Combined Pre- and Postharvest Melatonin Treatments Improve the Functional Quality of the Sweet Cherry cv. ‘Sunburst’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Sample Processing
2.3. Extraction and Quantification of Melatonin Content
2.4. Qualitative and Quantitative Analysis of Individual Phenolic Compounds
2.5. Statistical Analysis
3. Results
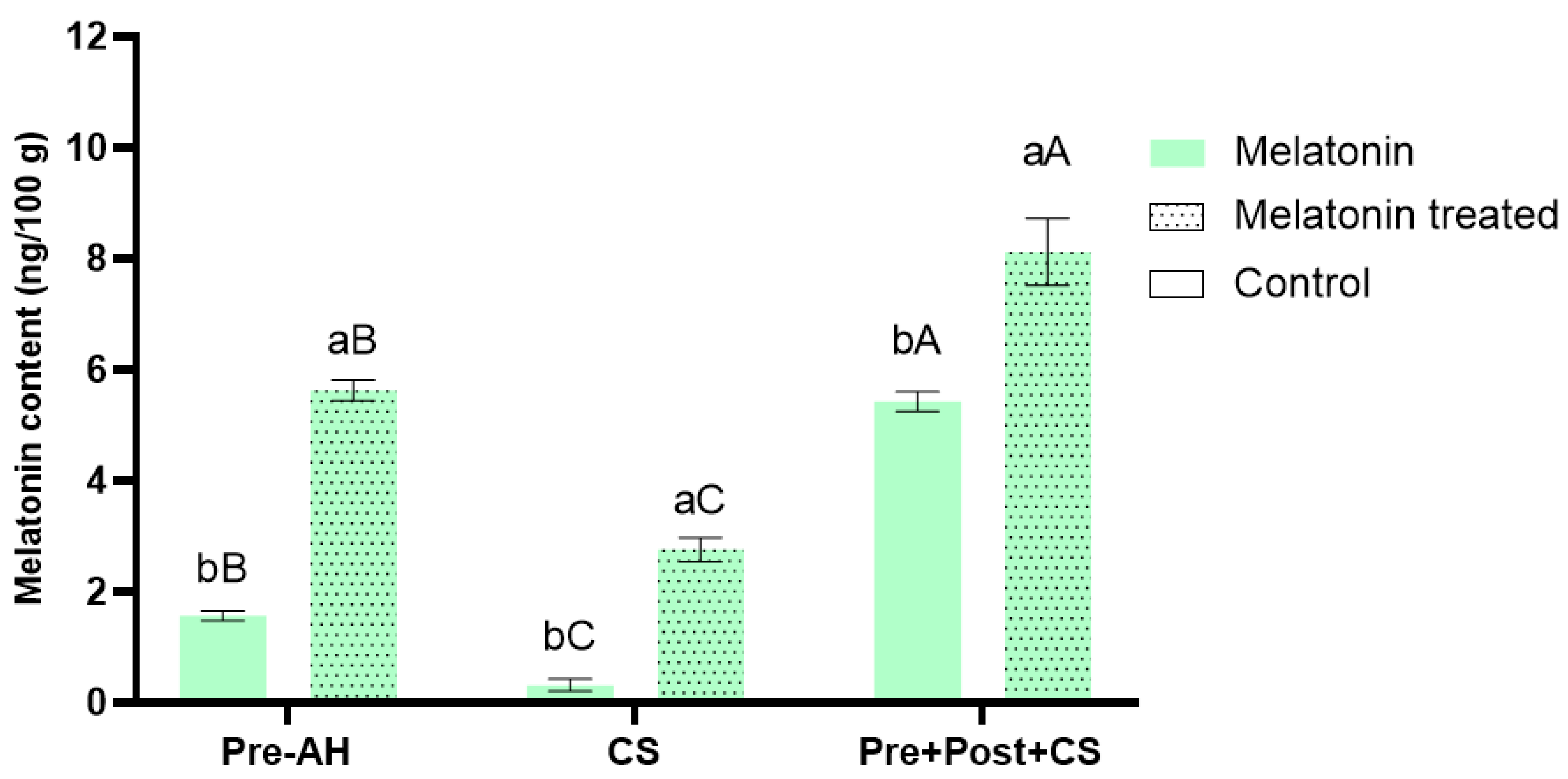
3.1. Impact of Melatonin Treatments on Melatonin Accumulation in Sweet Cherry Fruit
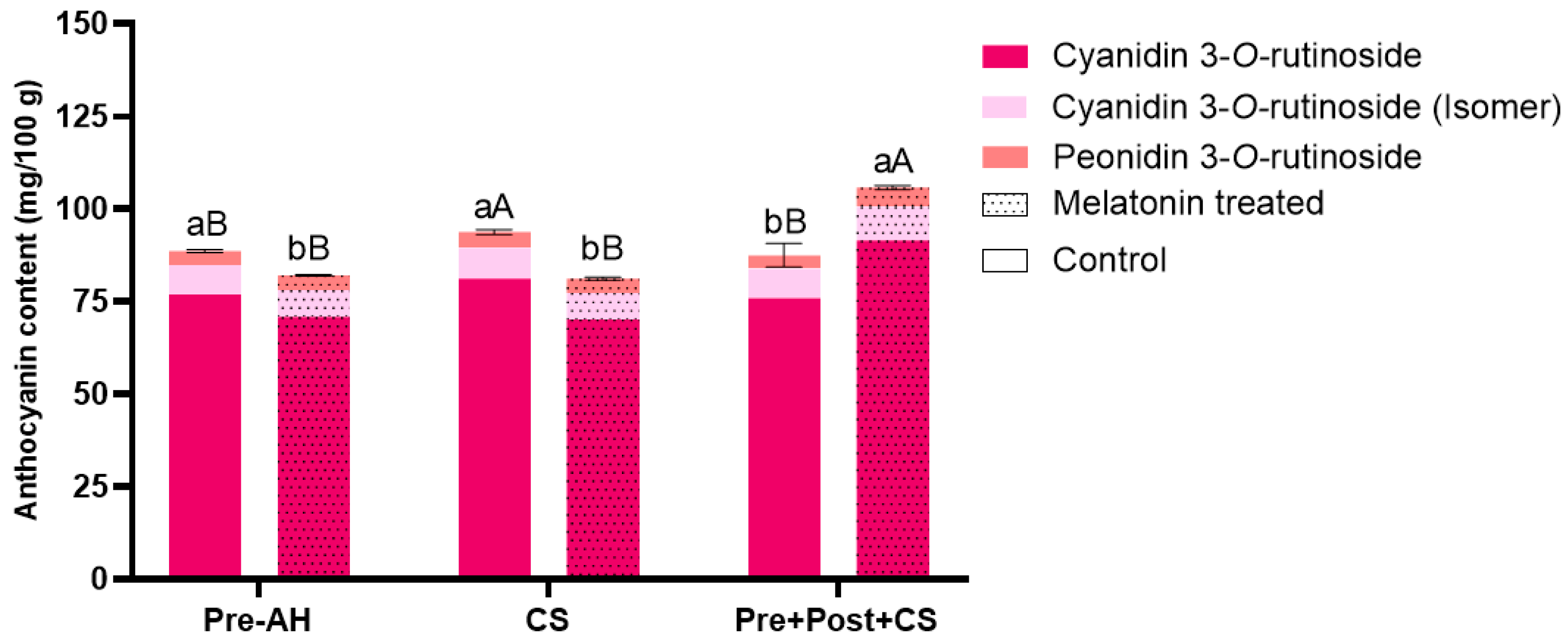
3.2. Impact of Melatonin Treatments on Anthocyanins Accumulation in Sweet Cherry Fruit
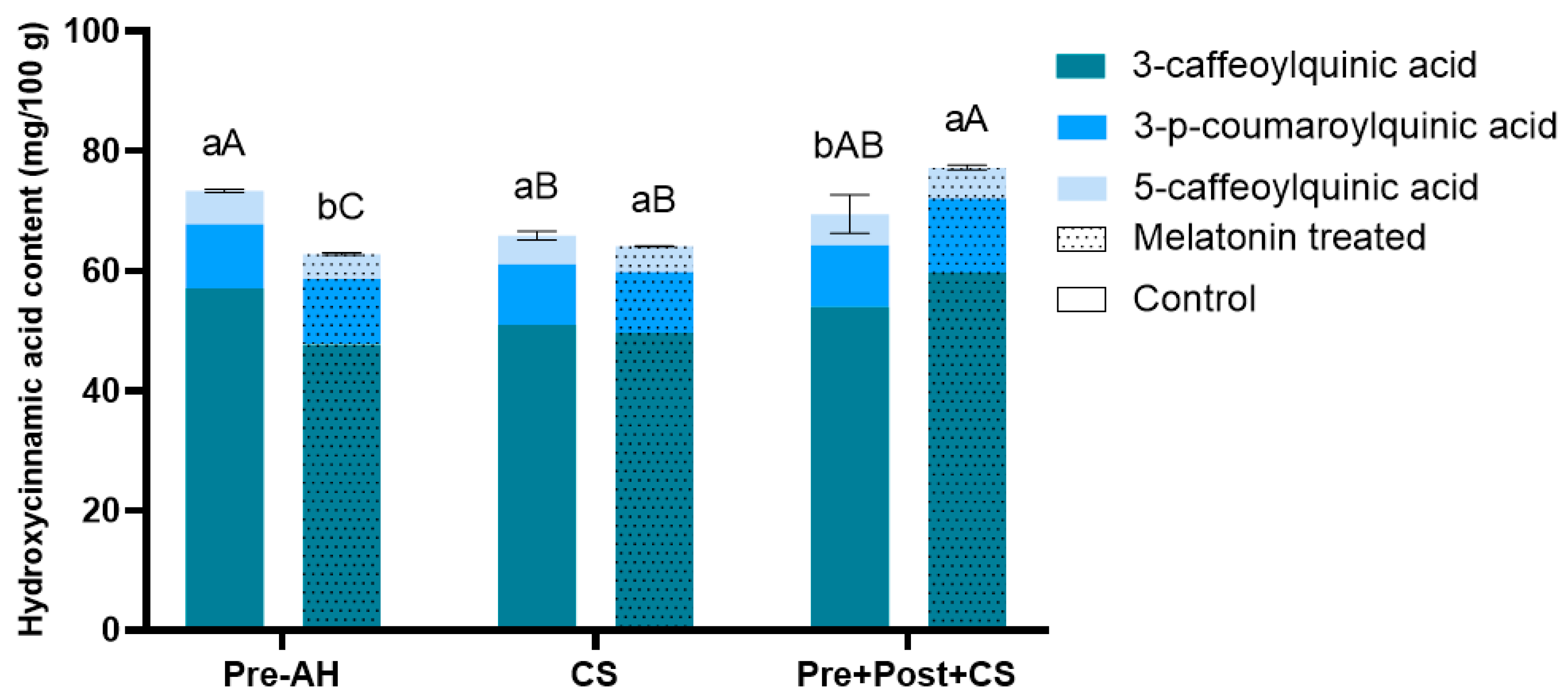
3.3. Impact of Melatonin Treatments on Hydroxycinnamic Acids Accumulation in Sweet Cherry Fruit
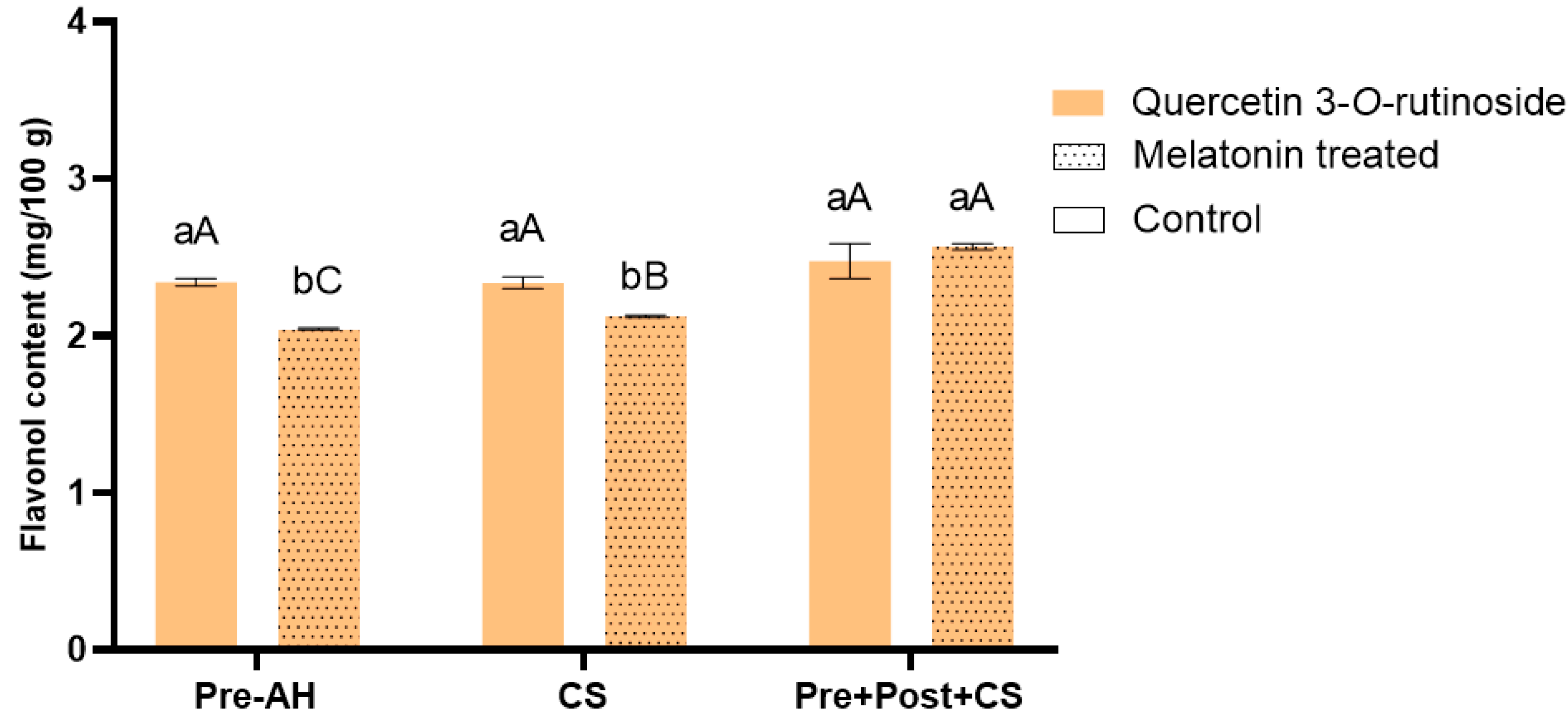
3.4. Impact of Melatonin Treatments on Flavonol Accumulation in Sweet Cherry Fruit
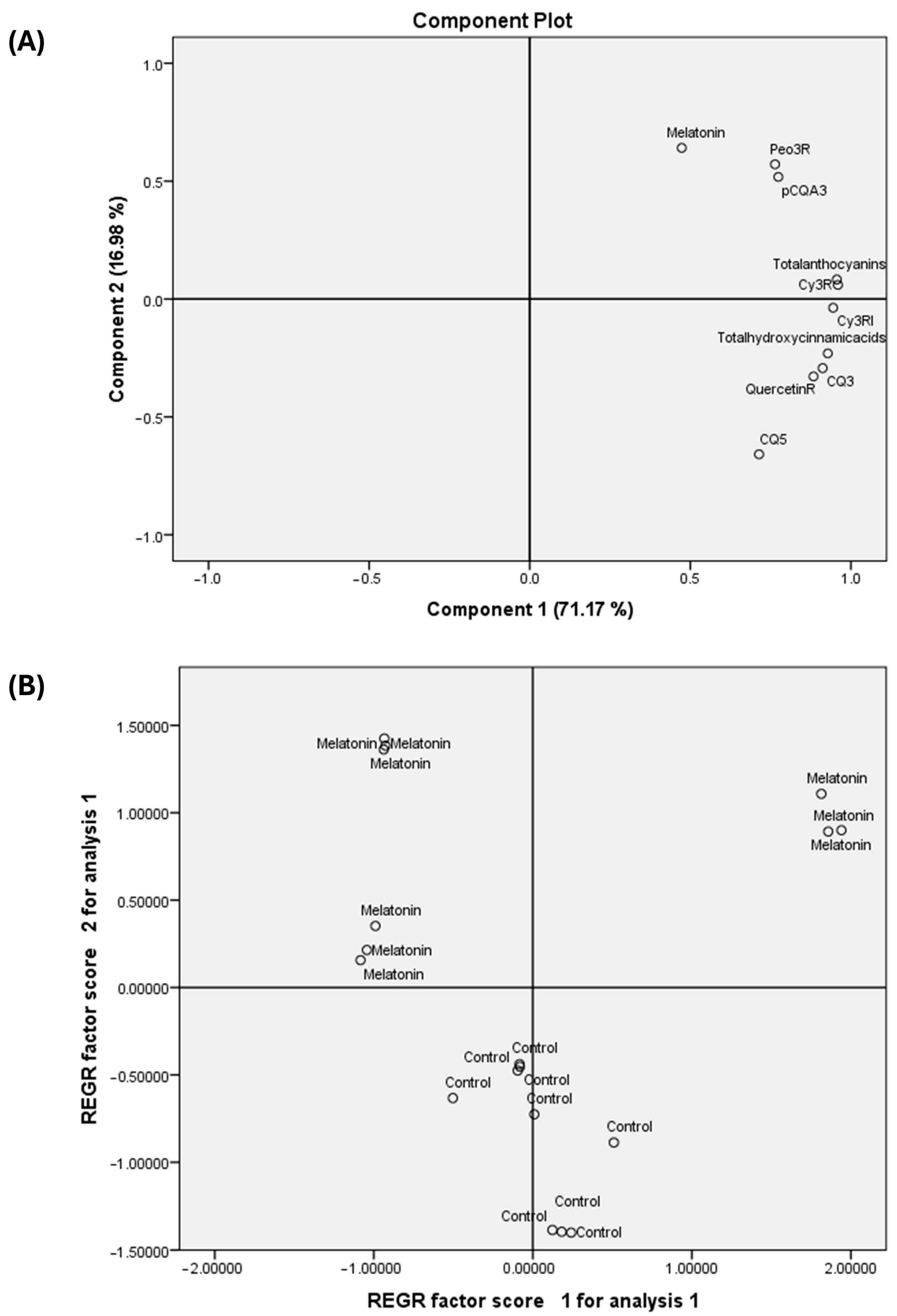
3.5. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirwan, D. Global health: Current issues, future trends and foreign policy. Clin. Med. 2009, 9, 247–253. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Evaluación de la capacidad nacional para la prevención y el control de enfermedades no transmisibles. In Informe de la Encuesta Mundial 2021; Organización Mundial de la Salud: Geneva, Switzerland, 2023; pp. 1–122. [Google Scholar]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sivapragasam, N.; Maurya, A.; Tiwari, S.; Dwivedy, A.K.; Thorakkattu, P.; Koirala, P.; Nirmal, N. Edible Berries- An Update on Nutritional Composition and Health Benefits-Part I. Curr. Nutr. Rep. 2025, 14, 7. [Google Scholar] [CrossRef]
- Fruta de Hueso. Campaña 2024. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/frutas-y-hortalizas/documentos-fruta-hueso-2024 (accessed on 12 August 2025).
