Active Targeting Strategies for Improving the Bioavailability of Curcumin: A Systematic Review
Abstract
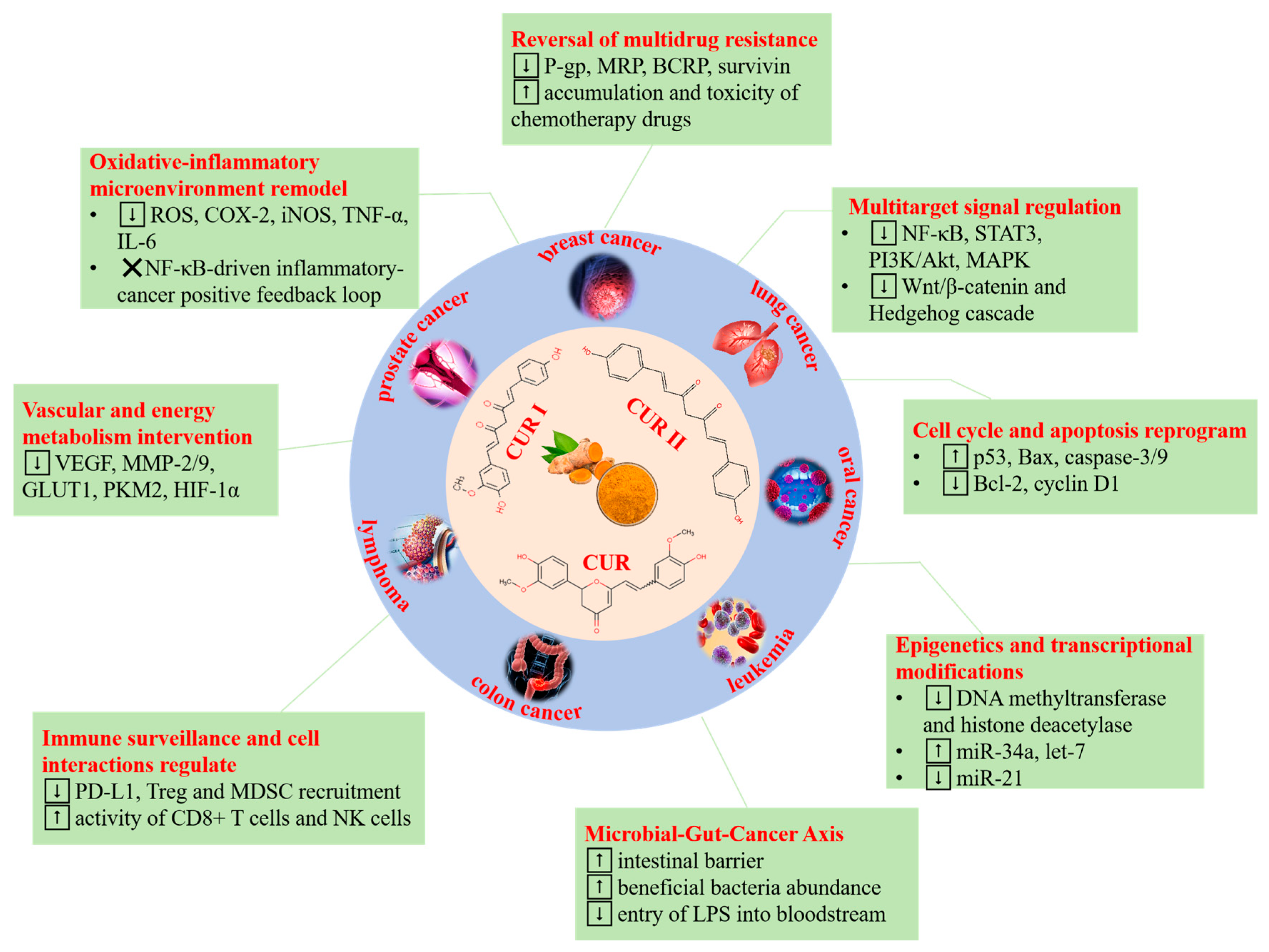
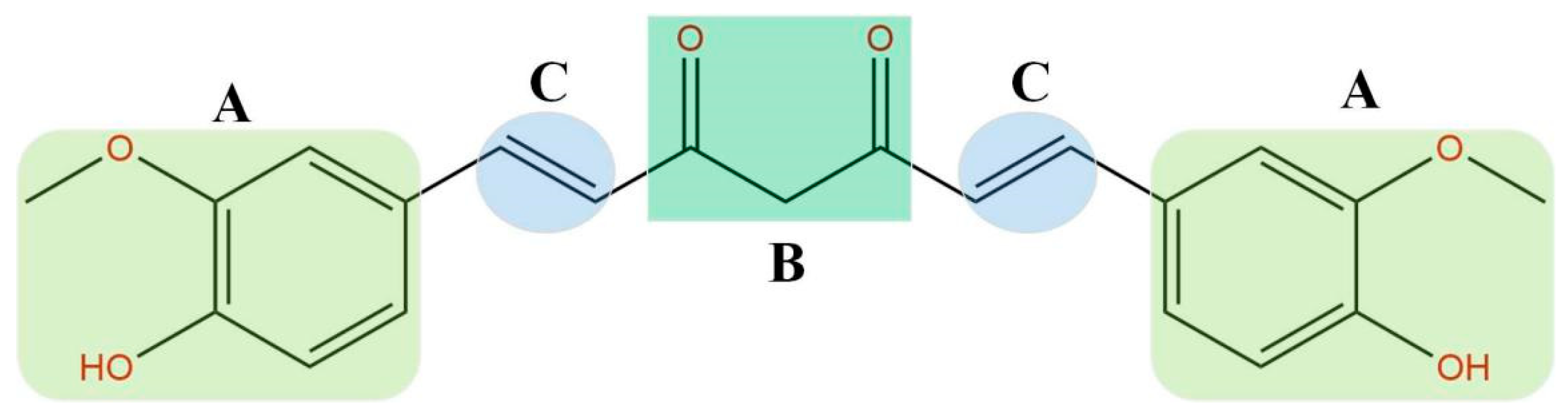
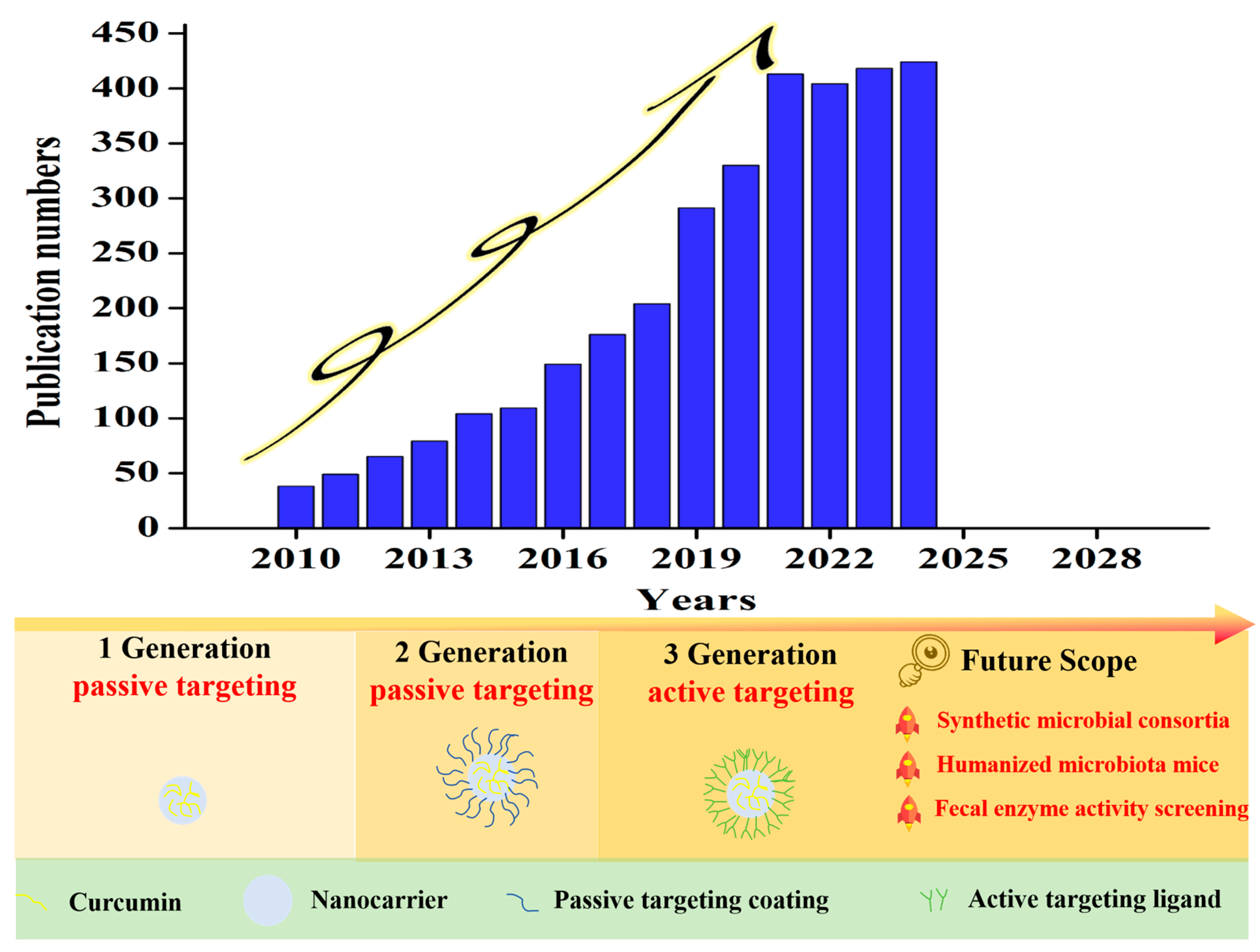
1. Introduction
2. Receptor-Mediated Targeting System
2.1. Folic Acid
2.2. Peptide
2.3. Antibody
2.4. Carbohydrate-Based Ligands
2.5. Glycoprotein/Glycosylamine
2.6. Combined Strategy
3. Colonic Environment-Responsive Targeting
3.1. pH-Responsive Delivery System
3.2. Microbial/Enzyme-Sensitive Delivery System
4. Evaluation Approaches for Active Targeting Nanocurcumin
4.1. Traditional Evaluation Methods
4.1.1. In Vitro Release Tests
In Vitro Dissolution Tests
Modified In Vitro Dissolution Tests
4.1.2. Cell Tests
4.1.3. In Vivo Tests
4.2. Emerging Evaluation Methods
5. Core Challenges of CUR Active Targeting
5.1. Antibody-Based Targeting: Cost, Stability and Immunogenicity
5.2. Translational Gap Between Proof-of-Concept and Validated In Vivo Efficacy
5.3. Microbiota Complexity and Inter-Individual Variability
5.4. Regulatory and Manufacturing Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Z.Y.; Wang, S.; Zhou, X.; Ouyang, L.; Chen, Z.; Deng, G. Harnessing the power of traditional Chinese medicine in cancer treatment: The role of nanocarriers. Int. J. Nanomed. 2025, 20, 3147–3174. [Google Scholar] [CrossRef] [PubMed]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a natural phenol and its therapeutic role in cancer and photodynamic therapy: A review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.K. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Hussain, Y.; Islam, L.; Khan, H.; Filosa, R.; Aschner, M.; Javed, S. Curcumin-cisplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytother. Res. 2021, 35, 6514–6529. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Kariznavi, E.; Jannati, M.; Elyasi, S.; Tayarani-Najaran, Z. Curcumin as a preventive or therapeutic measure for chemotherapy and radiotherapy induced adverse reaction: A comprehensive review. Food Chem. Toxicol. 2021, 145, 111699. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Z.X.; Fu, H.L.; Yu, G.Q.; Luo, X.; Zhu, K.W. Enhanced bioavailability of curcumin amorphous nanocomposite prepared by a green process using modified starch. Int. J. Biol. Macromol. 2024, 270, 132210. [Google Scholar] [CrossRef]
- Fan, Y.T.; Gan, C.; Zhang, H.L.; Yi, J. Characteristics, physicochemical stability and in vitro release of curcumin-loaded glycated bovine serum albumin nanofibrils: Effects of molecular weight of saccharide. Food Hydrocol. 2024, 155, 110210. [Google Scholar] [CrossRef]
- Chen, Z.F.; Liu, W.L.; Zeng, Z.J.; Yan, Z.H.; Ma, L.H.; Liu, Y.; Cao, X.S. Construction of active-passive dual-targeted drug-loaded micelle nanoparticles with modified dopamine molecules for efficient anti-tumor therapy. Int. J. Nanomed. 2025, 20, 10089–10100. [Google Scholar] [CrossRef]
- Bayomi, S.M.; El-Kashef, H.A.; El-Ashmawy, M.B.; Nasr, M.N.A.; El-Sherbeny, M.A.; Badria, F.A.; Abou-Zeid, L.A.; Ghaly, M.A.; Abdel-Aziz, N.I. Synthesis and biological evaluation of new curcumin derivatives as antioxidant and antitumor agents. Med. Chem. Res. 2013, 22, 1147–1162. [Google Scholar] [CrossRef]
- Kuzminska, J.; Szyk, P.; Mlynarczyk, D.T.; Bakun, P.; Muszalska-Kolos, I.; Dettlaff, K.; Sobczak, A.; Goslinski, T.; Jelinska, A. Curcumin Derivatives in Medicinal Chemistry: Potential Applications in Cancer Treatment. Molecules 2024, 29, 5321. [Google Scholar] [CrossRef]
- Wilar, G.; Suhandi, C.; Fukunaga, K.; Shigeno, M.; Kawahata, I.; Abdulah, R.; Sasaki, T. Effects of nanocurcumin supplementation on metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2025, 213, 107641. [Google Scholar] [CrossRef]
- Ahmad, E.; Ali, A.; Fatima, M.T.; Nimisha; Apurva; Kumar, A.; Sumi, M.P.; Sattar, R.S.A.; Mahajan, B.; Saluja, S.S. Ligand decorated biodegradable nanomedicine in the treatment cancer. Pharmacol. Res. 2021, 167, 105544. [Google Scholar] [CrossRef]
- Wang, Y.D.; Li, Z.Y.; Bao, Y.W.; Cui, H.J.; Li, J.X.; Song, B.G.; Wang, M.Z.; Li, H.K.; Cui, X.Y.; Chen, Y. Colon-targeted delivery of polyphenols: Construction principles, targeting mechanisms and evaluation methods. Crit. Rev. Food Sci. Nutr. 2025, 65, 64–86. [Google Scholar] [CrossRef]
- Hegde, M.; Kumar, A.; Girisa, S.; Aswani, B.S.; Vishwa, R.; Sethi, G.; Kunnumakkara, A.B. Nanoformulations of curcumin: An alliance for effective cancer therapeutics. Food Biosci. 2023, 56, 103095. [Google Scholar] [CrossRef]
- Wang, L.C.; Yu, M.; Yang, H. Recent progress in the diagnosis and precise nanocarrier-mediated therapy of inflammatory bowel disease. J. Inflamm. Res. 2021, 14, 170176. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.J.; Donadkar, A.D.; Nair, B.; Kumar, A.R.; Sabitha, M.; Sethi, G.; Chauhan, A.S.; Nath, L.R. Smart polymer-based delivery systems for curcumin in colon cancer therapy: A review. Phytother. Res. 2025, 39, 698–713. [Google Scholar] [CrossRef]
- Azehaf, H.; Benzine, Y.; Tagzirt, M.; Skiba, M.; Karrout, Y. Microbiota-sensitive drug delivery systems based on natural polysaccharides for colon targeting. Drug Discov. Today 2023, 28, 103606. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhang, X.N. Advances in receptor modulation strategies for flexible, efficient, and enhanced antitumor efficacy. J. Control. Release 2021, 333, 418–447. [Google Scholar] [CrossRef] [PubMed]
- Yaman, Y.T.; Vural, O.A.; Bolat, G.; Abaci, S. Fabrication of trastuzumab conjugated curcumin nanoparticles based impedimetric cytosensor for the cancer cell detection. Microchem. J. 2023, 191, 108773. [Google Scholar] [CrossRef]
- Li, H.J.; Zhang, M.Z.; He, J.L.; Liu, J.; Sun, X.W.; Ni, P.H. A CD326 monoclonal antibody modified core cross-linked curcumin-polyphosphoester prodrug for targeted delivery and cancer treatment. J. Mater. Chem. B 2023, 11, 9467–9477. [Google Scholar] [CrossRef]
- Huang, A.G.; Chen, C.; Liu, T.Q.; Wang, G.X. scFv antibody-mediated targeted drug delivery system improves the antiviral activity of geniposidic acid against WSSV. Aquaculture 2022, 560, 738496. [Google Scholar] [CrossRef]
- Navarro-Marchal, S.A.; Martín-Contreras, M.; Castro-Santiago, D.; del Castillo-Santaella, T.; Graván, P.; Jódar-Reyes, A.B.; Marchal, J.A.; Peula-García, J.M. Effect of the protein corona formation on antibody functionalized liquid lipid nanocarriers. Int. J. Mol. Sci. 2023, 24, 16759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, L.; Dong, Y.; Dong, B.Z.; Wang, X.L.; Liu, X.; Hu, Z.X.; Fang, G.C.; Wang, G.Y.; Qin, J.X.; et al. Co-delivery of siape1 and melatonin by 125I-loaded PSMA-targeted nanoparticles for the treatment of prostate cancer. Recent Pat. Anti-Cancer 2024, 19, 503–515. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, K.M.; Yao, X.; Chen, Y.W.; Ma, N.N.; Feng, Q.Q.; Zhu, F.; Ma, X.T.; Zhang, T.J.; Yue, Y.L.; et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundam. Res. 2022, 2, 23–26. [Google Scholar] [CrossRef]
