Drying Model and Mechanism of Sugar Beet Pulp Based on Its Crosslinking with Ca2+ and Cu2+
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Modification of SBP
2.3. Drying Experiment
2.3.1. Effect of Ca2+ Concentration and Cu2+ Concentration on the SBP-Drying Process Through a Crosslinking Modification
2.3.2. Effect of Water Bath Temperatures on the SBP-Drying Process with a Crosslinking Modification
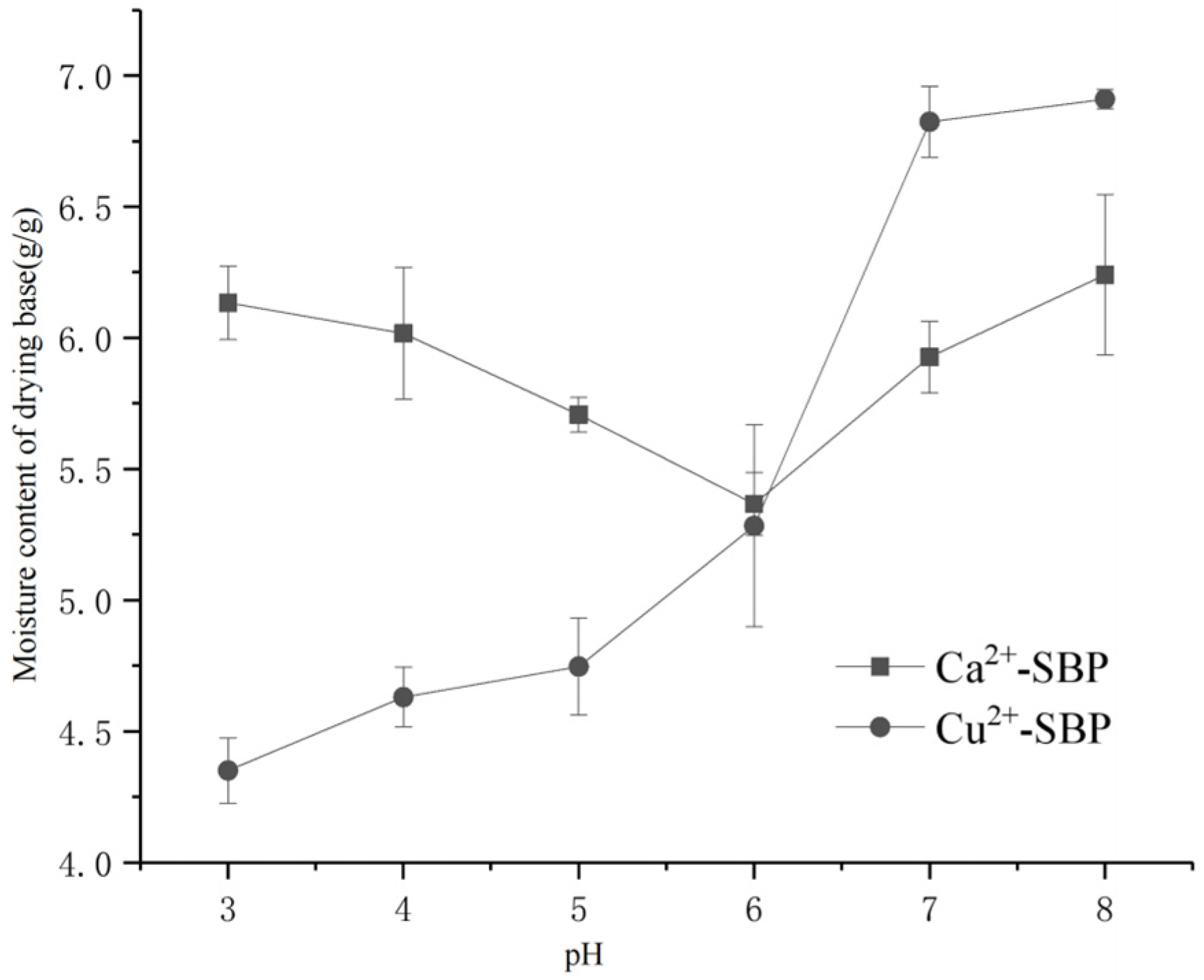
2.3.3. Effect of pH Value on the SBP-Drying Process Through a Crosslinking Modification
2.4. Parameter Calculation Method
2.4.1. Moisture Content of Drying Base
2.4.2. Ratio of Moisture
2.4.3. Drying Rate
2.5. Theoretical Analysis Using Theory Chemical Calculation
2.6. Structural Characterization of SBP, Ca2+-SBP, and Cu2+-SBP Using FTIR
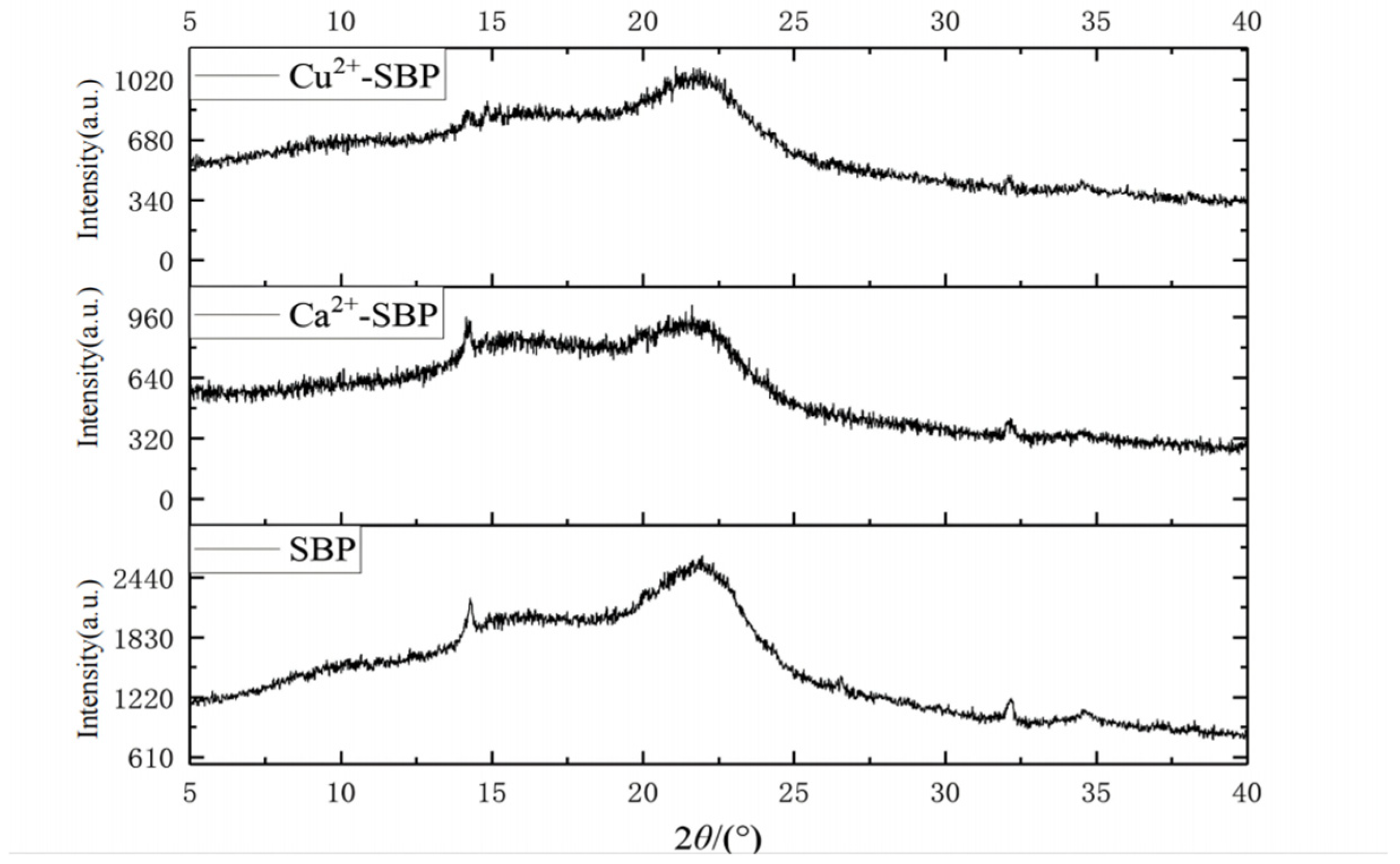
2.7. Structural Characterization of SBP, Ca2+-SBP, and Cu2+-SBP Using XRD
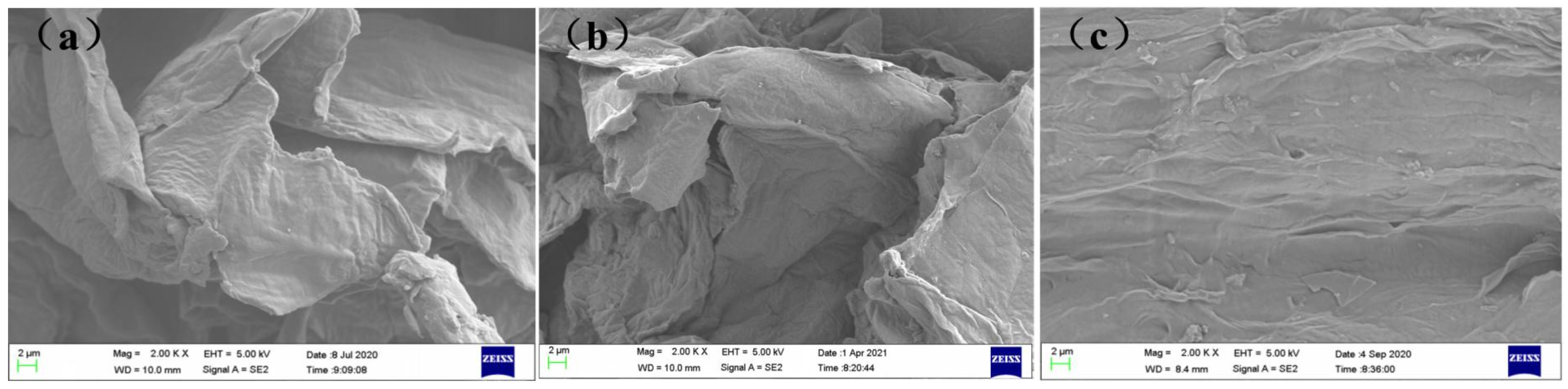
2.8. Structural Characterization of SBP, Ca2+-SBP, and Cu2+-SBP Using SEM
3. Results and Discussion
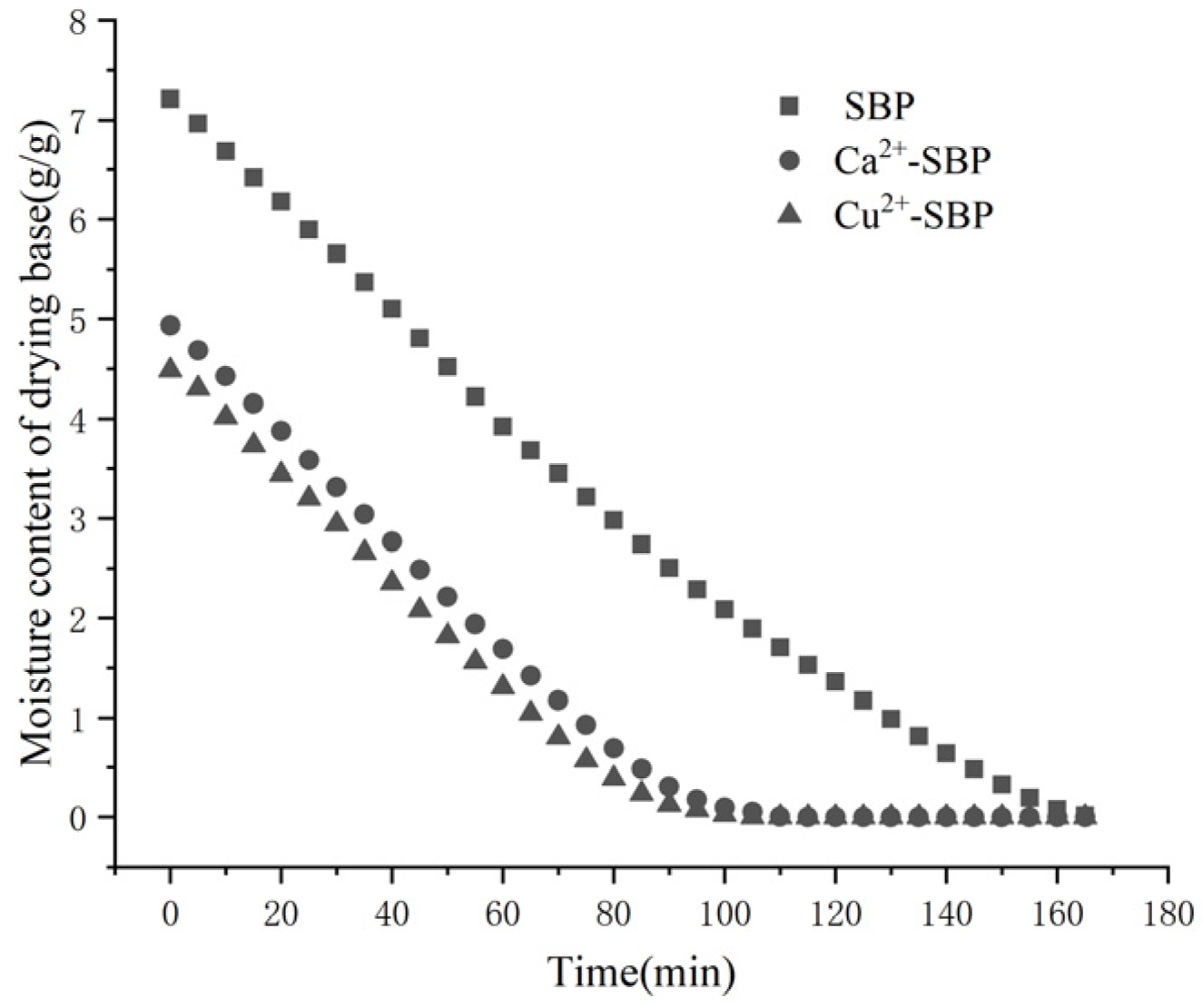
3.1. Effects of Different Crosslinking Metal Ions on SBP Dryingness
