Ultrasound-Assisted Green Natural Deep Eutectic Solvent Extraction of Flavonoids from Wild Blueberry: Process Optimization, Composition Identification, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of NADESs
2.3. Screening of NADESs
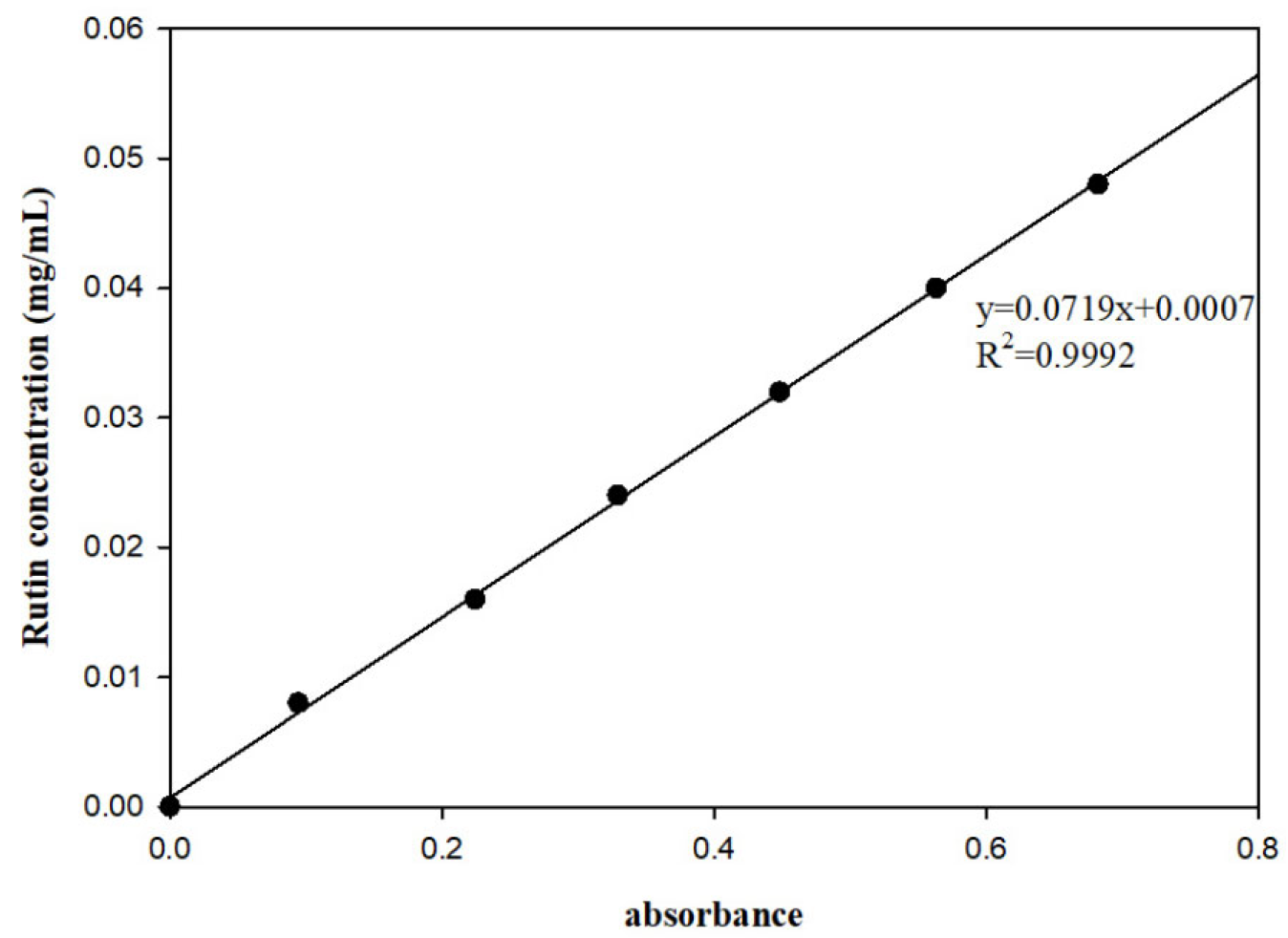
2.4. Extraction and Determinationof Total Flavonoids from Blueberries
2.5. Optimization of UANE Conditions for Total Flavonoid Content
2.5.1. Single Factor Experiments Design
2.5.2. Response Surface Methodology Experiments
2.6. Identification of Main Flavonoids Relative Content Using LC-MS/MS Untargeted Metabolomics
2.7. FT-IR Spectroscopy and Differential Scanning Calorimeter (DSC)
2.8. In Vitro Antioxidant Activity
2.8.1. Hydroxyl Radical Scavenging Capacity
2.8.2. DPPH Radical Scavenging Activity Measurements
2.8.3. ABTS Radical Scavenging Activity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Selection of NADESs for the Extraction of Total Flavonoid Content from Wild Blueberries
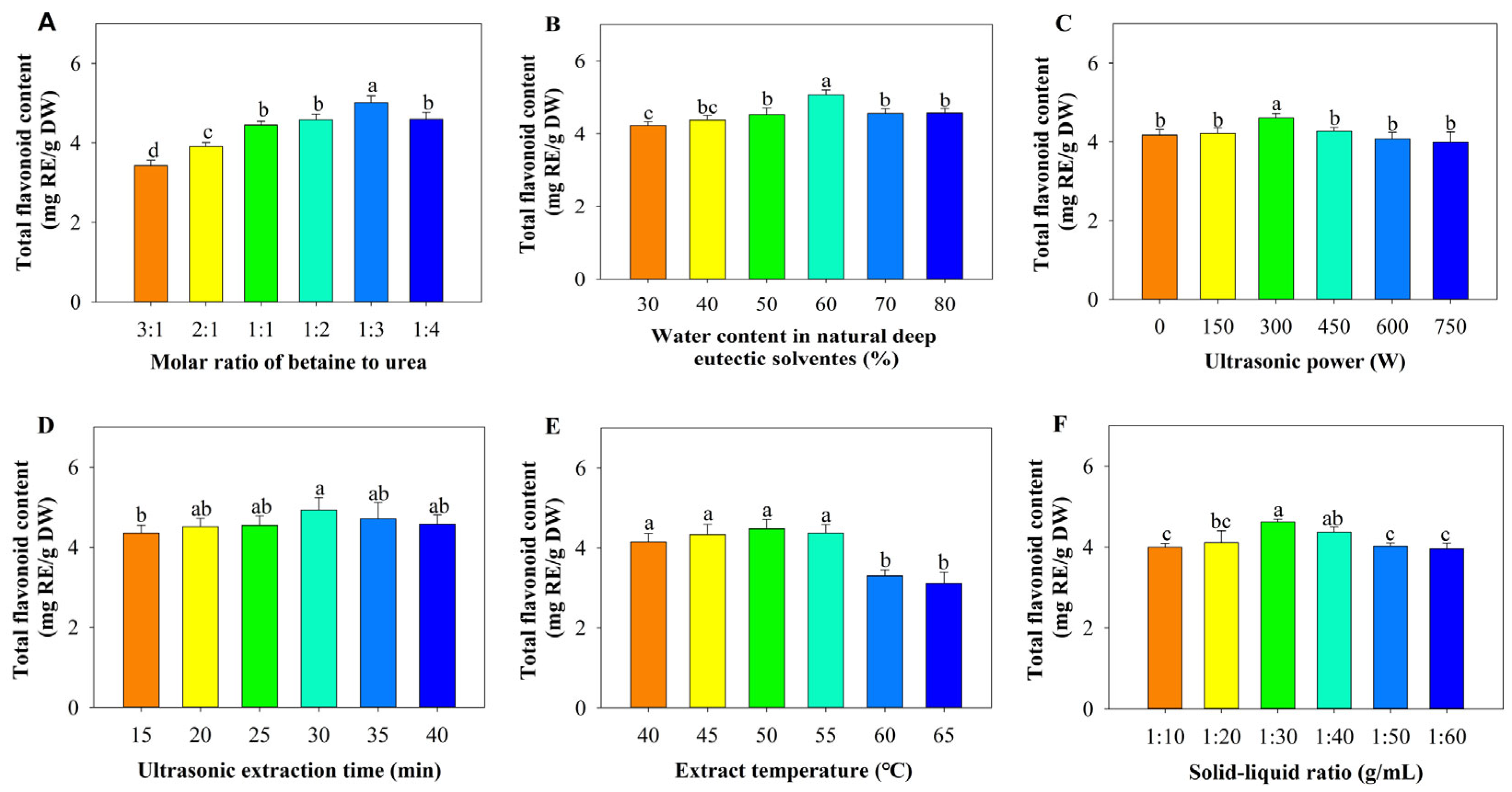
3.2. Single Factor Experiments
3.3. Response Surface Optimization of Total Flavonoid Extraction
3.4. Utilizing LC-MS/MS-Based Untargeted Metabolomics for the Identification and In-Depth Analysis of Primary Flavonoid Compounds
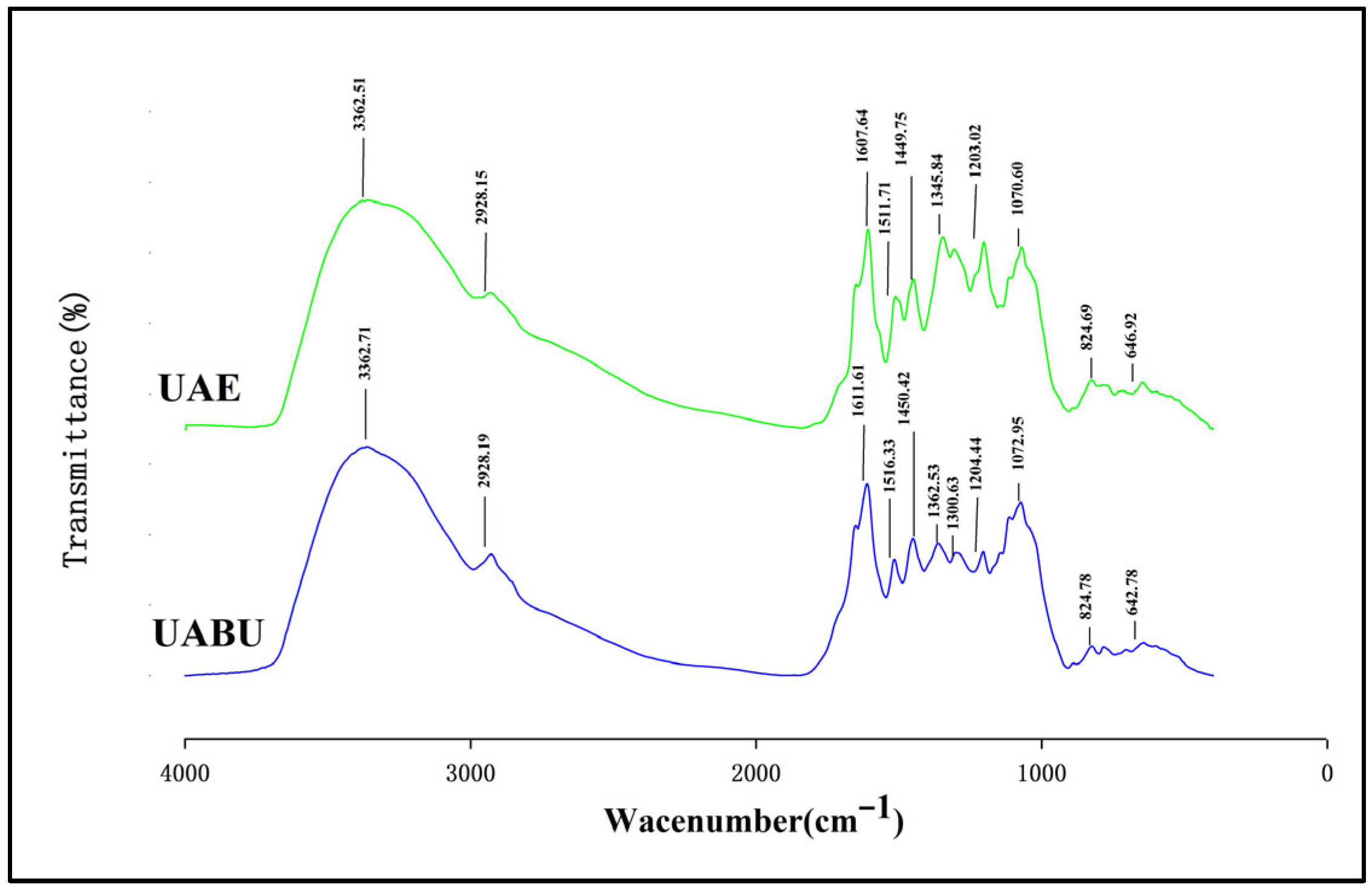
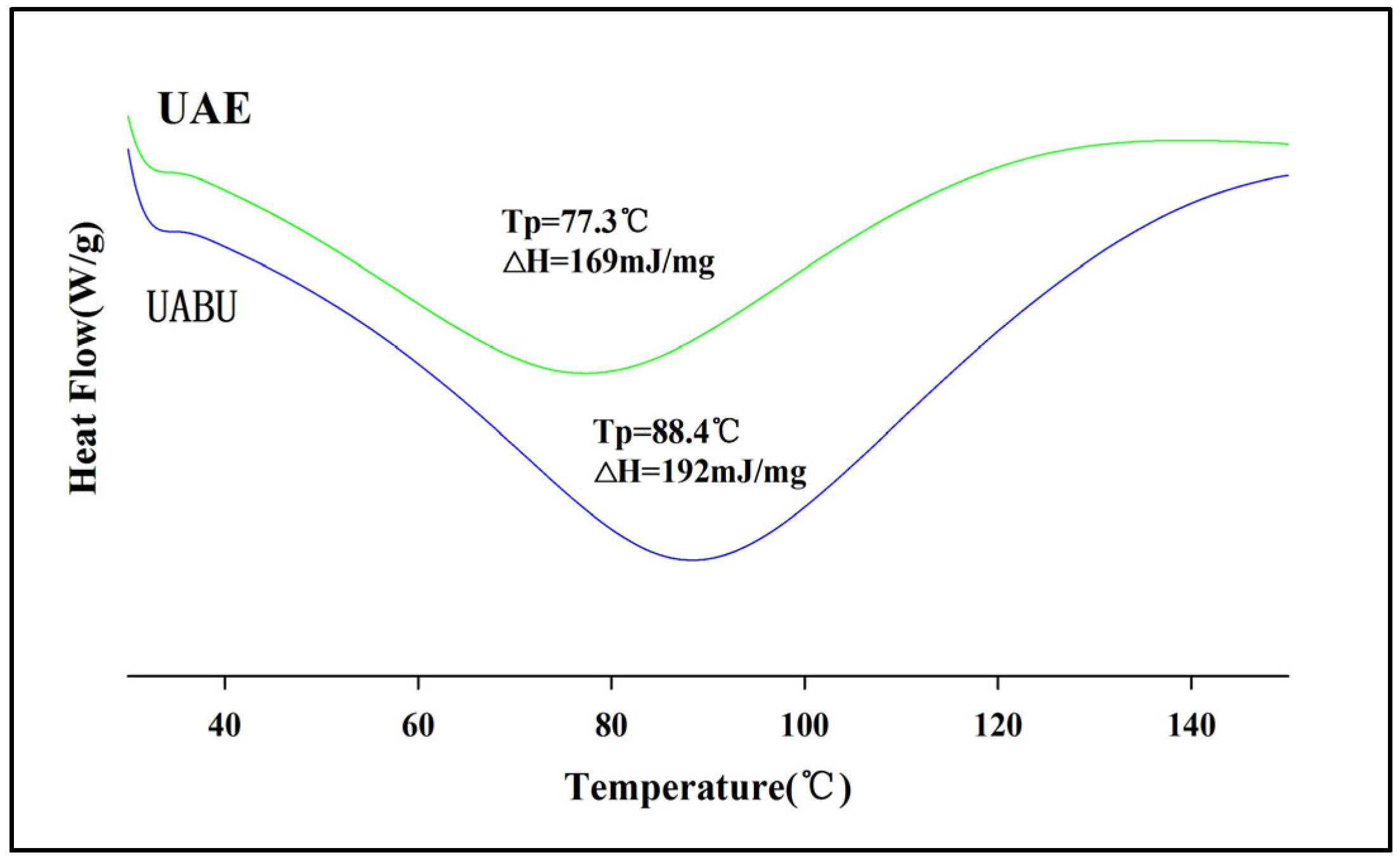
3.5. FTIR Spectra and DSC Analysis
3.6. In Vitro Antioxidant Activities
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The research progress of extraction, purification and analysis methods of phenolic compounds from Blueberry: A comprehensive review. Molecules 2023, 28, 3610. [Google Scholar] [CrossRef]
- Adhikari, J.; Araghi, L.R.; Singh, R.; Adhikari, K.; Patil, B.S. Continuous-flow high-pressure homogenization of Blueberry juice enhances anthocyanin and ascorbic acid stability during cold storage. J. Agric. Food Chem. 2024, 72, 11629–11639. [Google Scholar] [CrossRef]
- Liu, Y.X.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.H.; Li, J.F. Flavonoids: Potential therapeutic agents for cardiovascular disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef]
- Franco, J.G.; Cefali, L.C.; Ataide, J.A.; Santini, A.; Souto, E.B.; Mazzola, P.G. Effect of nanoencapsulation of blueberry (Vaccinium myrtillus): A green source of flavonoids with antioxidant and photoprotective properties. Sustain. Chem. Pharm. 2021, 23, 100515. [Google Scholar] [CrossRef]
- Huang, W.Y.; Wu, H.; Li, D.J.; Song, J.F.; Xiao, Y.D.; Liu, C.Q.; Sui, Z.Q. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef]
- Du, J.; Leng, J.Y.; Zhang, L.; Bai, G.X.; Yang, D.; Lin, H.; Qin, J.J. Angiotensin II-induced apoptosis of human umbilical vein endothelial cells was inhibited by blueberry anthocyanin through Bax- and Caspase 3-dependent pathways. Med. Sci. Monitor. 2016, 22, 3223–3228. [Google Scholar] [CrossRef][Green Version]