- Ahlgren, S.; Fondell, A.; Gedda, L.; Edwards, K. Egf-targeting lipodisks for specific delivery of poorly water-soluble anticancer agents to tumor cells. RSC Adv. 2017, 7, 22178–22186. [Google Scholar] [CrossRef]
- McDaid, W.J.; Greene, M.K.; Johnston, M.C.; Pollheimer, E.; Smyth, P.; McLaughlin, K.; Van Schaeybroeck, S.; Straubinger, R.M.; Longley, D.B.; Scott, C.J. Repurposing of cetuximab in antibody-directed chemotherapy-loaded nanoparticles in EGFR therapy-resistant pancreatic tumors. Nanoscale 2019, 11, 2026120273. [Google Scholar] [CrossRef]
- Ehrbar, M.; Rossi, F.; Cellesi, F. Editorial: Nanosized drug delivery systems: Colloids and gels for site specific targeting. Front. Bioeng. Biotechnol. 2020, 8, 803. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Du, C.; Zhao, Y.; Nie, G.J.; Yang, Y.M. Trap and kill strategy for nonBRCA mutant pancreatic cancer by codelivery of olaparib and JQ1 with plectin1 targeting peptide nanoparticles. Nano Today 2020, 33, 100877. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, R.; Zhang, D.; Zhang, C.; Zhang, Z.; Fu, X.; Luo, Y.; Chen, S.; Wu, A.; Zeng, W.; et al. Cyclic RGD-decorated liposomal gossypol AT-101 targeting for enhanced antitumor effect. Int. J. Nanomed. 2022, 17, 227–244. [Google Scholar] [CrossRef]
- Tang, H.L.; Pan, Y.H.; Zhang, Y.F.; Tang, H.T. Challenges for the application of EGFR-targeting peptide GE11 in tumor diagnosis and treatment. J. Control. Release 2022, 349, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Khan, R.A.; Alhowail, A.H.; Alqasoumi, A.; Sajid, S.M.; Mohammed, A.M.; Alsharidah, M.; Al Rugaie, O.; Mousa, A.M. Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model. Nanotechnol. Rev. 2022, 11, 266–283. [Google Scholar] [CrossRef]
- An, C.J.; Wei, S.; Dao, Y.K.; Wang, X.Y.; Dong, W.D.; You, X.; Tian, C.; Zhang, Z.L.; Dong, S.W. Discovery of endosomalytic cell-penetrating peptides based on bacterial membrane-targeting sequences. Bioorg. Chem. 2023, 134, 106424. [Google Scholar] [CrossRef]
- Gonzalez-Cruz, A.O.; Hernandez-Juarez, J.; Ramirez-Cabrera, M.A.; BalderasRenteria, I.; Arredondo-Espinoza, E. Peptide-based drug-delivery systems: A new hope for improving cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 72, 103362. [Google Scholar] [CrossRef]
- Li, J.R.; Zhang, Z.X.; Zhang, B.L.; Yan, X.Y.; Fan, K.L. Transferrin receptor 1 targeted nanomedicine for brain tumor therapy. Biomater. Sci. 2023, 11, 3394–3413. [Google Scholar] [CrossRef]
- Fard, M.G.; Khabir, Z.; Reineck, P.; Cordina, N.M.; Abe, H.; Ohshima, T.; Dalal, S.; Gibson, B.C.; Packer, N.H.; Parker, L.M. Targeting cell surface glycans with lectin-coated fluorescent nanodiamonds. Nanoscale Adv. 2022, 4, 1551–1564. [Google Scholar] [CrossRef]
- Baig, M.M.F.A.; Ma, J.W.; Gao, X.L.; Khan, M.A.; Ali, A.; Farid, A.; Zia, A.W.; Noreen, S.; Wu, H.K. Exploring the robustness of DNA nanotubes framework for anticancer theranostics toward the 2D/3D clusters of hypopharyngeal respiratory tumor cells. Int. J. Biol. Macromol. 2023, 236, 123988. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yang, H.Q.; Li, D.D.; Li, F.; Ma, J.; Liu, P.D. The effect of AS1411 surface density on the tumor targeting properties of PEGylated silver nanotriangles. Nanomedicine 2022, 17, 289–302. [Google Scholar] [CrossRef]
- Shahrad, S.; Rajabi, M.; Javadi, H.; Zarchi, A.A.K.; Darvishi, M.H. Targeting lung cancer cells with MUC1 aptamer functionalized PLA-PEG nanocarriers. Sci. Rep. 2022, 12, 4718. [Google Scholar] [CrossRef]
- Mikled, P.; Chavasiri, W.; Khongkow, M. Dual folate/biotin-decorated liposomes mediated delivery of methylnaphthazarin for anti-cancer activity. Sci. Rep. 2024, 14, 21796. [Google Scholar] [CrossRef] [PubMed]
- Varvarà, P.; Drago, S.E.; Esposito, E.; Campolo, M.; Mauro, N.; Calabrese, G.; Conoci, S.; Morganti, D.; Fazio, B.; Giammona, G.; et al. Biotinylated polyaminoacid-based nanoparticles for the targeted delivery of lenvatinib towards hepatocarcinoma. Int. J. Pharm. 2024, 662, 124537. [Google Scholar] [CrossRef]
- Fatima, M.; Karwasra, R.; Almalki, W.H.; Sahebkar, A.; Kesharwani, P. Galactose engineered nanocarriers: Hopes and hypes in cancer therapy. Eur. Polym. J. 2023, 183, 111759. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, K.; Zhang, X.-S.; Zhou, S.; Zou, J.-H.; Tian, M.-Y.; Hou, X.-L.; Hu, Y.-G.; Yuan, J.; Fan, J.-X.; et al. Cascade-catalysed nanocarrier degradation for regulating metabolism homeostasis and enhancing drug penetration on breast cancer. J. Nanobiotechnol. 2024, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, Z.Z.; Liang, J.L.; Li, N.; Song, R.J.; Luo, L.; Ai, Y.L.; Li, X.; Tang, S.Q. An oral delivery vehicle based on konjac glucomannan acetate targeting the colon for inflammatory bowel disease therapy. Front. Bioeng. Biotech. 2022, 10, 1025155. [Google Scholar] [CrossRef]
- Farjadian, F.; Faghih, Z.; Fakhimi, M.; Iranpour, P.; Mohammadi-Samani, S.; Doroudian, M. Glucosamine-modified mesoporous silica-coated magnetic nanoparticles: A “Raisin-Cake”-like structure as an efficient theranostic platform for targeted methotrexate delivery. Pharmaceutics 2023, 15, 2491. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, X.; Liu, Y.; Li, H.; Zhang, Z.Y. Physiologically driven nanodrug delivery system for targeted lung cancer treatment. Explor. Med. 2024, 5, 280–311. [Google Scholar] [CrossRef]
- Li, X.; Diao, W.; Xue, H.; Wu, F.; Wang, W.; Jiang, B.; Bai, J.; Lian, B.; Feng, W.; Sun, T.; et al. Improved efficacy of doxorubicin delivery by a novel dual-ligand-modified liposome in hepatocellular carcinoma. Cancer Lett. 2020, 489, 163–173. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, W.; Huang, C.; Long, J.; Zhou, Y.; Liu, Y.; Tang, S.; He, D.-X.; Tan, X.-W.; Wei, H.; et al. Facile fabrication of nanoparticles with dual-targeting ligands for precise hepatocellular carcinoma therapy in vitro and in vivo. Mol. Pharm. 2020, 17, 3223–3235. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineagehoming cell-penetrating peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, W.; Hu, L.; Xiao, Z.; Yang, X.; Cao, Z.; Cao, J. Long Circulating Cancer Cell-Targeted Bionic Nanocarriers Enable Synergistic Combinatorial Therapy in Colon Cancer. ACS Appl. Mater. Interfaces 2023, 15, 22843–22853. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.H.; Han, D.; He, X.Y.; Guo, T.; Chen, X.S.; Pang, X.; Cheng, S.W. Multitargeting nano-systems targeting heterogeneous cancer cells for therapeutics and biomarker detection. Adv. Healthc. Mater. 2023, 12, e220215. [Google Scholar] [CrossRef]
- Gao, G.Y.; Zhou, W.H.; Jiang, X.; Ma, J. Bovine serum albumin and folic acid-modified aurum nanoparticles loaded with paclitaxel and curcumin enhance radiotherapy sensitization for esophageal cancer. Int. J. Radiat. Biol. 2024, 100, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, I.; Yaraki, M.T.; Ahmadi, S.; Chiani, M.; Nourouzian, D. Folic acidfunctionalized niosomal nanoparticles for selective dual-drug delivery into breast cancer cells: An in-vitro investigation. Adv. Powder Technol. 2020, 31, 40644071. [Google Scholar] [CrossRef]
