | C7H10O3 | − | − | − | + | − | − | Brown Sugar; Cream Candy; Caramel; Nutty; Condiment; Spicy; Sweet |
| 9 | Tridecane | 629-50-5 | C13H28 | − | − | − | + | − | − | Alkanes; Oranges; Fruits; Heteroalcohols |
| 10 | Carvone | 6485-40-1 | C10H14O | − | − | − | + | − | − | Basil; Bitter; Coriander; Fennel; Minty; Peppermint; Ruminal; Sweet |
| 11 | 3-Methyl-dodecane | 17312-57-1 | C13H28 | − | − | − | + | − | − | / |
| 12 | Anisyl alcohol | 105-13-5 | C8H10O2 | − | − | − | + | − | − | Floral; Vegetative; Powdery |
| 13 | E-Tetradec-7-ene | 41446-63-3 | C14H28 | − | − | − | + | ↓ | − | Freshly cut grass |
| 14 | 2-Methyltetradecane | 1560-95-8 | C15H32 | − | − | − | + | − | − | / |
| 15 | 3-Ethyltridecane | 13286-73-2 | C15H32 | − | − | − | + | − | − | / |
| 16 | 3-Methyltetradecane | 18435-22-8 | C15H32 | − | − | − | + | − | − | / |
| 17 | Pentadecane | 629-62-9 | C15H32 | − | − | − | + | − | − | Alkanes; Heteroalcohols; Freshly cut grass |
| 18 | Propylenglycol | 57-55-6 | C3H8O2 | − | − | − | − | + | − | Alcohol; Caramel; Flavourless |
| 19 | 2,3-Butanediol | 513-85-9 | C4H10O2 | − | − | − | − | + | − | Creamy; Fruit; Flavourless; Onion |
| 20 | Methylcyclohexane | 108-87-2 | C7H14 | − | − | − | − | − | + | Fuzzy, Dizzy; Fruit; Sweet |
| 21 | 5-Methylfurfural | 620-02-0 | C6H6O2 | − | − | − | − | − | + | Acidic; Almond; Caramel; Coffee; Spicy |
| 22 | Cis-Decalin | 493-01-6 | C10H18 | − | − | − | − | − | + | / |
| Models | PCA | OPLS-DA | |||
|---|---|---|---|---|---|
| R2X | Q2 | R2X | R2Y | Q2 | |
| 0 vs. 1 | 0.845 | 0.614 | 0.844 | 0.992 | 0.903 |
| 0 vs. 2 | 0.861 | 0.647 | 0.857 | 0.995 | 0.977 |
| 0 vs. 3 | 0.911 | 0.776 | 0.911 | 0.999 | 0.992 |
| 0 vs. 4 | 0.942 | 0.853 | 0.940 | 0.994 | 0.979 |
| 0 vs. 5 | 0.953 | 0.881 | 0.906 | 0.996 | 0.987 |
| NO. | Gene | Sensors | Degree |
|---|---|---|---|
| 1 | EGFR | Epidermal Growth Factor Receptor | 33 |
| 2 | ESR1 | Estrogen Receptor 1 | 37 |
| 3 | SERPINE1 | Serpin Family E Member 1 | 23 |
| 4 | ERBB2 | Erb-B2 Receptor Tyrosine Kinase 2 | 26 |
| 5 | PPARG | Peroxisome Proliferator Activated Receptor Gamma | 37 |
| 6 | MMP9 | Matrix Metallopeptidase 9 | 32 |
| 7 | ACE | Angiotensin I-Converting Enzyme | 29 |
| 8 | HSP90AA1 | Heat Shock Protein 90 Alpha Family Class A Member 1 | 28 |
| 9 | AKT1 | AKT Serine/Threonine Kinase 1 | 46 |
| 10 | PPARA | Peroxisome Proliferator Activated Receptor Alpha | 20 |
| Energy | AKT1-Luteolin | MMP9-Genistein | ESR1-Daidzein |
|---|---|---|---|
| Van der Waals Energy (KJ/mol) | −141.890 | −178.102 | −152.691 |
| Electrostatic energy (KJ/mol) | −32.798 | −43.121 | −36.634 |
| Polar solvation energy (KJ/mol) | 141.124 | 149.786 | 129.209 |
| Nonpolar solvation Energy (KJ/mol) | −20.120 | −17.975 | −18.206 |
| Total Binding Energy (KJ/mol) | −53.684 | −89.412 | −78.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Li, L.; Li, Q.; Li, Z.; Dong, H.; Zhang, H.; Pan, H.; Zhu, W.; Zang, Z.; Guan, Y. Dynamics of Physicochemical Properties, Flavor, and Bioactive Components in Lactobacillus-Fermented Pueraria lobata with Potential Hypolipidemic Mechanisms. Foods 2025, 14, 3425. https://doi.org/10.3390/foods14193425
Tang Y, Li L, Li Q, Li Z, Dong H, Zhang H, Pan H, Zhu W, Zang Z, Guan Y. Dynamics of Physicochemical Properties, Flavor, and Bioactive Components in Lactobacillus-Fermented Pueraria lobata with Potential Hypolipidemic Mechanisms. Foods. 2025; 14(19):3425. https://doi.org/10.3390/foods14193425
Chicago/Turabian StyleTang, Ye, Liqin Li, Qiong Li, Zhe Li, Huanhuan Dong, Hua Zhang, Huaping Pan, Weifeng Zhu, Zhenzhong Zang, and Yongmei Guan. 2025. "Dynamics of Physicochemical Properties, Flavor, and Bioactive Components in Lactobacillus-Fermented Pueraria lobata with Potential Hypolipidemic Mechanisms" Foods 14, no. 19: 3425. https://doi.org/10.3390/foods14193425
APA StyleTang, Y., Li, L., Li, Q., Li, Z., Dong, H., Zhang, H., Pan, H., Zhu, W., Zang, Z., & Guan, Y. (2025). Dynamics of Physicochemical Properties, Flavor, and Bioactive Components in Lactobacillus-Fermented Pueraria lobata with Potential Hypolipidemic Mechanisms. Foods, 14(19), 3425. https://doi.org/10.3390/foods14193425







