Grifola frondosa Polysaccharides Alleviated Cyclophosphamide—Induced Intestinal Injury Based on Microbiota, Metabolite and Immune Axis Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Polysaccharides from Grifola frondosa
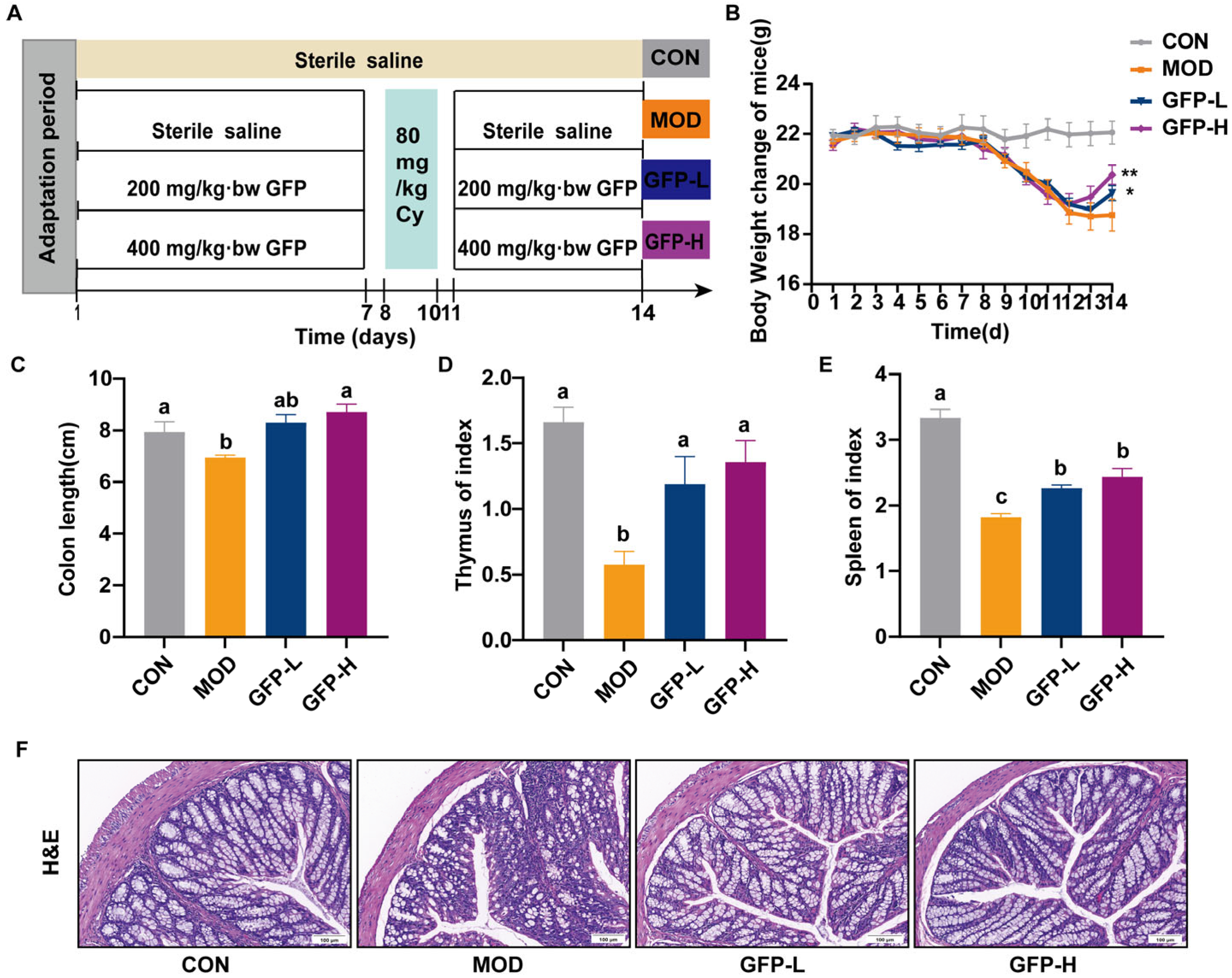
2.3. Animal and Experimental Design
2.4. Evaluation of Spleen and Thymus Indices
2.5. Measurement of Relevant Indicators in Serum
2.6. Histopathological Analysis
2.7. qRT-PCR Analysis
2.8. Immunofluorescence Analysis
2.9. Determination of SCFAs Content in Cecal Contents
2.10. 16S rDNA Sequencing
2.11. Untargeted Metabolomics Analysis of Fecal
2.12. Statistical Analysis
3. Results
3.1. GFP Alleviated Symptoms of Intestinal Barrier Injury
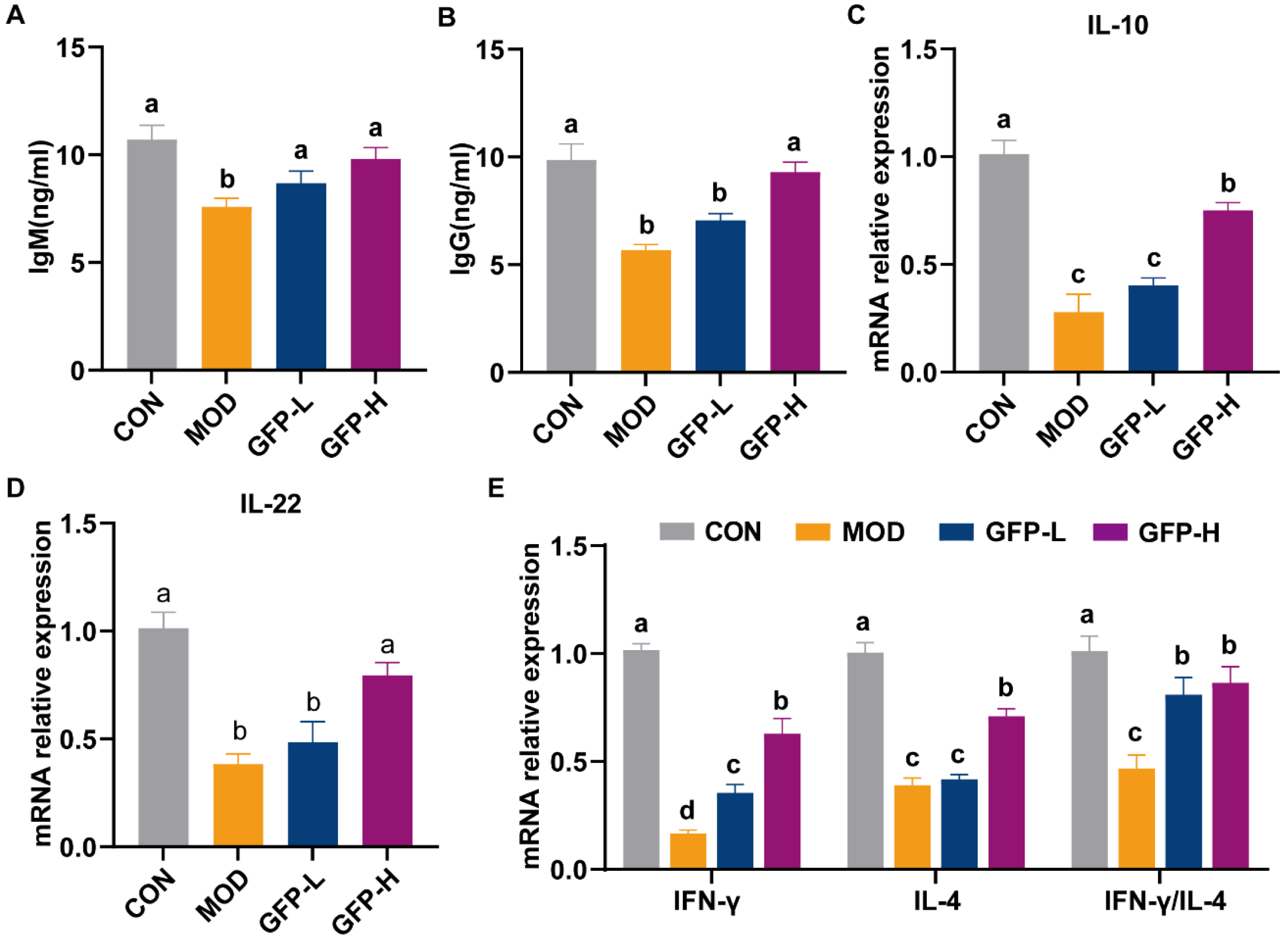
3.2. GFP Increased Immunoglobulin Levels and Cytokine mRNA Expression
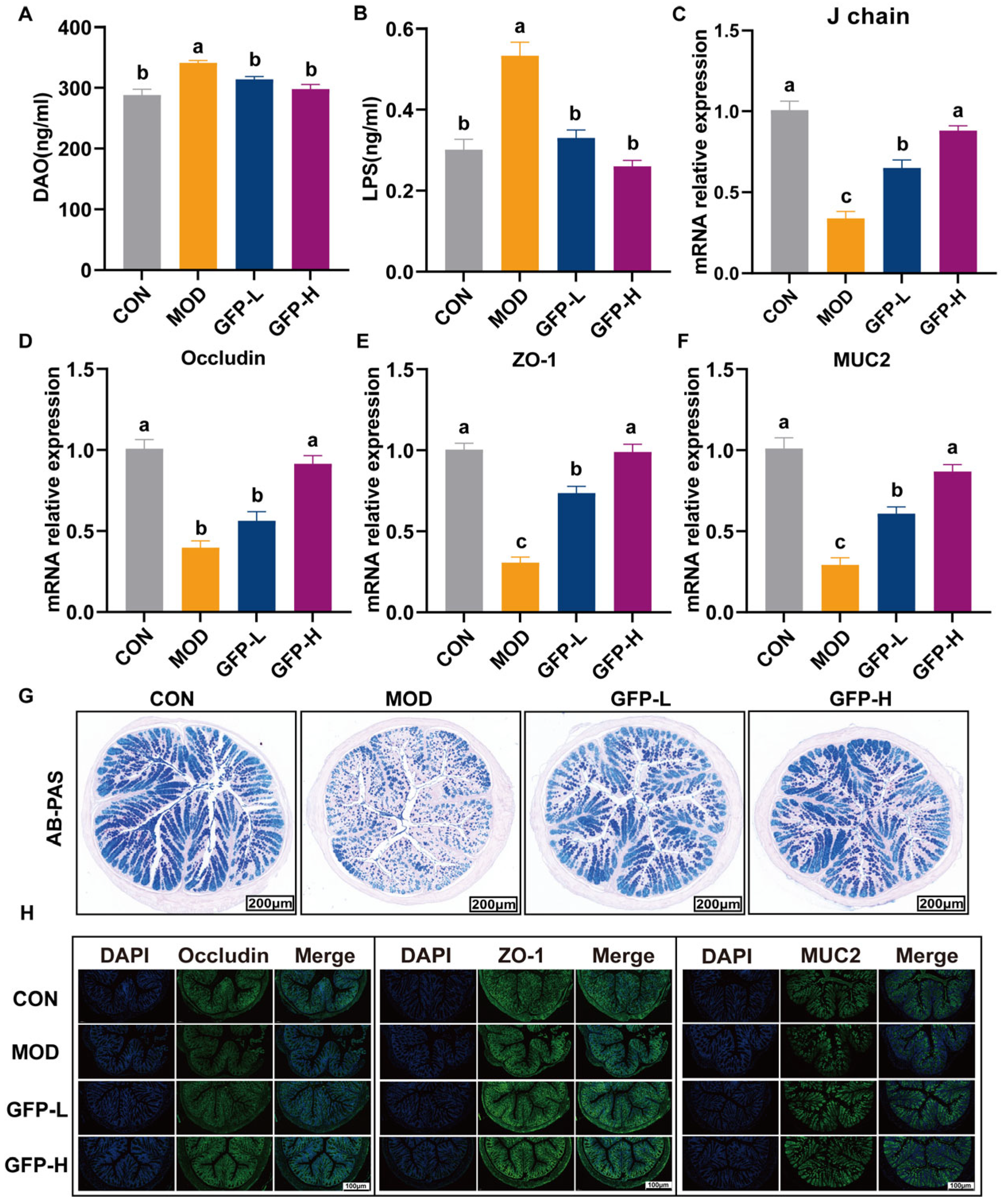
3.3. GFP Improved Intestinal Barrier Function
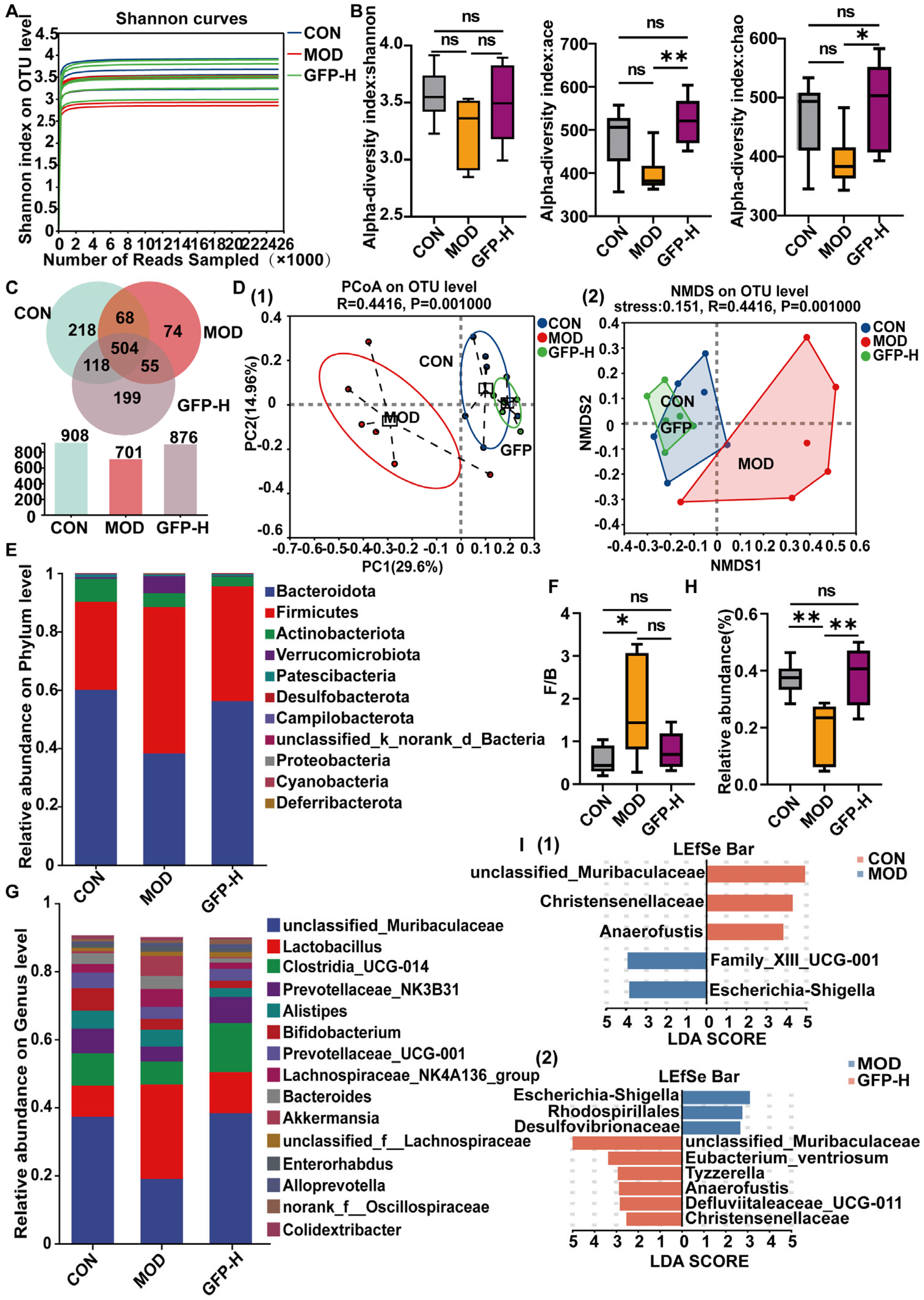
3.4. GFP Restored Gut Microbiota Diversity and Composition
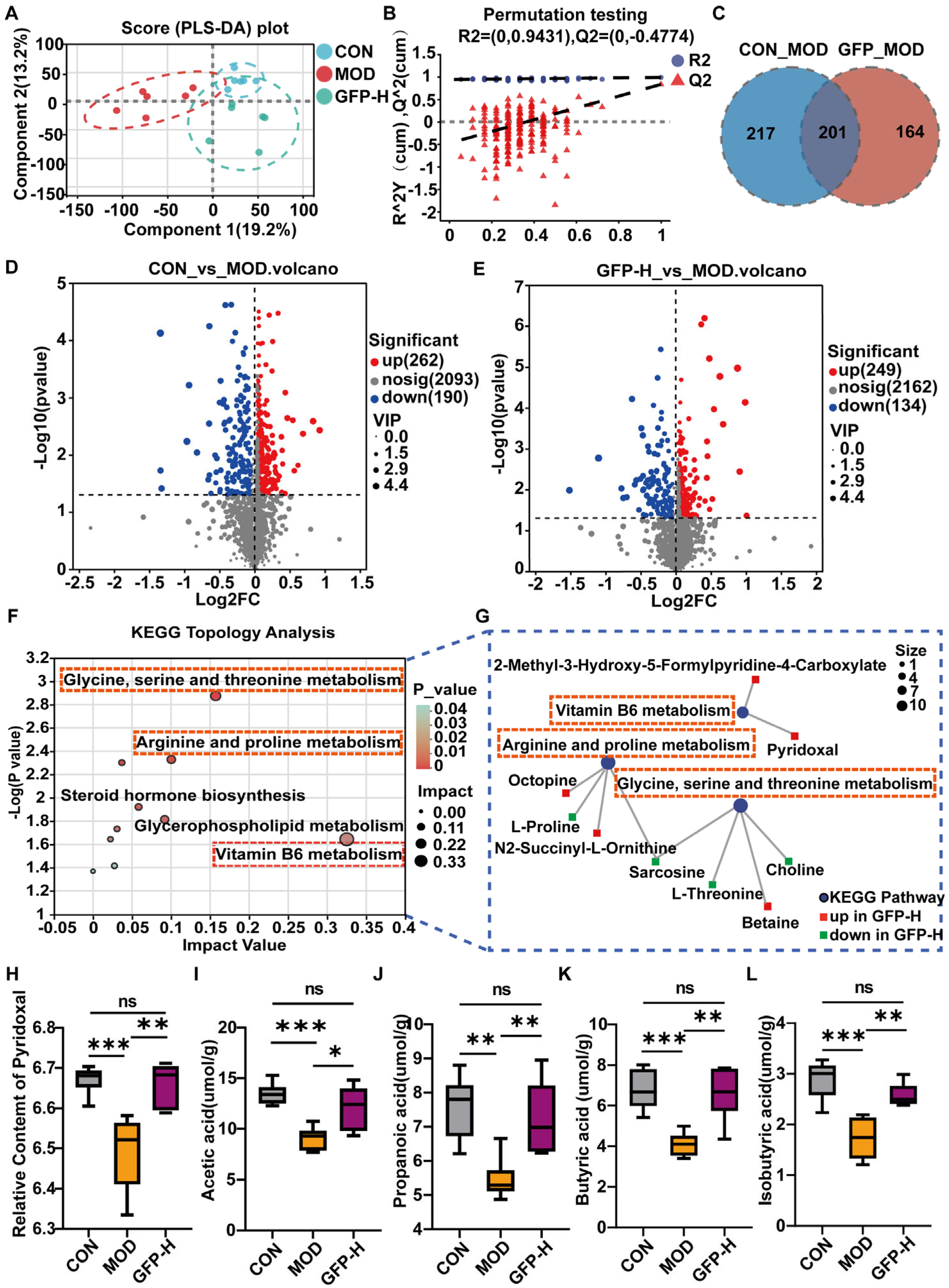
3.5. GFP Modulated Gut Microbiota Metabolism
3.6. GFP Increased the Production of SCFAs
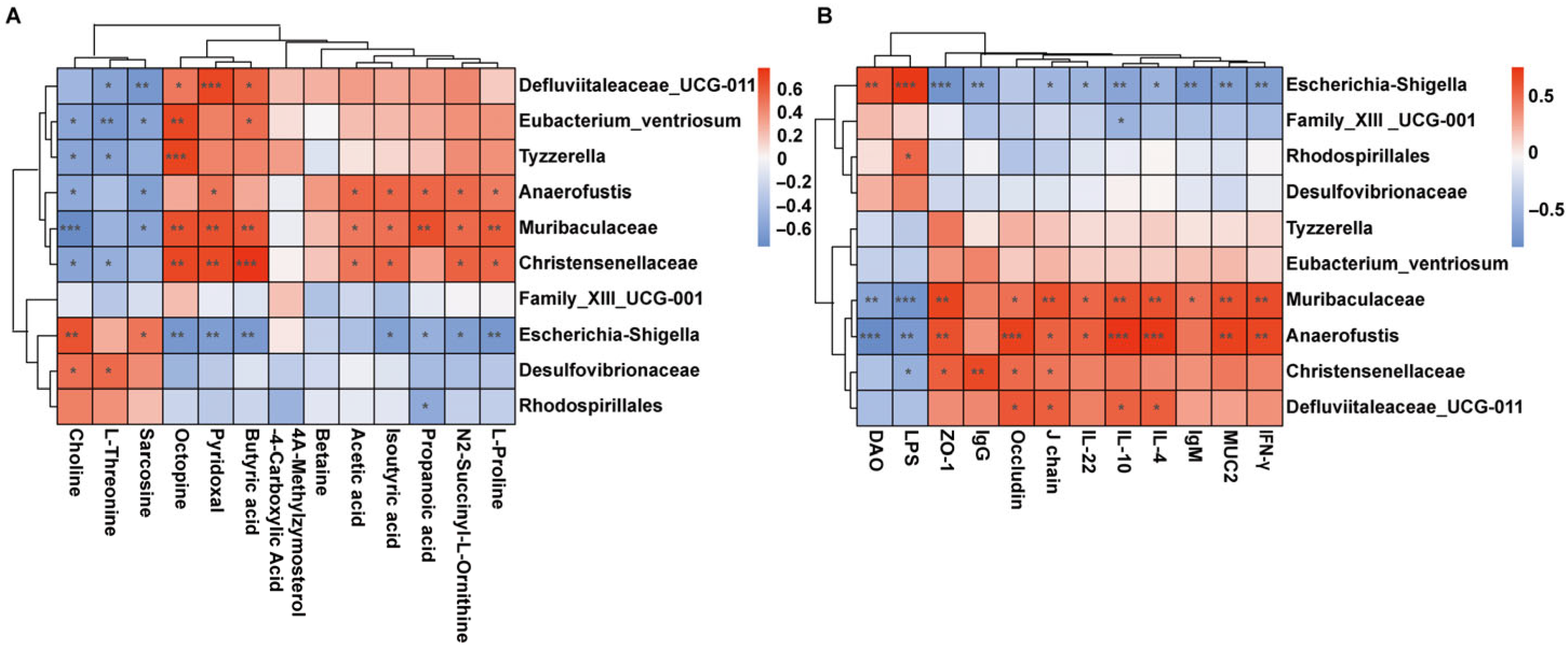
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GFP | Grifola frondosa polysaccharides |
| CTX | Cyclophosphamide |
| Ig G | Immunoglobulin G |
| Ig M | Immunoglobulin M |
| IFN-γ | Interferon-γ |
| IL-4 | interleukin 4 |
