Abstract
Nanoplastics have emerged as significant global pollutants, drawing worldwide concern. Due to their small particle size, large specific surface area, and high surface activity, nanoplastics can combine with other environmental contaminants, including environmental nanoparticles, persistent organic pollutants, antibiotics, and endocrine-disrupting chemicals. This review summarizes recent progress on the environmental behavior of nanoplastics and their complex effects on food safety when co-exposed to various contaminants. These composite pollutants accumulate in foods and the environment, and are ultimately taken up by humans, posing potential toxic effects on human health. In the future, the interaction mechanisms between environmental NPs and various co-contaminants, as well as their transfer routes from food to humans, should be addressed.
1. Introduction
Since the 1950s, plastics have been widely used in packaging, construction, healthcare, and agriculture due to their excellent properties and low production costs. However, the massive production and improper disposal of plastic products have led to a continuous increase in their environmental residues, making plastic pollution an increasingly severe global issue [1].
Under the influence of natural forces and human activities, large plastic debris gradually degrades into microplastics (MPs, size of 1 µm−5 mm) and nanoplastics (NPs, with size generally < 1 µm) [2], which are contaminants of emerging concern in the environment. Beyond the fragmentation of environmental plastic debris, microplastics and nanoplastics (MNPs) in the environment also originate from inadequate disposal of specific products produced at a micro/nanoscale size [3]. MNPs were found in various foods originating from ocean, freshwater, and terrestrial environments [4]. MNPs can be generated from both conventional fossil-based plastics (e.g., polystyrene (PS), polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), polyvinyl chloride (PVC), etc.) [5] and biodegradable plastics (e.g., polylactic acid (PLA), poly(ε-caprolactone) (PCL), etc.) [6].
Compared with MPs, the smaller NPs show enhanced mobility and bioavailability [7]. NPs have been detected in various environmental media, including water, sediment, soil, and air. The NPs transferred in the food web and those generated during food processing are accumulated in food products, which will eventually be taken up by humans, posing potential threats to food security and human health [8,9,10]. NPs can enter various organisms and cross critical physiological barriers, such as the placental barrier and blood–brain barrier, inducing adverse effects including inflammatory responses, immunosuppression, oxidative stress, and genotoxicity [11].
The physicochemical properties of NPs determine their environmental transport behaviors and fate. NPs exhibit strong adsorption capacities and act as vectors for environmental co-contaminants, such as heavy metals and persistent organic pollutants (POPs), consequently facilitating their diffusion and transportation in the environment [12,13]. Compared with single contaminants, NPs in combination with other environmental pollutants exhibit complex effects, including synergistic, antagonistic, and independent effects [14,15] on the risks of food security and human health. Furthermore, NPs and co-contaminants can enter the human body through food, drinking water, and air, potentially causing chronic toxicity effects across multiple organ systems. However, prospective studies on their complex effects on human health are still lacking.
This work reviews the latest progress on the food security and human health risks associated with exposure to NPs and environmental co-contaminants, emphasizing their complicated effects. Further research elucidating the interaction mechanisms of environmental NPs and various contaminants, as well as their transfer mechanisms from food to humans, will contribute to a deep understanding of their complicated effects on humans.
2. Environmental Behavior of Nanoplastics
2.1. Distribution and Transportation in Environments
NPs are ubiquitously distributed in marine, terrestrial, and atmospheric sinks (Figure 1), such as seawater [16,17,18], sediment [19], river water [20,21], ground water [22], potable waters [23], ice [24], snow [25], air [26], soil [27] and even human brains [28].
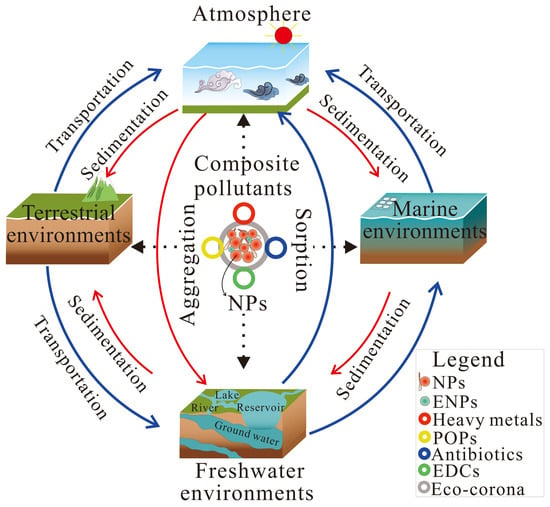
Figure 1.
The possible distribution and transport of nanoplastics and co-contaminants in the environment. The term composite pollutants refers to nanoplastics (NPs) and co-contaminants, which include environmental nanoparticles (ENPs), persistent organic pollutants (POPs), and endocrine-disrupting chemicals (EDCs).
The concentrations and polymer types of NPs vary in these sinks. For example, in the water column of the North Atlantic, about 1.5–32.0 mg m−3 of PET, PS, and PVC-NPs (filtered through a 1 µm PTFE syringe filter) were detected with thermal-desorption proton-transfer-reaction mass spectrometry (TD-PTR-MS) [18]. And the total mass concentrations of NPs (10 nm–1 μm) in surface water from the Fuhe River and groundwater, China were 0.283–0.793 μg L−1 and 0.021–0.203 μg L−1, respectively, by pyrolysis gas chromatography−mass spectrometry (Py-GC/MS) [22]. And NPs (<1 μm) were detected in bottled water at about 2.16 × 105 particles per liter through a hyperspectral stimulated Raman scattering (SRS) imaging platform [29]. Also, for the Antarctic sea ice samples, the average concentrations of NPs (<200 nm) at the top and bottom were 67.0 and 37.7 ng/mL, respectively, based on TD-PTR-MS [24]. Further, NPs (10 nm–1 μm) in municipal water (0.55 and 0.61 µg L−1), bottled water (0.21 and 0.46 µg L−1), and reservoir water (1.5 µg L−1) from Australia were tested, and polymer types of PE, PET, PP, PS, polymethylmethacrylate (PMMA), and Nylon 6 were found by Py-GC/MS [23]. In addition, the concentration of NPs in surface snow after 0.2 μm filtration was 4.6–23.6 ng/mL, while the concentration of NPs in a snow pit at a depth of 2.8 m after 0.2 μm filtration was 18.5 ng/mL in snow samples, and only PET was found, while PET, polypropylene carbonate (PPC), and PVC were found before filtration by TD-PTR-MS [25]. While in a high-altitude alpine area, the concentration of NPs (<200 nm) in melted surface snow was 46.5 ng/mL, with a mean deposition rate of 42 kg km−2 year−1 detected by TD-PTR-MS [30]. And the determined concentrations of PS-NPs (<1 μm) were 6.5–8.5, 1.4–1.8, and 0.7–1.0 μg L−1 for water samples from a river, a mariculture farm, and a beach, respectively, by the optical manipulation–surface-enhanced Raman scattering approach [19]. The highest amount of NPs (<200 nm) was 180–1588 μg L−1, detected in freshwater at a Swedish sampling site analyzed by TD-PTR-MS [31]. Further, the highest concentrations of PS-NPs, 0.17 µg g−1 (<1 μm) in soil from Guangzhou and 0.73 µg g−1 in Pearl River sediment in Guangzhou, were detected by Py-GC/MS [32].
NPs can be transported far away from their source. The unique characteristics of NPs, such as their small size and buoyancy, enable them to remain suspended in water columns or be transported over long distances by wind and ocean currents [33]. In marine and freshwater systems, NPs can remain in suspension, aggregate with natural colloids, or settle into sediments depending on their density and environmental conditions [34]. In addition, NPs in soils may interact with clay minerals, organic matter, and soil microbiota, which affect their transportation in the terrestrial environment [35]. They can be transported via leaching into groundwater systems or taken up by plant roots, potentially entering terrestrial food webs [36,37]. Moreover, NPs can become airborne through wind erosion of contaminated soils or resuspension from water surfaces, and they can be transported globally, contributing to long-range environmental pollution [38]. The transportation of NPs is determined by their habits, physicochemical properties, and environmental behaviors, such as aggregation and sedimentation.
2.2. Aggregation and Sedimentation
Aggregation refers to the process by which NPs and environmental particles accumulate together and form larger clusters or aggregates. Their high surface-to-volume ratio makes NPs easily form homogeneous or heterogeneous aggregates [39], while in the natural environment, NPs usually undergo heteroaggregation because of their relatively low concentrations [40].
NPs can aggregate with various minerals (e.g., the clay colloids [41], coastal sand [17], and kaolinite [42]) and environmental nanoparticles (ENPs), such as nano-Fe2O3 [43], nano-TiO2 [44], and nano-GO [45]. For example, PS-NPs and nano-Fe2O3 aggregate at appropriate pH and relative abundances (0.1–0.4) [43]. Furthermore, NPs can heteroaggregate more minerals or organo-mineral-complex-coated sand than bare quartz sand [46].
