Effect of Pectin on the Quality Attributes and Phenolic Composition of Blackberry Jam from Wild and Cultivated Fruits at Different Altitudes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Jam Formulation and Preparation Procedures
2.3. Physicochemical Analysis
2.4. Individual Phenolic Compounds
2.5. Total Phenolics, Total Flavonoids and Total Monomeric Anthocyanin
2.6. DPPH and FRAP Antioxidant Activity
2.7. Color Characteristics
2.8. Texture Characteristics
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties and Color Profile
3.2. Results of Total Phenolics, Flavonoids, Anthocyanins and Antioxidant Assays
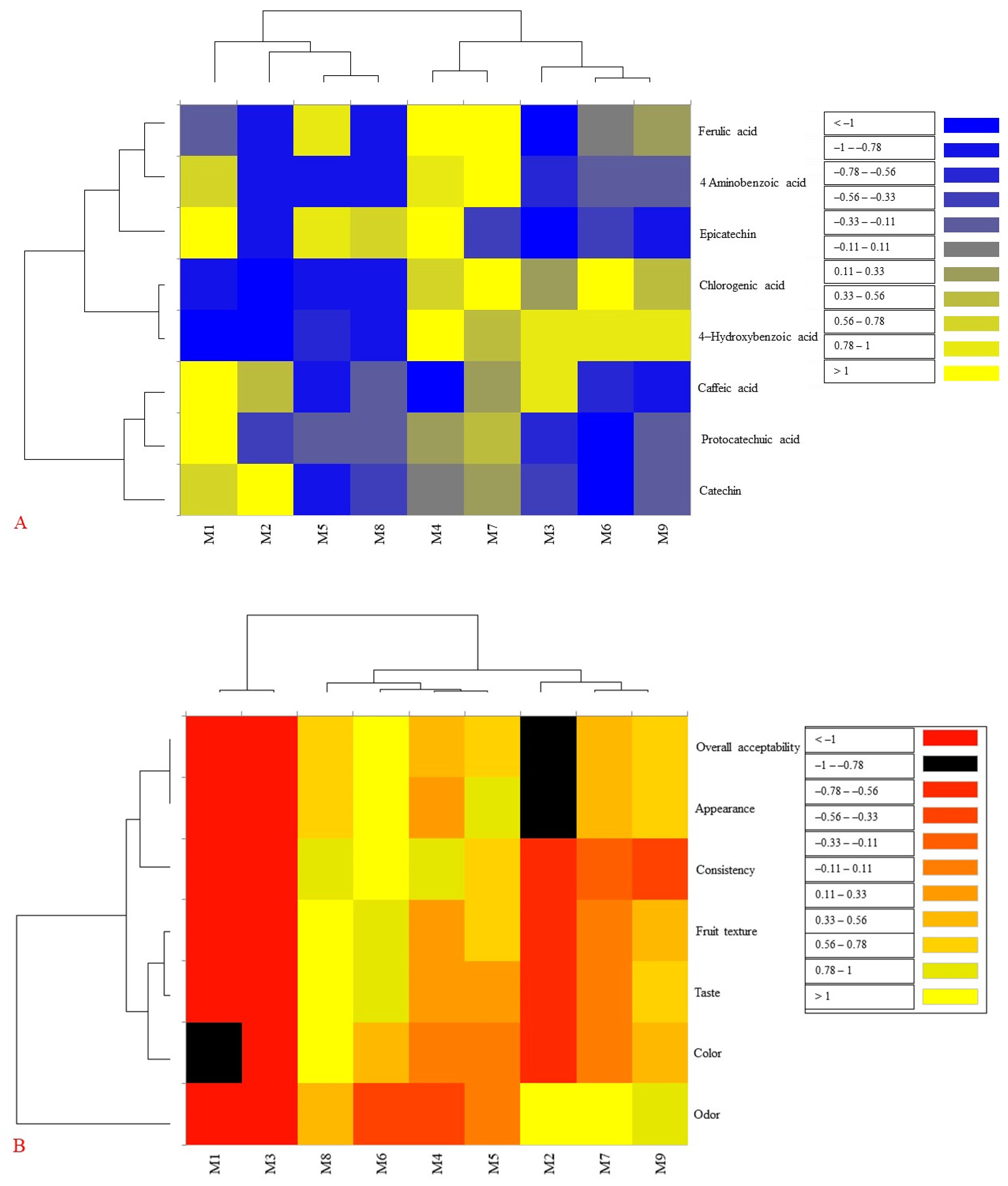
3.3. Results of Individual Phenolic Compounds
3.4. Results of Textural and Sensory Analysis of Blackberry Jam
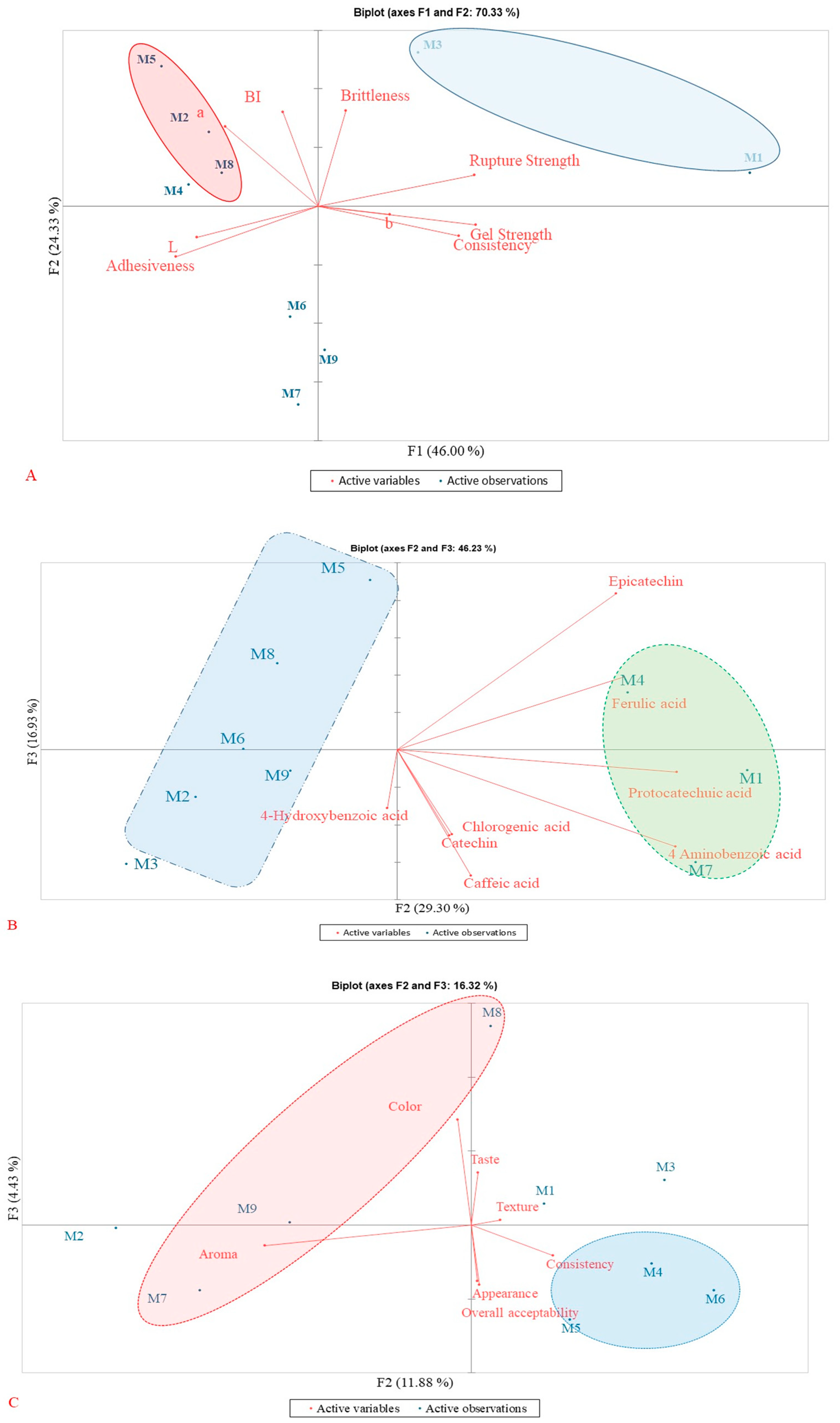
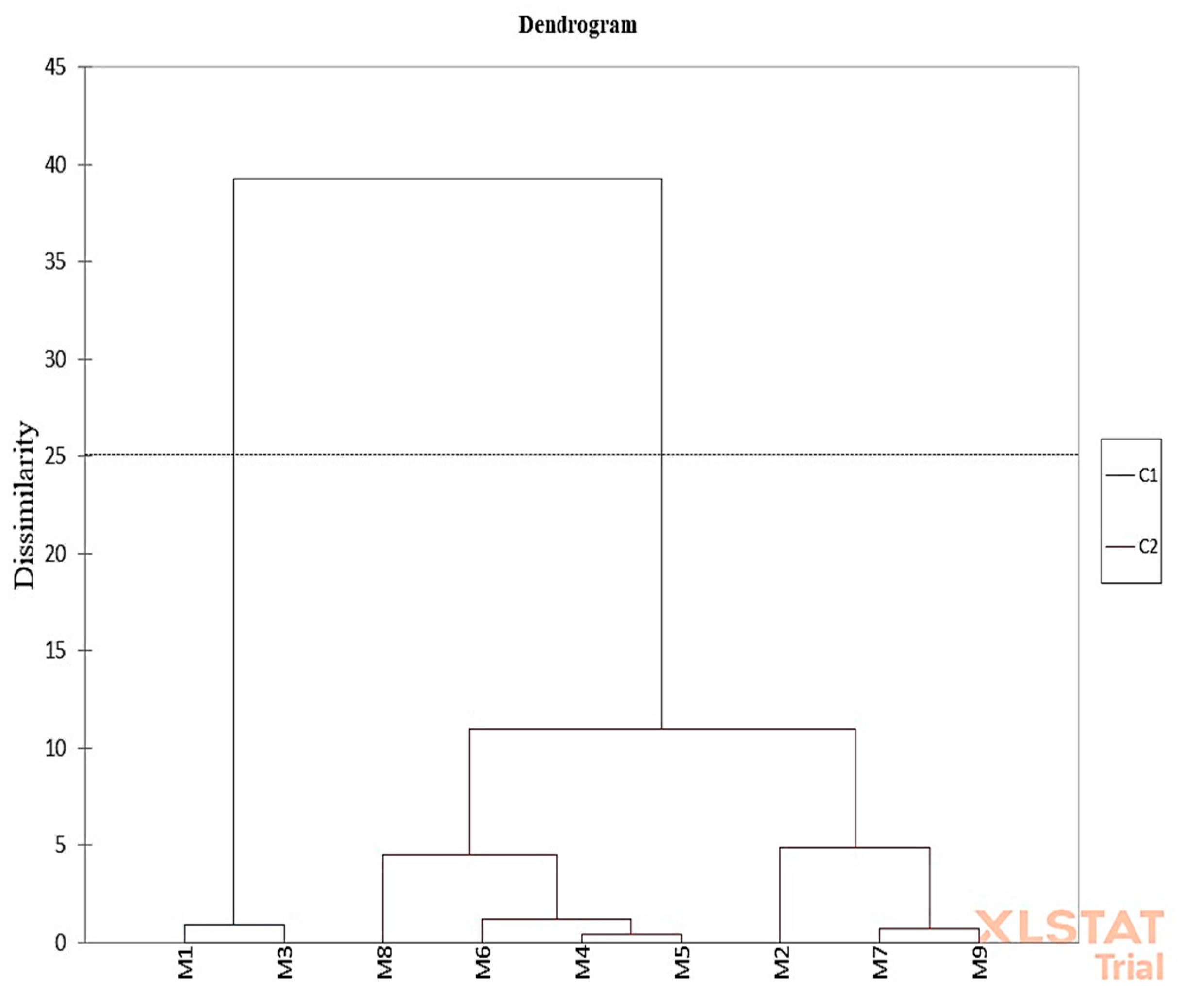
3.5. Principal Component Analysis (PCA) Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noila, S.J.; Flora, D.K.; Alisher, N.K.; Alimjon, D.M. Biochemical composition of blackberry varieties cultivated under the climatic conditions of the Samarkand region. Plant Sci. Today 2025, 12, 3. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Leporini, M.; D’Urso, G.; Gagliano Candela, R.; Falco, T.; Piacente, S.; Bruno, M.; Sottile, F. Almond (Prunus dulcis cv. Casteltermini) skin confectionery by-products: New opportunity for the development of a functional blackberry (Rubus ulmifolius Schott) jam. Antioxidants 2021, 10, 1218. [Google Scholar] [CrossRef]
- Karaklajic-Stajic, Z.; Tomic, J.; Pesakovic, M.; Paunovic, S.M.; Stampar, F.; Mikulic-Petkovsek, M.; Grohar, M.C.; Hudina, M.; Jakopic, J. Black queens of fruits: Chemical composition of blackberry (Rubus subg. Rubus Watson) and black currant (Ribes nigrum L.) cultivars selected in Serbia. Foods 2023, 12, 2775. [Google Scholar] [CrossRef]
- Salanță, L.C.; Uifălean, A.; Iuga, C.A.; Tofană, M.; Crăpotova, J.; Pop, O.L.; Socaci, S.A.; Rotar, A.M.; Mureșan, C.I. Valuable food molecules with potential benefits for human health. In The Health Benefits of Foods—Current Knowledge and Further Development; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef][Green Version]
- Da Silva, D.F.; Itoda, C.; Rosa, C.I.L.F.; Pelaes-Vital, A.C.; Yamamoto, L.N.; Yamamoto, L.Y.; Vasconcelos-Botelho, R.; Matumoto-Pintro, P.T. Effects of blackberries (Rubus sp., cv. Xavante) processing on its physicochemical properties, phenolic contents and antioxidant activity. J. Food Sci. Technol. 2018, 55, 4642–4649. [Google Scholar] [CrossRef]
- Finn, C.E.; Strik, B.C.; Yorgey, B.M.; Peterson, M.E.; Lee, J.; Martin, R.R.; Hall, H.K. ‘Columbia Star’ thornless trailing blackberry. HortScience 2014, 49, 1108–1112. [Google Scholar] [CrossRef]
- Veberic, R.; Stampar, F.; Schmitzer, V.; Cunja, V.; Zupan, A.; Koron, D.; Mikulic-Petkovsek, M. Changes in the contents of anthocyanins and other compounds in blackberry fruits due to freezing and long-term frozen storage. J. Agric. Food Chem. 2014, 62, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Zuman, D.; Chun, C.; Xiong, F. Digestive property and bioactivity of blackberry polysaccharides with different molecular weights. J. Agric. Food Chem. 2019, 67, 12428–12440. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Trends and Challenges. 2017. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/0b6fdfa4-5a48-4289-99f5-2e1d898e5c60/content (accessed on 10 August 2025).