- Bharat, D.; Cavalcanti, R.R.M.; Petersen, C.; Begaye, N.; Cutle, B.R.; Costa, M.M.A.; Ramos, R.K.L.G.; Ferreira, M.R.; Li, Y.Y.; Bharath, L.P.; et al. Blueberry metabolites attenuate lipotoxicity-Induced endothelial dysfunction. Mol. Nutr. Food Res. 2017, 62, 1700601. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, M.; Sun, Q.; Mujumdar, A.S.; Yu, D. Extraction of functional extracts from berries and their high quality processing: A comprehensive review. Crit. Rev. Food Sci. 2022, 63, 7108–7125. [Google Scholar] [CrossRef]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs): Designer solvents for green extraction of anthocyanin. Sustain Chem Pharm. 2023, 34, 101168. [Google Scholar] [CrossRef]
- Doldolova, K.; Bener, M.; Lalikoglu, M.; Asci, Y.S.; Arat, R.; Apak, R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; House, J.D.; Aluko, R.E.; Bandara, N. Improved protein extraction technology using deep eutectic solvent system for producing high purity fava bean protein isolates at mild conditions. Food Hydrocoll. 2024, 147, 109283. [Google Scholar] [CrossRef]
- Fu, X.Z.; Belwal, T.; He, Y.H.; Xu, Y.Q.; Li, L.; Luo, Z.S. UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: Composition, extraction mechanism and in vitro biological activities. Food Chem. 2022, 370, 131042. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.H.; Feng, S.M.; Liu, G.D.; Chen, B.L.; Li, Z.H.; Shao, P. Deep eutectic solvent on extraction of flavonoid glycosides from Dendrobium officinale and rapid identification with UPLC-triple-TOF/MS. Food Chem. 2023, 401, 134054. [Google Scholar] [CrossRef] [PubMed]
- Foroutani, Z.; Mogaddam, M.R.A.; Ghasempour, Z.; Ghareaghajlou, N. Application of deep eutectic solvents in the extraction of anthocyanins: Stability, bioavailability, and antioxidant property. Trends Food Sci. Technol. 2024, 144, 104324. [Google Scholar] [CrossRef]
- Xia, B.; Liu, Q.; Sun, D.; Wang, Y.; Wang, W.J.; Liu, D.H. Ultrasound-assisted deep eutectic solvent extraction of polysaccharides from Anji white tea: Characterization and comparison with the conventional method. Foods 2023, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.L.; Wang, X.G.; Wang, W.M.; Wang, Y.Y.; Huang, J.J.; Zhou, R.G.; Bo, R.N.; Liu, M.J.; Yin, S.J.; Li, J.G. Comparison, optimization and antioxidant activity of ultrasound-assisted natural deep eutectic solvents extraction and traditional method: A greener route for extraction of flavonoid from Moringa oleifera Lam. Leaves. Ultrason Sonochem. 2024, 109, 107003. [Google Scholar] [CrossRef]