The aggregation behavior of NPs in the environment is influenced by ionic strength, surface properties of NPs, natural organic matter (NOM), and environmental pH. For example, CaCl2 destabilizes both PE-NPs and PS-NPs more aggressively than NaCl and MgCl2, and PS-NPs are more stable in the aquatic environment than PE-NPs [47]. In addition, weathering of NPs alters their physicochemical properties and, consequently, their aggregation behavior [39]. For example, ultraviolet (UV) aged PS-NPs exhibited higher stability in a monovalent cation-dominated solution and lower stability in a divalent salt solution compared with sulfide-aged PS-NPs [48]. NPs are most stable in groundwater, then natural river water, and lastly seawater [40,41]. Moreover, at pH 5.0, antibiotics of both ciprofloxacin (CIP) and sulfamethoxazole (SMX) significantly promote nanoplastics aggregation, with the former being stronger through charge shielding and sulfamethoxazole through molecular bridging [49].
Furthermore, NOM is vital for the environmental behavior of NPs. NOM interacts with NPs rapidly when they enter aquatic environments. Generally, NOM could reduce the aggregation and sedimentation of NPs, exhibiting an excellent stabilization effect. While NOM, like humic acid, can promote the aggregation of PS-NPs when suspended sediments exist with or without NaCl, NaCl can also improve aggregation [50]. NOM and NPs interact through various mechanisms, including intermolecular forces [43] and the Ca2+ bridging effect [51]. And the interaction is affected by concentrations, sources [52], and properties of NOM [53]. Exposed to the natural environment, the surfaces of NPs are covered by various environmental coronas, including the ‘hard corona’ and ‘soft corona’ [54]. The ‘eco-corona’ modifies the particle sizes and surface properties of NPs [55,56], influencing their uptake by organisms and sedimentation. For example, the environmental corona on PS-NPs can alter their intracellular locations, activate new internalization pathways in both keratinocytes and fibroblasts, and modify cellular responses of keratinocytes [54].
Sedimentation occurs when aggregated NPs become sufficiently dense to settle onto the bottom of a water column [40]. The properties of NPs, such as polymer types and shapes, also affect their sedimentation in the environment. For example, with NOM, polymorphic NPs showed greater retention than spherical NPs in porous media [57].
2.3. Sorption and Carrier Effect for Co-Contaminants
NPs can sorb and carry various environmental co-contaminants (Figure 1), such as heavy metal ions, POPs (e.g., polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), pesticides, etc.), antibiotics, and endocrine-disrupting chemicals (EDCs) [58]. Co-contaminants can attach to the surface of NPs by adsorption [59,60] or penetrate the solid matrix of NPs by absorption because of partitioning [61]. For example, lead (Pb) can be adsorbed onto NPs as complexes of monoligands and biligands [62]. And electrostatic and dispersion interactions dominate the adsorption of NP–neonicotinoid-insecticide complexes through physisorption onto the surface of all plastic (PET, PS, PE) matrices [63].
The adsorption capacities of NPs are affected by the physicochemical properties of NPs, such as the polymer type, size, and surface properties. For example, weathered PET, PS, and PP-NPs (<200 nm) showed strong adsorption of heavy metal ions, including Mn2+, Co2+, Zn2+, and Cd2+, with PP-NPs showing the highest adsorption capacity [64]. The adsorption capacities of functionalized PS-NPs (i.e., PS-COOH-NPs, PS-NH2-NPs) were higher than those of pristine PS-NPs [65]. Compared with pristine PS-NPs, aged PS-NPs showed a higher adsorption capacity for heavy metals, such as Pb and cadmium (Cd) [39].
Also, as particle size decreases, the adsorption capacity of NPs increases, while the diffusion of co-contaminants inside NPs decreases [66]. For example, the adsorption capacity of NPs for butyltin increases with decreasing particle size and salinity [12]. And the adsorption capacity of 20 nm carboxylated PS-NPs for perfluorooctanoic acid (PFOA) is twice that of 500 nm PS-NPs in wastewater [67]. Also, the adsorption equilibrium constant (kd) for triclosan (TCS) increased from 1.36 L⋅g−1 of 900 nm PS-NPs to 4.39 L⋅g−1 for 50 nm PS-NPs [65].
The adsorption capacities of NPs are affected by the types of co-contaminants. For example, their adsorption of hydrophilic and negatively charged contaminants is lower than that of hydrophobic and cationic ones, and the adsorption capacity of PS-NPs for different co-contaminants follows glyphosate < PFOA < methyl parathion < phenanthrene < fluoxetine [67].
Environmental factors, such as salinity, pH [65], and NOM [68], can also affect the interactions of NPs and co-contaminants. For example, humic acid impedes the sorption of benzo[a]pyrene and copper (Cu) on anionic NPs, while facilitating that process on neutral and cationic NPs [69]. In addition, humic acid improved the sorption capacity for a certain antibiotic (ciprofloxacin, CIP) and endocrine disruptor (bisphenol-A, BPA) of 40 nm PS-NPs and 1 μm PVC-NPs [70]. A strong adsorption for sulfadiazine (SDZ) occurred when the relative abundance ratio of PS-NPs to nano-Fe2O3 at 0.2 was achieved through charge neutralization [43]. In addition, after aggregation with kaolinite, the PS-NPs showed an increase in the sorption capacity for Pb2+ [42].
2.4. Degradation and Persistence in the Environment
NPs are highly persistent in the environment due to their chemical stability and resistance to degradation. Degradation processes include photodegradation, oxidative degradation, and biodegradation, but these processes are generally slow in natural environments [55]. For photodegradation, UV radiation can break down NPs and fragment polymers into smaller particles. However, this process is limited in deep water and sediment environments where light penetration is low [71]. For biodegradation, the degradation efficiency of various microorganisms in natural environments, such as fungi and bacteria on NPs, is limited [55].
In addition, the degradation of biodegradable plastic is also limited. For example, although the PCL film is embedded with an enzyme, the released MPs and NPs after hydrolysis could not be completely degraded after up to 130 days [72]. Significantly, while the amorphous phase of the semicrystalline NPs continues to degrade, crystal fragments do not, and hence they temporally persist in the environment [73]. In addition, NPs are more stable and have longer residence times in freshwater compared with brackish water or seawater [40]. Furthermore, the presence of additives and stabilizers further complicates biodegradation [55].
3. Food Security and Human Health Risks of Nanoplastics and Co-Contaminants
3.1. Food Security Risks of Nanoplastics and Co-Contaminants
3.1.1. Nanoplastics in Foods
Food security is at risk due to NPs in the environment, food web, and food processing and food packing, which will eventually threaten the health of humans. Due to the small size of NPs and the detection limits of conventional spectroscopic techniques, detecting NPs is quite challenging [74], although simulated NPs in various foods, beverages, and condiments can be detected using Py-GC-MS or surface-enhanced Raman spectroscopy (SERS) methods [75]. However, the direct dictation of NPs in field samples of foods has not been reported yet, whereas the generation of NPs during food preparation and food packaging through various food contact articles, such as bottles, bags, and pans, has been verified [76,77,78]. These NPs can potentially enter into foods (Figure 2).
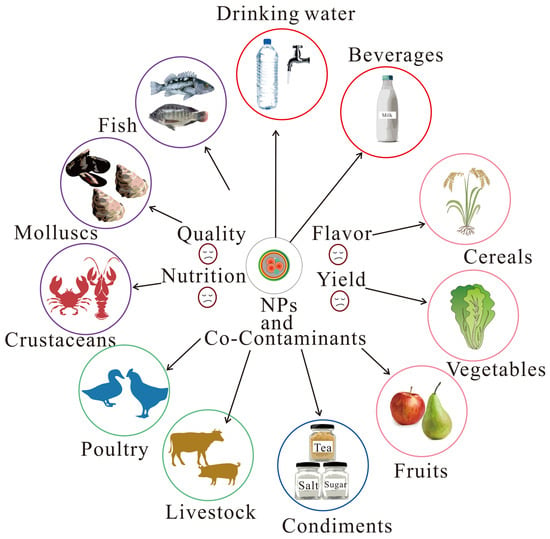
Figure 2.
Risks of nanoplastics and co-contaminants on food security.
In the aquatic environment, crustaceans, mollusks, and fish are common food sources for humans. They are generally exposed to NPs by ingestion, which results in toxicological effects on their behavior, growth, and reproduction [79]. NPs can exacerbate oxidative stress, DNA damage [5], and neurotoxicity [80] in aquatic species, while also negatively impacting their behavior, antioxidant defense system, reproduction, and survival [81], and are associated with changes in crucial metabolic pathways [82]. The biological effects of NPs depend on their concentrations and physicochemical properties, such as size and surface charges [83].
Once exposed to the NPs, they could affect the nutritional quality of plants over time and gradually accumulate in fruits as plants grow, thereby reducing taste and flavor [84], and pose a risk to the security of foods [85].