- Nguyen, V.-L.; Tran, M.-T.; Nguyen-Thi, T.-D.; Nguyen, M.-A.; Le, M.-T.; Nguyen, T.-M.; Nguyen, Q.-D. Valorization of cocoa (Theobroma cacao L.) pod husks as a fruit pulp substitute in mango jam formulations: Effects on jam qualities during storage and sensory discrimination. Sustain. Food Technol. 2025, 3, 333. [Google Scholar] [CrossRef]
- De Jager, K.; Augustyn, W.; Regnier, T.; Meiring, B. Investigating the nutritional and sensory potential of selected indigenous South African fruits: Physicochemical properties, jam production and quality evaluation. Food Sci. Nutr. 2025, 13, e70287. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current advancements in pectin: Extraction, properties and multifunctional applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Dranca, F.; Mircea, O. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef]
- Barrera-Chamorro, L.; Fernandez-Prior, Á.; Rivero-Pino, F.; Montserrat-de la Paz, S. A comprehensive review on the functionality and biological relevance of pectin and the use in the food industry. Carbohydr. Polym. 2025, 348, 122794. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.S.; Sharma, S.; Singh, P. Pectin content in apricot pulp and its impact on jam quality. J. Food Sci. Technol. 2015, 52, 1234–1240. [Google Scholar]
- Lara-Espinoza, C.; Moreno-Escamilla, J.; Pacheco-López, J. Pectin levels in cherries for food processing applications. Food Chem. 2014, 165, 45–50. [Google Scholar]
- Stanisavljević, S.; Milovanović, J.; Popović, V. Determination of pectin content in raspberries during ripening. Acta Sci. Pol. Technol. Aliment. 2016, 15, 75–82. [Google Scholar]
- Owolade, S.; Babalola, S.; Popoola, F.; Akinrinola, A.; Olabod, I. Study on physico-chemical properties and shelf-life of watermelon jam under ambient storage. Agroaliment. Process. Technol. 2016, 22, 487–503. [Google Scholar]
- Uddin, R.; Siddique, A.B.; Sultana, S.; Bithi, U.H.; Akter, N.; Idris, A.M.; Al Mansur, M.A.; Jamal, A.S.I.M.; Khandaker, M.U. Techno-economic assessment and innovative production of nutrient-rich jam, jelly, and pickle from Sonneratia apetala fruit. PLoS ONE 2024, 19, e022. [Google Scholar] [CrossRef]
- Amaar, F.E.; Khallaf, M.; Ibrahim, M.; Yasin, N. Utilization of Egyptian golden berry fruit for producing sugar-preserved functional products. Egypt. J. Chem. 2025, 68, 79–89. [Google Scholar] [CrossRef]
- Sosa, A.V.; Povilonis, I.S.; Borroni, V.; Pérez, E.; Radice, S.; Arena, M.E. Unveiling the potential of southern elderberry (Sambucus australis): Characterization of physicochemical properties, carbohydrates, organic acids and biophenols. Plant Foods Hum. Nutr. 2025, 80, 45. [Google Scholar] [CrossRef]
- Schulz, M.; Seraglio, S.K.T.; Della Betta, F.; Nehring, P.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int. 2019, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Yildiz, K.; Ozturk, B.; Karakaya, O.; Gun, S.; Uzun, S.; Gundogdu, M. Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT-Food Sci. Technol. 2019, 111, 117–124. [Google Scholar] [CrossRef]
- Leporini, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.M.; Reitano, A.; Dugay, A.; Deguin, B.; Tundis, R. Citrus clementina Hort. juice enriched with its by-products (peels and leaves): Chemical composition, in vitro bioactivity, and impact of processing. Antioxidants 2020, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Karaklajić-Stajić, Ž.; Tomić, J.; Pešaković, M.; Paunović, S.M.; Rilak, B.; Milinković, M. Influence of a new growing technology on antioxidant capacity and phenolic composition of blackberry ‘Čačanska Bestrna’. J. Mount. Agric. Balkans 2019, 22, 132–148. [Google Scholar]