3.2. Effects of Metal Ion Concentrations, Operating Temperatures, and Solution pH on the Drying Process
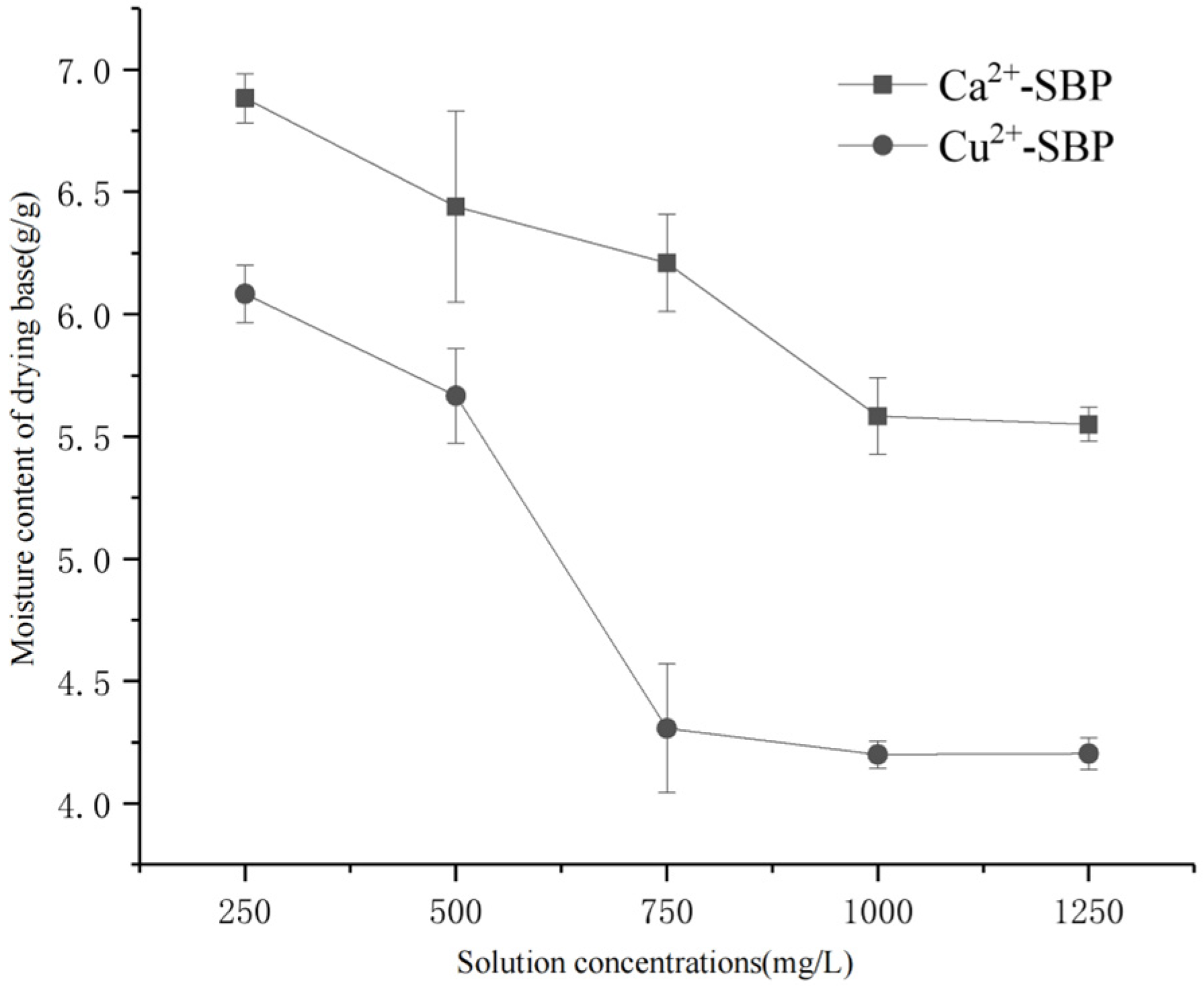
3.2.1. Effect of Ca2+ or Cu2+ Concentrations on the Drying Process of SBP
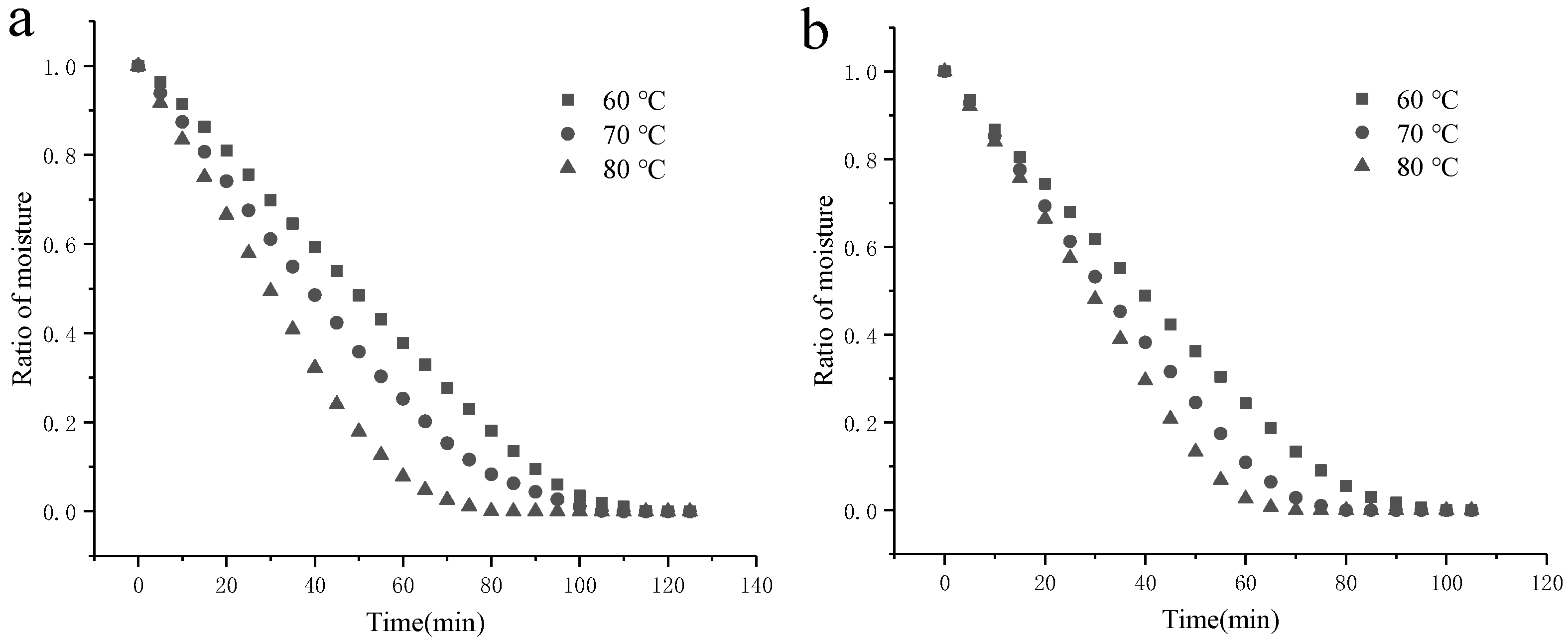
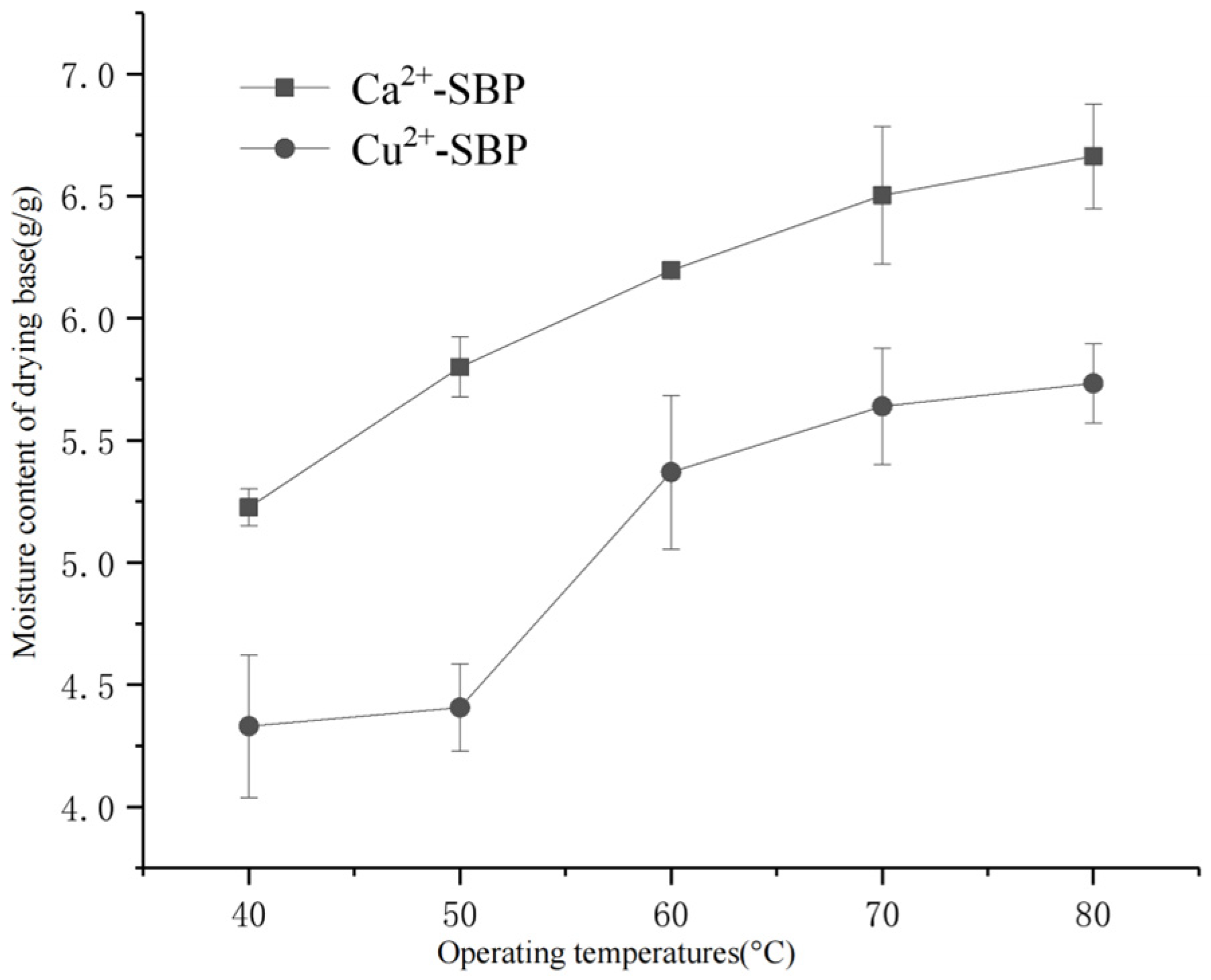
3.2.2. Effect of Operating Temperatures on the Drying Process of SBP
3.2.3. Effect of Solution pH on the Drying Process of SBP
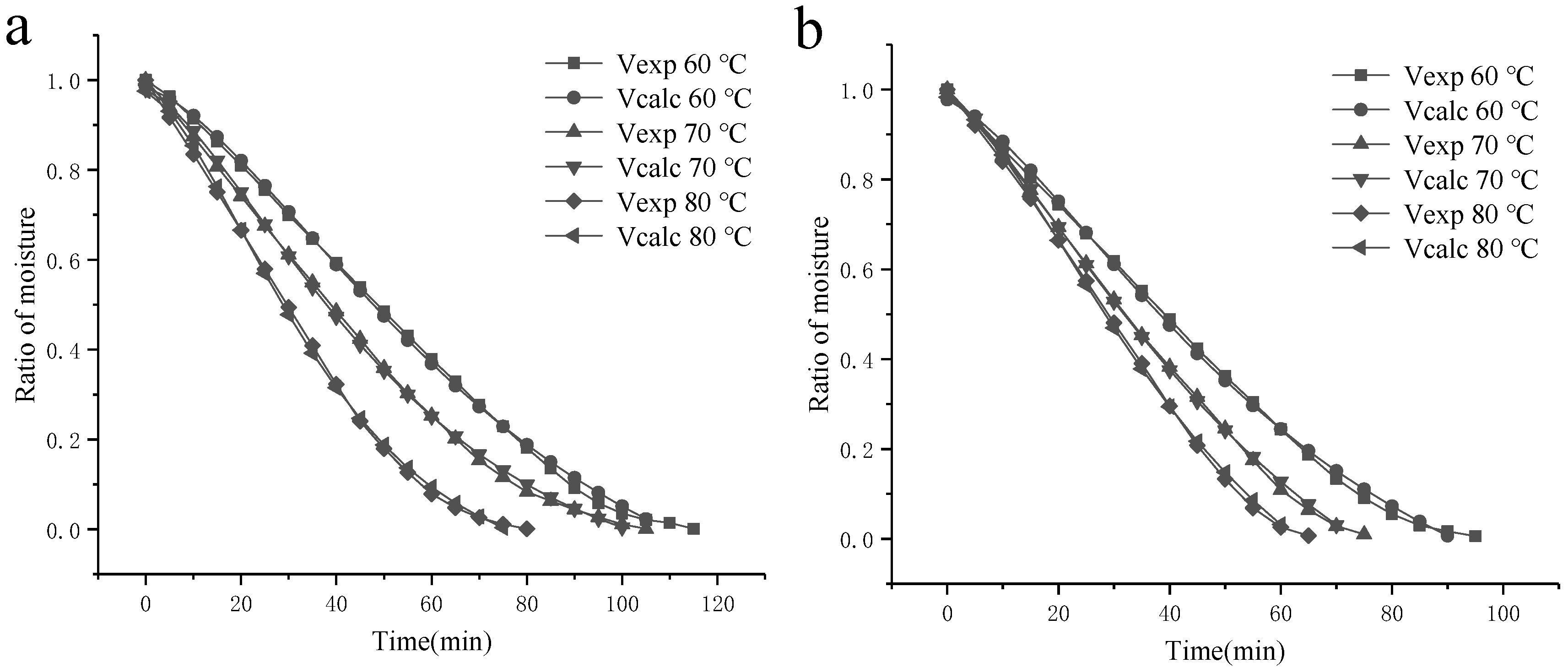
3.3. Fitting to Drying Mathematical Models
3.4. Characterization of SBP, Ca2+-SBP, and Cu2+-SBP Using FTIR, XRD, and SEM
3.5. Drying Mechanism
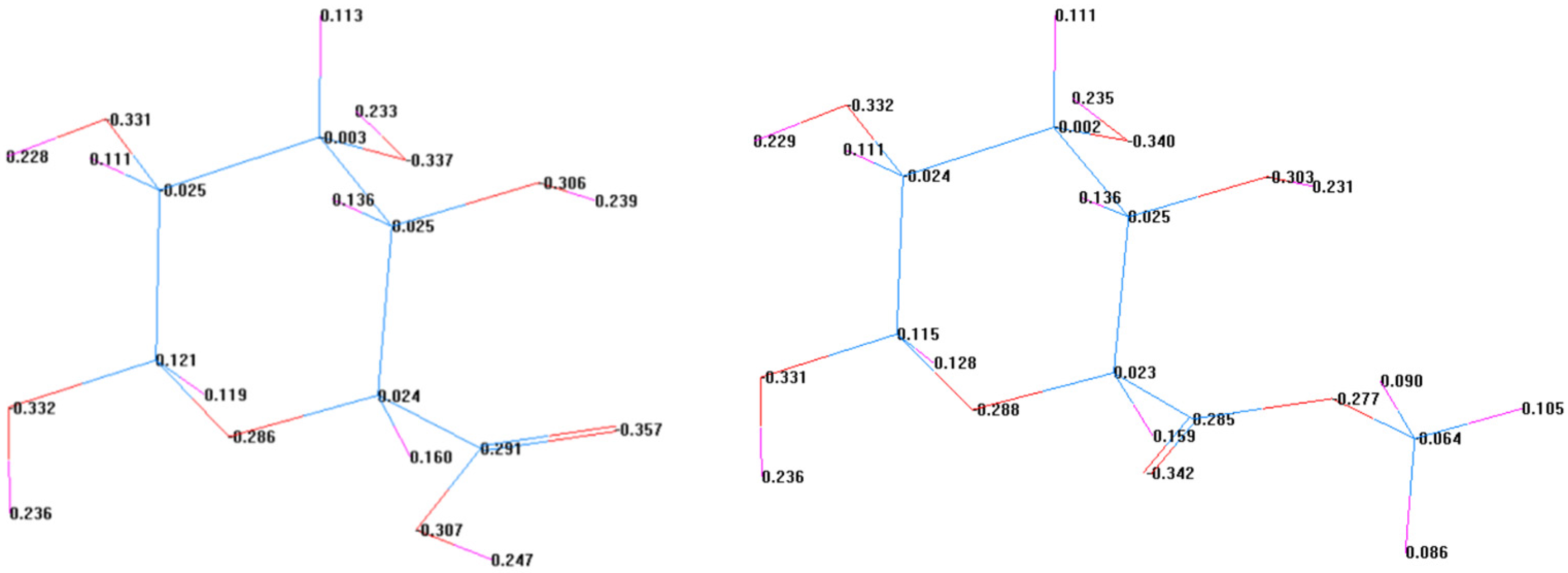
3.5.1. Electronegativity Calculation
3.5.2. Chemical Reaction Process and Possible Dryness–Strengthening Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasquini, D.; Teixeira, E.d.M.; Curvelo, A.A.d.S.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforcing agent in natural rubber. Ind. Crops Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Ma, S.; Yu, S.-j.; Zheng, X.-l.; Wang, X.-x.; Bao, Q.-d.; Guo, X.-m. Extraction, characterization and spontaneous emulsifying properties of pectin from sugar beet pulp. Carbohydr. Polym. 2013, 98, 750–753. [Google Scholar] [CrossRef]