NPs mainly accumulate in the root and leaves of crops [86,87]. NPs can enter plants by roots, apoplastic transport, foliar uptake, and transpirational pull [88]. For example, PS-NPs (50 nm) could enter the xylem vessel of the root and eventually be transported to the leaf, affecting the growth of pakchoi by disturbing the homeostasis of endogenous hormones [89]. In addition, PS-NPs (100–500 nm) can also transfer from root to shoot, accumulating in intercellular spaces and changing in phenolic compounds [90]. While PS-NPs (200 nm) primarily accumulated in the peels, cortex, and xylem of radish roots [91], they may be directly taken up by humans. Exposure to PS-NPs could trigger plant defense systems by upregulating gene expression and metabolites and downregulating genes associated with plant hormone signal transduction, which finally changes the nutrition and flavor of crops [91]. Moreover, NPs can be transferred to subsequent generations of pea plants (Pisum sativum) [92], indicating they can be continuously circulated in terrestrial ecosystems and have a lasting effect on food yield and quality. The toxicity of NPs can be affected by organism species, toxicity metrics, physicochemical properties of NPs, concentrations, exposure time, and medium.
NPs can affect the health of poultry and livestock. For example, when exposed to PS-NPs (100 nm) for 120 days, chickens showed histopathological liver injury with metabolic disturbances [93]. In addition, PS-NPs (100 nm) inhibited muscle fiber formation on porcine myoblasts derived from skeletal muscle satellite cells in vitro [94].
3.1.2. Exposure to Nanoplastics and Co-Contaminants in Foods
Beyond NPs, foods are also exposed to various co-contaminants, such as phthalates and bisphenol A [95]. Exposure to NPs and co-contaminants has complex toxicity effects on aquatic food products, such as invertebrates, fish, and crops (Figure 2). Co-exposure to NPs and a single type of heavy metal, such as Cd, As, Pb, Cu, or Hg, has been frequently studied. For example, simultaneous exposure to PS-NPs (100 nm) and Cd synergistically led to more severe histopathological damage to liver tissue and increased liver transaminases activities of Prussian carp in 21 days, resulting in decreased superoxide dismutase (SOD) activity, with increased malondialdehyde (MDA) and total antioxidant capacity (T-AOC), and upregulated transcriptional levels of immune-associated genes [96]. PS-NPs could also enhance the bioaccumulation and toxicity of a heavy metal cocktail (HMC) in shrimp [97].
Exposure to NPs with different co-existing contaminants could cause different effects. For example, PS-NPs (100 nm or 80 nm) mitigate the intestinal toxicity of Cd [98] but aggravate the hepatopancreatic toxicity of phoxim (PHO) [99] and hepatotoxicity of perfluorooctanoic acid (PFOA) [100] in Chinese mitten crabs, which may be related to different interactions between NPs and co-contaminants.
Co-exposure to different kinds of contaminants for both freshwater fishes [101,102,103] and marine fishes [104,105] caused intestinal damage and hepatotoxicity, while fish intestines and liver usually being abandoned during food processing. Combined exposure to NPs and mercury (Hg) increased toxic effects on rare minnows, Gobiocypris rarus, compared with individual exposures, although NPs had limited effects on methylmercury (MeHg) content and its proportional distribution in muscle tissue of rare minnows [106], which may be linked to microbial dysbiosis with systemic metabolic dysfunction as well as oxidative stress and inflammatory responses triggered by NPs and/or Hg exposure.
Exposure to NPs and co-contaminants (e.g., heavy metals and POPs) also has a complex effect on crops [107], for example, increased bioaccumulation of PS (20 and 1000 nm) and As in edible tissues [108], as well as PS-NP (500 nm) and Cd [109] in shoots of lettuce, show increased effects in combination with smaller NPs [108] and saline conditions [110], which is related to an energy-intensive oxidative stress response [109]. In addition, although PS-NPs (100 nm) primarily accumulate in the roots of lettuce, they amplify the toxic effect of tebuconazole on auxin biosynthesis, inactivation, and signaling [111].
3.2. Human Health Risks of Nanoplastics and Co-Contaminants
Humans can be exposed to environmental NPs through ingestion, inhalation, and dermal exposure [112]. NPs in contaminated food and water can be ingested by humans, while airborne NPs can be inhaled or dermally absorbed by humans (Figure 3). NPs potentially pose a toxic effect on the human body through mechanisms such as oxidative stress, mitochondrial dysfunction, immune responses, and the gut–brain axis [113]. The potential toxic effects of NPs and co-contaminants have been shown on multiple physiological systems of humans.
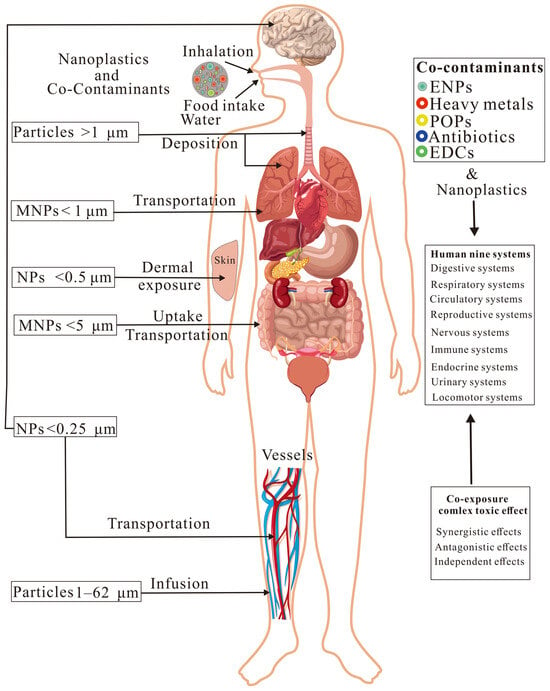
Figure 3.
Potential health risks of nanoplastics and co-contaminants on humans. The terms composite pollutants refer to nanoplastics (NPs) and co-contaminants, environmental nanoparticles (ENPs), persistent organic pollutants (POPs), and endocrine-disrupting chemicals (EDCs).
3.2.1. Ingestion and Infusions
Humans can ingest NPs through contaminated food and drinking water [80]. MNPs smaller than 5 μm can be directly taken up by intestinal epithelial Caco-2 cells [114]. In addition, MPs (1–62 μm, mainly PP, with NPs possibly existing) can be directly injected into the human bloodstream through infusions [115], which may deposit in various organs of humans. Once ingested, NPs can enter the gastrointestinal tract, where they may cross biological barriers, such as the intestinal lining, and possibly accumulate in the livers, kidneys, and even the brains of humans [28]. Similarly, PP and PET-NPs showed modest genotoxicity in DNA strand breaks, although they did not show significant cytotoxicity in human liver cancer cells HepG2 and intestinal epithelial cells Caco-2 [116]. However, NPs may change lipid digestion in the gastrointestinal system of humans, threatening the assimilation of nutrients [68].
A limited co-exposure study showed that PS-NPs (100 nm and 700 nm) and the heavy metals Cd, Pb, As, and chromium (Cr) synergistically increased the expression of inflammatory cytokines in the human intestinal epithelial cell line Caco-2 [117], indicating the ‘Trojan horse’ effects of NPs [118]. In addition, the combined toxicity of PS-NPs and perfluorooctanesulfonic acid (PFOS) on Caco-2 cells seems to be independent (no effects), with combined exposure disrupting RNA, endoplasmic reticulum, and mitochondria, with NPs as the primary driver of toxicity [119], which may indicate cross-talk between cellular structure and metabolism in response to the co-exposure.
3.2.2. Inhalation and Dermal Exposure
Airborne NPs can be directly inhaled by humans and deposit in lung tissue, significantly influencing human respiratory health [120]. And 1 μm PS-NPs can be taken up by human lung alveolar A549 cells [121]. In addition, NPs can penetrate the epidermis and enter the human body through dermal exposure [122]. For example, after 16 h of incubation, fluorescently labeled PS-NPs (100 nm and 500 nm) were taken up by human skin keratinocytes and fibroblast cells [122]. PET-NPs internalized in the endosomes and nuclei of human primary nasal epithelial cells induced intracellular ROS induction and mitochondrial membrane potential loss [123]. In addition, both PVC-NPs and PS-NPs (250 nm) showed a strong affinity for phosphatidylcholines and lysophosphocholines from human plasma, with more PVC-NPs crossing the human blood–brain barrier than PS-NPs [124]. Also, amino-modified PS-NPs showed significant cytotoxicity on normal human dermal fibroblast cells, with the cytotoxicity decreasing as PS-NH2-NPs > PS-COOH-NPs > PS-NPs [125].
Co-exposure to NPs and other contaminants can have synergistic and antagonistic effects on human lung cells. For example, PS-NH2-NPs (68.6 nm) and spherical Nano-ZnO showed a synergistic effect on toxicity in human lung BEAS-2B epithelial cells, while PS-NH2-NPs and triangular pyramid of Nano-ZnO exhibited an antagonistic effect [126], owing to change in cell membrane integrity and oxidative stress. In addition, co-exposure to PET-NPs and cigarette smoke condensate (CSC) for 4 weeks synergistically exacerbated oxidative stress, genotoxicity, and tumorigenic transformation of human lung BEAS-2B cells compared with individual exposures [127], which may be attributed to enhanced carcinogenic traits through oxidative stress, genomic instability, and disruption of tumor-suppressive pathways.