- Yu, K.; Zhou, L.; Sun, Y.; Zeng, Z.; Chen, H.; Liu, J.; Zou, L.; Liu, W. Anti-browning effect of Rosa roxburghii on apple juice and identification of polyphenol oxidase inhibitors. Food Chem. 2021, 359, 129855. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Zorenc, Z.; Koron, D.; Senica, M. Fruit quality characteristics and biochemical composition of fully ripe blackberries harvested at different times. Foods 2021, 10, 1581. [Google Scholar] [CrossRef]
- Siddiqui, N.H.; Azhar, I.; Tarar, O.M.; Masood, S.; Mahmood, Z.A. Influence of pectin concentrations on physicochemical and sensory qualities of jams. World J. Pharm. Pharm. Sci. 2015, 4, 68–77. [Google Scholar]
- Ramadhan, W.; Trilaksani, W. Formulasi hidrokolid-agar, sukrosa dan acidulant pada pengembangan produk selai lembaran. J. Pengol. Hasil Perikanan Ind. 2017, 20, 95–108. [Google Scholar]
- Prisacaru, A.E.; Ghinea, C.; Albu, E.; Pădureţ, S. Storage impact on the physicochemical and microbiological stability of apricot, cherry, raspberry, and strawberry jams. Foods 2025, 14, 1695. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Ferranti, P.; De Cunzo, F.; Sacco, E.; Volpe, M.G. Polyphenol patterns to trace sweet (Prunus avium) and tart (Prunus cerasus) varieties in cherry jam. J. Food Sci. Technol. 2017, 54, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef]
- Mahdi, Z.E. Chemical, sensory and microbiological assessment of some local and imported jam in the Egyptian market. Ann. Agric. Sci. Moshtohor 2019, 57, 405–418. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Bravo-Villar, M.; Igual, M.; Savall, C.; García-Segovia, P.; Martínez-Monzó, J. Sugar and no sugar added fruit microalgae-enriched jams: A study about their physicochemical, rheological, and textural properties. Eur. Food Res. Technol. 2021, 247, 2565–2578. [Google Scholar] [CrossRef]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of formulation variables on rheology, texture, colour, and acceptability of apple jelly: Modelling and optimization. LWT-Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.D.; Velioglu, S.D. Partial replacement of sugar with Rebaudioside A in blackberry jam production using response surface methodology. In Proceedings of the International Conference on Raw Material to Processed Foods, Spice Hotel, Antalya, Turkey, 11–13 April 2018. [Google Scholar]
- Marjanović, A.; Djedjibegović, J.; Lugusić, A.; Djedjibegović, J.; Soldo, B.; Soldo, S.; Brkić, S. Multivariate analysis of polyphenolic content and in vitro antioxidant capacity of wild and cultivated berries from Bosnia and Herzegovina. Sci. Rep. 2021, 11, 19259. [Google Scholar] [CrossRef]
- Paunović, D.; Kalušević, A.; Ćinović, D.; Petrović, T.; Rajić, J.; Cvetković, M.; Nedović, V. Antioksidativna svojstva različitih proizvoda od kupine (Rubus fruticosus). Voćarstvo 2016, 50, 39–45. (In Serbian) [Google Scholar]
- Benedek, C.; Bodor, Z.; Merrill, V.T.; Kókai, Z.; Gere, A.; Kovacs, Z.; Dalmadi, I.; Abrankó, L. Effect of sweeteners and storage on compositional and sensory properties of blackberry jams. Eur. Food Res. Technol. 2020, 246, 2187–2204. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Zorenc, Z.; Veberic, R. Do optimally ripe blackberries contain the highest levels of metabolites? Food Chem. 2017, 215, 41–49. [Google Scholar] [CrossRef]