- Finkenstadt, V.L. A Review on the Complete Utilization of the Sugarbeet. Sugar Technol. 2013, 16, 339–346. [Google Scholar] [CrossRef]
- GB 5749-2022; Standards for Drinking Water Quality. State Market Regulatory Administration: Beijing, China, 2022.
- Zhang, X.Y.; Li, Y.; Zhu, S.M. Saponification modification and Ca2+-loaded treatment improve the calcium adsorption performance and feed quality of sugar beet pulp. Feed Res. 2021, 4, 61–66. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Hameed, B.H.; Ok, Y.S.; Omirou, M. A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J. Mol. Liq. 2017, 240, 179–188. [Google Scholar] [CrossRef]
- Aksu, Z.; İşoğlu, İ.A. Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005, 40, 3031–3044. [Google Scholar] [CrossRef]
- Reddad, Z.; Gérente, C.; Andrès, Y.; Ralet, M.C.; Thibault, J.F.; Le Cloirec, P. Ni (II) and Cu (II) binding properties of native and modified sugar beet pulp. Carbohydr. Polym. 2002, 49, 23–31. [Google Scholar] [CrossRef]
- Chio, C.-P.; Lin, M.-C.; Liao, C.-M. Low-cost farmed shrimp shells could remove arsenic from solutions kinetically. J. Hazard. Mater. 2009, 171, 859–864. [Google Scholar] [CrossRef]
- Ćirić, M.; Popović, V.; Prodanović, S.; Živanović, T.; Ikanović, J.; Bajić, I. Sugar Beet: Perspectives for the Future. Sugar Technol. 2024, 26, 1208–1219. [Google Scholar] [CrossRef]
- Bao, S.F.; Windisch, W.; Kirchgessner, M. Calcium bioavailability of different organic and inorganic dietary Ca sources (citrate, lactate, acetate, oyster-shell, eggshell, β-tri-Ca phosphate). J. Anim. Physiol. Anim. Nutr. 1997, 78, 154–160. [Google Scholar] [CrossRef]
- Dancea, Z. Nutritia Animala si Elemente de Nutritie a Omului; Tedesco: Cluj Napoca, Romania, 2005. [Google Scholar]
- Ministry of Agriculture. Announcement No. 2625 of the Ministry of Agriculture of the People’s Republic of China. Hunan Feed 2018, 1, 17. [Google Scholar] [CrossRef]
- Yang, D. Analysis of Adding Calcium to the Ration on the Transl Atomics of Pig Muscle Tissue. Master’s Thesis, Guangxi University, Nanning, China, 2021. [Google Scholar]