- Rashid, R.; Wani, S.M.; Manzoor, S.; Masoodi, F.A.; Dar, M.M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kočar, D.; Kolar, M. Extraction of bioactive metabolites from Achillea millefolium L. with choline chloride based natural deep eutectic solvents: A study of the antioxidant and antimicrobial activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Niu, D.B.; Wang, R.; Xu, F.Y.; Chen, B.R.; Lin, J.W.; Tang, Z.S.; Zeng, X.A. Efficient and green strategy based on pulsed electric field coupled with deep eutectic solvents for recovering flavonoids and preparing flavonoid aglycones from noni-processing wastes. J. Clean Prod. 2022, 368, 133019. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.Z.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.H.; Zhao, Y.; Qin, X.H.; Li, J.F.; Park, J.H.; et al. Ultrasonic-assisted extraction of flavonoids from peanut leave and stem using deep eutectic solvents and its molecular mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, S.; Hu, J.; Liu, G.; Peng, S.; Chen, H.; Jiang, Z.; Wang, T.; Ye, Q.; Zhu, H. Natural deep eutectic solvent-based microwave-assisted extraction of total flavonoid compounds from spent sweet potato (Ipomoea batatas L.) leaves: Optimization and antioxidant and bacteriostatic activity. Molecules 2022, 27, 5985. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Huang, R.; Wu, W.; Shen, S.; Fan, J.; Chang, Y.; Chen, S.; Ye, X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods 2018, 10, 2575–2587. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.X.; Li, N.; Hou, X.G.; Wang, D.M.; Li, D.W.; Liu, J.J. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, K.H.; Jiang, Y.; Yang, Z.W.; Hu, W.B.; Xiong, L.; Wang, N.; Liu, X.; Wang, W.J. Constituent analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on α-glucosidase activity. Int. J. Biol. Macromol. 2017, 98, 829–836. [Google Scholar] [CrossRef]
- Sun, X.J.; Fu, M.; Lou, S.H.; Li, D.D.; Han, X.; Gao, S.; Xiu, J.H.; Wang, J.F.; Ren, Y.Y. Optimization of flavonoids extracted from hawthorn (Crataegus pinnatifida) by ultrasonic-assisted deep eutectic solvent. Food Biosci. 2024, 59, 103767. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wang, D.D.; Fang, J.X.; Song, Z.X.; Geng, J.M.; Zhao, J.F.; Fang, Y.F.; Wang, C.T.; Li, M. Green and efficient extraction of flavonoids from Perilla frutescens (L.) Britt. leaves based on natural deep eutectic solvents: Process optimization, component identification, and biological activity. Food Chem. 2024, 452, 139508. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhao, M.T.; Wang, X.W.; Li, C.; Wang, J.M.; Liu, Z.Y.; Shen, X.R.; Zhou, D.Y. Response surface methodology-optimized extraction of flavonoids with antioxidant and antimicrobial activities from the exocarp of three genera of coconut and characterization by HPLC-IT-TOF-MS/MS. Food Chem. 2022, 391, 132966. [Google Scholar] [CrossRef]
- Yang, Y.H.; Liang, Q.M.; Zhang, B.; Zhang, J.M.; Fan, L.; Kang, J.H.; Lin, Y.Q.; Huang, Y.; Tan, T.C.; Ho, L.H. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef]
- SYARPIN; Permatasari, S.; Pujianto, D.A. Analysis of phytochemical constituents and antioxidant activity from the fractions of Luvunga sarmentosa root extract using LCMS/MS. Biodiversitas J. Biol. Divers. 2023, 24, 733–740. [Google Scholar] [CrossRef]