3.2.3. Potential Health Risks
Studies have shown that NPs can alter hormone levels by interacting with estrogen and thyroid hormone receptors. For example, NPs can affect the homeostasis of breast cells [128]. In addition, PS-Pd NPs can translocate into and interact with human primary immune cell subpopulations from human blood after exposure [129]. Furthermore, PS-NPs can influence the dysfunction of cellular components and the development of atrioventricular heart valves in human-induced pluripotent stem cells hiPSCs [130]. Furthermore, carboxylated PS-NPs (40 nm and 200 nm) affected early-pregnancy trophoblast phenotype and function of human trophoblast cells [131]. NPs can interfere with hormonal systems in humans, leading to endocrine disruption, which poses potential risks, especially for children and pregnant women, which can be potentially transferred to the next generation [132].
The ‘Trojan horse’ effect of NPs can increase the bioaccessibility of environmental contaminants to humans [133], enhancing their toxicity or even carcinogenic risk to humans [134]. For example, PS-NPs and the heavy metals Cd, Pb, As, and Cr synergistically increased the inflammation in human monocytic leukemia cell (THP-1)-differentiated macrophages [117]. PS-NPs (500 nm) significantly reduced the availability and uptake of Cd, reducing its cytotoxicity on human hepatocytes (HepG2) hepatoma cells in a dose-dependent manner [135], suggesting that cadmium might play an active role in the interaction between NPs and lipids, promoting tighter and more stable binding. In addition, co-exposure to NPs and the pharmaceutical and personal care product triclosan synergistically increased the production of reactive oxygen species (ROS) in human KGN ovarian granulosa cells [136], activating the antioxidant stress pathway. In addition, PS-NPs enhanced the binding of triclosan to human serum albumin, promoting its accessibility to the binding sites [137], which may be attributed to the alteration of human serum albumin conformation and microenvironment of the amino acid residues induced by NPs.
4. Research Trends
Although research on the ecological influence of NPs and co-contamination has made some progress, it remains in its infancy and faces numerous challenges. The direct detection of NPs in environmental samples of foods is still lacking, which indicates an urgent need for sound separation and analytical methods for NPs. Co-exposure studies are mostly conducted under laboratory conditions using a single type of NP and co-contaminant, which is different from environmental conditions, in which NPs are complicated, heterogeneous, and dispersed with long-term interaction.
Also, the study on how co-exposure to composite pollutants affects the taste, nutrition, and quality of various foods is still insufficient. In addition, the mechanisms of interaction between environmental NPs and novel pollutants and their molecular pathways on the aquatic and terrestrial organisms of food sources are underexplored.
Furthermore, studies evaluating human exposure to environmental NPs and co-contaminants are very limited. And studies are limited to co-exposure of human cells, making it difficult to achieve a deep understanding of the risks of NPs and co-contaminants to human health. Moreover, there are large gaps in evaluating exposure to NPs and co-contaminants through foods without looking into their transfer routes from foods to humans.
5. Conclusions
Environmental nanoplastics can sorb and aggregate with various environmental pollutants, forming composite pollution systems with enhanced mobility and bioavailability. Composite pollutants widely distributed in the environment can pose a risk to a variety of foods and will eventually be transported to humans. The carrier effects of NPs for co-contaminants are complicated and pose a risk of toxicity on food security and human health. Yet research on these complex effects is still in a very early stage and needs to be further explored.
Author Contributions
Conceptualization, S.X. and J.W.; methodology, M.C.; validation, J.L. and J.W.; formal analysis, S.X.; investigation, M.C.; resources, J.L.; writing—original draft preparation, S.X. and M.C.; writing—review and editing, J.L. and J.W.; visualization, M.C. and J.W.; supervision, J.W.; project administration, M.C. and J.W.; funding acquisition, M.C. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangxi Young Talents Development Program, grant number 2024QMJH-1901, ‘Light of Bagui’ Visiting Scholar Program, Guangdong S&T Program, grant number 2024B1111150001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We thank the editors and reviewers for their kind suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MPs | microplastics |
| NPs | nanoplastics |
| MNPs | microplastics and nanoplastics |
| PS | polystyrene |
| PE | polyethylene |
| PP | polypropylene |
| PET | polyethylene terephthalate |
| PVC | polyvinyl chloride |
| PLA | polylactic acid |
| PCL | poly(ε-caprolactone) |
| PMMA | polymethylmethacrylate |
| PPC | polypropylene carbonate |
| POPs | persistent organic pollutants |
| ENPs | environmental nanoparticles |
| EDCs | endocrine-disrupting chemicals |
| TD-PTR-MS | thermal-desorption proton-transfer-reaction mass spectrometry |
| Py-GC/MS | pyrolysis gas chromatography−mass spectrometry |
| SRS | stimulated Raman scattering |
| SERS | surface-enhanced Raman spectroscopy |
| NOM | natural organic matter |
| UV | ultraviolet |
| PAHs | polycyclic aromatic hydrocarbons |
| PCBs | polychlorinated biphenyls |
| PFOA | perfluorooctanoic acid |
| TCS | triclosan |
| CIP | ciprofloxacin |
| BPA | bisphenol-A |
| SDZ | sulfadiazine |
| IBU | ibuprofen |
| As | arsenic |
| Cd | cadmium |
| Cr | chromium |
| Cu | copper |
| Hg | mercury |
| Pb | lead |
| Zn | zinc |
| MeHg | methylmercury |
| DBDPE | decabromodiphenyl ethane |
| PCB-18 | 2,2’,5-Trichlorobiphenyl |
| DEHP | di(2-ethylhexyl) phthalate |
| PBDE-47 | 2,2′,4,4′-tetrabromodiphenyl ether |
| TDCIPP | tris (1,3-dichloro-2-propyl) phosphate |
| DES | diethylstilbestrol |
| APAP | acetaminophen |
| TPT | triphenyltin |
| DBP | dibutyl phthalate |
| Phe | phenanthrene |
| PYR | pyraclostrobin |
| F-53B | 6:2 chlorinated polyfluorinated ether sulfonate |
| PFOS | perfluorooctanesulfonic acid |
| CSC | cigarette smoke condensate |
| CIP | ciprofloxacin |
| SMX | sulfamethoxazole |
| PHO | phoxim |
| HMC | heavy metal cocktail |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| T-AOC | total antioxidant capacity |
| ROS | reactive oxygen species |
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.Y.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of nanoplastics in the aquatic environment and possible impacts on aquatic organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef]
- Antonelli, P.; Fazion, J.P.; Marzoli, F.; Losasso, C.; Belluco, S. Routes of human exposure to Micro- and Nanoplastics through the food chain: What do literature reviews say? Eur. Food Res. Technol. 2024, 250, 2697–2709. [Google Scholar] [CrossRef]
- Ducoli, S.; Kalcíková, G.; Velimirovic, M.; Depero, L.E.; Federici, S. Production, characterization, and toxicology of environmentally relevant nanoplastics: A review. Environ. Chem. Lett. 2025, 23, 649–675. [Google Scholar] [CrossRef]
- Chen, M.Y.; Chen, F.; Li, Z.; Haider, M.R.; Wei, J.X.; Chen, G.L.; Wang, W.J.; Wang, J. Environmental risk assessment of microplastics and nanoplastics generated from biodegradable plastics in marine ecosystem. TrAC Trends Anal. Chem. 2023, 169, 117381. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V.; Khan, S.A.; Patri, A.K.; Wiggins, S. Regulatory Science Perspective on the Analysis of Microplastics and Nanoplastics in Human Food. Anal. Chem. 2024, 96, 4343–4358. [Google Scholar] [CrossRef]
