Metabolite Signatures and Particle Size as Determinants of Anti-Inflammatory and Gastrointestinal Smooth Muscle Modulation by Chlorella vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Particle-Size Analysis
2.3. GC–MS Sample Preparation and Analysis
2.4. Inhibition of Albumin Denaturation
2.5. Spasmolytic Effect
2.5.1. Ex Vivo Experiments
2.5.2. Gastric Smooth Muscle Preparations and Evaluation of Spontaneous Contractile Activity
2.6. Immunohistochemistry
2.6.1. Staining Protocol
2.6.2. Quantitative Analysis
2.7. Statistical Analysis
3. Results
3.1. Particle Size and Distribution
3.2. Chemical Composition of the C. vulgaris Samples
3.3. In Vitro Inhibition of Albumin Denaturation
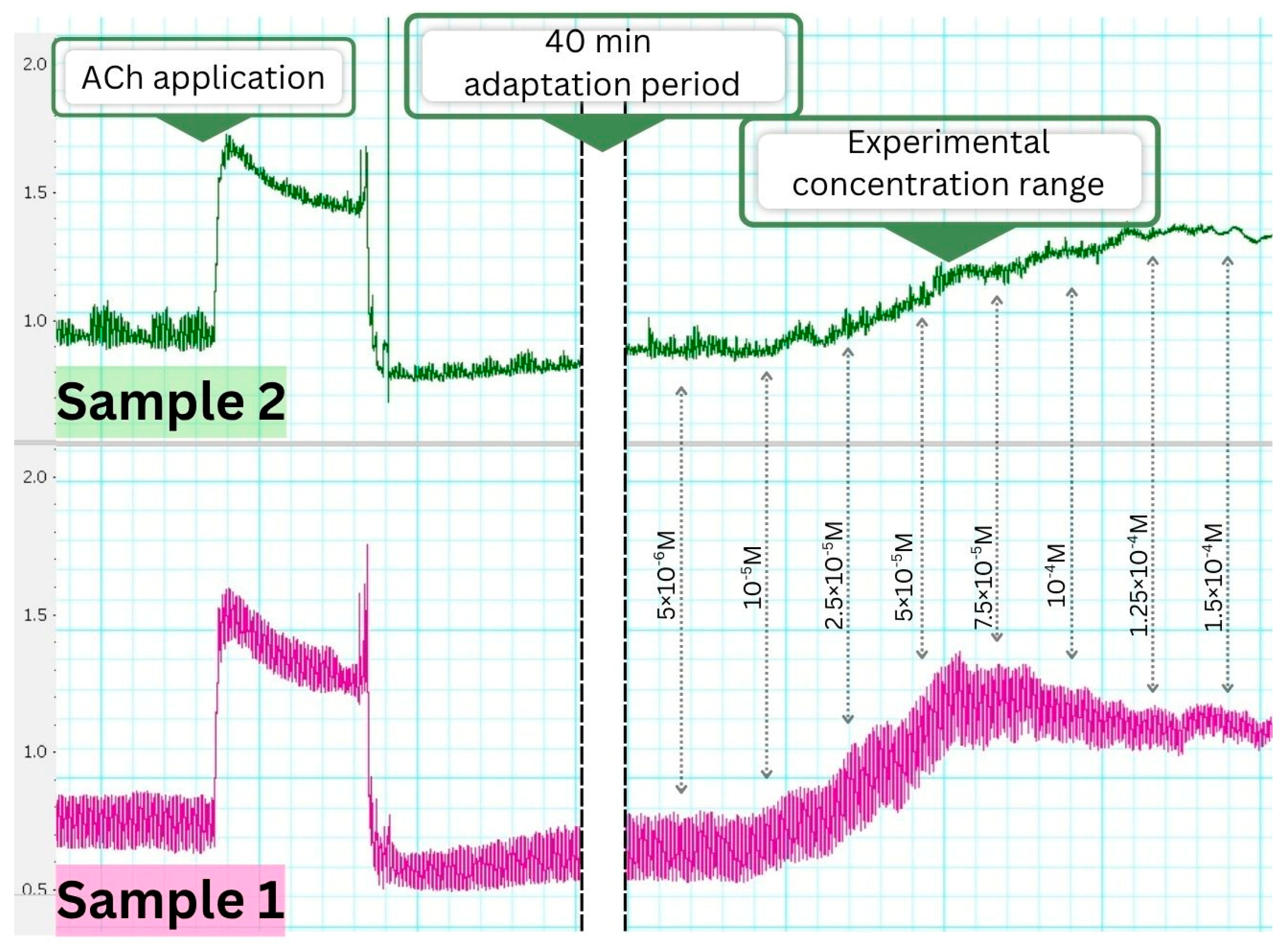
3.4. Evaluation of Ex Vivo Spasmolytic Effect
3.4.1. Effects on Spontaneous Smooth Muscle Contractile Activity
3.4.2. Pharmacological Modulation of the Contractile Response
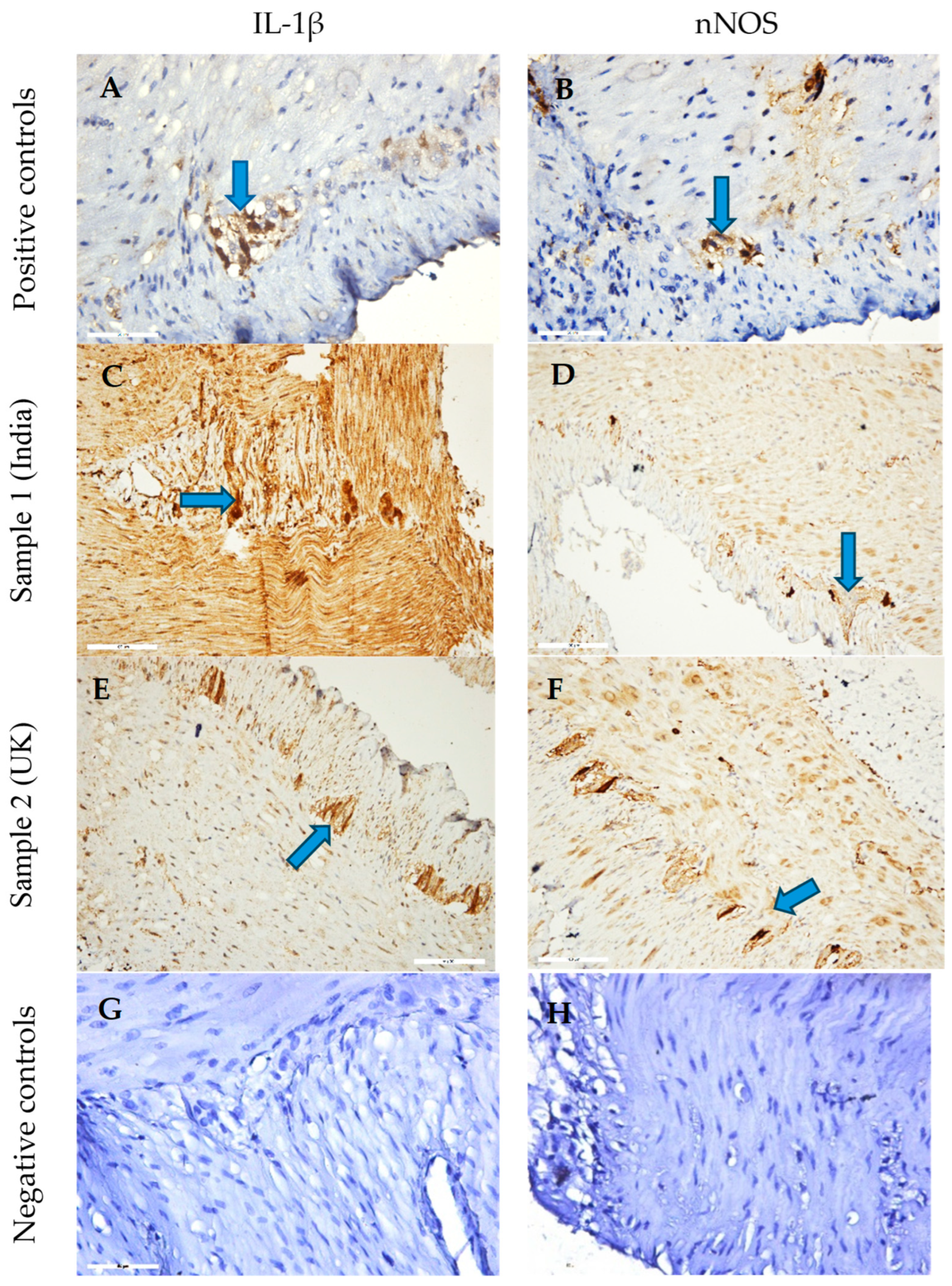
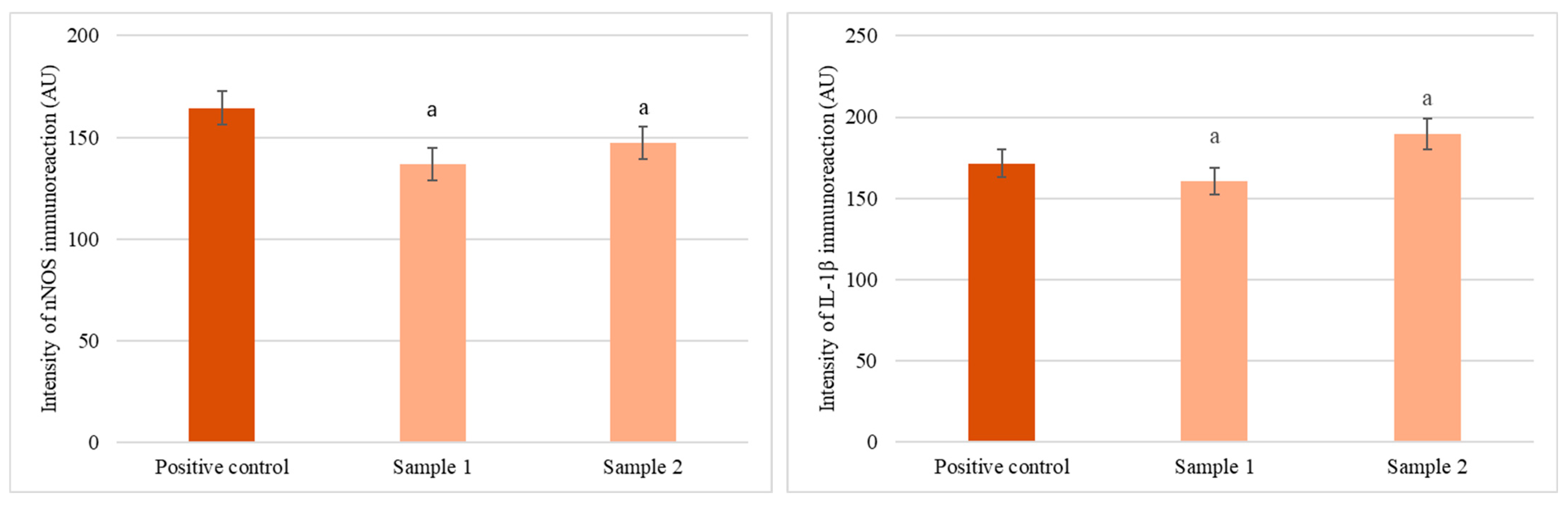
3.5. Ex Vivo Immunohistochemical Analysis
4. Discussion
4.1. Particle Size and Functional Food Relevance
4.2. Metabolite Profile and Functional Implications
4.3. In Vitro Anti-Inflammatory Activity
4.4. Smooth Muscle Contractility and Its Relation to Anti-Inflammatory Activity
4.5. Contractile Activity and Calcium Modulation in Smooth Muscle
4.6. Immunohistochemical Evaluation of IL-1β and nNOS Expression
4.7. Integrated Interpretation and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mTOR | Mammalian target of rapamycin |
| SM | Smooth muscle |
| NO | Nitric oxide |
| nNOS | Enzyme neuronal nitric oxide synthase |
| MP | Myenteric plexus |
References
- Wang, C.A.; Onyeaka, H.; Miri, T.; Soltani, F. Chlorella vulgaris as a Food Substitute: Applications and Benefits in the Food Industry. J. Food Sci. 2024, 89, 8231–8247. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Prates, J.A.M. Microalgae Bioactives for Functional Food Innovation and Health Promotion. Foods 2025, 14, 2122. [Google Scholar] [CrossRef] [PubMed]
- Fekete, G.; Klátyik, S.; Sebők, A.; Dálnoki, A.B.; Takács, A.; Gulyás, M.; Czinkota, I.; Székács, A.; Gyuricza, C.; Aleksza, L. Optimization of a Chlorella vulgaris-Based Carbon Sequestration Technique Using an Alkaline Medium of Wood Biomass Ash Extract. Water 2024, 16, 3696. [Google Scholar] [CrossRef]
- Feng, Y.; Ge, J.; Show, P.L.; Song, C.; Wu, L.; Ma, Z.; Gao, G. Using High CO2 Concentrations to Culture Microalgae for Lipid and Fatty Acid Production: Synthesis Based on a Meta-Analysis. Aquaculture 2025, 594, 741386. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Advances in Bioprocess Engineering for Optimising Chlorella vulgaris Fermentation: Biotechnological Innovations and Applications. Foods 2024, 13, 4154. [Google Scholar] [CrossRef] [PubMed]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Yin, L.; Rao, H.; Zheng, H.; Xun, C.; Hao, J. Application and Prospect of Microbial Food Chlorella. Heliyon 2024, 10, e37025. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Abdel-Moatamed, B.R.; El-Fakhrany, A.M.A.; Elneairy, N.A.A.; Shaban, M.M.; Roby, M.H.H. The Impact of Chlorella vulgaris Fortification on the Nutritional Composition and Quality Characteristics of Beef Burgers. Foods 2024, 13, 1945. [Google Scholar] [CrossRef]
- Mtaki, K.; Kyewalyanga, M.S.; Mtolera, M.S.P. Assessment of Antioxidant Contents and Free Radical-Scavenging Capacity of Chlorella vulgaris Cultivated in Low Cost Media. Appl. Sci. 2020, 10, 8611. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Nurdin, A.; Ismail, S.; Yusof, Z.N.B. Evaluation of the Antioxidant and Cytotoxic Activities of Crude Extracts from Marine Chlorella sp. Biocatal. Agric. Biotechnol. 2023, 47, 102551. [Google Scholar] [CrossRef]
- Tarsitano, M.; Ming, C.L.C.; Bennar, L.; Mahmodi, H.; Wyllie, K.; Idais, D.; Al Shamery, W.; Paolino, D.; Cox, T.R.; Kabakova, I.; et al. Chlorella-Enriched Hydrogels Protect against Myocardial Damage and Reactive Oxygen Species Production in an In Vitro Ischemia/Reperfusion Model Using Cardiac Spheroids. Biofabrication 2025, 17, 015006. [Google Scholar] [CrossRef]
- Elbarbary, N.B.; Abd El-Wahab, R.A.; Gad, W.M.; Zayed, S.; Basiony, S.; Darwish, W.S.; Abdallah, M.S. Anti-Inflammatory, Antioxidant, and Immunomodulatory Effect of Chlorella vulgaris in Chicken Experimentally Infected with Eimeria tenella and Clostridium perfringens. Egypt. J. Vet. Sci. 2025, 56, 211–223. [Google Scholar] [CrossRef]
- Capek, P.; Matulová, M.; Šutovská, M.; Barboríková, J.; Molitorisová, M.; Kazimierová, I. Chlorella vulgaris α-L-Arabino-α-L-Rhamno-α,β-D-Galactan Structure and Mechanisms of Its Anti-Inflammatory and Anti-Remodelling Effects. Int. J. Biol. Macromol. 2020, 162, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.; Ramos-Pinto, L.; Cunha, S.A.; Pintado, M.; da Silva, J.L.; Dias, J.; Conceição, L.; Matos, E.; Costas, B. Chlorella vulgaris Extracts as Modulators of the Health Status and the Inflammatory Response of Gilthead Seabream Juveniles (Sparus aurata). Mar. Drugs 2022, 20, 407. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, M.; Sahoo, S.; Sahoo, D.P.; Sethi, G.; Singh, S.; Lee, H.-K.; Pradhan, B.; Shin, D. Computational Identification of Dual COX-1 and NIK Inhibitors from Marine Microalga Chlorella vulgaris. J. Genet. Eng. Biotechnol. 2025, 23, 100531. [Google Scholar] [CrossRef]
- Jo, S.W.; Velankanni, P.; Cam, N.D.T.; Nguyen, C.H.B.; Jeon, J.Y.; Kim, B.R.; Lee, D.H.; Lee, C.G.; Park, J.S. Chemical Profiling and Immune-Stimulating Activity of Solvent Fractions Derived from Dietary Chlorella. J. Microbiol. Biotechnol. 2025, 35, e2503021. [Google Scholar] [CrossRef]