- Nagy, E.M.; Coţa, C.; Gyorgy, Z.; Deac, T. Mathematical modelling of the drying process of sugar beet pulp with macro and microelement additions in order to improve the feed quality. E3S Web Conf. 2020, 180, 03020. [Google Scholar] [CrossRef]
- Zhang, Y.X. Study on Improvement of Drying and Adsorption Properties of Sugar Beet Pulp by Esterification and Metal Ion Crosslinking. Master’s Thesis, Kashi University, Kashi, China, 2021. [Google Scholar]
- Henderson, S.M.; Pabis, S. Grain drying theory I. Temperature effect on drying coefficient. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar] [CrossRef]
- El-Beltagy, A.; Gamea, G.R.; Essa, A.H.A. Solar drying characteristics of strawberry. J. Food Eng. 2007, 78, 456–464. [Google Scholar] [CrossRef]
- Yaldiz, O.; Ertekin, C.; Uzun, H.I. Mathematical modeling of thin layer solar drying of sultana grapes. Energy 2001, 26, 457–465. [Google Scholar] [CrossRef]
- Doymaz, İ. Drying of Pomegranate Arils and Selection of a Suitable Drying Model. Food Biophys. 2011, 6, 461–467. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; McLoughlin, C.M.; Magee, T.R.A. Thin-Layer Modeling of Microwave, Microwave-Convective, and Microwave-Vacuum Drying of Pharmaceutical Powders. Dry. Technol. 2005, 23, 513–532. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers. Master’s Thesis, Purdue University, Lafayette, IN, USA, 1949. [Google Scholar]
- Bantle, M.; Kolsaker, K.; Eikevik, T.M. Modification of the Weibull Distribution for Modeling Atmospheric Freeze-Drying of Food. Dry. Technol. 2011, 29, 1161–1169. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A New Model for Single-Layer Drying. Dry. Technol. 2007, 20, 1503–1513. [Google Scholar] [CrossRef]
- Yoshiharu, N.; Junji, S.; Henri, C.; Paul, L. Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Dronnet, V.M.; Renard, C.M.G.C.; Axelos, M.A.V.; Thibault, J.-F. Binding of divalent metal cations by sugar-beet pulp. Carbohydr. Polym. 1997, 34, 73–82. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xie, L.J.; Zhang, Y.X.; Guo, L.J.; Wang, Y.T. Adsorptive capacity of methylene blue by saponification and esterification modification of sugar beet pulp. Biomass Chem. Eng. 2024, 58, 39–46. [Google Scholar] [CrossRef]
