High-Porosity Hydrogel Microneedles for Rapid and Efficient Extraction of Imidacloprid Residues in Peach Fruits
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of HMN
2.3. Characterization
2.3.1. In Vitro Extraction Property of HMN in a Simulated Tissue Matrix
2.3.2. In Vitro Extraction Property of HMN in the Crushed Peach Flesh
2.3.3. In Vivo Penetration HMN Elution for HPLC
2.3.4. Cell Activity Detection
2.3.5. Applicator Fabrication, Assembly and Usage
2.3.6. Large Instruments Used in the Experiment
3. Results and Discussion
3.1. Preparation and Structure Characterization of HMN Patch
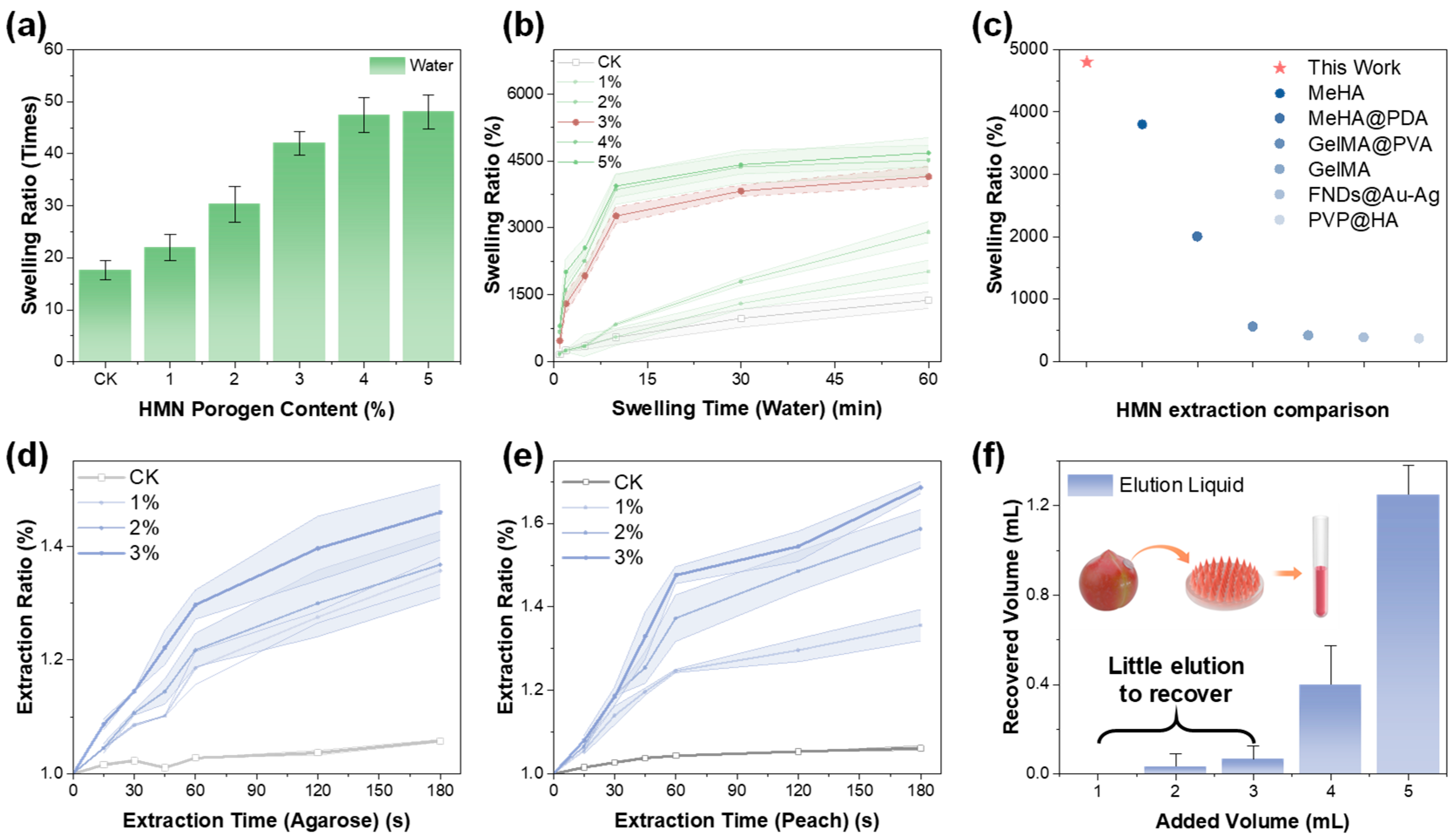
3.2. Swelling and Extraction Properties of HMN Patch
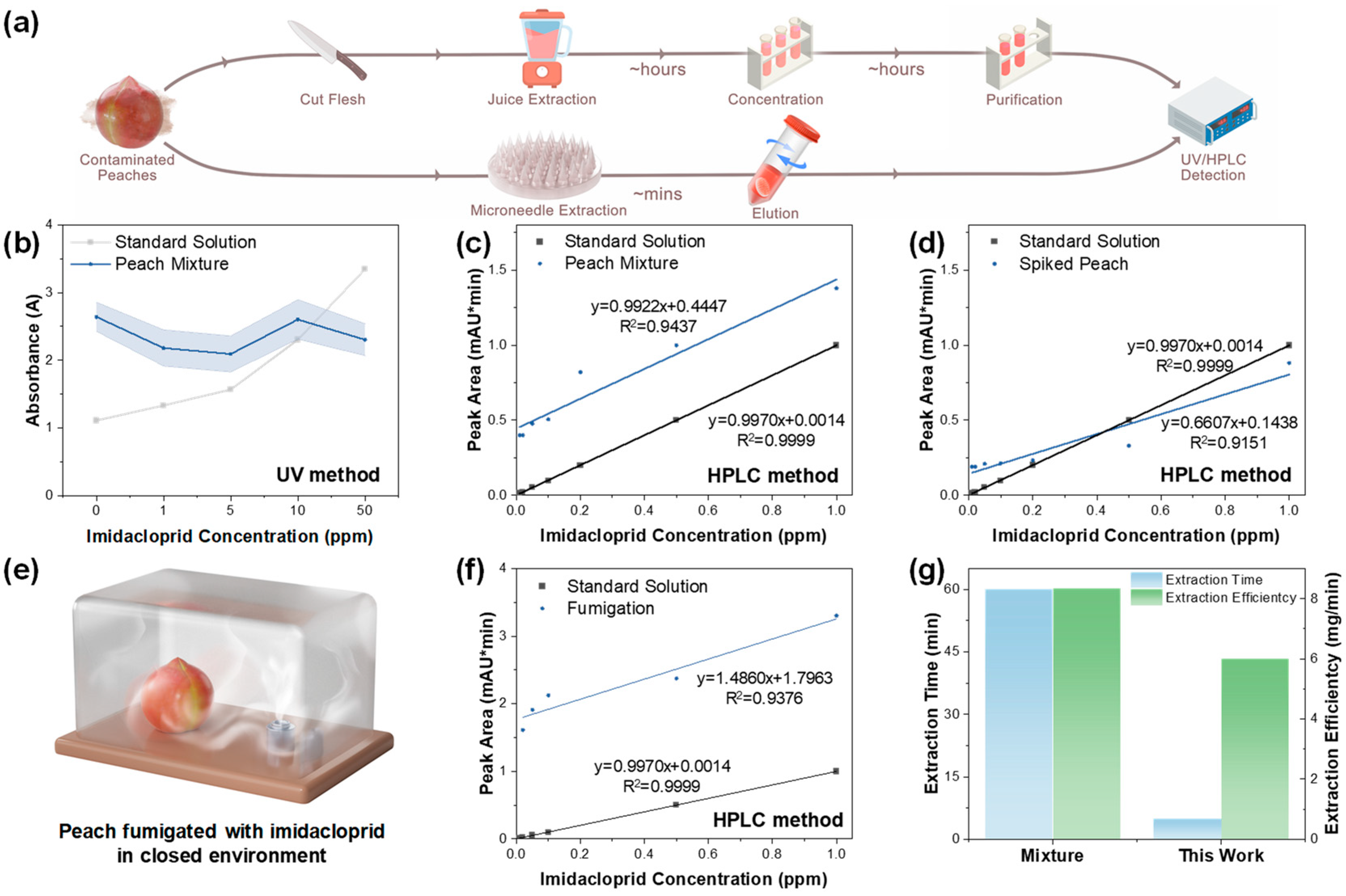
3.3. Practical Extraction Performance of HMN Patch for Peach Juice Detection
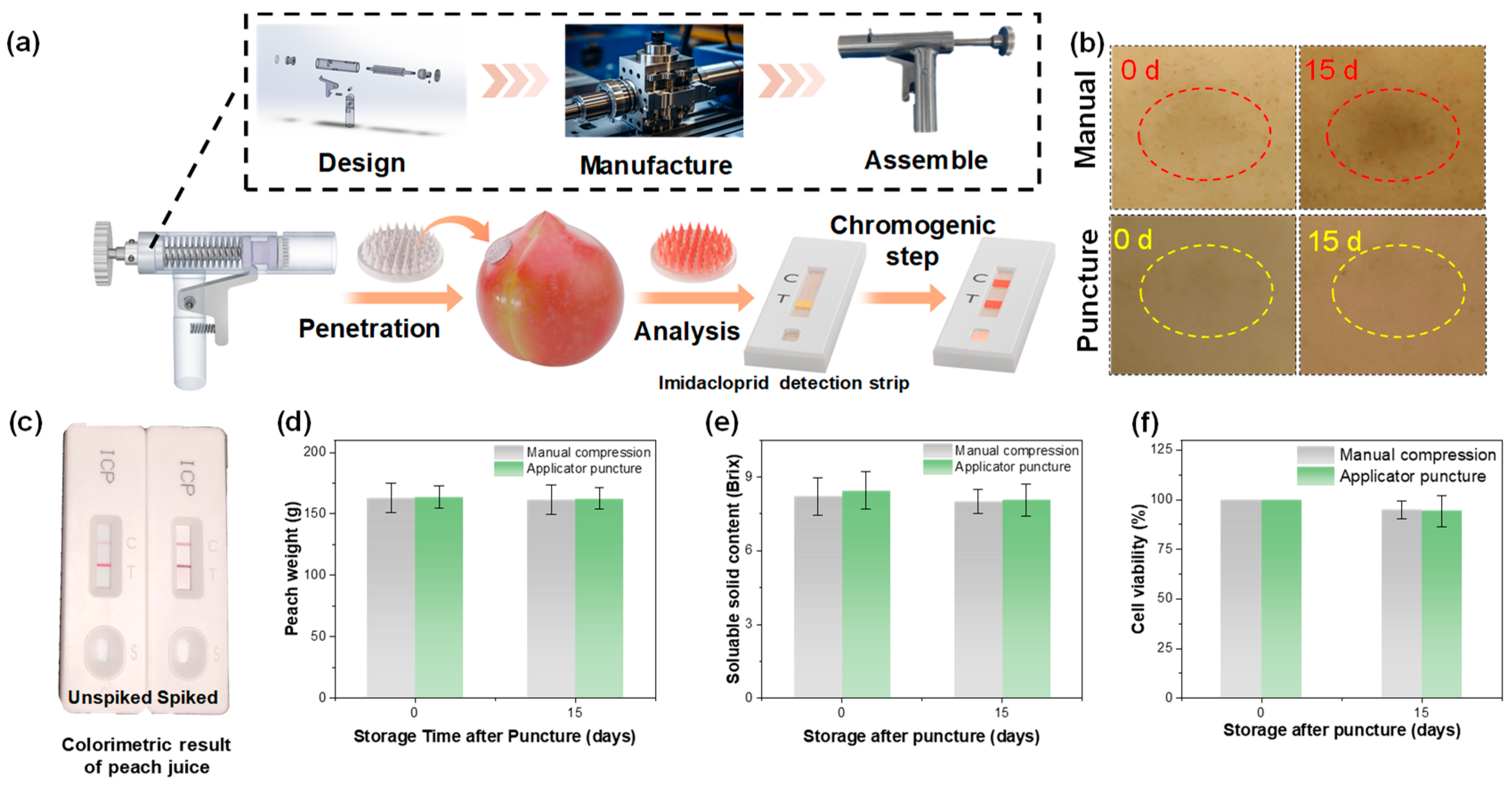
3.4. Design of HMN Patch Insertion Applicator and Rapid Detection System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Yu, H.; Pan, K.; Xu, Z.; Lei, Y.; Mo, Q.; Lei, H.; Guan, T. Sample pretreatment-free rapid determination of difenoconazole in vegetables by swellable hydrogel microneedles. J. Hazard. Mater. 2025, 497, 139712. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Chia, S.Y.; Dofuor, A.K.; Antwi-Agyakwa, A.K.; Okyere, H.; Gyan, M.; Edusei, G.; Ninsin, K.D.; Duker, R.Q.; et al. Remediation of pesticide residues using ozone: A comprehensive overview. Sci. Total Environ. 2023, 894, 164933. [Google Scholar] [CrossRef]
- Shim, J.-H.; Eun, J.-B.; Zaky, A.A.; Hussein, A.S.; Hacimüftüoğlu, A.; El-Aty, A.M.A. A comprehensive review of pesticide residues in peppers. Foods 2023, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, L.; Li, M.; Wang, M.; Liu, G.; Ping, J. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. Trends Anal. Chem. 2023, 165, 117152. [Google Scholar] [CrossRef]