- Kargar, B.; Fazeli, M.; Sobhani, Z.; Hosseinzadeh, S.; Solhjoo, A.; Akbarizadeh, A.R. Exploration of the photothermal role of curcumin-loaded targeted carbon nanotubes as a potential therapy for melanoma cancer. Sci. Rep. 2024, 14, 10117. [Google Scholar] [CrossRef]
- Dutta, D.; Pajaniradje, S.; Nair, A.S.; Chandramohan, S.; Bhat, S.A.; Manikandan, E.; Rajagopalan, R. An in-vitro study of active targeting & anti-cancer effect of folic acid conjugated chitosan encapsulated indole curcumin analogue nanoparticles. Int. J. Biol. Macromol. 2024, 282, 136990. [Google Scholar]
- Kavya, K.; Vargheese, S.; Shukla, S.; Khan, I.; Dey, D.K.; Bajpai, V.K.; Thangavelu, K.; Vivek, R.; Kumar, R.R.; Han, Y.-K.; et al. A cationic amino acid polymer nanocarrier synthesized in supercritical CO2 for co-delivery of drug and gene to cervical cancer cells. Colloids Surf. R Biointerfaces 2022, 216, 112584. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Naeimi, H.; Goliaei, B.; Bigdeli, B.; Sadighi, A.; Dehghani, S.; Lotfabadi, A.; Hosseini, M.; Nezamtaheri, M.S.; Amanlou, M.; et al. Magnetic biometal-organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mater. Sci. Eng. C 2019, 99, 805–815. [Google Scholar] [CrossRef]
- Jalaladdiny, S.-S.; Badoei-Dalfard, A.; Karami, Z.; Sargazi, G. Co-delivery of doxorubicin and curcumin to breast cancer cells by a targeted delivery system based on Ni/Ta core-shell metalorganic framework coated with folic acid-activated chitosan nanoparticles. J. Iran. Chem. Soc. 2022, 19, 4287–4298. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Aboushoushah, S.F.; Mohamed, N. Bioinspired synthesis of protein/polysaccharide-decorated folate as a nanocarrier of curcumin to potentiate cancer therapy. Int. J. Pharm. 2022, 613, 121420. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, E.S.; Ghanbari, N.; Salehi, Z.; Derakhti, S.; Amoabediny, G.; Akbari, M.; Tokmedash, M.A. Smart pH-responsive magnetic graphene quantum dots nanocarriers for anticancer drug delivery of curcumin. Mater. Chem. Phys. 2023, 297, 127336. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tang, L.; Lu, X.H.; Liu, J.T.; Wang, Y.Y.; Geng, H.X.; Li, X.T.; An, Q. Efficacy of epi-1 modified epirubicin and curcumin encapsulated liposomes targeting-EpCAM in the inhibition of epithelial ovarian cancer cells. J. Liposome Res. 2023, 33, 197–213. [Google Scholar] [CrossRef]
- Fatih, S.; Soner, C. Fabrication of curcumin-loaded magnetic PEGylated-PLGA nanocarriers tagged with GRGDS peptide for improving anticancer activity. MethodsX 2023, 10, 102229. [Google Scholar]
- Hou, J.W.; Cong, Y.Y.; Ji, J.; Liu, Y.X.; Hong, H.; Han, X.D. Spatial targeting of fibrosis-promoting macrophages with nanoscale metal-organic frameworks for idiopathic pulmonary fibrosis therapy. Acta Biomater. 2024, 174, 372–385. [Google Scholar] [CrossRef]
- Dai, Y.M.; Jiang, Z.L.; Li, J.Y.; Wang, M.F.; Liu, C.; Qi, W.; Su, R.X.; He, Z.M. Coassembly of curcumin and a cystine bridged peptide to construct tumorresponsive nano-micelles for efficient chemotherapy. J. Mater. Chem. B 2020, 8, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Q.; Song, B.; Yang, J.Y.; Wang, B.; Ma, Z.Q.; Yu, L.; Li, Y.H.; Xu, H.J.; Qiao, M.Q. Curcumin-encapsulated fusion protein-based nanocarrier demonstrated highly efficient epidermal growth factor receptor-targeted treatment of colorectal cancer. J. Agric. Food Chem. 2022, 70, 15461–15473. [Google Scholar] [CrossRef]
- Huang, M.; Zhai, B.T.; Fan, Y.; Sun, J.; Shi, Y.J.; Zhang, X.F.; Zou, J.B.; Wang, J.W.; Guo, D.Y. Targeted drug delivery systems for curcumin in breast cancer therapy. Int. J. Nanomed. 2023, 18, 4275–4311. [Google Scholar] [CrossRef]
- Mukerjee, A.; Ranjan, A.P.; Vishwanatha, J.K. Targeted nanocurcumin therapy using annexin a2 antibody improves tumor accumulation and therapeutic efficacy against highly metastatic breast cancer. J. Biomed. Nanotechnol. 2016, 12, 1374–1392. [Google Scholar] [CrossRef]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagnosis Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Moulahoum, H.; Ghorbanizamani, F.; Barlas, F.B.; Yesiltepe, O.; Gumus, Z.P.; Meral, K.; Demirkol, D.O.; Timur, S. Carbon dots and curcumin-loaded CD44-Targeted liposomes for imaging and tracking cancer chemotherapy: A multi-purpose tool for theranostics. J. Drug Deliv. Sci. Technol. 2021, 62, 102363. [Google Scholar] [CrossRef]
- Varshosaz, J.; Jandaghian, S.; Mirian, M.; Sajjadi, S.E. Co-delivery of rituximab targeted curcumin and imatinib nanostructured lipid carriers in non-hodgkin lymphoma cells. J. Liposome Res. 2020, 31, 64–78. [Google Scholar] [CrossRef]
- Wang, T.; Lin, M.; Mao, J.; Tian, L.; Gan, H.; Hu, X.; Yan, L.; Long, H.; Cai, J.; Zheng, X.; et al. Inflammation-regulated nanodrug sensitizes hepatocellular carcinoma to checkpoint blockade therapy by reprogramming the tumor micro-environment. ACS Appl. Mater. Interfaces 2022, 14, 49542–49554. [Google Scholar] [CrossRef]
- Nguyen, V.; You, D.G.; Kim, C.H.; Kwon, S.; Um, W.; Oh, B.H.; An, J.Y.; Jeon, J.; Park, J.H. An anti-DR5 antibodycurcumin conjugate for the enhanced clearance of activated hepatic stellate cells. Int. J. Biol. Macromol. 2021, 192, 1231–1239. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Ghalehkhondabi, V.; Fazlali, A.; Soleymani, M. Preparation of hyaluronic acid-decorated hollow meso-organosilica/poly (methacrylic acid) nanospheres with redox/pH dual responsivity for delivery of curcumin to breast cancer cells. Mater. Today Chem. 2023, 34, 101780. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.W.; Liu, Y.R.; Ren, Y.; Wang, J.H.; Niu, B.L.; Li, W.F. Preparation of curcumin loaded hyaluronic acid-poly (lactic-co-glycolic acid) micelles with pH response and tumor targeting. Eur. Polym. J. 2022, 177, 111450. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Xia, Q.; Li, Y.Y.; He, Z.H.; Li, Z.; Guo, T.; Wu, Z.H.; Feng, N.P. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: A new strategy for clustering drug in inflammatory skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, T.; Yu, Y.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Shen, Y.; et al. Dual targeting curcumin loaded alendronate-hyaluronan-octadecanoic acid micelles for improving osteosarcoma therapy. Int. J. Nanomed. 2019, 14, 6425–6437. [Google Scholar] [CrossRef]
- Mokhtari, S.; Solati-Hashjin, M.; Khosrowpour, Z.; Gholipourmalekabadi, M. Layered double hydroxide-galactose as an excellent nanocarrier for targeted delivery of curcumin to hepatocellular carcinoma cells. Appl. Clay Sci. 2021, 200, 105891. [Google Scholar] [CrossRef]
- Gupta, B.; Sadaria, D.; Warrier, V.U.; Kirtonia, A.; Kant, R.; Awasthi, A.; Baligar, P.; Pal, J.K.; Yuba, E.; Sethi, G.; et al. Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin. Cancer Biol. 2022, 80, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Jani, P.U.; Florence, A.T. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm. Res. 1997, 14, 613–618. [Google Scholar] [CrossRef]