- Ifie, I.; Abrankó, L.; Villa-Rodriguez, J.A.; Papp, N.; Ho, P.; Williamson, G.; Marshall, L.J. The effect of ageing temperature on the physicochemical properties, phytochemical profile and α-glucosidase inhibition of Hibiscus sabdariffa (roselle) wine. Food Chem. 2018, 267, 263–270. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Pistanty, M.; Kiki, N. Formulasi asam sitrat pada kualitas selai terong ungu (Solanum melongena) sebagai upaya diversifikasi pangan. J. Shine Cahaya Dunia S-1 Keperawatan 2021, 6, 40–48. [Google Scholar]
- Nguyen, M.T.; Huynh, M.T.; Ngo, V.T. Optimization of ingredient levels of reduced-calorie blackberry jam using response surface methodology. J. Appl. Biol. Biotechnol. 2022, 10, 68–75. [Google Scholar] [CrossRef]
- Pebriawati, N.W.A.; Sudiarta, N.P.; Pujawan, A.A.K.A.; Martadjaya, I.G.M.I.D.; Rumadana, I.M. Quality of seaweed jam (Caulerpa racemosa). Indones. J. Appl. Ind. Sci. 2024, 3, 691–704. [Google Scholar] [CrossRef]
- Sharma, A.; Meghwal, M.L.; Sharma, J.; Kumawat, S.L. Standardization of recipe for fruit jam prepared from ripe banana. Int. J. Agric. Sci. 2025, 21, 58–62. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Drusch, S.; Acevedo, F.; Castro-Alvarez, A.; Benie, A.; Poncelet, D.; Dragosavac, M.M.; Tesoriero, M.V.D.; Löwenstein, P.; Yonaha, V. Structure, controlled release mechanisms and health benefits of pectins as an encapsulation material for bioactive food components. Food Funct. 2022, 13, 10870–10881. [Google Scholar] [CrossRef] [PubMed]
- Ropartz, D.; Ralet, M.-C. Pectin structure. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer: Cham, Switzerland, 2020; pp. 17–36. [Google Scholar]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectinin biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. Relationships between the behavior of three different sources of pectin at the oil-water interface and the stability of the emulsion. Food Hydrocoll. 2022, 128, 107566. [Google Scholar] [CrossRef]
- Shahin, L.; Zhang, L.; Mohnen, D.; Urbanowicz, B.R. Insights in to Pectin O-acetylation in plant cell wall: Structure, synthesis, and modification. Cell Surf. 2023, 9, 100099. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of pectin structural properties on interactions with divalent cations and its associated functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Cacao podhusks as a source of low-methoxyl, highly acetylated pectins able to gel in acidic media. Int. J. Biol. Macromol. 2017, 101, 146–152. [Google Scholar] [CrossRef]

| Jam Samples | Code | Blackberries | Sugar | Citric Acid | Pectin |
|---|---|---|---|---|---|
| J-F1-0P J-F2-0P J-F3-0P | M1 M2 M3 | 62 | 37 | 1 | - |
| J-F1-0.1P J-F2-0.1P J-F3-0.1P | M4 M5 M6 | 62 | 37 | 0.9 | 0.1 |
| J-F1-0.5P J-F2-0.5P J-F3-0.5P | M7 M8 M9 | 62 | 37 | 0.5 | 0.5 |
| Fruit Samples | pH | Titratable Acidity (g Malic Acid 100 g−1) | Vitamin C (mg 100 g−1) |
|---|---|---|---|
| F1 | 2.90 ± 0.02 a | 0.73 ± 0.08 a | 239.1 ± 0.20 a |
| F2 | 2.89 ± 0.01 a | 1.12 ± 0.25 a | 266.4 ± 2.95 a |
| F3 | 3.09 ± 0.01 b | 1.06 ± 0.03 a | 449.5 ± 2.44 b |
| Jam samples | |||
| J-F1-0P | 3.52 ± 0.02 f | 1.01 ± 0.00 b | 162.33 ± 3.21 f |
| J-F2-0P | 3.08 ± 0.03 a | 1.01 ± 0.00 b | 69.67 ± 5.03 a |
| J-F3-0P | 3.28 ± 0.01 c | 1.32 ± 0.02 f | 125.33 ± 1.53 d |
| J-F1-0.1P | 3.40 ± 0.02 e | 1.10 ± 0.01 d | 175.33 ± 5.03 g |