- Jeong, K.-M.; Choi, Y.-J.; Jeong, H.-J. Immunomodulatory and Energy-Enhancing Effects of Modified SOUL-Tang. CellMed 2025, 15, 1–6. [Google Scholar] [CrossRef]
- Ewert, A.M.; McMenamin, A.; Adjaye, D.; Rainey, V.; Ricigliano, V. Microalgae Functional Feed Additives Strengthen Immunity and Increase Longevity in Honey Bees. J. Invertebr. Pathol. 2025, 211, 108352. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Khan, S.A.; Shakoor, A.; Uddin, S.; Nazarudin, M.F.; Zia, A.W. Chlorella spp. as an Emerging Source for Anticancer Remedy and Nutraceuticals: An Advance Study. Food Rev. Int. 2025, 1–29. [Google Scholar] [CrossRef]
- Almutairi, L.A.; Abdelghaffar, E.G.; Hafney, H.A.; Ebaid, H.M.; Alkhodair, S.A.; Shaalan, A.A.M.; El-Hak, H.N.G. Protective Impacts of Chlorella vulgaris on Cisplatin-Induced Toxicity in Liver, Kidney, and Spleen of Rats: Role of Oxidative Stress, Inflammation, and Nrf2 Modulation. Life 2025, 15, 934. [Google Scholar] [CrossRef]
- Mustafa, G.; Islam, M.; Ahmad, I.; Shakir, H.A.; Khan, M.; Irfan, M. Harnessing the Anticancer Potential of Algae: A Comprehensive Review. ChemBioEng Rev. 2025, 12, e70011. [Google Scholar] [CrossRef]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Barghchi, H.; Dehnavi, Z.; Nattagh-Eshtivani, E.; Alwaily, E.R.; Almulla, A.F.; Kareem, A.K.; Barati, M.; Ranjbar, G.; Mohammadzadeh, A.; Rahimi, P.; et al. The Effects of Chlorella vulgaris on Cardiovascular Risk Factors: A Comprehensive Review on Putative Molecular Mechanisms. Biomed. Pharmacother. 2023, 162, 114624. [Google Scholar] [CrossRef]
- Kaeoboon, S.; Songserm, R.; Suksungworn, R.; Duangsrisai, S.; Sanevas, N. In Vitro Antioxidant Potential, Antidiabetic Activities, and GC-MS Analysis of Lipid Extracts of Chlorella Microalgae. BioTech 2025, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Kolonas, A.; Stagos, D.; Gortzi, O. A Review of the Sustainability, Chemical Composition, Bioactive Compounds, Antioxidant and Antidiabetic Activity, Neuroprotective Properties, and Health Benefits of Microalgae. Biomass 2025, 5, 11. [Google Scholar] [CrossRef]
- Shah, Z.; Iqbal, A.; Badshah, S.L.; Rauf, K.; Nabi, G.; Khan, H.; Ullah, A.; Ali, S.; Bibi, S.; Haider, S.; et al. Macroalgae Polysaccharides Enhance Brain Health by Mitigating Scopolamine-Induced Oxidative Stress and Inflammation via Nrf2/TLR4/NF-κB Pathways. J. Neuroimmune Pharmacol. 2025, 20, 74. [Google Scholar] [CrossRef]
- Gochhi, M.; Dash, P.; Kar, B.; Pradhan, D.; Halder, J.; Das, C.; Rai, V.K.; Rout, S.K.; Ghosh, G.; Rath, G. Anti-Hypertensive Function of Plant-Derived Bioactive Peptides: A Review. Curr. Pharm. Des. 2025, 31, 2742–2762. [Google Scholar] [CrossRef]