| IL-10 | interleukin 10 |
| IL-22 | interleukin 22 |
| ZO-1 | Zonula occludens–1 |
| MUC-2 | Mucin2 |
| SCFAs | Short-chain fatty acids |
| MAPK | Mitogen-activated protein kinase |
| DAO | Diamine oxidas |
| LPS | Lipopolysaccharide |
| ELISA | Enzyme-linked immunosorbent assay |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| H&E | Hematoxylin and eosin |
| AB-PAS | Alcian Blue-Periodic Acid Schiff |
| sIgA | Secretory IgA |
| pIgR | Polymeric immunoglobulin receptor |
| IF | Immunofluorescence |
| GC-MS | Gas Chromatography–Mass Spectrometer |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| PCoA | Principal co-ordinates analysis |
| LEfSe | Linear discriminant analysis of effect size |
| GALT | Gut-associated lymphoid tissue |
| Th1/Th2 | T helper 1 and T helper 2 |
| TJ | Tight junction |
References
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Tomas-Hernandez, S.; Blanco, J.; Garcia-Vallvé, S.; Pujadas, G.; Ojeda-Montes, M.J.; Gimeno, A.; Arola, L.; Minghetti, L.; Beltrán-Debón, R.; Mulero, M. Anti-Inflammatory and Immunomodulatory Effects of the Grifola frondosa Natural Compound o-Orsellinaldehyde on LPS-Challenged Murine Primary Glial Cells. Roles of NF-κβ and MAPK. Pharmaceutics 2021, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, L.; Qi, X.; Zhou, T.; Li, Y.; Cai, M.; Yan, Y.; Qian, J.Y.; Peng, D. Immunostimulatory and antioxidant activities