- Panghal, A.; Kumar, N.; Kumar, S.; Kumari, A.; Chhikara, N. Food Function and Health Benefits of Functional Foods. Funct. Foods 2021, 1, 419–441. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Costa, A.R.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Anti-Inflammatory and Antiproliferative Properties of Sweet Cherry Phenolic-Rich Extracts. Molecules 2022, 27, 268. [Google Scholar] [CrossRef]
- Lane, W.D.; Schmid, H. Lapins and sunburst sweet cherry. Can. J. Plant Sci. 1983, 64, 211–214. [Google Scholar] [CrossRef]
- Jiménez, S.; Garín, A.; Albá, E.S.; Betrá, J.A.; Gogorcena, Y.; Moreno, M.A. Effect of several rootstocks on fruit quality of “sunburst” sweet cherry. Acta Hortic. 2004, 658, 353–358. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1–21. [Google Scholar] [CrossRef]
- Gonçalves, B.; Aires, A.; Oliveira, I.; Baltazar, M.; Cosme, F.; Afonso, S.; Pinto, T.; Anjos, M.R.; Inês, A.; Morais, M.C.; et al. From Orchard to Wellness: Unveiling the Health Effects of Sweet Cherry Nutrients. Nutrients 2024, 16, 3660. [Google Scholar] [CrossRef]
- Rossi, I.; Mignogna, C.; Del Rio, D.; Mena, P. Health effects of 100% fruit and vegetable juices: Evidence from human subject intervention studies. Nutr. Res. Rev. 2024, 37, 194–238. [Google Scholar] [CrossRef]
- Serrano, M.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valverde, J.M.; Valero, D. Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef]
- Boskov, D.; Milatovic, D.; Rakonjac, V.; Zec, G.; Hudina, M.; Veberic, R.; Mikulic-Petkovsek, M. The Phenolic Profile of Sweet Cherry Fruits Influenced by Cultivar/Rootstock Combination. Plants 2023, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, Y.; Jannatizadeh, A.; Fard, J.R.; Aghdam, M.S. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 2021, 288, 110304. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, Z.; Zhang, Y.; Shi, X.; Chen, F.; Liu, K. Effect of temperature fluctuation on colour change and softening of postharvest sweet cherry. RSC Adv. 2021, 11, 22969–22982. [Google Scholar] [CrossRef]