- Velankanni, P.; Go, S.H.; Jin, J.B.; Park, J.S.; Park, S.; Lee, S.B.; Kwon, H.K.; Pan, C.H.; Cha, K.H.; Lee, C.G. Chlorella vulgaris Modulates Gut Microbiota and Induces Regulatory T Cells to Alleviate Colitis in Mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef]
- Bañares, C.; Paterson, S.; Gómez-Garre, D.; Ortega-Hernández, A.; Sánchez-González, S.; Cueva, C.; de la Fuente, M.Á.; Hernández-Ledesma, B.; Gómez-Cortés, P. Modulation of Gut Microbiota and Short-Chain Fatty Acid Production by Simulated Gastrointestinal Digests from Microalga Chlorella vulgaris. Int. J. Mol. Sci. 2025, 26, 2754. [Google Scholar] [CrossRef]
- Brizzi, A.; Rispoli, R.M.; Autore, G.; Marzocco, S. Anti-Inflammatory Effects of Algae-Derived Biomolecules in Gut Health: A Review. Int. J. Mol. Sci. 2025, 26, 885. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nomaguchi, T.; Mori, Y.; Ito, M.; Nakamura, Y.; Fujishima, M.; Murakami, S.; Yamada, T.; Fukuda, S. The Nutritional Efficacy of Chlorella Supplementation Depends on the Individual Gut Environment: A Randomised Control Study. Front. Nutr. 2021, 8, 648073. [Google Scholar] [CrossRef]
- Feng, S.; Hernández-Olivas, E.; Sahin, A.W.; Giblin, L.; Brodkorb, A. Semi-Dynamic In Vitro Digestion of Honey Chlorella vulgaris Reveals Biochemical and Structural Insights during Gastrointestinal Transit. Food Res. Int. 2025, 208, 116037. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V.; Melnyk, M.I.; Dryn, D.O. Molecular Mechanisms of Cholinergic Neurotransmission in Visceral Smooth Muscles with a Focus on Receptor-Operated TRPC4 Channel and Impairment of Gastrointestinal Motility by General Anaesthetics and Anxiolytics. Neuropharmacology 2024, 242, 109776. [Google Scholar] [CrossRef] [PubMed]
- Sampaio Moura, N.; Schledwitz, A.; Alizadeh, M.; Kodan, A.; Njei, L.-P.; Raufman, J.-P. Cholinergic Mechanisms in Gastrointestinal Neoplasia. Int. J. Mol. Sci. 2024, 25, 5316. [Google Scholar] [CrossRef]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural Antispasmodics: Source, Stereochemical Configuration, and Biological Activity. Biomed Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Crespo, F.J.; Aragón-Gastélum, J.L.; Gutiérrez-Alcántara, E.J.; Zamora-Crescencio, P.; Gómez-Galicia, D.L.; Alatriste-Kurzel, D.R.; Alvarez, G.; Hernández-Núñez, E. β-Sitosterol Mediates Gastrointestinal Smooth Muscle Relaxation Induced by Coccoloba uvifera via Muscarinic Acetylcholine Receptor Subtype 3. Sci. Pharm. 2024, 92, 19. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, R.; Chen, T.; Guo, Z.; Peng, T.; Zhao, L. Antispasmodic Effects and Potential Mechanism of the Traditional Chinese Medicine Shaoyao–Gancao Decoction on Intestinal Spasm in Rats. Pharmacol. Res. Mod. Chin. Med. 2025, 14, 100570. [Google Scholar] [CrossRef]
- Standard’s ISO 13320:2009; Particle Size Analysis—Laser Diffraction Methods. International Organization for Standardization: Geneva, Switzerland, 2009.