- Sun, S.Y.; Du, X.Y.; Fu, M.F.; Khan, A.R.; Ji, J.B.; Liu, W.D. Galactosamine-modified PEG-PLA/TPGS micelles for the oral delivery of curcumin. Int. J. Pharm. 2021, 595, 120227. [Google Scholar] [CrossRef]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A.; Farzaneh, F. Tryptophan-functionalized graphene quantum dots with enhanced curcumin loading capacity and pH-sensitive release. J. Drug Deliv. Sci. Technol. 2021, 61, 102137. [Google Scholar] [CrossRef]
- Guo, C.J.; Hou, X.Y.; Liu, Y.H.; Zhang, Y.C.; Xu, H.Y.; Zhao, F.; Chen, D.Q. Novel Chinese angelica polysaccharide biomimetic nanomedicine to curcumin delivery for hepatocellular carcinoma treatment and immunomodulatory effect. Phytomedicine 2020, 80, 153356. [Google Scholar] [CrossRef]
- Wang, K.L.; Guo, C.J.; Zou, S.H.; Yu, Y.M.; Fan, X.X.; Wang, B.B.; Liu, M.N.; Fang, L.; Chen, D.Q. Synthesis, characterization and in vitro/in vivo evaluation of novel reduction-sensitive hybrid nano-echinus-like nanomedicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 659–667. [Google Scholar] [CrossRef]
- Tian, C.H.; Asghar, S.; Hu, Z.Y.; Qiu, Y.; Zhang, J.W.; Shao, F.; Xiao, Y.Y. Understanding the cellular uptake and biodistribution of a dual-targeting carrier based on redox-sensitive hyaluronic acid-ss-curcumin micelles for treating brain glioma. Int. J. Biol. Macromol. 2019, 136, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zou, S.H.; Guo, C.J.; Wang, K.L.; Zhao, F.; Fan, H.Y.; Yin, J.G.; Chen, D.Q. Multifunctional redoxresponsive and cd44 receptor targeting polymer-drug nanomedicine based curcumin and alendronate: Synthesis, characterization and in vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 46, 168–177. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Hadadzadeh, H.; Rezakhani, S.; Amirghofran, Z. Design and synthesis of gatekeeper coated dendritic silica/titania mesoporous nanoparticles with sustained and controlled drug release properties for targeted synergetic chemo-sonodynamic therapy. ACS Biomater. Sci. Eng. 2019, 5, 4405–4415. [Google Scholar] [CrossRef]
- Telang, N. Drug-resistant stem cells: Novel approach for colon cancer therapy. Int. J. Mol. Sci. 2022, 23, 2519. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, Y.R.; Lu, Y.; Quan, H.; Wang, Y.Q.; Song, S.J.; Guo, H.Y. Advanced oral drug delivery systems for gastrointestinal targeted delivery: The design principles and foundations. J. Nanobiotechnol. 2025, 23, 400. [Google Scholar] [CrossRef]
- Shao, H.; Liu, M.; Jiang, H.; Zhang, Y. Polysaccharide-based drug delivery targeted approach for colon cancer treatment: A comprehensive review. Int. J. Biol. Macromol. 2025, 302, 139177. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lan, H.R.; Jin, K.T.; Chen, Y. Responsive nanosystems for targeted therapy of ulcerative colitis: Current practices and future perspectives. Drug Deliv. 2023, 30, 2219427. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hu, Q.; Sun, Z.; Yu, Y.; Li, X.; Tian, T.; Bi, X.; Li, Y.; Niu, B.; Zhang, Z. Colon targeting pH-responsive coacervate microdroplets for treatment of ulcerative colitis. Small 2024, 20, e2311890. [Google Scholar] [CrossRef] [PubMed]
- Khatik, R.; Mishra, R.; Verma, A.; Dwivedi, P.; Kumar, V.; Gupta, V.; Paliwal, S.K.; Mishra, P.R.; Dwivedi, A.K. Colon specific delivery of curcumin by exploiting Eudragit-decorated chitosan nanoparticles in vitro and in vivo. J. Nanoparticle Res. 2013, 15, 1893. [Google Scholar] [CrossRef]
- Lertpairod, J.; Tiyaboonchai, W. pH-sensitive beads containing curcumin loaded nanostructured lipid carriers for a colon targeted oral delivery system. J. Pharm. Investig. 2022, 52, 387–396. [Google Scholar] [CrossRef]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Rieux, A.D.; Préat, V. pH sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharmacol. 2014, 473, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mashaqbeh, H.; Obaidat, R.; Alsmadi, M.M.; Bardaweel, S.; Hailat, N. Characterization and optimization of colon specific nanosponges immobilized polymeric microbeads formulation for the combined delivery of 5-fluorouracil and curcumin. J. Drug Deliv. Sci. Technol. 2024, 99, 105968. [Google Scholar] [CrossRef]
- Li, H.; Gao, Z.X.; Xu, J.J.; Sun, W.; Wu, J.R.; Zhu, L.; Gao, M.J.; Zhan, X.B. Encapsulation of polyphenols in pH-responsive micelles self-assembled from octenyl-succinylated curdlan oligosaccharide and its effect on the gut microbiota. Colloids Surf. B Biointerfaces 2022, 219, 112857. [Google Scholar] [CrossRef]
- Moideen, M.M.J.; Karuppaiyan, K.; Kandhasamy, R.; Seetharaman, S. Skimmed milk powder and pectin decorated solid lipid nanoparticle containing soluble curcumin used for the treatment of colorectal cancer. J. Food Process Eng. 2019, 43, e13246. [Google Scholar] [CrossRef]
- Zhang, G.S.; Han, W.; Zhao, P.X.; Wang, Z.J.; Li, M.; Sui, X.F.; Liu, Y.H.; Tian, B.C.; He, Z.G.; Fu, Q. Programmed pHresponsive core-shell nanoparticles for precisely targeted therapy of ulcerative colitis. Nanoscale 2023, 15, 19371946. [Google Scholar]
- Guo, X.; Liu, H.Y.; Hou, R.Y.; Chen, G.J.; Xiao, H.; Liu, L.Y.; Ciftci, O.N.; Liu, L.L. Design strategies of polysaccharide, protein and lipid-based nano-delivery systems in improving the bioavailability of polyphenols and regulating gut homeostasis. Int. J. Biol. Macromol. 2024, 283, 137463. [Google Scholar] [CrossRef]
- Huang, D.; Zou, M.L.; Xu, C.L.; Wang, Y.M.; Xu, Z.J.; Zhang, W.C.; Tang, S.J.; Weng, Z.Q. Colon-targeted oral delivery of hydroxyethyl starch–curcumin microcapsules loaded with multiple drugs alleviates DSS-induced ulcerative colitis in mice. Macromol. Biosci. 2024, 24, e2300465. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Jin, M.F.; Wu, Y.H.; Jung, S.; Li, D.D.; He, N.N.; Lee, M.S. An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice. Drug Deliv. 2021, 28, 1120–1131. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.J.; He, Y.N.; Xian, J.; Luo, R.F.; Zheng, C.; Zhang, J.M. Oral colon-targeting core-shell microparticles loading curcumin for enhanced ulcerative colitis alleviating efficacy. Chin. Med. 2021, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, N.; Buyukkileci, A.O.; Gulec, S.; Polat, M.; Polat, H. Designing robust xylan/chitosan composite shells around drug-loaded MSNs: Stability in upper GIT and degradation in the colon microbiota. J. Drug Deliv. Sci. Technol. 2023, 79, 103983. [Google Scholar] [CrossRef]
- Wen, Z.; Kang, L.; Fu, H.; Zhu, S.; Ye, X.; Yang, X.; Zhang, S.; Hu, J.; Li, X.; Chen, L.; et al. Orall delivery of porous starch-loaded bilayer microgels for controlled drug delivery and treatment of ulcerative colitis. Carbohydr. Polym. 2023, 314, 120887. [Google Scholar] [CrossRef]
- Meng, F.B.; Zhang, Q.; Li, Y.C.; Li, J.J.; Peng, L.X. Konjac glucomannan octenyl succinate as a novel encapsulation wall material to improve curcumin stability and bioavailability. Carbohydr. Polym. 2020, 238, 116193. [Google Scholar] [CrossRef]