- Li, N.; Zhai, K.; Yin, Q.; Gu, Q.; Zhang, X.; Melencion, M.G.; Chen, Z. Crosstalk between melatonin and reactive oxygen species in fruits and vegetables post-harvest preservation: An update. Front. Nutr. 2023, 10, 1143511. [Google Scholar] [CrossRef] [PubMed]
- Agulló, V.; García-Pastor, M.E.; Valero, D. Melatonin Elicitation Differentially Enhances Flavanone and Its Endogenous Content in Lemon Tissues Through Preharvest and Postharvest Applications. Agronomy 2025, 15, 1233. [Google Scholar] [CrossRef]
- Garrido-Auñón, F.; Padilla-González, P.A.; Serrano, M.; Valero, D.; Agulló, V. Melatonin Boosts the Phytochemical Profile of Blood Oranges, Enhancing (Poly)phenol and Endogenous Melatonin Content, Through Pre- and Postharvest Treatments. J. Pineal Res. 2025, 77, e70078. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Velardo-Micharet, B.; Serrano, M.; Serradilla, M.J. Impact of Pre-Storage Melatonin Application on the Standard, Sensory, and Bioactive Quality of Early Sweet Cherry. Foods 2023, 12, 1723. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of melatonin treatment on sweet cherry tree yield and fruit quality. Agronomy 2022, 12, 3. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Hernández, I.; Ponce, E.; Vidal, J.; Chirinos, R.; Campos, D.; Pedreschi, R.; Fuentealba, C. Metabolomics Reveals Specific Metabolic Changes in Sweet Cherries (Prunus avium L.) Subjected to Postharvest Treatment with Melatonin after Mechanical Stress. Horticulturae 2023, 9, 940. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Michailidis, M.; Tanou, G.; Sarrou, E.; Karagiannis, E.; Ganopoulos, I.; Martens, S.; Molassiotis, A. Pre- and Post-harvest Melatonin Application Boosted Phenolic Compounds Accumulation and Altered Respiratory Characters in Sweet Cherry Fruit. Front. Nutr. 2021, 8, 695061. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin Pre-harvest Treatments Leads to Maintenance of Sweet Cherry Quality During Storage by Increasing Antioxidant Systems. Front. Plant Sci. 2022, 13, 863467. [Google Scholar] [CrossRef] [PubMed]
- Badiche-El Hilali, F.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds. Foods 2023, 12, 2979. [Google Scholar] [CrossRef]
- Badiche-El Hilali, F.; Medeiros-Fonseca, B.; Silva, J.; Silvestre-Ferreira, A.C.; Pires, M.J.; Gil da Costa, R.M.; Peixoto, F.; Oliveira, P.A.; Valero, D. The Effect of Lemon Juice (Citrus limon L.) Treated with Melatonin on the Health Status and Treatment of K14HPV16 Mice. Antioxidants 2024, 13, 588. [Google Scholar] [CrossRef]
- Guirao, A.; Martínez-Romero, D.; Solana-Guilabert, A.; Agulló, V.; Díaz-Mula, H.M.; Valverde, J.M. Influence of Preharvest sorbitol and calcium-sorbitol applications on the ripening process and anthocyanin biosynthesis in blood Orange (Citrus sinensis cv. Sanguinelli). Food Chem. 2025, 481, 144105. [Google Scholar] [CrossRef]
- Agulló, V.; Domínguez-Perles, R.; Moreno, D.A.; Zafrilla, P.; García-Viguera, C. Alternative sweeteners modify the urinary excretion of flavanones metabolites ingested through a new maqui-berry beverage. Foods 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Agulló, V.; González-Trujano, M.E.; Hernandez-Leon, A.; Estrada-Camarena, E.; Pellicer, F.; García-Viguera, C. Antinociceptive effects of maqui-berry (Aristotelia chilensis (Mol.) Stuntz). Int. J. Food Sci. Nutr. 2021, 72, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kamfar, W.W.; Khraiwesh, H.M.; Ibrahim, M.O.; Qadhi, A.H.; Azhar, W.F.; Ghafouri, K.J.; Alhussain, M.H.; Aldairi, A.F.; AlShahrani, A.M.; Alghannam, A.F.; et al. Comprehensive review of melatonin as a promising nutritional and nutraceutical supplement. Heliyon 2024, 10, e24266. [Google Scholar] [CrossRef] [PubMed]