- Dincheva, I.; Badjakov, I.; Georgiev, V.; Semerdjieva, I.; Vrancheva, R.; Ivanov, I.; Pavlov, A. Comprehensive GC-MS Characterization and Histochemical Assessment of Various Parts of Three Colchicum Species from Bulgarian Flora. Plants 2025, 14, 270. [Google Scholar] [CrossRef]
- NIST08; NIST Standard Reference Database 1A: NIST/EPA/NIH Mass Spectral Library (NIST 08) and NIST Mass Spectral Search Program (Version 20f) Manual. U.S. Department of Commerce, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision Tree-Supported Substructure Prediction of Metabolites from GC-MS Profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef]
- Milusheva, M.; Todorova, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Pencheva, M.; Nedialkov, P.; Tumbarski, Y.; Yanakieva, V.; Tsoneva, S. Novel Anthranilic Acid Hybrids—An Alternative Weapon against Inflammatory Diseases. Pharmaceuticals 2023, 16, 1660. [Google Scholar] [CrossRef]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Mihaylova, R.; Nedialkov, P.; Cherneva, E.; Tumbarski, Y.; Tsoneva, S.; Todorova, M. Synthesis, Molecular Docking, and Biological Evaluation of a Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics. Int. J. Mol. Sci. 2023, 24, 13855. [Google Scholar] [CrossRef]
- Shu, Y.; Li, J.; Yang, X.; Dong, X.; Wang, X. Effect of Particle Size on the Bioaccessibility of Polyphenols and Polysaccharides in Green Tea Powder and Its Antioxidant Activity after Simulated Human Digestion. J. Food Sci. Technol. 2019, 56, 1127–1133. [Google Scholar] [CrossRef]
- Wang, E.; Yin, Z.; Zeng, M. Microalgae as a Promising Structure Ingredient in Food: Obtained by Simple Thermal and High-Speed Shearing Homogenization. Food Hydrocoll. 2022, 131, 107743. [Google Scholar] [CrossRef]
- Pantami, H.A.; Ahamad Bustamam, M.S.; Lee, S.Y.; Ismail, I.S.; Mohd Faudzi, S.M.; Nakakuni, M.; Shaari, K. Comprehensive GC-MS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Krivina, E.; Degtyaryov, E.; Tebina, E.; Temraleeva, A.; Savchenko, T. Comparative Analysis of the Fatty Acid Profiles of Selected Representatives of Chlorella-Clade to Evaluate Their Biotechnological Potential. Int. J. Plant Biol. 2024, 15, 837–854. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Involvement of Fatty Acids and Their Metabolites in the Development of Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1308. [Google Scholar] [CrossRef]