of the selenized polysaccharide from edible Grifola frondosa. Food Sci. Nutr. 2022, 10, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Lu, M.K.; Lu, T.J.; Lai, M.N.; Ng, L.T. A (1→6)-Branched (1→4)-β-d-Glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264.7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. J. Nat. Prod. 2020, 83, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.H.; Ren, Y.; Feng, W.W.; Li, Q.; Wu, H.Y.; Jin, D.; Zhao, T.; Xu, C.Q.; Yang, L.Q.; Wu, X.Y. Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 2015, 134, 406–412. [Google Scholar] [CrossRef]
- Ni, J.; Zheng, J.; Mo, G.; Chen, G.; Li, J.; Cao, L.; Hu, B.; Liu, H. Structural characterization and immunomodulatory effect of a starch-like Grifola frondosa polysaccharides on cyclophosphamide-induced immunosuppression in mice. Carbohydr. Res. 2024, 535, 109011. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Yu, J.; Liu, A.-J. Structural characterization of a low molecular weight polysaccharide from Grifola frondosa and its antitumor activity in H22 tumor-bearing mice. J. Funct. Foods 2019, 61, 103472. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q.; Jia, S.; Zhao, M. Gut immune microenvironment and autoimmunity. Int. Immunopharmacol. 2023, 124, 110842. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ohno, H. Reciprocal regulation of IgA and the gut microbiota: A key mutualism in the intestine. Int. Immunol. 