- Wang, L.H.; Xiao, J.X.; Li, X.D.; Huang, G.Q. Carboxymethyl konjac glucomannan coating on multilayered emulsions for improved bioavailability and targeted delivery of curcumin. Food Funct. 2021, 12, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Peng, H.H.; Guan, Y.X.; Yao, S.J. Supercritical CO2 assisted micronization of curcumin-loaded oil-inwater emulsion promising in colon targeted delivery. J. CO2 Util. 2022, 59, 101966. [Google Scholar] [CrossRef]
- Kotla, N.G.; Burke, O.; Pandit, A.; Rochev, Y. An Orally administrated hyaluronan functionalized polymeric hybrid nanoparticle system for colon-specific drug delivery. Nanomaterials 2019, 9, 1246. [Google Scholar] [CrossRef]
- Borah, P.K.; Das, A.S.; Mukhopadhyay, R.; Sarkar, A.; Duary, R.K. Macromolecular design of folic acid functionalized amylopectin-albumin core-shell nanogels for improved physiological stability and colon cancer cell targeted delivery of curcumin. J. Colloid Interface Sci. 2020, 580, 561–572. [Google Scholar] [CrossRef]
- Li, H.; He, W.J.; Wang, Z.J.; Zhang, Q.; Hu, D.; Ding, K.; Xie, Q.T.; Xu, Y.Q.; Shan, Y.; Ding, S.H. Improving the prebiotic activity and oxidative stability of carboxymethyl curdlan-quercetin conjugates stabilized Pickering emulsions for the colonic targeting delivery of curcumin. Food Res. Int. 2025, 201, 115641. [Google Scholar] [CrossRef]
- Izadi, Z.; Rashidi, M.; Derakhshankhah, H.; Dolati, M.; Kermanshahi, M.G.; Adibi, H.; Samadian, H. Curcumin-loaded porous particles functionalized with pH-responsive cell-penetrating peptide for colorectal cancer targeted drug delivery. RSC Adv. 2023, 13, 34587. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.X.; Lin, J.Q.; Shen, M.Y.; Wang, Y.X.; Sarkar, A.; Wen Hl Xie, J.H. Co-delivery of resveratrol and curcumin based on Mesona chinensis polysaccharides/zein nanoparticle for targeted alleviation of ulcerative colitis. Food Biosci. 2024, 59, 104060. [Google Scholar] [CrossRef]
- Zeybek, N.; Polat, H.; Gulec, S.; Buyukkileci, A.O. Development of xylan-coated acid-resistant micellar drug carriers for colon-targeted oral delivery. Int. J. Polym. Mater. 2025, 74, 474–483. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.X.; Xiao, M.; Wang, D.; Qu, Y.; Zou, L.; Zheng, C.A.; Zhang, J.M. Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 2022, 33, 4924–4929. [Google Scholar] [CrossRef]
- Hua, Y.J.; Wei, Z.H.; Xue, C.H.; Si, J.Y. Stability and programmed sequential release of Lactobacillus plantarum and curcumin encapsulated in bilayer-stabilized W1/O/W2 double emulsion: Effect of pectin as protective shell. Int. J. Biol. Macromol. 2024, 165, 130805. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ping, H.; Wang, K.; Ding, H.; Zhang, M.; Yang, Z.; Du, Q. Oral delivery of pectin-chitosan hydrogels entrapping macrophage-targeted curcumin-loaded liposomes for the treatment of ulcerative colitis. Int. J. Pharm. 2023, 647, 123510. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Xu, C.; Zou, M.; Ming, Y.; Luo, F.; Xu, Z.; Miao, Y.; Wang, N.; Lin, Z.; et al. Colon-targeted hydroxyethyl starch-curcumin microspheres with high loading capacity ameliorate ulcerative colitis via alleviating oxidative stress, regulating inflammation, and modulating gut microbiota. Int. J. Biol. Macromol. 2024, 266, 131107. [Google Scholar] [CrossRef] [PubMed]
- Kurra, P.; Narra, K.; Orfali, R.; Puttugunta, S.B.; Alam Khan, S.; Meenakshi, D.U.; Francis, A.P.; Asdaq, S.M.B.; Imran, M. Studies on jackfruit-okra mucilage-based curcumin mucoadhesive tablet for colon targeted delivery. Front. Pharmacol. 2022, 13, 902207. [Google Scholar] [CrossRef]
- Hales, D.; Muntean, D.-M.; Neag, M.A.; Kiss, B.; Ștefan, M.-G.; Tefas, L.R.; Tomuță, I.; Sesărman, A.; Rațiu, I.-A.; Porfire, A. Curcumin-loaded microspheres are Effective in preventing oxidative stress and intestinal inflammatory abnormalities in experimental ulcerative colitis in rats. Molecules 2022, 27, 5680. [Google Scholar] [CrossRef]
- Cai, R.; Pan, S.Y.; Li, R.X.; Xu, X.Y.; Pan, S.Y.; Liu, F.X. Curcumin loading and colon release of pectin gel beads: Effect of different de-esterification method. Food Chem. 2022, 389, 133130. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.S.; Shen, X.F.; Li, T.T.; Deng, Y.; Xiao, H.L.; Li, Z.P.; Bai, S.C.; Ma, X.Y.; Liao, X.P.; Zhao, D.H. A bionic yeast for the colon-targeted delivery of curcumin in the treatment of inflammatory bowel disease. Chem. Eng. J. 2025, 516, 164121. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Yang, L.Y.; Ouyang, X.K.; Huang, F. Folic acid and PEI modified mesoporous silica for targeted delivery of curcumin. Pharmaceutics 2019, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, X.P.; Ding, J.S.; Zhou, W.H.; Zheng, X.; Tang, G.T. Mechanisms of drug release in pH-sensitive micelles for tumor targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253260. [Google Scholar] [CrossRef]
- Emami, J. In vitro-in vivo correlation: From theory to applications. J. Pharm. Pharm. Sci. 2006, 9, 169–189. [Google Scholar]
- Feng, K.; Li, C.; Wei, Y.S.; Zong, M.H.; Han, S.Y. Development of a polysaccharide based multi-unit nanofiber mat for colon-targeted sustained release of salmon calcitonin. J. Colloid Interface Sci. 2019, 552, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Bokkhim, H.; Bansal, N.; Grondahl, L.; Bhandari, B. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2015, 52, 231–242. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.S.; Wen, P.; Feng, K.; Zong, Z.M.; Wu, H. Preparation and characterization of an electrospun colonspecific delivery system for salmon calcitonin. RSC Adv. 2018, 8, 9762–9769. [Google Scholar] [CrossRef]
- Singh, S.K.; Yadav, A.K.; Prudhviraj, G.; Gulati, M.; Kaur, P.; Vaidya, Y. A novel dissolution method for evaluation of polysaccharide based colon specific delivery systems: A suitable alternative to animal sacrifice. Eur. J. Pharm. Sci. 2015, 73, 72–80. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Yazdani, Y.; Mohammadi, Z.; Alivirdiloo, V.; Nikzad, B.; Mohammadzadeh, M. Fabrication of poly (lactic-co-glycolic acid)/mesoporous silica composite nanofibers for controllable co-delivery of 5-fluorouracil and curcumin against HT-29 colon cancer cells. J. Mater. Sci. 2024, 59, 2104–2120. [Google Scholar] [CrossRef]
- Abouaitah, K.; Swiderska-Sroda, A.; Farghali, A.A.; Wojnarowicz, J.; Lojkowski, W. Folic acid-conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget 2018, 9, 26466–26490. [Google Scholar] [CrossRef]
- Duan, D.Y.; Wang, A.P.; Ni, L.; Zhang, L.P.; Yan, X.J.; Jiang, Y.; Mu, H.J.; Wu, Z.M.; Sun, K.X.; Li, Y.X. Trastuzumaband Fab’ fragment-modified curcumin PEG-PLGA nanoparticles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2018, 13, 1831–1840. [Google Scholar] [CrossRef]
- Feng, R.L.; Deng, P.Z.; Song, Z.M.; Chu, W.; Zhu, W.X.; Teng, F.F.; Zhou, F.L. Glycyrrhetinic acid-modified PEG-PCL copolymeric micelles for the delivery of curcumin. React. Funct. Polym. 2017, 111, 30–37. [Google Scholar] [CrossRef]
- Chen, G.Q.; Li, J.L.; Cai, Y.B.; Zhan, J.; Gao, J.; Song, M.C.; Shi, Y.; Yang, Z.M. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci. Rep. 2017, 7, 44210. [Google Scholar] [CrossRef] [PubMed]