- Zahid, S.; Malik, A.; Waqar, S.; Zahid, F.; Tariq, N.; Khawaja, A.I.; Safir, W.; Gulzar, F.; Iqbal, J.; Ali, Q. Countenance and Implication of β-Sitosterol, β-Amyrin and Epiafzelechin in Nickel-Exposed Rats: In-Silico and In-Vivo Approach. Sci. Rep. 2023, 13, 21351. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; An, L.; Yuan, X.; Yao, L. Polyol and Sugar Osmolytes Can Shorten Protein Hydrogen Bonds to Modulate Function. Commun. Biol. 2020, 3, 528. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Vieira, A.P.; Lelis, C.A.; Ochioni, A.C.; Rosário, D.K.A.; Rosario, I.L.S.; Vieira, I.R.S.; Carvalho, A.P.A.; Janeiro, J.M.; da Costa, M.P.; Lima, F.R.S.; et al. Estimating the Therapeutic Potential of NSAIDs and Linoleic Acid-Isomers Supplementation against Neuroinflammation. Biomed. Pharmacother. 2024, 177, 116884. [Google Scholar] [CrossRef]
- Seufert, A.L.; Napier, B.A. A New Frontier for Fat: Dietary Palmitic Acid Induces Innate Immune Memory. Immunometabolism 2023, 5, e00021. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.P.; Baviskar, D.T. Anti-Inflammatory Activity of Chlorella vulgaris in Experimental Models of Rats. Int. J. Pharm. Investig. 2021, 11, 358–361. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Georgiopoulou, I.; Antoniou, N.; Tzima, S.; Kontou, M.; Louli, V.; Fatouros, C.; Magoulas, K.; Kolisis, F.N. Extracts from Chlorella vulgaris Protect Mesenchymal Stromal Cells from Oxidative Stress Induced by Hydrogen Peroxide. Plants 2023, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Yan, D.; Ye, S.; He, Y.; Wang, S.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty Acids and Lipid Mediators in Inflammatory Bowel Disease: From Mechanism to Treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef]
- Hossain, R.; Kim, K.I.; Jin, F.; Lee, H.J.; Lee, C.J. Betulin, an Anti-Inflammatory Triterpenoid Compound, Regulates MUC5AC Mucin Gene Expression through NF-κB Signaling in Human Airway Epithelial Cells. Biomol. Ther. 2022, 30, 540–705. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, J.G.; Kim, S.J.; Song, D.K.; Lee, S.G.; Kim, E.S.; Cho, K.B.; Jang, B.I.; Kim, D.H.; Sin, J.-I.; et al. Alterations of Colonic Contractility in an Interleukin-10 Knockout Mouse Model of Inflammatory Bowel Disease. J. Neurogastroenterol. Motil. 2014, 21, 51–61. [Google Scholar] [CrossRef]
- Ohama, T.; Hori, M.; Ozaki, H. Mechanism of Abnormal Intestinal Motility in Inflammatory Bowel Disease: How Smooth Muscle Contraction Is Reduced? J. Smooth Muscle Res. 2007, 43, 43–54. [Google Scholar] [CrossRef]
- Malykhina, A.P.; Akbarali, H.I. Inflammation-Induced “Channelopathies” in the Gastrointestinal Smooth Muscle. Cell Biochem. Biophys. 2004, 41, 319–330. [Google Scholar] [CrossRef]
- Gougeon, P.-Y.; Lourenssen, S.; Han, T.Y.; Nair, D.G.; Ropeleski, M.J.; Blennerhassett, M.G. The Pro-Inflammatory Cytokines IL-1β and TNFα Are Neurotrophic for Enteric Neurons. J. Neurosci. 2013, 33, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Hąc-Wydro, K.; Wydro, P. The Influence of Fatty Acids on Model Cholesterol/Phospholipid Membranes. Chem. Phys. Lipids 2007, 150, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Palmgren, H.; Henricsson, M.; Devkota, R.; Jaiswal, H.; Maresca, M.; Bohlooly, Y.M.; Peng, X.-R.; Borén, J.; Pilon, M. Extensive Transcription Mis-Regulation and Membrane Defects in AdipoR2-Deficient Cells Challenged with Saturated Fatty Acids. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 158884. [Google Scholar] [CrossRef]