- Savage, R.A.; Zafar, N.; Yohannan, S.; Miller, J.-M.M. Melatonin; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Melhuish Beaupre, L.M.; Brown, G.M.; Gonçalves, V.F.; Kennedy, J.L. Melatonin’s neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef]
- Bocheva, G.; Bakalov, D.; Iliev, P.; Tafradjiiska-Hadjiolova, R. The Vital Role of Melatonin and Its Metabolites in the Neuroprotection and Retardation of Brain Aging. Int. J. Mol. Sci. 2024, 25, 5122. [Google Scholar] [CrossRef]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodríguez, A.B. Detection and quantification of melatonin and serotonin in eight Sweet Cherry cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Rosado, T.; Henriques, I.; Gallardo, E.; Duarte, A.P. Determination of melatonin levels in different cherry cultivars by high-performance liquid chromatography coupled to electrochemical detection. Eur. Food Res. Technol. 2017, 243, 1749–1757. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef]
- Vuletić, M.V.; Dugalić, K.; Mihaljević, I.; Tomaš, V.; Vuković, D.; Zdunić, Z.; Puškar, B.; Jurković, Z. Season, location and cultivar influence on bioactive compounds of sour cherry fruits. Plant Soil Environ. 2017, 63, 389–395. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Palomino-Vasco, M.; Serradilla, M.J.; Velardo-Micharet, B. Effect of preharvest melatonin applications at dusk on quality and bioactive compounds content of early sweet cherries. J. Sci. Food Agric. 2024, 104, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin Accumulation in Sweet Cherry and Its Influence on Fruit Quality and Antioxidant Properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Tijero, V.; Muñoz, P.; Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef]