- Almenhali, A.Z.; Eissa, S. Aptamer-based biosensors for the detection of neonicotinoid insecticides in environmental samples: A systematic review. Talanta 2024, 275, 126190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, Y.; Wang, Y.; Lu, Q.; Ruan, H.; Luo, J.; Yang, M. A comprehensive review on the pretreatment and detection methods of neonicotinoid insecticides in food and environmental samples. Food Chem. X 2022, 15, 100375. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Jiao, S.; Zhao, Y.; Guo, Y.; Wang, M.; Zhu, G. Quantum-dot-based lateral flow immunoassay for detection of neonicotinoid residues in tea leaves. J. Agric. Food Chem. 2017, 65, 10107–10114. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Zhang, Y.; Zhao, Z.; Xu, T.; Chen, Y.; Luo, J.; Yang, M. Residue, distribution and degradation of neonicotinoids and their metabolites in chrysanthemum plants and cultivated soils. Microchem. J. 2023, 194, 109315. [Google Scholar] [CrossRef]
- Wei, F.; Cheng, F.; Li, H.; You, J. Imidacloprid affects human cells through mitochondrial dysfunction and oxidative stress. Sci. Total Environ. 2024, 951, 175422. [Google Scholar] [CrossRef]
- GB 2763-2019; National Food Safety Standard—Maximum Residue Limits for Pesticides in Food. National Health Commission, Ministry of Agriculture and Rural Affairs, State Administration for Market Regulation: Beijing, China, 2019.
- Guo, S.; Zhang, S.; Zhang, J.; Lei, J.; Chen, H. Occurrence, residue level, distribution and risk assessment of pesticides in the typical polder areas of Lake Dongting. J. Hazard. Mater. 2025, 496, 139530. [Google Scholar] [CrossRef]
- Wang, M.; Tian, Q.; Li, H.; Dai, L.; Wan, Y.; Wang, M.; Han, B.; Huang, H.; Zhang, Y.; Chen, J. Visualization and metabolome for the migration and distribution behavior of pesticides residue in after-ripening of banana. J. Hazard. Mater. 2022, 446, 130665. [Google Scholar] [CrossRef]