- Nandy, B.C.; Verma, V.; Dey, S.; Mazumder, B. Three levels face centered central composite design of colon targeted micro-particulates system of celecoxib: Screening of vehicles variables and in vivo studies. Curr. Drug Del. 2014, 11, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Sawarkar, S.P.; Deshpande, S.G.; Bajaj, A.N.; Soni, P.S.; Nikam, V.S. Potential of low molecular weight natural polysaccharides for colon targeted formulation and its evaluation in human by gamma scintigraphy. J. Pharm. Investig. 2019, 50, 173–187. [Google Scholar] [CrossRef]
- Handali, S.; Moghimipour, E.; Kouchak, M.; Ramezani, Z.; Dorkoosh, F.A. In vitro and in vivo evaluation of coated capsules for colonic delivery. J. Drug Deliv. Sci. Technol. 2018, 47, 492–498. [Google Scholar] [CrossRef]
- Du, B.; Du, Q.; Bai, Y.M.; Yu, L.L.; Wang, Y.H.; Huang, J.; Zheng, M.; Shen, G.P.; Zhou, J.; Yao, H.C. Chemotherapy based on “domino-effect” combined with immunotherapy amplifying the efficacy of an anti-metastatic treatment. Mater. Chem. B 2020, 8, 9139–9150. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhou, J.; Yang, C.; Wang, W.; Chu, L.; Huang, F.; Deng, L.; Kong, D.; Liu, J.; et al. Folic acid-targeted disulfide-based cross-linking micelle for enhanced drug encapsulation stability and site-specific drug delivery against tumors. Int. J. Nanomed. 2016, 11, 1119–1130. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 23, 6. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jäger, C.; Seguin-Devaux, C.; et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yu, Y.; Tian, N.; Huang, J.; Zhang, X.; Yu, R. Human microbiota-associated animal models: A review. Front. Cell. Infect. Microbiol. 2025, 15, 1644187. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Perri, A.; Di Agostino, S. New Therapeutic perspectives in prostate cancer: Patient-derived organoids and patient-derived xenograft models in precision medicine. Biomedicines 2023, 11, 2743. [Google Scholar] [CrossRef]
- Ranjit, R. Advancing Monoclonal Antibody Manufacturing: Process Optimization, Cost Reduction Strategies, and Emerging Technologies. Biol. Targets Ther. 2025, 19, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wagh, A.; Song, H.T.; Zeng, M.; Tao, L.; Das, T.K. Challenges and new frontiers in analytical characterization of antibody-drug conjugates. mAbs 2018, 10, 222–243. [Google Scholar] [CrossRef]
- Ben Mkaddem, S.; Benhamou, M.; Monteiro, R.C. Understanding Fc receptor involvement in inflammatory diseases: From mechanisms to new therapeutic tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Sripetthong, S.; Nalinbenjapun, S.; Basit, A.; Surassmo, S.; Sajomsang, W.; Ovatlarnporn, C. Preparation of self-assembled, curcumin-loaded nano-micelles using quarternized chitosan-vanillin imine (QCS-Vani Imine) conjugate and evaluation of synergistic anticancer effect with cisplatin. Funct. Biomater. 2023, 14, 525. [Google Scholar] [CrossRef]
- Ndeh, D.A.; Nakjang, S.; Kwiatkowski, K.J.; Sawyers, C.; Koropatkin, N.M.; Hirt, R.P.; Bolam, D.N. A Bacteroides thetaiotaomicron genetic locus encodes activities consistent with mucin O-glycoprotein processing and N-acetylgalactosamine metabolism. Nat. Commun. 2025, 16, 3485. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, H.J.; Zhu, P.; Liu, Y.J.; Zhang, Y.J.; Zhang, W.; Zhou, H.H.; Li, X.; Li, Q. Effect of gut microbiota on the pharmacokinetics of nifedipine in spontaneously hypertensive rats. Pharmaceutics 2023, 15, 2085. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K. Solid in Oil/Water Emulsion-Diffusion-Evaporation Formulation for Preparing Curcumin-Loaded PLGA Nanoparticles. U.S. Patent 20100290982A1, 18 November 2010. [Google Scholar]
- Sinko, P.J.; Gao, J.; Deshmukh, M.; Zhang, X.; Palombo, M.S.; Ibrahim, S. Synergistic Combinations to Reduce Particle Dose for Targeted Treatment of Cancer and Its Metastases. U.S. Patent 20120183621A1, 19 July 2012. [Google Scholar]
- Chauhan, S.; Jaggi, M.; Yallapu, M.M. Magnetic Nanoparticle Formulations, Methods for Making Such Formulations, and Methods for Their Use. U.S. Patent 20130245357A1, 19 September 2013. [Google Scholar]
- Braden, A.R.C.; Vishwanatha, J.K. Formulation of Active Agent Loaded Activated PLGA Nanoparticles for Targeted Cancer Nano-Therapeutics. U.S. Patent 9023395B2, 5 May 2015. [Google Scholar]
- Chang, R.; Sun, L.; Webster, T.J.; Mi, G. Amphiphilic Peptide Nanoparticles for Use as Hydrophobic Drug Carriers and Antibacterial Agents. U.S. Patent 20170202783A1, 20 July 2017. [Google Scholar]
- Huang, W.T.; Chiang, Y.C.; Liu, D.M. Nanocomposite, a Preparation Method Thereof and Method for Treating Cancer Using the Same. U.S. Patent 20180280517A1, 4 October 2018. [Google Scholar]
- Yin, J.; Qiao, Z.; Liu, H.; Mao, X.; Zha, J. Preparation and Application of a Reactive Oxygen Species-Responsive Polycurcumin Prodrug-Based Nanocarrier with Ultrahigh Drug Loading Capacity. CN110051855A, 26 July 2019. [Google Scholar]
- Wen, R.; Feng, L.; Lan, Y.; Liu, Y. A Nanocarrier Material for Co-Delivery of Curcumin and miRNA in Prostate Cancer Therapy with GSH Responsiveness and CT Imaging Capability. CN111686249A, 22 September 2020. [Google Scholar]
- Zhang, H.-G. Edible Plant Exosome-like Nanovectors for Vaccination. U.S. Patent 20210236612A1, 5 August 2021. [Google Scholar]
- Li, G.-C.; Huang, X.-Z.; Lü, L.; Shi, Y.-H.; Xu, J.-J.; Mao, Y.-P.; Meng, N.; Deng, Z.-C. Amphiphilic Block Copolymer-Based Nano-Carrier Simultaneously Targeting Tumor Cells and Cancer Stem Cells. CN116731325A, 12 September 2023. [Google Scholar]
- Liu, Y.; Chen, Z.; Zhang, X.; Li, H.; Yan, Z.; Wu, T. Targeted Nano-Carrier System Constructed from Dopamine Derivatives for Enhanced Delivery of Hydrophobic Drugs. Chinese Patent CN116925337B, 1 December 2023. [Google Scholar]
- Sun, C.C.; Liu, D.M. Injectable Nanocomposite Gel Composition for Co-Delivery of Multiple Medicines or Drugs. U.S. Patent 20240226027A1, 11 July 2024. [Google Scholar]
- Xiao, J.X.; Huang, G.Q.; Cao, Y.Q.; Li, K.Y. Curcumin-Loaded Pickering Emulsion with Colon-Targeted Delivery Function and Preparation Method and Application Thereof. Chinese Patent CN111000799B, 30 November 2021. [Google Scholar]
- Xiao, J.X.; Wang, L.H.; Huang, G.Q.; Zhang, X.R. Curcumin Double-Layer Emulsion with Colon-Targeted Delivery Function and Preparation Method and Application Thereof. Chinese Patent CN111096950B, 30 November 2021. [Google Scholar]
- Zhan, X.B.; Li, H.; Zhu, L.; Gao, M.J.; Jiang, Y. pH- and Microbe-Dual-Responsive Colon-Targeted Micelles and Preparation Method Thereof. Chinese Patent CN115364051B, 28 May 2024. [Google Scholar]
- Li, B.; Yuan, Y.; Zhang, Y.; Li, L.; Zhang, X.; Guo, Q.Y.; Zheng, Q.S. Colon-Targeted Oil-in-Water Pickering Emulsion Based on Shellac Nanoparticles and Chitosan and Its Preparation and Application. Chinese Patent CN115364054B, 24 November 2023. [Google Scholar]
| Targeting Ligand | Overexpressed Receptor | Targeted Cells | Nanocarrier (NC) | Effect | References | |
|---|---|---|---|---|---|---|
| Antibody | Trastuzumab | HER-2 | Breast cancer cells (BT-474 cells) | CUR nanoparticles prepared by wet milling–solvent evaporation process | Improved cell diagnosis | [19] |