- Son, J.W.; Nam, Y.; Kim, C. Nanoplastics from disposable paper cups and microwavable food containers. J. Hazard. Mater. 2024, 464, 133014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, H.T.; Niu, S.Y.; Guo, M.H.; Xue, Y.Y. Mechanisms of micro- and nanoplastics on blood-brain barrier crossing and neurotoxicity: Current evidence and future perspectives. Neurotoxicology 2025, 109, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Felipe-Sotelo, M.; Bance-Soualhi, R.; Crean, C.; Al-Sid-Cheikh, M. Sub-100 nm nanoplastics: Potent carriers of tributyltin in marine water. Environ. Sci.-Nano 2024, 11, 241–252. [Google Scholar] [CrossRef]
- Borriello, L.; Scivicco, M.; Cacciola, N.A.; Esposito, F.; Severino, L.; Cirillo, T. Microplastics, a Global Issue: Human Exposure through Environmental and Dietary Sources. Foods 2023, 12, 3396. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef]
- Sun, N.; Shi, H.; Li, X.; Gao, C.; Liu, R. Combined toxicity of micro/nanoplastics loaded with environmental pollutants to organisms and cells: Role, effects, and mechanism. Environ. Int. 2023, 171, 107711. [Google Scholar] [CrossRef]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef]
- Davranche, M.; Lory, C.; Le Juge, C.; Blancho, F.; Dia, A.; Grassl, B.; El Hadri, H.; Pascal, P.Y.; Gigault, J. Nanoplastics on the coast exposed to the North Atlantic Gyre: Evidence and traceability. Nanoimpact 2020, 20, 100262. [Google Scholar] [CrossRef]
- ten Hietbrink, S.; Materic, D.; Holzinger, R.; Groeskamp, S.; Niemann, H. Nanoplastic concentrations across the North Atlantic. Nature 2025, 643, 412–416. [Google Scholar] [CrossRef]
- Shi, X.; Mao, T.; Huang, X.; Shi, H.; Jiang, K.; Lan, R.; Zhao, H.; Ma, J.; Zhao, J.; Xing, B. Capturing, enriching and detecting nanoplastics in water based on optical manipulation, surface-enhanced Raman scattering and microfluidics. Nat. Water 2025, 3, 449–460. [Google Scholar] [CrossRef]
- Slaveykova, V.I.; Marelja, M. Progress in Research on the Bioavailability and Toxicity of Nanoplastics to Freshwater Plankton. Microplastics 2023, 2, 389–410. [Google Scholar] [CrossRef]
- Tamayo-Belda, M.; Pulido-Reyes, G.; Rosal, R.; Fernández-Piñas, F. Nanoplastic toxicity towards freshwater organisms. Water Emerg. Contam. Nanoplastics 2022, 1, 19. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Jiao, M.; Liu, G.; van der Hoek, J.P. Identification and Quantification of Nanoplastics in Surface Water and Groundwater by Pyrolysis Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2022, 56, 4988–4997. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Thomas, K. Quantitative analysis of nanoplastics in environmental and potable waters by pyrolysis-gas chromatography-mass spectrometry. J. Hazard. Mater. 2024, 464, 133013. [Google Scholar] [CrossRef]
- Materić, D.; Kjær, H.A.; Vallelonga, P.; Tison, J.L.; Röckmann, T.; Holzinger, R. Nanoplastics measurements in Northern and Southern polar ice. Environ. Res. 2022, 208, 112741. [Google Scholar] [CrossRef]
- Materić, D.; Kasper-Giebl, A.; Kau, D.; Anten, M.; Greilinger, M.; Ludewig, E.; van Sebille, E.; Röckmann, T.; Holzinger, R. Micro- and Nanoplastics in Alpine Snow: A New Method for Chemical Identification and (Semi)Quantification in the Nanogram Range. Environ. Sci. Technol. 2020, 54, 2353–2359. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, H.; Jones, R.; Feng, Y.; Gong, K.; Li, K.; Fang, X.; Tahir, M.A.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy Facilitates the Detection of Microplastics <1 μm in the Environment. Environ. Sci. Technol. 2020, 54, 15594–15603. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of microplastics in decedent human brains. Nat. Med. 2025, 31, 1367. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef] [PubMed]
- Materić, D.; Ludewig, E.; Brunner, D.; Röckmann, T.; Holzinger, R. Nanoplastics transport to the remote, high-altitude Alps. Environ. Pollut. 2021, 288, 117697. [Google Scholar] [CrossRef]
- Materić, D.; Peacock, M.; Dean, J.; Futter, M.; Maximov, T.; Moldan, F.; Röckmann, T.; Holzinger, R. Presence of nanoplastics in rural and remote surface waters. Environ. Res. Lett. 2022, 17, 054036. [Google Scholar] [CrossRef]
- Li, Z.C.; Gao, Y.; Wu, Q.H.; Yan, B.; Zhou, X.X. Quantifying the occurrence of polystyrene nanoplastics in environmental solid matrices via pyrolysis-gas chromatography/mass spectrometry. J. Hazard. Mater. 2022, 440, 129855. [Google Scholar] [CrossRef]
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N.; et al. Microplastics and nanoplastics in the marine-atmosphere environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Jebashalomi, V.; Charles, P.E.; Rajaram, R.; Sadayan, P. A critical review on nanoplastics and its future perspectives in the marine environment. Environ. Monit. Assess. 2023, 195, 1186. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; Alvarez-Méndez, S.J.; González-Sálamo, J.; Socas-Hernández, C.; Díaz-Peña, F.J.; Hernández-Sánchez, C.; Hernández-Borges, J. Nanoplastics in the soil environment: Analytical methods, occurrence, fate and ecological implications. Environ. Pollut. 2023, 317, 120788. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zuo, R.; Shang, J.H.; Wu, G.L.; Dong, Y.N.; Zheng, S.D.; Xu, Z.R.; Liu, J.C.; Xu, Y.X.; Wu, Z.Y.; et al. Nano- and micro-plastic transport in soil and groundwater environments: Sources, behaviors, theories, and models. Sci. Total Environ. 2023, 904, 166641. [Google Scholar] [CrossRef]
- Zhang, D.M.; Chen, Q.Q.; Xu, T.; Yin, D.Q. Current research status on the distribution and transport of micro (nano) plastics in hyporheic zones and groundwater. J. Environ. Sci. 2025, 151, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Gedik, K.; Gaga, E.O. Atmospheric micro (nano) plastics: Future growing concerns for human health. Air Qual. Atmos. Health 2023, 16, 233–262. [Google Scholar] [CrossRef]
- Permana, R.; Chakraborty, S.; Valsami-Jones, E. Nanoplastics in aquatic environments: The hidden impact of aging on fate and toxicity. Environ. Chem. Ecotoxicol. 2025, 7, 429–444. [Google Scholar] [CrossRef]
- Pradel, A.; Catrouillet, C.; Gigault, J. The environmental fate of nanoplastics: What we know and what we need to know about aggregation. Nanoimpact 2023, 29, 100453. [Google Scholar] [CrossRef]
- Singh, N.; Tiwari, E.; Khandelwal, N.; Darbha, G.K. Understanding the stability of nanoplastics in aqueous environments: Effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals. Environ. Sci.-Nano 2019, 6, 2968–2976. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ye, Q.Y.; Chen, M.Q.; Sun, L.Y.; Liu, S.; Liu, L.Q.; Wu, P.X.; Zhu, N.W. Nanoplastics enhance the stability of kaolinite and affect the sorption of Pb2in aquatic environments. Chem. Eng. J. 2024, 491, 152123. [Google Scholar] [CrossRef]
- Zhou, X.P.; Li, A.Z.; Cerne, M.; Macrae, S.; Eggleston, I.; Qiao, H.T.; Li, X.Y.; Huang, G.Y.; Wang, P.; Zhao, J.; et al. Nanoplastic-mineral heteroaggregation under varying degrees of plastic pollution: Implications for antibiotic adsorption in aquatic systems. Chem. Eng. J. 2025, 503, 158444. [Google Scholar] [CrossRef]
- Natarajan, L.; Jenifer, M.A.; Chandrasekaran, N.; Suraishkumar, G.K.; Mukherjee, A. Polystyrene nanoplastics diminish the toxic effects of Nano-TiO2 in marine algae Chlorella sp. Environ. Res. 2022, 204, 112400. [Google Scholar]
- Wang, Y.; Wang, K.Y.; Yang, J.B.; Dai, M.Q.; Zeng, D.J.; Wang, X.H.; Du, J.J.; Pu, G.Z. Synergistic effects of nanoplastics and graphene oxides on microbe-driven litter decomposition in streams. J. Hazard. Mater. 2025, 494, 138613. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Darbha, G.K. Impact of Minerals (Ferrihydrite and Goethite) and Their Organo-Mineral Complexes on Fate and Transport of Nanoplastics in the Riverine and Terrestrial Environments. Environ. Sci. Technol. 2025, 59, 11205–11215. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Chowdhury, I. Aggregation and stability of nanoscale plastics in aquatic environment. Water Res. 2020, 171, 115401. [Google Scholar] [CrossRef]