- Christopher, F.C.; Kumar, P.S.; Christopher, F.J.; Joshiba, G.J.; Madhesh, P. Recent advancements in rapid analysis of pesticides using nano biosensors: A present and future perspective. J. Clean. Prod. 2020, 269, 122356. [Google Scholar] [CrossRef]
- Domínguez, I.; González, R.R.; Liébanas, F.J.A.; Vidal, J.L.M.; Frenich, A.G. Automated and semi-automated extraction methods for GC–MS determination of pesticides in environmental samples. Trends Environ. Anal. Chem. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2016, 90, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Mishra, R.K.; Tang, X.; Marty, J.L. Detection of antibiotics in food: New achievements in the development of biosensors. Trends Anal. Chem. 2020, 127, 115883. [Google Scholar] [CrossRef]
- Tsiasioti, A.; Zacharis, C.K.; Tzanavaras, P.D. Derivatization strategies for the determination of histamine in food samples: A review of recent separation-based methods. Trends Anal. Chem. 2023, 168, 117302. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S.; Fan, P.; Li, X.; Ying, Y.; Ping, J.; Pan, Y. Implantable electrochemical microsensors for in vivo monitoring of animal physiological information. Nano-Micro Lett. 2023, 16, 49. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Chen, J.; Zhou, J.; Fan, K.; Pan, Y.; Ping, J. An integrated plant glucose monitoring system based on microneedle-enabled electrochemical sensor. Biosens. Bioelectron. 2023, 248, 115964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, X.; Yao, S.; Shao, Y.; Zhang, C.; Zhou, S.; Ping, S.; Ying, Y. An implantable and self-powered sensing system for the in vivo monitoring of dynamic H2O2 level in plants. Engineering, 2024; in press. [Google Scholar]