| Anti-CD326 | mAb-CD326 | Breast cancer tumors | Glutathione-stimulated responsive nanocarrier | Improved cell targeting capacity | [20,21,22,23] | |
| Single-chain fragment variable (scFv) antibody | VP28 | White spot syndrome virus (WSVV) | Carbon nanotubes | |||
| Anti-HER2-FITC | Anti-HER2-FITC antibody | Several tumor cells | Lipid liquid nanoemulsion | |||
| PSMA monoclonal antibody | PSMA | Prostate cancer tumor cells | Peptide-based conjugated nanoparticles | |||
| αDEC205 antibody | αDEC205 | Dendritic cells | Bacteria-derived outer membrane vesicles | Remodeled dendritic cell uptake pattern | [24] | |
| Growth factor | EGF | VEGF receptor | Human A-431 tumor cells | Lipodisks | Improved cell targeting capacity | [25,26,27,28] |
| Cetuximab | VEGF receptor | Mutant KRAS PANC-1 tumors | Lipodisks | |||
| K237/RGD/cRGD/LyP-1/Bombesin | Integrin/Annexin1/Integrin β6 | Glioma/melanoma/ovarian cancer | Chitosan nanoparticles/polytyrosine nanoparticles | |||
| Plectin-1-targeting peptide | Plectin-1 | Pancreatic tumor cells | Peptide nanoparticle platform | |||
| Peptide/Protein | Cyclic RGD | Integrins | Various types of cancer cells | Liposome | Improved blood–retinal barrier permeability | [29] |
| GE11 Peptides | Epidermal growth factor receptor (EGFR) | Liver tumor SMMC7721 cells | Polymersome/liposome | Increased cellular accumulation and antitumor activity | [30,31,32,33,34,35,36,37,38,39,40,41,42] | |
| Octreotide | Somatostatin receptor | MCF-7 cell lines | Silver nanoparticles | |||
| Cell-penetrating peptides (e.g., Penetratin, Polyarginine, Transportan, Pep-7, HIV-1TAT) | TFR | Numerous cancer cells | Liposome | |||
| Tumor-homing peptide | Neuropilin-1 | Tumor blood vessel | Lipid–polymer hybrid nanoparticles | |||
| Transferrin | Transferrin receptor | Tumor in intracranial orthotopic models/gliomas | DSPE-PEG2k nanoparticles/PEGylated liposome | |||
| Aptamer | Lectin | Cell surface glycans | Glioblastoma phenotype astrocyte cells | Lectin-coated fluorescent nanodiamonds | ||
| Anti-EGFR aptamer | EGFR | Various tumor cells | DNA nanotube framework | |||
| AS1411 | Nucleolin receptor | Breast cancer cell | Silver nanotriangles | |||
| Mucin 1 (MUC1) aptamer | MUC1 | Lung cancer cells | PLA-PEG nanocarriers | |||
| Vitamin | Folic acid | Folic acid receptor | Various tumor cells | Liposome | ||
| Biotin | biotin receptor | Hepatocellular carcinoma | Brush copolymer nanocarrier | |||
| Sugar | Galactose | Asialoglycoprotein receptors | Various cancer cells | Micelles, polymeric nanoparticles, SLN, liposomes, etc. | ||
| Hyaluronic acid | Glycoprotein CD44 | Cu-doped zeolite imidazole framework-8 | ||||
| Acetylated konjac glucomannan | Mannose receptors | Macrophages | AceKGM nanoparticles | Improved colonic macrophage targeting | [43] | |
| Glycosylamine | Glucosamine | Glucose transporter 2 | Breast cancer cells | Mesoporous silica-coated magnetic nanoparticle | Increased tumor targeting and MRI visualization | [44] |
| N-acetyl-glycamide | Lung cancer cells | Micelles | Increased cellular accumulation and antitumor activity | [45,46,47,48,49,50] | ||
| Dual/multi-ligand | Glycyrrhetinic acid and peanut agglutinin | Glycyrrhetinic acid receptor, MUC1 | Hepatoma carcinoma cells | Liposomes | ||
| Folic acid and lactobionic acid | asialoglycoprotein receptors and folate receptors | Galactosylated chitosan nanoparticles | ||||
| Anti-carbonic anhydrase IX (anti-CA IX) antibody and CPP33 | Carbonic anhydrase IX | Lung cancer | Liposome | |||
| Anti-CD133 and folic acid | CD133 and folate receptor | Colon cancer cells | Hybrid biomimetic nanomedicine | |||
| Hyaluronic acid, SYL3C and CL4 | CD44/epithelial cell adhesion molecule/EGFR | Stem tumor cells/epithelial cancer cells | Multi-targeting nanosystem |
| Category | Targeting Stimuli | Formulation Strategy | Key Findings | References |
|---|---|---|---|---|
| pH-responsive | Gradual pH increase from stomach to colon | Core–shell microparticles with shellac (pH-sensitive polymer) coating | Shellac coating delayed CUR release before colon Burst release occurred at colonic pH Enhanced colonic accumulation and anti-inflammatory efficacy in DSS-induced colitis mice | [102] |
| Carboxymethyl curdlan–quercetin conjugate-stabilized Pickering emulsion | Stable in acidic gastric fluid Released CUR in simulated colonic fluid | [110] | ||
| Tumor microenvironment (acidic pH) | CUR-loaded aerogel functionalized with pH-responsive cell-penetrating peptide (PLP) and coated with trehalose | Trehalose coating prevented drug release in acidic pH PLP enhanced cellular uptake at tumor site Showed superior cytotoxicity against HT29 | [111] | |
| Microbiota-active | Gut microbiota metabolism | Curcumin and resveratrol-co-loaded Mesona chinensis polysaccharides/zein nanoparticles | Enhanced gastrointestinal stability Promoted short-chain fatty acid production Modulated gut microbiota to alleviate UC | [112] |
| Xylanase-producing Bacteroides spp. | Xylan–chitosan-coated P-123 micelles | ≤85% CUR retained in upper GIT; 27–33% triggered release by B. ovatus in colon | [113] | |
| Enzyme-responsive | Anaerobic azo-reductase | Mixed micelles | 76% CUR released in rat colon fluid Strong mucoadhesion and anti-UC efficacy | [114] |
| Pectinase in colon | Bilayer W1/O/W2 emulsion with pectin outer shell | 90% CUR in colon | [115] | |
| Pectin–chitosan hydrogel encapsulating folate-modified CUR liposomes (FA-NLC@MPs) | FA-NLC@MPs protected CUR in upper GI tract Pectin degraded in colon, releasing FA-NLC for CD44-targeted uptake and enhanced colitis treatment | [116] | ||
| α-amylase overexpressed in UC mucosa | Hydroxyethyl starch–CUR microspheres | 80% cargo released in α-amylase-rich colon fluid 2.5-fold higher accumulation in inflamed colon | [117] | |
| β-glucanase in colon | Mucoadhesive tablets using jackfruit–okra mucilage blend | Tablets remained intact in stomach and small intestine Swelling and degradation occurred in colon due to microbial enzymes Enhanced CUR release in presence of cecal content | [118] | |
| Redox-responsive | Inflamed colon environment (elevated ROS, α-amylase) | Microcapsules based on hydroxyethyl starch–CUR | Microcapsules released drugs in response to α-amylase in colon Preferentially accumulated in inflamed tissue via EPR effect Significantly reduced inflammation and restored colon length in DSS-induced colitis mice | [100] |
| pH/mucoadhesive dual-responsive | pH gradient and mucus layer adhesion | CUR-loaded microparticles using Eudragit® FS and polycaprolactone | Colon-specific release Reduced oxidative stress and inflammation in acetic acid-induced UC rat model | [119] |
| Microbiota/enzyme dual-responsive | Colonic microbiota and pectinase enzymes | Low-methoxyl pectin (LMP) prepared through hydrostatic pressure-assisted enzymatic reaction (HHP) gelled with calcium | HHP-LMP beads resisted premature release in upper GI tract Degraded by colonic enzymes, enabling site-specific release | [120] |