- Cao, T.C.; Zhao, M.T.; Zhang, T.; Chen, W. Weathering pathways differentially affect colloidal stability of nanoplastics. Environ. Sci.-Nano 2025, 12, 232–240. [Google Scholar] [CrossRef]
- Zhou, X.; Eggleston, I.; MacRae, S.; Cerne, M.; Ma, C.; Li, X.; Qiao, H.; Zhao, J.; Xing, B. Interactions between Nanoplastics and Antibiotics: Implications for Nanoplastics Aggregation in Aquatic Environments. Environ. Sci. Technol. 2025, 59, 11261–11274. [Google Scholar] [CrossRef]
- Ali, I.; Tan, X.; Li, J.Y.; Peng, C.S.; Naz, I.; Duan, Z.P.; Ruan, Y.L. Interaction of microplastics and nanoplastics with natural organic matter (NOM) and the impact of NOM on the sorption behavior of anthropogenic contaminants—A critical review. J. Clean. Prod. 2022, 376, 134314. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Z.; Xi, M.; Ma, H.; Qin, J.; Jia, H. Molecular modeling to elucidate the dynamic interaction process and aggregation mechanism between natural organic matters and nanoplastics. Eco-Environ. Health 2025, 4, 100122. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Jiang, R.F.; Liu, Q.L.; Ouyang, G.F. Impact of different modes of adsorption of natural organic matter on the environmental fate of nanoplastics. Chemosphere 2021, 263, 127967. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Wang, X.T.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Natural Organic Matter Stabilizes Pristine Nanoplastics but Destabilizes Photochemical Weathered Nanoplastics in Monovalent Electrolyte Solutions. Environ. Sci. Technol. 2025, 59, 1822–1834. [Google Scholar] [CrossRef]
- Simpson, K.; Martin, L.; O’Leary, S.L.; Watt, J.; Moon, S.; Luo, T.F.; Xu, W. Environmental protein corona on nanoplastics altered the responses of skin keratinocytes and fibroblast cells to the particles. J. Hazard. Mater. 2025, 494, 138722. [Google Scholar] [CrossRef]
- Zhou, J.L.; Chen, M.Y.; Li, Y.; Wang, J.J.; Chen, G.L.; Wang, J. Microbial bioremediation techniques of microplastics and nanoplastics in the marine environment. TrAC Trends Anal. Chem. 2024, 180, 117971. [Google Scholar] [CrossRef]
- ter Halle, A.; Ghiglione, J.F. Nanoplastics: A Complex, Polluting Terra Incognita. Environ. Sci. Technol. 2021, 55, 14466–14469. [Google Scholar] [CrossRef]
- Gigault, J.; Davranche, M. Nanoplastics in focus: Exploring interdisciplinary approaches and future directions. Nanoimpact 2025, 37, 100544. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics-Organic Chemical Contaminants Interactions: A Review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Prajapati, A.; Vaidya, A.N.; Kumar, A.R. Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environ. Sci. Pollut. Res. 2022, 29, 49490–49512. [Google Scholar] [CrossRef]
- Yu, Y.M.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Trevisan, R.; Ranasinghe, P.; Jayasundara, N.; Di Giulio, R.T. Nanoplastics in Aquatic Environments: Impacts on Aquatic Species and Interactions with Environmental Factors and Pollutants. Toxics 2022, 10, 326. [Google Scholar] [CrossRef]
- Blancho, F.; Davranche, M.; Léon, A.; Marsac, R.; Reynauld, S.; Grassl, B.; Gigault, J. Mechanistic description of lead sorption onto nanoplastics. Environ. Sci.-Nano 2024, 11, 1671–1681. [Google Scholar] [CrossRef]
- García-Hernández, E.; Torres, F.J.; Cortés-Arriagada, D.; Nochebuena, J. Understanding the co-adsorption mechanism between nanoplastics and neonicotinoid insecticides from an atomistic perspective. J. Mol. Model. 2025, 31, 140. [Google Scholar] [CrossRef]
- Pokhrel, A.; Islam, M.S.; Mitra, S. Generation of Eroded Nanoplastics from Real World Wastes and Their Capacity for Heavy Metal Adsorption. ACS EST Water 2025, 5, 2291–2299. [Google Scholar] [CrossRef]
- Chen, C.Z.; Sun, C.X.; Wang, B.; Zhang, Z.G.; Yu, G. Adsorption behavior of triclosan on polystyrene nanoplastics: The roles of particle size, surface functionalization, and environmental factors. Sci. Total Environ. 2024, 906, 167430. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P.; Duval, J.F.L. Sorption kinetics of metallic and organic contaminants on micro- and nanoplastics: Remarkable dependence of the intraparticulate contaminant diffusion coefficient on the particle size and potential role of polymer crystallinity. Environ. Sci.-Process. Impacts 2025, 27, 634–648. [Google Scholar] [CrossRef]
- Nurain, A.; Zhang, Y.; Meier, D.; Farner, J.M.; Goss, G.; Arlos, M.J. Sorption Behavior of Trace Organic Chemicals on Carboxylated Polystyrene Nanoplastics. ACS EST Water 2024, 4, 4018–4027. [Google Scholar] [CrossRef]
- Oliveira, Y.M.; Vernin, N.S.; Maia Bila, D.; Marques, M.; Tavares, F.W. Pollution caused by nanoplastics: Adverse effects and mechanisms of interaction via molecular simulation. PeerJ 2022, 10, e13618. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, Y.; Xu, Y.; Li, S.; Liu, X.; Dai, Y.; Zhao, J.; Yue, T. Benzo[a]pyrene and heavy metal ion adsorption on nanoplastics regulated by humic acid: Cooperation/competition mechanisms revealed by molecular dynamics simulations. J. Hazard. Mater. 2022, 424, 127431. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.C.; Zhao, J.H.; Li, L.Q.; Wang, Y.Y.; Dai, X.H.; Yu, F.; Ma, J. Interfacial interaction between micro/nanoplastics and typical PPCPs and nanoplastics removal via electrosorption from an aqueous solution. Water Res. 2020, 184, 116100. [Google Scholar] [CrossRef]
- Xu, Y.H.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of Micro- and Nanoplastics: Physical, Chemical, and Biological Effects in Environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef]
- Ma, L.; Fan, Z.Y.; Lian, W.Q.; Wei, X.F.; Bao, R.Y.; Yang, W. Nanoplastics and microplastics released from an enzyme-embedded biodegradable polyester during hydrolysis. J. Hazard. Mater. 2025, 489, 137640. [Google Scholar] [CrossRef]
- Mendez, N.F.; Sharma, V.; Valsecchi, M.; Pai, V.; Lee, J.K.; Schadler, L.S.; Muller, A.J.; Watson-Sanders, S.; Dadmun, M.; Kumaraswamy, G.; et al. Mechanism of quiescent nanoplastic formation from semicrystalline polymers. Nat. Commun. 2025, 16, 3051. [Google Scholar] [CrossRef]
- Süssmann, J.; Walz, E.; Hetzer, B.; Greiner, R.; Fischer, E.K.; Rohn, S.; Fritsche, J. Pressure-assisted isolation of micro- and nanoplastics from food of animal origin with special emphasis on seafood. J. Consum. Prot. Food Saf. 2025, 20, 141–154. [Google Scholar] [CrossRef]
- Kousheh, S.; Lin, M.S. Recent advancements in SERS-based detection of micro- and nanoplastics in food and beverages: Techniques, instruments, and machine learning integration. Trends Food Sci. Tech. 2025, 159, 104940. [Google Scholar] [CrossRef]
- Zimmermann, L.; Geueke, B.; Parkinson, L.V.; Schuer, C.; Wagner, M.; Muncke, J. Food contact articles as source of micro- and nanoplastics: A systematic evidence map. Npj Sci. Food 2025, 9, 111. [Google Scholar] [CrossRef]
- Duda, A.; Petka, K. The Presence of Micro-and Nanoplastics in Food and the Estimation of the Amount Consumed Depending on Dietary Patterns. Molecules 2025, 30, 3666. [Google Scholar] [CrossRef]
- Palanisamy, S.; Kumar, B.K.S.; Vetrivel, A.; Michael, R.J.; Babu, N.; Nallamuthu, S.S.; Saravanan, K.; Venkatachalam, S.; Kumar, R.J.N.; Selvaraju, G.D. Nanoplastics in heat-sensitive food packaging: A review of migration, detection, health, and environmental impacts. Food Control 2025, 169, 111002. [Google Scholar] [CrossRef]
- Atugoda, T.; Piyumali, H.; Wijesekara, H.; Sonne, C.; Lam, S.S.; Mahatantila, K.; Vithanage, M. Nanoplastic occurrence, transformation and toxicity: A review. Environ. Chem. Lett. 2023, 21, 363–381. [Google Scholar] [CrossRef]