- Zhang, C.G.; Xie, Y.T.; Liu, D.Y.; Liu, R.X.; Han, J.C. Effects of Drying Process and High Hydrostatic Pressure on Extraction of Antioxidant Ergothioneine from Pleurotus citrinopileatus Singer. Foods 2024, 13, 878. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, B.G.; Che, K.D.; Gao, W.; Luo, H.Y.; Yang, J.L.; Chen, Z.J.; Hu, W.Z. Torreya grandis seed polyphenols protect RAW264.7 macrophages by inhibiting oxidative stress and inflammation. Food Sci. Nutr. 2025, 13, e70682. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zhang, Y.X.; Zhang, B.W.; Bao, Y.; Xu, S.T.; Tang, X.; Zhao, Q.; Li, J.; Li, R. In vitro antioxidative activity of Fritillaria cirrhosa D. Don straw ethanolic extract and its effect on lipid, protein oxidation, and quality of Chinese-style sausage. J. Food Sci. 2023, 8, 4745–4772. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhe, W.; Zhang, R.F.; Peng, Z.T.; Wang, Y.X.; Gao, H.Q.; Guo, Z.Q.; Xiao, J. Ultrasonic-assisted extraction of polyphenolic compounds from Paederia scandens (Lour.) Merr. using deep eutectic solvent: Optimization, identification, and comparison with traditional methods. Ultrason. Sonochem. 2022, 86, 106005. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (NADES): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- El-Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Qu, H.; Wu, Y.; Luo, Z.S.; Dong, Q.Y.; Yang, H.L.; Dai, C.Y. An efficient approach for extraction of polysaccharide from abalone (Haliotis Discus Hannai Ino) viscera by natural deep eutectic solvent. Int. J. Biol. Macromol. 2023, 244, 125336. [Google Scholar] [CrossRef]
- Liu, C.; Qiao, L.; Gao, Q.; Zhang, F.; Zhang, X.; Lei, J.; Ren, M.D.; Xiao, S.J.; Kuang, J.X.; Deng, S.X.; et al. Total biflavonoids extraction from Selaginella chaetoloma utilizing ultrasound-assisted deep eutectic solv ent: Optimization of conditions, extraction mechanism, and biological activity in vitro. Ultrason. Sonochem. 2023, 98, 106491. [Google Scholar] [CrossRef]
- Li, M.C.; Zhu, C.Y.; Fu, T.T.; Gao, X.Q.; Ma, Y.G. Effect of water on amine-based deep eutectic solvents (choline chloride + monoethanolamine): Structure and physicochemical properties. J. Environ. Chem. Eng. 2022, 10, 106952. [Google Scholar] [CrossRef]
- Xue, H.; Li, J.P.; Wang, G.Y.; Zuo, W.M.; Zeng, Y.; Liu, L.K. Ultrasound-assisted extraction of flavonoids from Potentilla fruticosa L. using natural deep eutectic solvents. Molecules 2022, 27, 5794. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.C.; Ding, G.J.; Quan, W.X. Artificial intelligence assisted ultrasonic extraction of total flavonoids from Rosa sterilis. Molecules 2021, 26, 3835. [Google Scholar] [CrossRef]
- Lai, J.J.; Zhou, P.; Li, X.Z.; Lu, Y.; Wang, Y.Q.; Yuan, H.; Yang, Y.H. Ultrasound-assisted deep eutectic solvent extraction of flavonol glycosides from Ginkgo Biloba: Optimization of efficiency and mechanism. Ultrason. Sonochem. 2025, 114, 107254. [Google Scholar] [CrossRef]