- Tovar, J.C.; Xu, J.J.; Savary, B.J. Plant pectin methylesterase treatments dramatically reduce water-binding in sugar beet pulp via calcium crosslinking. In Proceedings of the 38th Biennial Meeting of the American Society of Sugar Beet Technologists (ASSBT 2015), Clearwater Beach, FL, USA, 23–27 February 2015. [Google Scholar]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 2002, 32, 195–198. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]




| Model Types | Model Equation | References |
|---|---|---|
| Henderson and Pabis | MR = a·exp (−k·t) | [17] |
| Exponential | MR = exp (−k·t) | [18] |
| Logarithmic | MR = c + a·exp (−k·t) | [19] |
| Parabolic | MR = a + b·t + c·t2 | [20] |
| Wang and sigh | MR = 1 + b·t + c·t2 | [21] |
| Page | MR = exp (−k·tn) | [22] |
| Weibull | MR = exp (−(t/α)β) | [23] |
| Midilli–Kucuk model | MR = a·exp (−ktn) + bt | [24] |
| Samples | Constants | 60 °C | 70 °C | 80 °C |
|---|---|---|---|---|
| Ca2+-SBP | a | 0.9841 | 0.9795 | 0.9755 |
| k | 0.0016 | 0.0033 | 0.0037 | |
| n | 1.5100 | 1.4390 | 1.5290 | |
| b | −0.0012 | −0.0008 | −0.0008 | |
| R2 | 0.9987 | 0.9989 | 0.9984 | |