- Pang, L.; Chen, L.; Jiang, Y.; Zhou, C.; Liang, F.; Duan, L. Role of exogenous melatonin in quality maintenance of sweet cherry: Elaboration in links between phenolic and amino acid metabolism. Food Biosci. 2023, 56, 103223. [Google Scholar] [CrossRef]
- Vanhee, C.; Degrève, C.; Boschmans, N.; Naïmi, Y.; Canfyn, M.; Deconinck, E.; Willocx, M. Validated UHPLC Methods for Melatonin Quantification Reveal Regulatory Violations in EU Online Dietary Supplements Commerce. Molecules 2025, 30, 2647. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; de Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H. Bin Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Yaghoobi, A.; Rezaee, M.; Hedayati, N.; Keshavarzmotamed, A.; Khalilzad, M.A.; Russel, R.; Asemi, Z.; Rajabi Moghadam, H.; Mafi, A. Insight into the cardioprotective effects of melatonin: Shining a spotlight on intercellular Sirt signaling communication. Mol. Cell. Biochem. 2025, 480, 799–823. [Google Scholar] [CrossRef]
- Casper, E.A.; Wakeel, L.E.; Sabri, N.A.; Khorshid, R.; Gamal, M.A.; Fahmy, S.F. Melatonin ameliorates inflammation and improves outcomes of ischemia/reperfusion injury in patients undergoing coronary artery bypass grafting surgery: A randomized placebo-controlled study. Apoptosis 2025, 30, 267–281. [Google Scholar] [CrossRef]
- Milošević, T.M.; Milošević, N.M. Fruit Quality Attributes of Sour Cherry Cultivars. Int. Sch. Res. Not. 2012, 2012, 593981. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2023, 103, 463–478. [Google Scholar] [CrossRef] [PubMed]