- Cai, B.W.; Mazahreh, J.; Ma, Q.Y.; Wang, F.; Hu, X. Ultrasound-assisted fabrication of biopolymer materials: A review. Int. J. Biol. Macromol. 2022, 209, 1613–1628. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.J.; Li, Y.; Gao, M.Z.; Meng, Y.; Li, J.; Zhang, S.D.; Wang, W. Negative pressure cavitation based ultrasound-assisted extraction of main flavonoids from Flos Sophorae Immaturus and evaluation of its extraction kinetics. Sep. Purif. Technol. 2020, 244, 115805. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Z.; Liu, Y.Z.; Dong, B.; Yang, C.Q.; Li, H.H. Ultrasound-assisted extraction, optimization, and purification of total flavonoids from Daphne Genkwa and analysis of their antioxidant, anti-inflammatory, and analgesic activities. Ultrason. Sonochem. 2024, 111, 107079. [Google Scholar] [CrossRef]
- Cao, Y.J.; Song, Z.Y.; Dong, C.J.; Ni, W.J.; Xin, K.Q.; Yu, Q.L.; Han, L. Green ultrasound-assisted natural deep eutectic solvent extraction of phenolic compounds from waste broccoli leaves: Optimization, identification, biological activity, and structural characterization. LWT 2023, 190, 115407. [Google Scholar] [CrossRef]
- Bin Mokaizh, A.A.; Nour, A.H.; Kerboua, K. Ultrasonic-assisted extraction to enhance the recovery of bioactive phenolic compounds from Commiphora Gileadensis Leaves. Ultrason. Sonochem. 2024, 105, 106852. [Google Scholar] [CrossRef]
- Jiang, W.J.; Liu, K.X.; Huan, W.W.; Wu, X.Y.; Zhu, M.W.; Tao, H.; Song, L.L.; Gao, F. Specific Extraction of bioactive flavonoids from Torreya Grandis pomace using magnetic nanoparticles modified with a ChCl/Acetamide deep eutectic solvent. LWT 2024, 211, 116914. [Google Scholar] [CrossRef]
- Lei, J.; Wang, Y.M.; Li, W.W.; Fu, S.B.; Zhou, J.Q.; Lu, D.M.; Wang, C.H.; Sheng, X.N.; Zhang, M.S.; Xiao, S.J.; et al. Natural green deep eutectic solvents-based eco-friendly and efficient extraction of flavonoids from Selaginella Moellendorffii: Process optimization, composition identification and biological activity. Sep. Purif. Technol. 2022, 283, 120203. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction ofphenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Sukor, N.F.; Jusoh, R.; Kamarudin, N.S.; Abdul Halim, N.A.; Sulaiman, A.Z.; Abdullah, S.B. Synergistic effect of probe sonication and ionic liquid for extraction of phenolic acids from oak galls. Ultrason. Sonochem. 2019, 62, 104876. [Google Scholar] [CrossRef]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Zhu, X. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason. Sonochem. 2019, 51, 249–257. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhang, Q.; Zhao, W.; Shi, F. Optimization of ultrasound parameters and its effect on the properties of the activity of beta-glucosidase in apricot kernels. Ultrason. Sonochem. 2019, 52, 468–476. [Google Scholar] [CrossRef]