- Haldar, S.; Muralidaran, Y.; Miguez, D.; Mulla, S.I.; Mishra, P. Eco-toxicity of nano-plastics and its implication on human metabolism: Current and future perspective. Sci. Total Environ. 2023, 861, 160571. [Google Scholar] [CrossRef]
- Thakur, R.; Joshi, V.; Sahoo, G.C.; Jindal, N.; Tiwari, R.R.; Rana, S. Review of mechanisms and impacts of nanoplastic toxicity in aquatic organisms and potential impacts on human health. Toxicol. Rep. 2025, 14, 102013. [Google Scholar] [CrossRef]
- Kazmi, S.; Tayyab, M.; Pastorino, P.; Barcelo, D.; Yaseen, Z.M.; Grossart, H.P.; Khan, Z.H.; Li, G. Decoding the molecular concerto: Toxicotranscriptomic evaluation of microplastic and nanoplastic impacts on aquatic organisms. J. Hazard. Mater. 2024, 472, 134574. [Google Scholar] [CrossRef]
- Li, J.C.; Zhao, Y.C. Bioeffects of Nanoplastics: DNA Damage and Mechanism. Nano Lett. 2025, 25, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Xu, D.H.; Zhao, Y.M.; Sheng, B.; Wu, Z.J.; Wen, X.B.; Zhou, J.; Chen, G.; Lv, J.; Wang, J.; et al. Micro/Nanoplastics in plantation agricultural products: Behavior process, phytotoxicity under biotic and abiotic stresses, and controlling strategies. J. Nanobiotechnol. 2025, 23, 231. [Google Scholar] [CrossRef]
- Ruggieri, F.; Battistini, B.; Sorbo, A.; Senofonte, M.; Leso, V.; Iavicoli, I.; Bocca, B. From food-to-human microplastics and nanoplastics exposure and health effects: A review on food, animal and human monitoring data. Food Chem. Toxicol. 2024, 196, 115209. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, M.Y.; Wang, W.J.; Liu, W.J.; Liao, H.P.; Li, Z.; Wang, J. The direct effects of micro- and nanoplastics on rice and wheat. Trac-Trends Anal. Chem. 2024, 180, 117976. [Google Scholar] [CrossRef]
- Lazar, N.N.; Calmuc, M.; Milea, S.A.; Georgescu, P.L.; Iticescu, C. Micro and nano plastics in fruits and vegetables: A review. Heliyon 2024, 10, e28291. [Google Scholar] [CrossRef] [PubMed]
- Boctor, J.; Hoyle, F.C.; Farag, M.A.; Ebaid, M.; Walsh, T.; Whiteley, A.S.; Murphy, D.V. Microplastics and nanoplastics: Fate, transport, and governance from agricultural soil to food webs and humans. Environ. Sci. Eur. 2025, 37, 68. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, M.; Li, R.; Peijnenburg, W.J.; Yang, L.; Liu, P.; Shi, Q. Transport Dynamics and Physiological Responses of Polystyrene Nanoplastics in Pakchoi: Implications for Food Safety and Environmental Health. J. Agric. Food. Chem. 2025, 73, 10923–10933. [Google Scholar] [CrossRef]
- Zytowski, E.; Mollavali, M.; Baldermann, S. Uptake and translocation of nanoplastics in mono and dicot vegetables. Plant Cell Environ. 2025, 48, 134–148. [Google Scholar] [CrossRef]
- Li, C.Y.; Ma, C.X.; Shang, H.P.; White, J.C.; Cai, Z.Y.; Hao, Y.; Xu, X.X.; Liang, A.Q.; Jia, W.L.; Cao, Y.N.; et al. Polystyrene Nanoplastics Compromise the Nutritional Value of Radish (Raphanus sativus L.). Environ. Sci. Technol. 2025, 59, 9730–9743. [Google Scholar] [CrossRef]
- Kim, D.; Kweon, H.S.; An, Y.J. Grandparental transfer of nanoplastics in pea plants (Pisum sativum): Transmission from soil to third generations. J. Hazard. Mater. 2025, 492, 138198. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.Y.; Ma, Y.P.; Zhang, J.; Ma, L.A.; Chen, W.Q.; Tang, X.J.; Lu, J.X.; Chen, L.Z.; Cai, G.D.; et al. Untargeted Metabolomics Uncovers Food Safety Risks: Polystyrene Nanoplastics Induce Metabolic Disorders in Chicken Liver. Foods 2025, 14, 2781. [Google Scholar] [CrossRef] [PubMed]
- Pause, F.C.; Baufeld, A.; Urli, S.; Crociati, M.; Stradaioli, G.; Vanselow, J.; Kalbe, C. Exploring the influence of polystyrene-nanoplastics on two distinct in vitro systems in farm animals: A pilot study. Sci. Total Environ. 2025, 976, 179378. [Google Scholar] [CrossRef]
- Seref, N.; Cufaoglu, G. Food Packaging and Chemical Migration: A Food Safety Perspective. J. Food Sci. 2025, 90, e70265. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.Z.; Ding, Y.L.; Zeng, H.X.; Zhong, Y.F.; Zhang, H.Y.; Chen, Y.X.; Xu, X.L.; Wei, W. Effects of chronic co-exposure polystyrene nanoplastics and cadmium on liver function in Prussian carp (Carassius gibelio). Ecotox Environ. Safe 2025, 302, 118687. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Beitsayah, A.; Multisanti, C.R.; Faggio, C. Combined Effects of Nano-Polystyrene and Heavy Metal Mixture on the Bioaccumulation of Heavy Metals and Physiological Changes in Macrobrachium rosenbergii. J. Xenobiot. 2025, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Che, S.L.; Huang, M.T.; Ma, H.Y.; Wan, Z.C.; Feng, J.B.; Ding, S.Q.; Li, X.L. Toxic effects of nanopolystyrene and cadmium on the intestinal tract of the Chinese mitten crab (Eriocheir sinensis). Ecotox Environ. Safe 2024, 270, 115936. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Y.; Fan, Q.; Che, S.; Zhang, J.; Ding, S.; Zhu, S.; Li, X. Effects of nanopolystyrene and/or phoxim exposure on digestive function of Eriocheir sinensis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 289, 110102. [Google Scholar] [CrossRef]
- Huang, P.; Cao, L.P.; Du, J.L.; Guo, Y.Q.; Li, Q.J.; Sun, Y.; Zhu, H.J.; Xu, G.C.; Gao, J.C. Polystyrene nanoplastics amplify the toxic effects of PFOA on the Chinese mitten crab (Eriocheir sinensis). J. Hazard. Mater. 2025, 488, 137488. [Google Scholar] [CrossRef]
- Shi, B.D.; Xu, T.; Chen, T.; Xu, S.W.; Yao, Y.J. Co-exposure of decabromodiphenyl ethane and polystyrene nanoplastics damages grass carp (Ctenopharyngodon idell) hepatocytes: Focus on the role of oxidative stress, ferroptosis, and inflammatory reaction. Sci. Total Environ. 2024, 940, 173575. [Google Scholar] [CrossRef]
- Chen, T.T.; Jiang, H.W.; He, Y.J.; Shen, Y.W.; Huang, Z.Q.; Gu, Y.F.; Wei, Q.; Zhao, J.L.; Chen, X.W. Nanoplastics and chrysene pollution: Potential new triggers for nonalcoholic fatty liver disease and hepatitis, insights from juvenile Siniperca chuatsi. Sci. Total Environ. 2024, 922, 171125. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.J.; Gao, J.C.; Jin, W.; Hu, J.W.; Sun, Y.; Zhu, H.J.; Xu, G.C. Apoptosis, MAPK signaling pathway affected in tilapia liver following nano-microplastics and sulfamethoxazole acute co-exposure. Comp. Biochem. Phys. D 2025, 53, 101370. [Google Scholar] [CrossRef]
- Dai, Y.H.; Zhang, X.X.; Chen, X.Y.; Sun, C.X.; Lan, R.Y.; Fan, H.Z.; Liu, Z.M.; Liu, X.; Yue, T.T.; Zhao, J. Antagonistic toxicity of nanoplastics and perfluorobutanoic acid to the behavior of black rockfish (Sebastes schlegelii). Environ. Pollut. 2025, 381, 126578. [Google Scholar] [CrossRef]
- Lin, P.; Liu, L.; Ma, Y.; Du, R.; Yi, C.; Li, P.; Xu, Y.; Yin, H.; Sun, L.; Li, Z.-H. Neurobehavioral toxicity induced by combined exposure of micro/nanoplastics and triphenyltin in marine medaka (Oryzias melastigma). Environ. Pollut. 2024, 356, 124334. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Xu, B.; Guo, F.; Zhu, M.; Yang, R. Co-exposure to polystyrene nanoplastics and mercury synergistically exacerbates toxicity in rare minnow (Gobiocypris rarus) compared to individual exposures. Aquat. Toxicol. 2025, 285, 107416. [Google Scholar] [CrossRef] [PubMed]
- Naziri, A.; Mina, T.; Manoli, K.; Beretsou, V.G.; Christou, A.; Michael, C.; Agathokleous, E.; Fatta-Kassinos, D. Looking into the effects of co-contamination by micro(nano)plastics in the presence of other pollutants on irrigated edible plants. Sci. Total Environ. 2023, 892, 164618. [Google Scholar] [CrossRef]
- Bui, T.H.; Zuverza-Mena, N.; Kendrick, E.; Tamez, C.; Yadav, M.; Alotaibi, S.; Dimkpa, C.; Deloid, G.; Sadik, O.; Demokritou, P.; et al. Micro-nanoscale polystyrene co-exposure impacts the uptake and translocation of arsenic and boscalid by lettuce (Lactuca sativa). Nanoimpact 2025, 37, 100541. [Google Scholar] [CrossRef]