2021, 33, 781–786. [Google Scholar] [CrossRef]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Z.; Zhu, L.; Ma, S.; Luo, Y.; Liang, H.; Liu, Q.; Chen, J.; Guli, S.; Chen, X. Orchestration of MUC2—The key regulatory target of gut barrier and homeostasis: A review. Int. J. Biol. Macromol. 2023, 236, 123862. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Guo, J.; Shen, J.; Jiang, S.; Han, S.; Li, L. Butyrate ameliorated the intestinal barrier dysfunction and attenuated acute pancreatitis in mice fed with ketogenic diet. Life Sci. 2023, 334, 122188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Abbas, S.; Ren, J.; Huang, H.; Song, Y.; Su, X.; Wu, Q.; Ma, Y.; Tang, H.; Gao, Y.-Z.; et al. Dextran from human feces-derived Weissella cibaria facilitates intestinal mucosal barrier function by modulating gut bacteria and propionate levels. Carbohydr. Polym. 2025, 354, 123300. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Bai, X.; Zhu, W.; Ali, I.; Ma, C.; Zheng, Z.; Qiao, X. Integrated Mechanism of Immune Response Modulation by Arctium Lappa L. Fructans Based on Microbiome and Metabolomics Technologies. J. Agric. Food Chem. 2024, 72, 10981–10994. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Ogino, M.H.; Tadi, P. Cyclophosphamide. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhang, L.; Cao, W.; Gao, Y.; Yang, R.; Zhang, X.; Xu, J.; Tang, Q. Astaxanthin (ATX) enhances the intestinal mucosal functions in immunodeficient mice. Food Funct. 2020, 11, 3371–3381. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Yu, L.; Shi, X.; Lu, X.; Zhao, X.; Yu, Y.; Fang, Y.; Wang, D. Rehmannia glutinosa polysaccharides alleviate cyclophosphamide-induced immunosuppression by enhancing immunity and restoring intestinal homeostasis. Int. J. Biol. Macromol. 2025, 311, 143692. [Google Scholar] [CrossRef]
- Tian, B.; Liu, R.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Yang, K.; Zeng, X.; Peilong, S. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J. Sci. Food Agric. 2023, 103, 3050–3064. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Bai, B.; Zhou, Q.; Niu, J.; Yuan, J.; Zhang, H.; Jia, J.; Zhao, W.; Chen, H. Dietary supplementation with polysaccharides from Ziziphus Jujuba cv. Pozao intervenes in immune response via regulating peripheral immunity and intestinal barrier function in cyclophosphamide-induced mice. Food Funct. 2020, 11, 5992–6006. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xin, Y. Review: Gut microbiota: Therapeutic targets of ginseng polysaccharides against multiple disorders. Int. J. Biol. Macromol. 2025, 287, 138527. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; Qin, H.; Chen, Z.; Zhou, Y.; Zhou, Z.; Wang, J.; Wang, K. Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 2025, 349, 122829. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef]