- Cao, Y.; Koh, S.S.; Han, Y.; Tan, J.J.; Kim, D.; Chua, N.; Urano, D.; Marelli, B. Drug delivery in plants using silk microneedles. Adv. Mater. 2022, 35, 2205794. [Google Scholar] [CrossRef]
- Cao, Y.; Lim, E.; Xu, M.; Weng, J.; Marelli, B. Precision delivery of multiscale payloads to tissue-specific targets in plants. Adv. Sci. 2020, 7, 1903551. [Google Scholar] [CrossRef]
- Paul, R.; Saville, A.C.; Hansel, J.C.; Ye, Y.; Ball, C.; Williams, A.; Chang, X.; Chen, G.; Gu, Z.; Ristaino, J.B.; et al. Extraction of plant DNA by microneedle patch for rapid detection of plant diseases. ACS Nano 2019, 13, 6540–6549. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kai, H.; Nagamine, K.; Ogawa, Y.; Nishizawa, M. Porous polymer microneedles with interconnecting microchannels for rapid fluid transport. RSC Adv. 2016, 6, 48630–48635. [Google Scholar] [CrossRef]
- Wu, X.; Pan, Y.; Li, X.; Shao, Y.; Peng, B.; Zhang, C.; Zhang, C.; Yao, S.; Ping, J.; Ying, Y. Rapid and in-field sensing of hydrogen peroxide in plant by hydrogel microneedle patch. Small 2024, 20, 2402024. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, C.; Ping, J.; Ying, Y. Recent advances in hydrogel microneedle-based biofluid extraction and detection in food and agriculture. Biosens. Bioelectron. 2024, 250, 116066. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.J.; Park, S.; Hong, H.; Lee, J.; Kim, S.I.; Lee, K.J.; Ryu, W. Rapid extraction and detection of biomolecules via a microneedle array of wet-crosslinked methacrylated hyaluronic acid. Adv. Mater. Technol. 2021, 7, 2100874. [Google Scholar] [CrossRef]