| J-F2-0.1P | 3.18 ± 0.02 b | 0.85 ± 0.00 a | 88.67 ± 0.58 b |
| J-F3-0.1P | 3.37 ± 0.01 d | 1.02 ± 0.01 b | 124.67 ± 1.53 d |
| J-F1-0.5P | 3.39 ± 0.01d e | 1.16 ± 0.00 e | 173.33 ± 1.53 g |
| J-F2-0.5P | 3.21 ± 0.02 b | 1.09 ± 0.01 c | 99.67 ± 6.81 c |
| J-F3-0.5P | 3.40 ± 0.02 e | 1.11 ± 0.01 d | 133.33 ± 1.15 e |
| Samples | L* | a* | b* | BI |
|---|---|---|---|---|
| J-F1-0P | 25.24 ± 0.21 a | 0.43 ± 0.17 a | 0.41 ± 0.07 b | 2.78 ± 0.19 ab |
| J-F2-0P | 26.90 ± 0.47 d | 0.85 ± 0.10 c | 0.23 ± 0.03 ab | 3.07 ± 0.33 ab |
| J-F3-0P | 25.76 ± 0.39 ab | 0.83 ± 0.13 d | 0.47 ± 0.06 c | 4.08 ± 0.63 c |
| J-F1-0.1P | 26.29 ± 0.24 bc | 0.87 ± 0.07 d | 0.32 ± 0.18 bc | 3.50 ± 0.61 bc |
| J-F2-0.1P | 25.87 ± 0.45 bc | 1.09 ± 0.08 e | 0.10 ± 0.08 a | 3.35 ± 0.15 a–c |
| J-F3-0.1P | 26.32 ± 0.25 bc | 0.32 ± 0.12 a | 0.50 ± 0.07 c | 2.72 ± 0.09 ab |
| J-F1-0.5P | 26.42 ± 0.40 cd | 0.58 ± 0.01 bc | 0.27 ± 0.10 a–c | 2.54 ± 0.29 a |
| J-F2-0.5P | 25.99 ± 0.26 bc | 0.68 ± 0.07 cd | 0.46 ± 0.20 c | 3.61 ± 0.87 bc |
| J-F3-0.5P | 25.99 ± 0.18 bc | 0.58 ± 0.11 a–c | 0.28 ± 0.07 a–c | 2.61 ± 0.26 a |
| Total Phenolics (g GAE kg−1) | Total Flavonoids (g QE kg−1) | Total Anthocyanins (g C3GE kg−1) | FRAP (mmol TE kg−1) | DPPH (mmol TE kg−1) | |
|---|---|---|---|---|---|
| J-F1-0P | 1.96 ± 0.21 d | 0.87 ± 0.01 e | 0.09 ± 0.01 b | 35.73 ± 0.08 d | 6.74 ± 0.06 e |
| J-F2-0P | 1.08 ± 0.05 a–c | 0.19 ± 0.07 a | 0.03 ± 0.00 a | 25.04 ± 0.45 a | 5.74 ± 0.07 b |
| J-F3-0P | 1.35 ± 0.10 ab | 0.37 ± 0.01 c | 0.03 ± 0.00 a | 32.73 ± 1.23 c | 6.86 ± 0.04 ef |
| J-F1-0.1P | 1.64 ± 0.00 b–d | 0.71 ± 0.04 d | 0.12 ± 0.00 c | 36.08 ± 0.48 d | 6.76 ± 0.01 e |
| J-F2-0.1P | 1.12 ± 0.01 a | 0.27 ± 0.00 b | 0.05 ± 0.00 ab | 23.79 ± 1.52 a | 5.49 ± 0.12 a |
| J-F3-0.1P | 1.35 ± 0.02 ab | 0.37 ± 0.01 c | 0.05 ± 0.01 ab | 31.18 ± 0.93 c | 6.56 ± 0.04 d |
| J-F1-0.5P | 1.77 ± 0.00 cd | 0.68 ± 0.01 d | 0.13 ± 0.01 c | 36.74 ± 0.36 d | 6.92 ± 0.05 f |
| J-F2-0.5P | 1.38 ± 0.00 a–c | 0.37 ± 0.03 c | 0.06 ± 0.00 b | 31.06 ± 0.83 c | 6.78 ± 0.08 ef |
| J-F3-0.5P | 1.28 ± 0.01 ab | 0.42 ± 0.01 c | 0.05 ± 0.00 ab | 28.46 ± 0.25 b | 6.37 ± 0.10 c |
| Samples | 4-aminobenzoic Acid (PABA) | Protocatechuic Acid | 4-hydroxybenzoic Acid | Catechin | Chlorogenic Acid | Caffeic Acid | Epicatechin | Ferulic Acid |
|---|---|---|---|---|---|---|---|---|
| J-F1-0P | 13.43 ± 0.40 d | 1.55 ± 0.08 e | 9.32 ± 0.21 b | 1.97 ± 0.14 f | 6.33 ± 0.14 b | 5.88 ± 0.31 f | 11.41 ± 0.36 g | 7.51 ± 0.25 c |
| J-F2-0P | 3.38 ± 0.29 a | 0.44 ± 0.08 b | 7.25 ± 0.17 a | 3.18 ± 0.12 g | 3.23 ± 0.13 a | 3.43 ± 0.17 d | 2.61 ± 0.21 b | 4.75 ± 0.09 b |
| J-F3-0P | 5.33 ± 0.35 b | 0.36 ± 0.05 a | 20.73 ± 0.37 f | 1.12 ± 0.10 c | 17.35 ± 0.36 d | 4.42 ± 0.15 e | 1.16 ± 0.08 a | 2.00 ± 0.11 a |
| J-F1-0.1P | 15.25 ± 0.35 e | 0.62 ± 0.05 c | 22.15 ± 0.40 g | 1.63 ± 0.08 d | 24.38 ± 0.22 g | 1.35 ± 0.06 a | 10.12 ± 0.34 f | 14.48 ± 0.53 g |
| J-F2-0.1P | 3.11 ± 0.27 a | 0.51 ± 0.03 b | 12.54 ± 0.40 d | 0.82 ± 0.05 b | 6.45 ± 0.27 b | 1.65 ± 0.08 b | 9.39 ± 0.23 e | 12.96 ± 0.40 f |
| J-F3-0.1P | 7.58 ± 0.21 c | 0.22 ± 0.04 a | 20.51 ± 0.39 f | 0.61 ± 0.03 a | 29.83 ± 0.52 b | 1.85 ± 0.07 b | 4.52 ± 0.16 c | 8.45 ± 0.24 d |
| J-F1-0.5P | 24.28 ± 0.68 f | 0.84 ± 0.07 e | 18.62 ± 0.41 e | 1.75 ± 0.06 e | 28.89 ± 0.24 a | 3.29 ± 0.08 d | 4.42 ± 0.27 c | 14.79 ± 0.41 g |
| J-F2-0.5P | 3.43 ± 0.26 a | 0.52 ± 0.03 b | 11.27 ± 0.26 c | 1.24 ± 0.07 c | 7.55 ± 0.24 c | 2.66 ± 0.06 c | 8.66 ± 0.29 d | 5.29 ± 0.14 b |