- Ramalingam, G.; Khan, J.M.; Jasmine, S.; Saminathan, G.; Manickan, E.; Rajagopal, P.; Veeraraghavan, V.P.; Jayaraman, S. Role of Th1/Th2 cytokine balance in predicting treatment outcomes and disease severity in tuberculosis. J. King Saud Univ. Sci. 2024, 36, 103538. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Li, G.; Xue, S.; Zhang, G.; Dang, Y.; Wang, H. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023, 15, 2276814. [Google Scholar] [CrossRef]
- Miyoshi, J.; Miyamoto, H.; Goji, T.; Taniguchi, T.; Tomonari, T.; Sogabe, M.; Kimura, T.; Kitamura, S.; Okamoto, K.; Fujino, Y.; et al. Serum diamine oxidase activity as a predictor of gastrointestinal toxicity and malnutrition due to anticancer drugs. J. Gastroenterol. Hepatol. 2015, 30, 1582–1590. [Google Scholar] [CrossRef]
- Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Interactions between gut microbes and host cells control gut barrier and metabolism. Int. J. Obes. Suppl. 2016, 6, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Tai, W.-C.; Liang, C.-M.; Wu, C.-K.; Tsai, M.-C.; Hu, W.-H.; Huang, P.-Y.; Chen, C.-H.; Kuo, Y.-H.; Yao, C.-C.; et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Gao, Q.; Shen, W.; Weng, P.; Wu, Z.; Qin, W.; Liu, Y. Combined analysis of gut microbiota and metabolomics in high-fat model mice fed with Chlorella pyrenoidosa peptides. J. Funct. Foods 2024, 121, 106410. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Han, C.; Wang, Y.; Liu, R.; Ran, B.; Li, W. Structural characterization and protective effect of Lonicerae flos polysaccharide on cyclophosphamide-induced immunosuppression in mice. Ecotoxicol. Environ. Saf. 2022, 230, 113174. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Ye, H.-Y.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Healthy Aging 2016, 4, 3–16. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Qian, B.; Shen, S.; Zhang, J.; Jing, P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017, 2017, 2197975. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhang, C.; Deng, C.; Wen, A.; Zhou, H.; You, J.; Li, G. Dietary vitamin B6 supplementation alleviates heat stress-induced intestinal barrier impairment by regulating the gut microbiota and metabolites in broilers. Poult. Sci. 2024, 103, 104202. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
| Gene | Froward Primer | Reverse Primer |
|---|---|---|
| β-actin | GAGAGGGAAATCGTGCGTGACAT | GCTCGTTGCCAATAGTGATGACCT |
| ZO-1 | AAGAAGCGATTCAGCAGCAACAGA | AAGGTCATCACTTGTAGCACCATCC |
| Occludin | TGGCTATGGCTATGGCGGATATACA | ACTAAGGAAGCGATGAAGCAGAAGG |
| MUC2 | ACCGAGCACATCACCTACCACAT | TCCAGAATCCAGCCAGCCAGTC |
| IL-4 | GCCATATCCACGGATGCGACAA | GGTGTTCTTCGTTGCTGTGAGGA |