- Harayama, T.; Antonny, B. Beyond Fluidity: The Role of Lipid Unsaturation in Membrane Function. Cold Spring Harb. Perspect. Biol. 2023, 15, a041409. [Google Scholar] [CrossRef]
- Mondal, D.; Dutta, R.; Banerjee, P.; Mukherjee, D.; Maiti, T.K.; Sarkar, N. Modulation of Membrane Fluidity Performed on Model Phospholipid Membrane and Live Cell Membrane: Revealing through Spatiotemporal Approaches of FLIM, FAIM, and TRFS. Anal. Chem. 2019, 91, 4337–4345. [Google Scholar] [CrossRef]
- Tanahashi, Y.; Komori, S.; Matsuyama, H.; Kitazawa, T.; Unno, T. Functions of Muscarinic Receptor Subtypes in Gastrointestinal Smooth Muscle: A Review of Studies with Receptor-Knockout Mice. Int. J. Mol. Sci. 2021, 22, 926. [Google Scholar] [CrossRef]
- Saponaro, A.; Lolicato, M. Editorial: The Key Role of Lipids in the Regulation of Ion Channels. Front. Physiol. 2022, 13, 1000082. [Google Scholar] [CrossRef]
- Ali, O.; Szabó, A. Review of Eukaryote Cellular Membrane Lipid Composition, with Special Attention to the Fatty Acids. Int. J. Mol. Sci. 2023, 24, 15693. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, Y.; Jiang, C.; Wang, Q.; Geng, X.; Chen, L.; Zhou, S.; Wang, X.; Han, M.; Du, D.; et al. Saturated Fatty Acid Promotes Calcification via Suppressing SIRT6 Expression in Vascular Smooth Muscle Cells. J. Hypertens. 2023, 41, 393–769. [Google Scholar] [CrossRef]
- Santos, F.A.; Carvalho, K.M.M.B.; Batista-Lima, F.J.; Nunes, P.I.G.; Viana, A.F.S.C.; de Carvalho Almeida da Silva, A.A.; da Cruz Fonseca, S.G.; Chaves, M.H.; Rao, V.S.; Magalhães, P.J.C.; et al. The Triterpenoid α,β-Amyrin Prevents the Impaired Aortic Vascular Reactivity in High-Fat Diet-Induced Obese Mice. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Hernández, E.A.G.; Galindo, G.C.; Chávez, R.S.M.; Moreno, P.C.; Barajas, M.I.; Duque, T.E.V.; Antúnez, A.P.H.; Mondragón, L.D.V.; Guerrero, G.A.M.; Cobos, D.S.; et al. Antioxidant, Antidiabetic, and Vasorelaxant Effects of Ethanolic Extract from the Seeds of Swietenia humilis. Int. J. Mol. Sci. 2025, 26, 2063. [Google Scholar] [CrossRef]
- Aida, K.; Mita, M.; Ishii-Nozawa, R. Difference in Contractile Mechanisms between the Early and Sustained Components of Ionomycin-Induced Contraction in Rat Caudal Arterial Smooth Muscle. Biol. Pharm. Bull. 2024, 47, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, B.; Christin, M.; Tessandier, N.; Sotoudeh, C.; Bretheau, F.; Turmel, R.; Pellerin, È.; Wang, F.; Bories, C.; Joly-Beauparlant, C. Neuronal Interleukin-1 Receptors Mediate Pain in Chronic Inflammatory Diseases. J. Exp. Med. 2020, 217, e20191430. [Google Scholar] [CrossRef]