| Cu2+-SBP | a | 0.9775 | 0.9899 | 0.9825 |
| k | 0.0031 | 0.0049 | 0.0034 | |
| n | 1.4240 | 1.3600 | 1.5200 | |
| b | −0.0016 | −0.0025 | −0.0025 | |
| R2 | 0.9983 | 0.9992 | 0.9986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Zhang, Y.; Luo, D.; Zhu, S.; Wang, Y.; Li, W. Drying Model and Mechanism of Sugar Beet Pulp Based on Its Crosslinking with Ca2+ and Cu2+. Foods 2025, 14, 3362. https://doi.org/10.3390/foods14193362
Jiang G, Zhang Y, Luo D, Zhu S, Wang Y, Li W. Drying Model and Mechanism of Sugar Beet Pulp Based on Its Crosslinking with Ca2+ and Cu2+. Foods. 2025; 14(19):3362. https://doi.org/10.3390/foods14193362
Chicago/Turabian StyleJiang, Guili, Yanxia Zhang, Donghui Luo, Siming Zhu, Yutao Wang, and Wanzhi Li. 2025. "Drying Model and Mechanism of Sugar Beet Pulp Based on Its Crosslinking with Ca2+ and Cu2+" Foods 14, no. 19: 3362. https://doi.org/10.3390/foods14193362
APA StyleJiang, G., Zhang, Y., Luo, D., Zhu, S., Wang, Y., & Li, W. (2025). Drying Model and Mechanism of Sugar Beet Pulp Based on Its Crosslinking with Ca2+ and Cu2+. Foods, 14(19), 3362. https://doi.org/10.3390/foods14193362