- Saifullah, K.M.; Mushtaq, A.; Azarikhah, P.; Prewett, P.D.; Davies, G.J.; Rad, Z.F. Micro-vibration assisted dual-layer spiral microneedles to rapidly extract dermal interstitial fluid for minimally invasive detection of glucose. Microsyst. Nanoeng. 2025, 11, 3. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, X.; Kim, H.; Qu, M.; Jiang, X.; Lee, K.; Ren, L.; Wu, Q.; Wang, C.; Zhu, X.; et al. Gelatin methacryloyl microneedle patches for minimally invasive extraction of skin interstitial fluid. Small 2020, 16, 1905910. [Google Scholar] [CrossRef]
- Wu, M.; Liu, L.; Xing, Y.; Wu, Z.; Huang, P.; Li, B. Plasmonic nanodiamond—Microneedle bioinspired system for ultrarapid sampling and quantum sensing of melanoma-related MiRNA. Adv. Funct. Mater. 2025, 35, 2422305. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Ling, Z.; Zheng, Y.; Qu, F.; Chang, H. An ultraswelling microneedle device for facile and efficient drug loading and transdermal delivery. Adv. Healthc. Mater. 2023, 13, 2302406. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, X.; Cai, T.; Ping, J.; Ying, Y. High-Porosity Hydrogel Microneedles for Rapid and Efficient Extraction of Imidacloprid Residues in Peach Fruits. Foods 2025, 14, 3423. https://doi.org/10.3390/foods14193423
Zhang C, Liu X, Cai T, Ping J, Ying Y. High-Porosity Hydrogel Microneedles for Rapid and Efficient Extraction of Imidacloprid Residues in Peach Fruits. Foods. 2025; 14(19):3423. https://doi.org/10.3390/foods14193423
Chicago/Turabian StyleZhang, Chi, Xin Liu, Tailong Cai, Jianfeng Ping, and Yibin Ying. 2025. "High-Porosity Hydrogel Microneedles for Rapid and Efficient Extraction of Imidacloprid Residues in Peach Fruits" Foods 14, no. 19: 3423. https://doi.org/10.3390/foods14193423
APA StyleZhang, C., Liu, X., Cai, T., Ping, J., & Ying, Y. (2025). High-Porosity Hydrogel Microneedles for Rapid and Efficient Extraction of Imidacloprid Residues in Peach Fruits. Foods, 14(19), 3423. https://doi.org/10.3390/foods14193423