| β-glucanase and microbial fermentation in colon | Yeast cell wall β-glucan capsules + alginate/chitosan layer (“bionic yeast”) | β-glucan core resists gastric digestion Enzymatic degradation in colon, sustained 50% CUR release at 4 h and 14-fold higher local concentration vs. CUR | [121] | |
| pH/enzyme dual-responsive | Colonic pH and pectinase | Guar gum-low methoxyl pectin/alginate–chitosan microgels loaded with porous starch–CUR | 24 h colon retention F/B ratio restored TNF-α in DSS mice | [104] |
| Type | Patent Number | Legal State | Key Innovation Points | Evaluation | References |
|---|---|---|---|---|---|
| Activing targeting to cancer cells | US20100290982A1 | Publication | CUR-NPs prepared by a novel S-O/W emulsion–diffusion–evaporation method. Dual-functional crosslinker conjugates CUR-NPs to targeting ligands for active tumor targeting. | In vitro uptake and cytotoxicity in breast cancer cell lines | [152] |
| US20120183621A1 | Publication | Nanocurcumin prepared via flash nanoprecipitation. Dual active CD44/CXCR4 targeting via DV3 surface modification of nanocurcumin. | In vitro cellular uptake, cytotoxicity, apoptosis, and metastasis inhibition assays In vivo orthotopic lung cancer mouse model | [153] | |
| US20130245357A1 | Publication | Loading CUR onto magnetic nanoparticles enables targeted delivery to tumor tissues under the guidance of an external magnetic field. | In vitro cell uptake, cytotoxicity In vivo biodistribution, anti-tumor efficacy. | [154] | |
| US09023395B2 | Grant | CUR-loaded PLGA NPs prepared via optimized double-emulsion solvent evaporation. Surface functionalized with bis-succinimidyl suberate and conjugated with Thy-1 antibodies for targeting. | In vitro uptake studies | [155] | |
| US20170202783A1 | Publication | CUR was loaded in amphiphilic peptide nanoparticles self-assembled from C18GR7RGDS peptide. RGDS motif targets αvβ3 integrins on osteosarcoma and other cancers. | In vitro cell uptake, cytotoxicity, and selectivity | [156] | |
| US20180280517A1 | Publication | Hydrophobic glycosaminoglycans form nanoparticles encapsulating CUR through self-assembly. The cancer cell-targeting molecule (e.g., EGFR antibody, CD-133 antibody, PD-L1 antibody) is bound to the nanoparticle through a crosslinking agent. | In vitro cytotoxicity assay In vivo tumor growth inhibition | [157] | |
| CN110051855A | Grant | CUR, as a copolymer monomer, is used to construct prodrug structures to achieve extremely high drug loading capacity. ROS-responsive cleavage of the oxalate ester bond enables precise drug release upon H2O2 stimulation. | In vitro release under H2O2 and/or NIR light | [158] | |
| CN111686249A | Grant | GSH-responsive DSPAMAM dendrimer was integrated with gold nanorods (GNRs) featuring exceptional optical properties. GNRs further encapsulated with a mesoporous silica shell to sequester residual cetyltrimethylammonium bromide and to load CUR. RGD peptide was conjugated to the targeting ligand, enabling efficient cancer diagnosis and synergistic chemo-gene combination therapy. | In vitro cytotoxicity | [159] | |
| US20210236612A1 | Publication | CUR was encapsulated in edible plant-derived exosome-like nanoparticles (EPELNs). Active targeting achieved by coating EPELNs with plasma membranes derived from tumor cells. | In vivo assay: assessing the inhibition of tumor growth and metastasis | [160] | |
| CN116731325A | Publication | An amphiphilic block copolymer (HA-b-PCDA) including poly(curcumin dicarboxylic anhydride) (hydrophobic segment) and hyaluronic acid (hydrophilic block) was prepared through a robust amide bond/. HA-b-PCDA exhibits dual CD44 targeting of tumor and cancer stem cells. | In vitro cellular uptake and cytotoxicity | [161] | |
| CN116925337B | Publication | A dopamine-derivative-based nanocarrier encapsulating CUR was constructed. Dopamine-derivative-based nanocurcumin extended CUR solubility and half-life. Dopamine-derivative-based nanocurcumin targeted FA, a receptor overexpressed in cancer cells. | In vitro drug release profiles at different pH values | [162] | |
| US20240226027A1 | Publication | CUR-loaded injectable hydrogel nanoparticles fabricated via self-assembly of oleic acid-conjugated alginate. 100% drug loading achieved without any excipients. Trastuzumab was covalently linked to the gel surface via EDC/NHS chemistry for breast cancer targeting. | In vivo evaluation using SKBR-3 xenograft mouse model. | [163] | |
| Colon targeted delivery | CN111000799B | Grant | Soft solid particles formed from ovalbumin–carboxymethyl konjac glucomannan complexes crosslinked with dihydromyricetin. CUR was loaded in a Pickering emulsion stabilized by the soft solid particles. | In vitro simulated gastrointestinal release assay In vivo oral administration in mice, assessing relative bioavailability of CUR | [164] |
| CN111096950B | Grant | CUR-loaded double-layer emulsion was constructed using whey protein isolate (WPI) and carboxymethyl konjac glucomannan (CMKGM) as the inner/outer emulsifiers. The outer CMKGM layer provides enzyme-responsive targeting, while the inner WPI layer offers emulsifying stability and pH-sensitive protection. | In vitro simulated gastrointestinal release assay In vivo oral administration in mice, assessing relative bioavailability of CUR | [165] | |
| CN115364051B | Grant | A novel amphiphilic octenyl succinic anhydride-modified curdlan oligosaccharide (OSA-COS) was synthesized as a nanocurcumin. The system showed colon-targeted release due to dual triggers: pH change and microbial degradation in the colon. | In vitro pH-dependent release assay In vitro fecal fermentation, indicating microbiota-triggered release and prebiotic-like effects | [166] | |
| CN115364054B | Grant | A novel oil-in-water Pickering emulsion was developed using shellac nanoparticles and chitosan as stabilizers. CUR was encapsulated into shellac nanoparticles via antisolvent precipitation. The system enabled colon-targeted release and alleviated ulcerative colitis symptoms. | In vitro pH-dependent release assay In vivo oral administration in DSS-induced ulcerative colitis mice, assessing disease activity index | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.-S.; Liu, K.-L.; Feng, K.; Wang, Y. Active Targeting Strategies for Improving the Bioavailability of Curcumin: A Systematic Review. Foods 2025, 14, 3331. https://doi.org/10.3390/foods14193331
Wei Y-S, Liu K-L, Feng K, Wang Y. Active Targeting Strategies for Improving the Bioavailability of Curcumin: A Systematic Review. Foods. 2025; 14(19):3331. https://doi.org/10.3390/foods14193331
Chicago/Turabian StyleWei, Yun-Shan, Kun-Lun Liu, Kun Feng, and Yong Wang. 2025. "Active Targeting Strategies for Improving the Bioavailability of Curcumin: A Systematic Review" Foods 14, no. 19: 3331. https://doi.org/10.3390/foods14193331
APA StyleWei, Y.-S., Liu, K.-L., Feng, K., & Wang, Y. (2025). Active Targeting Strategies for Improving the Bioavailability of Curcumin: A Systematic Review. Foods, 14(19), 3331. https://doi.org/10.3390/foods14193331