- Bryant, M.T.; Rossi, L.; Mu, R.P.; Cao, Z.Y.; Ma, X.M. Synergistic Effects of Polystyrene Nanoplastics and Cadmium on the Metabolic Processes and Their Accumulation in Hydroponically Grown Lettuce (Lactuca sativa). J. Agric. Food. Chem. 2025, 73, 16157–16164. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.T.; Ren, J.; Sharma, V.K.; Ma, X. Mutual Effects and Uptake of Organic Contaminants and Nanoplastics by Lettuce in Co-Exposure. ACS Agric. Sci. Technol. 2024, 4, 463–470. [Google Scholar] [CrossRef]
- Liang, Y.B.; Liu, X.K.; Jiang, J.G.; Zhai, W.J.; Guo, Q.Q.; Guo, H.M.; Xiao, S.C.; Ling, F.; Zhou, Z.Q.; Liu, D.H.; et al. Nanoplastics enhance tebuconazole toxicity in lettuce by promoting its accumulation and disrupting phenylalanine metabolism: Importance of Trojan horse effect. J. Hazard. Mater. 2025, 489, 137538. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; Maestri, G.; Tochetto, G.A.; de Oliveira, J.L.; Stiegelmaier, E.; Fischer, T.V.; Immich, A.P.S. Nanoplastics: Unveiling Contamination Routes and Toxicological Implications for Human Health. Curr. Anal. Chem. 2024, 21, 175–190. [Google Scholar] [CrossRef]
- Ma, Q.; Lei, J.; Pang, Y.; Shen, Y.; Zhang, T. Neurotoxicity of Micro-and Nanoplastics: A Comprehensive Review of Central Nervous System Impacts. Environ. Health 2025, 87. [Google Scholar] [CrossRef]
- Stock, V.; Laurisch, C.; Franke, J.; Donmez, M.H.; Voss, L.; Bohmert, L.; Braeuning, A.; Sieg, H. Uptake and cellular effects of PE, PP, PET and PVC microplastic particles. Toxicol. Vitr. 2021, 70, 105021. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Y.; Wang, L.; Ruan, X.; Ge, Q.; Ma, M.; Wang, W.; You, W.; Zhang, L.; Valev, V.K. MPs Entering Human Circulation through Infusions: A Significant Pathway and Health Concern. Environ. Health 2025, 3, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Roursgaard, M.; Rothmann, M.H.; Schulte, J.; Karadimou, I.; Marinelli, E.; Moller, P. Genotoxicity of Particles From Grinded Plastic Items in Caco-2 and HepG2 Cells. Front. Public Health 2022, 10, 906430. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.X.; Min, W.C.; Zhong, H.Q.; Yan, X.L.; Gao, Y.; Wang, J.Q.; Zhou, H.Y.; Yan, B. Inflammatory responses induced by synergistic actions between nanoplastics and typical heavy metal ions in human cells. Environ. Sci.-Nano 2023, 10, 1599–1613. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Yu, Z.; He, F.; Zhao, X.; Liu, R. New Evidence for the Mechanisms of Nanoplastics Amplifying Cadmium Cytotoxicity: Trojan Horse Effect, Inflammatory Response, and Calcium Imbalance. Environ. Sci. Technol. 2025, 59, 9471–9485. [Google Scholar] [CrossRef]
- Alijagic, A.; Särndahl, E.; Kotlyar, O.; Karlsson, P.; Duberg, D.; Scherbak, N.; Pinsino, A.; Engwall, M.; Hyötyläinen, T. Nanoplastics drive toxicity under co-exposure with perfluorooctanesulfonic acid in human intestinal cells. Environ. Chem. Lett. 2025, 23, 1161–1169. [Google Scholar] [CrossRef]
- Gou, Z.X.; Wu, H.A.; Li, S.Y.; Liu, Z.Y.; Zhang, Y. Airborne micro- and nanoplastics: Emerging causes of respiratory diseases. Part. Fibre Toxicol. 2024, 21, 50. [Google Scholar] [CrossRef]
- Lagana, A.; Visalli, G.; Facciola, A.; Celesti, C.; Iannazzo, D.; Di Pietro, A. Uptake of Breathable Nano- and Micro-Sized Polystyrene Particles: Comparison of Virgin and Oxidised nPS/mPS in Human Alveolar Cells. Toxics 2023, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Simpson, K.; Brzezinski, M.; Watt, J.; Xu, W. Cellular response of keratinocytes to the entry and accumulation of nanoplastic particles. Part. Fibre Toxicol. 2024, 21, 22. [Google Scholar] [CrossRef]
- Annangi, B.; Villacorta, A.; Vela, L.; Tavakolpournegari, A.; Marcos, R.; Hernández, A. Effects of true-to-life PET nanoplastics using primary human nasal epithelial cells. Environ. Toxicol. Pharmacol. 2023, 100, 104140. [Google Scholar] [CrossRef]
- Monikh, F.A.; Lehtonen, S.; Kekäläinen, J.; Karkossa, I.; Auriola, S.; Schubert, K.; Zanut, A.; Peltonen, S.; Niskanen, J.; Bandekar, M.; et al. Biotransformation of nanoplastics in human plasma and their permeation through a model in vitro blood-brain barrier: An in-depth quantitative analysis. Nano Today 2024, 59, 102466. [Google Scholar] [CrossRef]
- Cheng, S.Q.; Hu, J.J.; Guo, C.; Ye, Z.C.; Shang, Y.Z.; Lian, C.; Liu, H.L. The effects of size and surface functionalization of polystyrene nanoplastics on stratum corneum model membranes: An experimental and computational study. J. Colloid. Interface Sci. 2023, 638, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, S.; Jia, X.; Zhu, X.; Cai, L.; Duan, M.; Wang, S.; Jiang, H.; Ji, M.; Wang, S.; et al. Combined toxicity evaluation of polystyrene nanoplastics and Nano-ZnO of distinctive morphology on human lung epithelial cells. Sci. Total Environ. 2025, 973, 179097. [Google Scholar] [CrossRef]
- Morataya-Reyes, M.; Villacorta, A.; Gutierrez-Garcia, J.; Egea, R.; Martin-Perez, J.; Barguilla, I.; Marcos, R.; Hernandez, A. The long-term in vitro co-exposure of polyethylene terephthalate (PET) nanoplastics and cigarette smoke condensate exacerbates the induction of carcinogenic traits. J. Hazard. Mater. 2025, 493, 138359. [Google Scholar] [CrossRef]
- Aloisi, M.; Poma, A.M.G. Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective. Int. J. Mol. Sci. 2025, 26, 2071. [Google Scholar] [CrossRef]
- Fusco, L.; Gazzi, A.; Giro, L.; Schefer, R.B.; D’Almeida, S.M.; Cagliani, R.; Zoccheddu, M.; Uyar, R.; Besbinar, O.; Çelik, D.; et al. Nanoplastics: Immune Impact, Detection, and Internalization after Human Blood Exposure by Single-Cell Mass Cytometry. Adv. Mater. 2025, 37, 2413413. [Google Scholar] [CrossRef]
- Bojic, S.; Falco, M.M.; Stojkovic, P.; Ljujic, B.; Jankovic, M.G.; Armstrong, L.; Markovic, N.; Dopazo, J.; Lako, M.; Bauer, R.; et al. Platform to study intracellular polystyrene nanoplastic pollution and clinical outcomes. Stem Cells 2020, 38, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Nacka-Aleksić, M.; Vilotić, A.; Pirković, A.; Živanović, M.; Ljujić, B.; Krivokuća, M.J. Nano-scale dangers: Unravelling the impact of nanoplastics on human trophoblast invasion. Chem. Biol. Interact. 2025, 405, 111317. [Google Scholar] [CrossRef]
- Balali, H.; Morabbi, A.; Karimian, M. Concerning influences of micro/nano plastics on female reproductive health: Focusing on cellular and molecular pathways from animal models to human studies. Reprod. Biol. Endocrinol. 2024, 22, 141. [Google Scholar] [CrossRef]
- Samaei, S.H.-A.; Mojahednia, P.; Chen, J.; Li, Z.; Jaszczyszyn, K.; Kiedrzyńska, E.; Xue, J. What Does the “Trojan Horse” Carry? The Pollutants Associated with Microplastics/Nanoplastics in Water Environments. ACS ES&T Water 2025, 5, 1530–1545. [Google Scholar]
- Zhang, M.; Xu, L.H. Transport of micro- and nanoplastics in the environment: Trojan-Horse effect for organic contaminants. Crit. Rev. Environ. Sci. Technol. 2022, 52, 810–846. [Google Scholar] [CrossRef]
- Mognetti, B.; Cecone, C.; Fancello, K.; Saraceni, A.; Cottone, E.; Bovolin, P. Interaction of Polystyrene Nanoplastics with Biomolecules and Environmental Pollutants: Effects on Human Hepatocytes. Int. J. Mol. Sci. 2025, 26, 2899. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Zhou, C.; Ma, Z.Q.; Zeng, L.J.; Wang, H.P.; Cheng, X.; Zhang, C.C.; Xue, Y.; Yuan, Y.Y.; Li, J.; et al. Co-exposure to polystyrene nanoplastics and triclosan induces synergistic cytotoxicity in human KGN granulosa cells by promoting reactive oxygen species accumulation. Ecotox Environ. Safe 2024, 273, 116121. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, Y.Y.; Liu, H.B.; Lan, J.; Li, Z.C.; Zong, W.S.; Zhao, Z.S. Co-Existing Nanoplastics Further Exacerbates the Effects of Triclosan on the Physiological Functions of Human Serum Albumin. Life 2025, 15, 112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).