- Wu, L.F.; Li, L.; Chen, S.J.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014. [Google Scholar] [CrossRef]
- Lin, S.X.; Meng, X.J.; Tan, C.; Tong, Y.Q.; Wan, M.Z.; Wang, M.Y.; Zhao, Y.; Deng, H.T.; Kong, Y.W.; Ma, Y. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa extracted using an ultrasonic-microwave-assisted natural deep eutectic solvent extraction method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef]
- Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a functional ingredient: Metabolism, health-promoting attributes, food sources, applications and analysis methods. Molecules 2023, 28, 4824. [Google Scholar] [CrossRef] [PubMed]
- Vicente, F.A.; Tkalec, N.; Likozar, B. Responsive deep eutectic solvents: Mechanisms, applications and their role in sustainable chemistry. Chem. Commun. 2024, 61, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Xu, Y.; Cai, C.Y.; Tan, Z.J. Extraction of Lycium barbarum polysaccharides using temperature-switchable deep eutectic solvents: A sustainable methodology for recycling and reuse of the extractant. J. Mol. Liq. 2023, 383, 122063. [Google Scholar] [CrossRef]





| NADESs NO. | HBA | HBD | Molar Ratio | Abbreviation |
|---|---|---|---|---|
| NADES-1 | Choline chloride | Sorbitol | 1:1 | ChSor |
| NADES-2 | Ethylene glycol | 1:1 | ChEG | |
| NADES-3 | Propylene glycol | 1:1 | ChPG | |
| NADES-4 | Butanediol | 1:1 | ChBut | |
| NADES-5 | Citric acid | 1:1 | ChCA | |
| NADES-6 | Malic acid | 1:1 | ChMA | |
| NADES-7 | Oxalic acid | 1:1 | ChOA | |
| NADES-8 | Lactic acid | 1:1 | ChLA | |
| NADES-9 | Tartaric acid | 1:1 | ChTA | |
| NADES-10 | Urea | 1:1 | ChU | |
| NADES-11 | Proline | 1:1 | ChPro | |
| NADES-12 | Betaine | Sorbitol | 1:1 | BSor |
| NADES-13 | Ethylene glycol | 1:1 | BEG | |
| NADES-14 | Propylene glycol | 1:1 | BPG | |
| NADES-15 | Butanediol | 1:1 | BBut | |
| NADES-16 | Citric acid | 1:1 | BCA | |
| NADES-17 | Malic acid | 1:1 | BMA | |
| NADES-18 | Oxalic acid | 1:1 | BOA | |
| NADES-19 | Lactic acid | 1:1 | BLA | |
| NADES-20 | Tartaric acid | 1:1 | BTA | |
| NADES-21 | Urea | 1:1 | BU | |
| NADES-22 | Proline | 1:1 | BPro |
| Time (min) | Flow-Rate (μL/min) | A (%) | B (%) |
|---|---|---|---|
| 0.00 | 400 | 95 | 5 |
| 1.50 | 400 | 95 | 5 |
| 2.50 | 400 | 90 | 10 |
| 14.00 | 400 | 60 | 40 |
| 22.00 | 400 | 5 | 95 |
| 25.00 | 400 | 5 | 95 |
| 26.00 | 400 | 95 | 5 |
| 30.00 | 400 | 95 | 5 |
| Run | Factor A | Factor B | Factor C | TFC |
|---|---|---|---|---|
| A: Molar Ratio of Urea to Betaine | B: Water Content in NADESs (%) | C: Ultrasound Power (W) | Experimental Values (mg_RE/g_DW) | |
| 1 | 3 | 60 | 300 | 6.13 |
| 2 | 3 | 60 | 300 | 6.21 |
| 3 | 3 | 60 | 300 | 6.02 |
| 4 | 2 | 50 | 300 | 5.13 |
| 5 | 3 | 50 | 150 | 5.23 |
| 6 | 3 | 70 | 150 | 5.51 |
| 7 | 2 | 70 | 300 | 5.39 |
| 8 | 2 | 60 | 450 | 5.42 |
| 9 | 4 | 50 | 300 | 5.69 |
| 10 | 3 | 70 | 450 | 5.50 |
| 11 | 3 | 60 | 300 | 5.95 |
| 12 | 4 | 60 | 450 | 5.79 |
| 13 | 3 | 50 | 450 | 5.57 |