- López, L.; Larrigaudière, C.; Giné-Bordonaba, J.; Echeverria, G. Defining key parameters and predictive markers of ‘Early Bigi’ cherry consumer satisfaction by means of differential storage scenarios. Postharvest Biol. Technol. 2023, 195, 112117. [Google Scholar] [CrossRef]
- Zhong, S.; Yang, Y.N.; Huo, J.X.; Sun, Y.Q.; Zhao, H.; Dong, X.T.; Feng, J.Y.; Zhao, J.; Wu, C.M.; Li, Y.G. Cyanidin-3-rutinoside from Mori Fructus ameliorates dyslipidemia via modulating gut microbiota and lipid metabolism pathway. J. Nutr. Biochem. 2025, 137, 109834. [Google Scholar] [CrossRef]
- Kongthitilerd, P.; Thilavech, T.; Marnpae, M.; Rong, W.; Yao, S.; Adisakwattana, S.; Cheng, H.; Suantawee, T. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells. Biomed. Pharmacother. 2022, 146, 112494. [Google Scholar] [CrossRef]
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Altern. Med. 2019, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef]
- Fu, K.; Dai, S.; Zhang, Y.; Gong, J.; Wang, C.; Yao, C.; Zhang, S.; Peng, C.; Li, Y. Natural product chlorogenic acid achieves pharmacological activity and health protection via regulating gut microbiota: A review. Food Sci. Hum. Wellness 2025, 14, 9250153. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Mi, S.; Fan, Q.; Sun, X.; Deng, B.; Wu, G.; Li, Y.; Zhou, Q.; Ruan, Z. Hepatoprotective effect of chlorogenic acid against chronic liver injury in inflammatory rats. J. Funct. Foods 2019, 62, 103540. [Google Scholar] [CrossRef]
- Saifur Rohman, M.; Lukitasari, M.; Nugroho, D.A.; Widodo, N.; Nugrahini, N.I.P. Cardiovascular protection effect of chlorogenic acid: Focus on the molecular mechanism. F1000Research 2020, 9, 1462. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Chu, L.; Zheng, W.; Wang, J.; Wang, Z.; Zhao, W.; Zhao, B.; Xu, G.; Xiao, M.; Lou, X.; Pan, F.; et al. Comparative analysis of the difference in flavonoid metabolic pathway during coloring between red-yellow and red sweet cherry (Prunus avium L.). Gene 2023, 880, 147602. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Physiological Function of Melatonin in Plants. Plant Signal. Behav. 2006, 1, 89. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; García, K.; Méndez, M.A.; González, M.; Meisel, L.A.; Defilippi, B.G.; del Pozo, T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, C.M.; Hernández, A.; Serradilla, M.J.; Moraga, C.; Martín, A.; Córdoba, M.d.G.; Ruiz-Moyano, S. Improvement of shelf-life of cherry (Prunus avium L.) by combined application of modified-atmosphere packaging and antagonistic yeast for long-distance export. J. Sci. Food Agric. 2023, 103, 4592–4602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Auñón, F.; García-Pastor, M.E.; Serrano, M.; Valero, D.; Agulló, V. Combined Pre- and Postharvest Melatonin Treatments Improve the Functional Quality of the Sweet Cherry cv. ‘Sunburst’. Foods 2025, 14, 3337. https://doi.org/10.3390/foods14193337
Garrido-Auñón F, García-Pastor ME, Serrano M, Valero D, Agulló V. Combined Pre- and Postharvest Melatonin Treatments Improve the Functional Quality of the Sweet Cherry cv. ‘Sunburst’. Foods. 2025; 14(19):3337. https://doi.org/10.3390/foods14193337
Chicago/Turabian StyleGarrido-Auñón, Fernando, María Emma García-Pastor, María Serrano, Daniel Valero, and Vicente Agulló. 2025. "Combined Pre- and Postharvest Melatonin Treatments Improve the Functional Quality of the Sweet Cherry cv. ‘Sunburst’" Foods 14, no. 19: 3337. https://doi.org/10.3390/foods14193337
APA StyleGarrido-Auñón, F., García-Pastor, M. E., Serrano, M., Valero, D., & Agulló, V. (2025). Combined Pre- and Postharvest Melatonin Treatments Improve the Functional Quality of the Sweet Cherry cv. ‘Sunburst’. Foods, 14(19), 3337. https://doi.org/10.3390/foods14193337