| J-F3-0.5P | 7.75 ± 0.45 c | 0.51 ± 0.06 b | 20.89 ± 0.26 f | 1.30 ± 0.04 c | 21.15 ± 0.26 e | 1.73 ± 0.04 b | 2.86 ± 0.06 b | 9.46 ± 0.25 e |
| Samples | Gel Strength (g) | Rupture Strength (g) | Brittleness (mm) | Adhesiveness (g.s) | Consistency (g.s) |
|---|---|---|---|---|---|
| J-F1-0P | 219.92 ± 13.38 d | 4486.65 ± 2621.92 b | 12.91 ± 2.95 c | −5203.23 ± 550.76 a | 27,196.79 ± 1367.30 e |
| J-F2-0P | 69.79 ± 13.33 ab | 1044.30 ± 66.45 a | 13.70 ± 0.41 c | −2631.53 ± 165.13 c | 7569.02 ± 357.06 b |
| J-F3-0P | 153.76 ± 73.26 b–d | 1375.04 ± 210.62 a | 11.57 ± 0.46 c | −3764.64 ± 505.43 b | 12,229.18 ± 1584.86 c |
| J-F1-0.1P | 80.20 ± 56.19 a–c | 543.97 ± 365.79 a | 8.67 ± 4.46 a–c | −526.76 ± 69.44 d | 7489.30 ± 1697.10 b |
| J-F2-0.1P | 80.13 ± 30.12 a–c | 398.71 ± 94.83 a | 13.22 ± 2.07 c | −828.46 ± 143.25 cd | 3337.89 ± 354.33 a |
| J-F3-0.1P | 108.99 ± 45.50 a–c | 417.45 ± 81.73 a | 10.70 ± 2.48 bc | −601.40 ± 77.21 d | 4317.53 ± 788.29 a |
| J-F1-0.5P | 122.24 ± 11.34 a–c | 294.17 ± 46.643 a | 4.69 ± 0.47 a | −727.83 ± 28.35 cd | 21,109.01 ± 4674.23 d |
| J-F2-0.5P | 66.20 ± 29.79 a | 407.26 ± 35.53 a | 9.89 ± 1.91 a–c | −667.66 ± 81.24 cd | 4291.41 ± 588.20 a |
| J-F3-0.5P | 159.68 ± 35.43 cd | 438.66 ± 217.84 a | 5.86 ± 2.34 ab | −1065.74 ± 236.18 d | 10,378.70 ± 1979.15 bc |
| Samples | Code | Odor | Color | Appearance | Taste | Consistency | Texture | Overall Acceptability |
|---|---|---|---|---|---|---|---|---|
| J-F1-0P | M1 | 2.22 a | 2.67 a | 2.33 ab | 1.89 a | 1.89 a | 1.33 a | 2.22 ab |
| J-F2-0P | M2 | 2.89 a | 2.78 ab | 2.44 a–c | 2.44 a–c | 2.33 ab | 2.00 a–c | 2.44 a–c |
| J-F3-0P | M3 | 2.11 a | 2.44 a | 2.00 a | 2.11 ab | 2.00 a | 1.67 ab | 2.00 a |
| J-F1-0.1P | M4 | 2.44 a | 3.00 ab | 3.00 b–d | 2.89 a–d | 3.33 bc | 2.78 cd | 3.00 bc |
| J-F2-0.1P | M5 | 2.56 a | 3.00 ab | 3.33 cd | 2.89 a–d | 3.22 bc | 3.11 d | 3.11 bc |
| J-F3-0.1P | M6 | 2.44 a | 3.11 ab | 3.44 d | 3.33 cd | 3.56 c | 3.22 d | 3.33 c |
| J-F1-0.5P | M7 | 2.89 a | 3.00 ab | 3.11 b–d | 2.78 a–d | 2.67 a–c | 2.56 b–d | 3.00 bc |
| J-F2-0.5P | M8 | 2.67 a | 3.67 b | 3.22 b–d | 3.67 d | 3.33 bc | 3.44 d | 3.11 bc |
| J-F3-0.5P | M9 | 2.78 a | 3.11 ab | 3.22 b–d | 3.22 b–d | 2.56 a–c | 2.89 cd | 3.11 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veliu, A.; Abdullahi, X.; Sulejmani, E.; Celik, O.F.; Olcer, M.A.; Ozturk, B. Effect of Pectin on the Quality Attributes and Phenolic Composition of Blackberry Jam from Wild and Cultivated Fruits at Different Altitudes. Foods 2025, 14, 3420. https://doi.org/10.3390/foods14193420
Veliu A, Abdullahi X, Sulejmani E, Celik OF, Olcer MA, Ozturk B. Effect of Pectin on the Quality Attributes and Phenolic Composition of Blackberry Jam from Wild and Cultivated Fruits at Different Altitudes. Foods. 2025; 14(19):3420. https://doi.org/10.3390/foods14193420
Chicago/Turabian StyleVeliu, Adis, Xhabir Abdullahi, Erhan Sulejmani, Omer Faruk Celik, Mehmet Ali Olcer, and Burhan Ozturk. 2025. "Effect of Pectin on the Quality Attributes and Phenolic Composition of Blackberry Jam from Wild and Cultivated Fruits at Different Altitudes" Foods 14, no. 19: 3420. https://doi.org/10.3390/foods14193420
APA StyleVeliu, A., Abdullahi, X., Sulejmani, E., Celik, O. F., Olcer, M. A., & Ozturk, B. (2025). Effect of Pectin on the Quality Attributes and Phenolic Composition of Blackberry Jam from Wild and Cultivated Fruits at Different Altitudes. Foods, 14(19), 3420. https://doi.org/10.3390/foods14193420