| 14 | 3 | 60 | 300 | 6.06 |
| 15 | 4 | 60 | 150 | 5.41 |
| 16 | 2 | 60 | 150 | 5.30 |
| 17 | 4 | 70 | 300 | 5.49 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1.73 | 9 | 0.1917 | 26.66 | 0.0001 | significant |
| A: Molar ratio of urea to betaine | 0.1645 | 1 | 0.1645 | 22.88 | 0.0020 | |
| B: Water content in NADESs | 0.0088 | 1 | 0.0088 | 1.22 | 0.3064 | |
| C: Ultrasonic power | 0.0843 | 1 | 0.0843 | 11.72 | 0.0111 | |
| AB | 0.0539 | 1 | 0.0539 | 7.49 | 0.0290 | |
| AC | 0.0153 | 1 | 0.0153 | 2.13 | 0.1877 | |
| BC | 0.0306 | 1 | 0.0306 | 4.26 | 0.0780 | |
| A2 | 0.4051 | 1 | 0.4051 | 56.33 | 0.0001 | |
| B2 | 0.4777 | 1 | 0.4777 | 66.44 | <0.0001 | |
| C2 | 0.3420 | 1 | 0.3420 | 47.57 | 0.0002 | |
| Residual | 0.0503 | 7 | 0.0072 | |||
| Lack of Fit | 0.0106 | 3 | 0.0035 | 0.3541 | 0.7899 | not significant |
| Pure Error | 0.0398 | 4 | 0.0099 | |||
| Cor Total | 1.78 | 16 | ||||
| R2 | 0.9717 | |||||
| Adjusted R2 | 0.9352 |
| Flavonoid | RT (min) | Ion Mode | Formula | Precursor (m/z) | RC (%) |
|---|---|---|---|---|---|
| Oenin | 5.8 | [M+H]+ | C23H24O12 | 493.1349 | 20.3 |
| 3′-METHOXY-4′,5,7-TRIHYDROXYFLAVONOL | 18.6 | [M+H]− | C16H12O7 | 315.0566 | 10.1 |
| Isorhamnetin-3-O-glucoside | 5.2 | [M+H]+ | C22H22O12 | 479.1201 | 9.9 |
| Isoquercitrin | 4.5 | [M+H]+ | C21H20O12 | 465.1058 | 8.8 |
| Isoquercetin | 7.6 | [M+H]+ | C21H20O12 | 465.103 | 3.4 |
| Myricetin 3-glucoside | 6.7 | [M+H]+ | C21H20O13 | 481.1002 | 3.3 |
| luteolin 4′-O-glucoside | 5.0 | [M+H]+ | C21H20O11 | 449.1081 | 2.5 |
| Quercetin | 7.6 | [M+H]+ | C15H10O7 | 303.052 | 2.1 |
| Syringetin-3-O-glucoside | 8.7 | [M+H]+ | C23H24O13 | 509.1299 | 1.8 |
| Peonidin 3-O-glucoside | 5.7 | [M+H]+ | C22H22O11 | 463.1252 | 1.8 |
| Laricitrin 3-galactoside | 7.8 | [M+H]+ | C22H22O13 | 495.1131 | 1.4 |
| Quercetin-3-O-glucoside | 3.6 | [M+H]+ | C21H20O12 | 465.105 | 1.3 |
| Quercetin-3-Arabinoside | 8.3 | [M+H]+ | C20H18O11 | 435.0935 | 1.2 |
| Limocitrin | 8.7 | [M+H]+ | C17H14O8 | 347.0782 | 1.2 |
| Myricetin-3-Xyloside | 7.3 | [M+H]+ | C20H18O12 | 451.0887 | 1.0 |
| quercetin 3-O-glucuronide | 7.8 | [M+H]+ | C21H18O13 | 479.0844 | 1.0 |
| Quercetin-3-O-galactoside | 1.0 | [M+H]+ | C21H20O12 | 465.1058 | 0.6 |
| Reynoutrin | 4.1 | [M+H]+ | C20H18O11 | 435.0934 | 0.6 |
| 4′,5,7-trihydroxy-3,6-dimethoxyflavone | 5.8 | [M+H]+ | C17H14O7 | 331.0808 | 0.5 |
| Kaempferol-3-O-alpha-L-arabinoside | 5.3 | [M+H]+ | C20H18O10 | 419.0987 | 0.5 |
| Isorhamnetin | 5.2 | [M+H]+ | C16H12O7 | 317.0671 | 0.4 |
| Myricetin | 9.3 | [M+H]+ | C15H10O8 | 319.0463 | 0.4 |
| Naringenin | 18.9 | [M+H]− | C15H12O5 | 271.0582 | 0.3 |
| Quercetin-4′-O-glucoside | 8.4 | [M+H]+ | C21H20O12 | 465.105 | 0.2 |
| Apigenin | 19.4 | [M+H]− | C15H10O5 | 269.0435 | 0.2 |
| Isorhamnetin 3-glucoside | 9.4 | [M+H]+ | C22H22O12 | 479.121 | 0.1 |
| Fisetin | 5.0 | [M+H]+ | C15H10O6 | 287.0542 | 0.1 |