| IL-10 | TCCCTGGGTGAGAAGCTGAAGAC | ACCTGCTCCACTGCCTTGCT |
| IL-22 | GTCCAACTTCCAGCAGCCATACAT | GGTAGCACTGATCCTTAGCACTGAC |
| IFN-γ | TCTTGGATATCTGGAGGAACTGGCA | ATGACGCTTATGTTGTTGCTGATGG |
| J-chain | TGTGGAAGTGGAGCTGGAAGATCA | AGGTCTCAGGAACACCATCGTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Chen, G.; Chen, D.; Zhang, H.; Lv, H.; Wen, Z. Grifola frondosa Polysaccharides Alleviated Cyclophosphamide—Induced Intestinal Injury Based on Microbiota, Metabolite and Immune Axis Modulation. Foods 2025, 14, 3376. https://doi.org/10.3390/foods14193376
Wu J, Chen G, Chen D, Zhang H, Lv H, Wen Z. Grifola frondosa Polysaccharides Alleviated Cyclophosphamide—Induced Intestinal Injury Based on Microbiota, Metabolite and Immune Axis Modulation. Foods. 2025; 14(19):3376. https://doi.org/10.3390/foods14193376
Chicago/Turabian StyleWu, Jindi, Guilu Chen, Dingfeng Chen, Haoran Zhang, Huirong Lv, and Zhengshun Wen. 2025. "Grifola frondosa Polysaccharides Alleviated Cyclophosphamide—Induced Intestinal Injury Based on Microbiota, Metabolite and Immune Axis Modulation" Foods 14, no. 19: 3376. https://doi.org/10.3390/foods14193376
APA StyleWu, J., Chen, G., Chen, D., Zhang, H., Lv, H., & Wen, Z. (2025). Grifola frondosa Polysaccharides Alleviated Cyclophosphamide—Induced Intestinal Injury Based on Microbiota, Metabolite and Immune Axis Modulation. Foods, 14(19), 3376. https://doi.org/10.3390/foods14193376