| petunidin-3-O-arabinoside | 9.3 | [M+H]+ | C21H20O11 | 449.1072 | 0.1 |
| Vitexin | 6.7 | [M+H]+ | C21H20O10 | 433.1147 | 0.1 |
| Flavonoid | RT (min) | Ion Mode | Formula | Precursor (m/z) | RC (%) |
|---|---|---|---|---|---|
| Isoquercetin | 7.6 | [M+H]+ | C21H20O12 | 465.1031 | 8.0 |
| Hirsutrin | 7.6 | [M+H]+ | C21H20O12 | 465.1121 | 5.5 |
| Oenin | 5.9 | [M+H]+ | C23H24O12 | 493.1356 | 5.2 |
| Syringetin-3-O-glucoside | 8.7 | [M+H]+ | C23H24O13 | 509.1307 | 4.5 |
| Limocitrin | 8.7 | [M+H]+ | C17H14O8 | 347.0788 | 3.0 |
| Laricitrin 3-galactoside | 7.8 | [M+H]+ | C22H22O13 | 495.1139 | 2.9 |
| Quercetin-3-Arabinoside | 8.3 | [M+H]+ | C20H18O11 | 435.0943 | 2.8 |
| Quercetin | 8.3 | [M+H]+ | C15H10O7 | 303.0503 | 2.6 |
| quercetin 3-O-glucuronide | 7.8 | [M+H]+ | C21H18O13 | 479.0821 | 1.2 |
| Procyanidin A2 | 7.2 | [M+H]+ | C30H24O12 | 577.1346 | 1.0 |
| Isorhamnetin-3-O-beta-D-Glucoside | 8.6 | [M+H]+ | C22H22O12 | 479.1183 | 0.6 |
| Quercetin-4′-O-glucoside | 8.4 | [M+H]+ | C21H20O12 | 465.103 | 0.5 |
| Isorhamnetin | 8.6 | [M+H]+ | C16H12O7 | 317.0679 | 0.5 |
| Isorhamnetin 3-glucoside | 9.4 | [M+H]+ | C22H22O12 | 479.1207 | 0.4 |
| Peonidin 3-O-glucoside | 5.8 | [M+H]+ | C22H22O11 | 463.1252 | 0.3 |
| Myricetin 3-glucoside | 6.7 | [M+H]+ | C21H20O13 | 463.1252 | 0.2 |
| Chrysanthemin | 9.3 | [M+H]+ | C21H20O11 | 449.1079 | 0.2 |
| Myricetin, 3-Galactopyranoside | 6.7 | [M+H]+ | C21H20O13 | 481.1073 | 0.1 |
| Malvidin | 5.8 | [M+H]+ | C17H14O7 | 331.0808 | 0.1 |
| Myricetin | 7.4 | [M+H]+ | C15H10O8 | 319.0471 | 0.1 |
| Myricetin-3-Xyloside | 7.4 | [M+H]+ | C20H18O12 | 451.0871 | 0.1 |
| Kaempferol-3-O-glucuronoside | 8.6 | [M+H]+ | C21H18O12 | 463.0876 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, L.; Liang, W.; Bian, C.; Shan, Y.; Wang, S. Ultrasound-Assisted Green Natural Deep Eutectic Solvent Extraction of Flavonoids from Wild Blueberry: Process Optimization, Composition Identification, and Antioxidant Activity. Foods 2025, 14, 3325. https://doi.org/10.3390/foods14193325
Ouyang L, Liang W, Bian C, Shan Y, Wang S. Ultrasound-Assisted Green Natural Deep Eutectic Solvent Extraction of Flavonoids from Wild Blueberry: Process Optimization, Composition Identification, and Antioxidant Activity. Foods. 2025; 14(19):3325. https://doi.org/10.3390/foods14193325
Chicago/Turabian StyleOuyang, Le, Weiwei Liang, Chun Bian, Yi Shan, and Shumei Wang. 2025. "Ultrasound-Assisted Green Natural Deep Eutectic Solvent Extraction of Flavonoids from Wild Blueberry: Process Optimization, Composition Identification, and Antioxidant Activity" Foods 14, no. 19: 3325. https://doi.org/10.3390/foods14193325
APA StyleOuyang, L., Liang, W., Bian, C., Shan, Y., & Wang, S. (2025). Ultrasound-Assisted Green Natural Deep Eutectic Solvent Extraction of Flavonoids from Wild Blueberry: Process Optimization, Composition Identification, and Antioxidant Activity. Foods, 14(19), 3325. https://doi.org/10.3390/foods14193325