- Dinarello, C.A. A Clinical Perspective of IL-1β as the Gatekeeper of Inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Huang, S.; Choi, I.W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-Mediated Secretion of IL-1β in Human Monocytes through TLR2 Activation; Modulation by Dietary Fatty Acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The Effect of Palmitic Acid on Inflammatory Response in Macrophages: An Overview of Molecular Mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Triantafilou, M.; Miyake, K.; Golenbock, D.T.; Triantafilou, K. Mediators of Innate Immune Recognition of Bacteria Concentrate in Lipid Rafts and Facilitate Lipopolysaccharide-Induced Cell Activation. J. Cell Sci. 2002, 115, 2603–2611. [Google Scholar] [CrossRef]
- Bodur, M.; Yilmaz, B.; Ağagündüz, D.; Ozogul, Y. Immunomodulatory Effects of Omega-3 Fatty Acids: Mechanistic Insights and Health Implications. Mol. Nutr. Food Res. 2025, 69, e202400752. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Jin, J.; Hu, W.; Chen, Q.; Yang, J.; Wu, Y.; Zhou, Y.; Sun, L.; Gao, W.; Zhang, X. Betulin Alleviates the Inflammatory Response in Mouse Chondrocytes and Ameliorates Osteoarthritis via AKT/Nrf2/HO-1/NF-κB Axis. Front. Pharmacol. 2021, 12, 754038. [Google Scholar] [CrossRef]
- Su, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Lee, H.-P.; Lo, L.-C.; Huang, W.-C.; Wu, Y.-C.; Hsieh, C.-L.; Tang, C.-H. Betulin Suppresses TNF-α and IL-1β Production in Osteoarthritis Synovial Fibroblasts by Inhibiting the MEK/ERK/NF-κB Pathway. J. Funct. Foods 2021, 86, 104729. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The Enteric Nervous System. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, I.; Gu, W.; Hyam, S.R.; Kim, D. β-Sitosterol Attenuates High-Fat Diet-Induced Intestinal Inflammation in Mice by Inhibiting the Binding of Lipopolysaccharide to Toll-like Receptor 4 in the NF-ΚB Pathway. Mol. Nutr. Food Res. 2014, 58, 963–972. [Google Scholar] [CrossRef]
- Reichardt, F.; Chassaing, B.; Nezami, B.G.; Li, G.; Tabatabavakili, S.; Mwangi, S.; Uppal, K.; Liang, B.; Vijay-Kumar, M.; Jones, D.; et al. Western Diet Induces Colonic Nitrergic Myenteric Neuropathy and Dysmotility in Mice via Saturated Fatty Acid- and Lipopolysaccharide-Induced TLR4 Signalling. J. Physiol. 2017, 595, 1831–1846. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated Fatty Acids Trigger TLR4-Mediated Inflammatory Response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Coniglio, S.; Shumskaya, M.; Vassiliou, E. Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology 2023, 12, 279. [Google Scholar] [CrossRef]
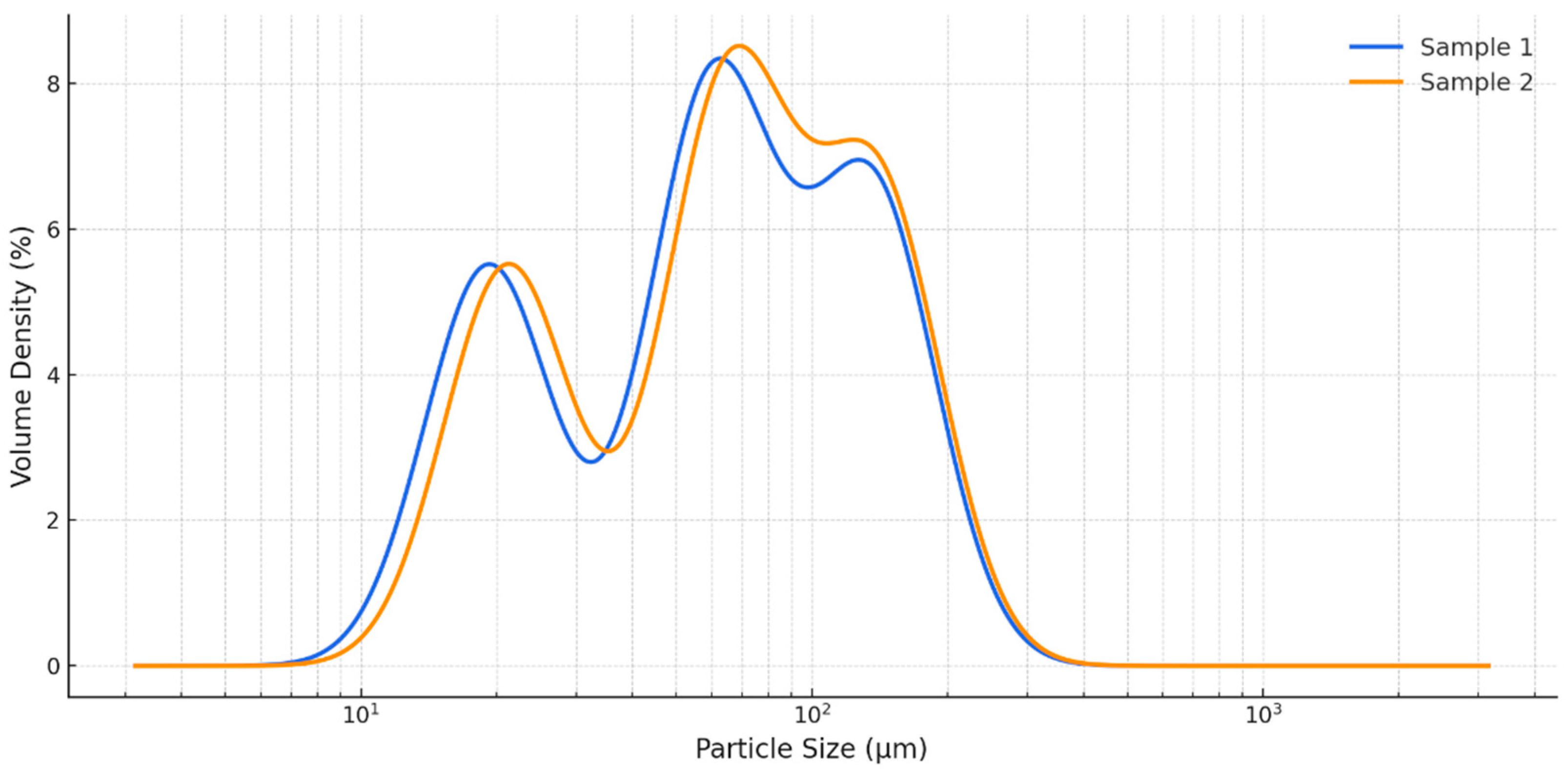
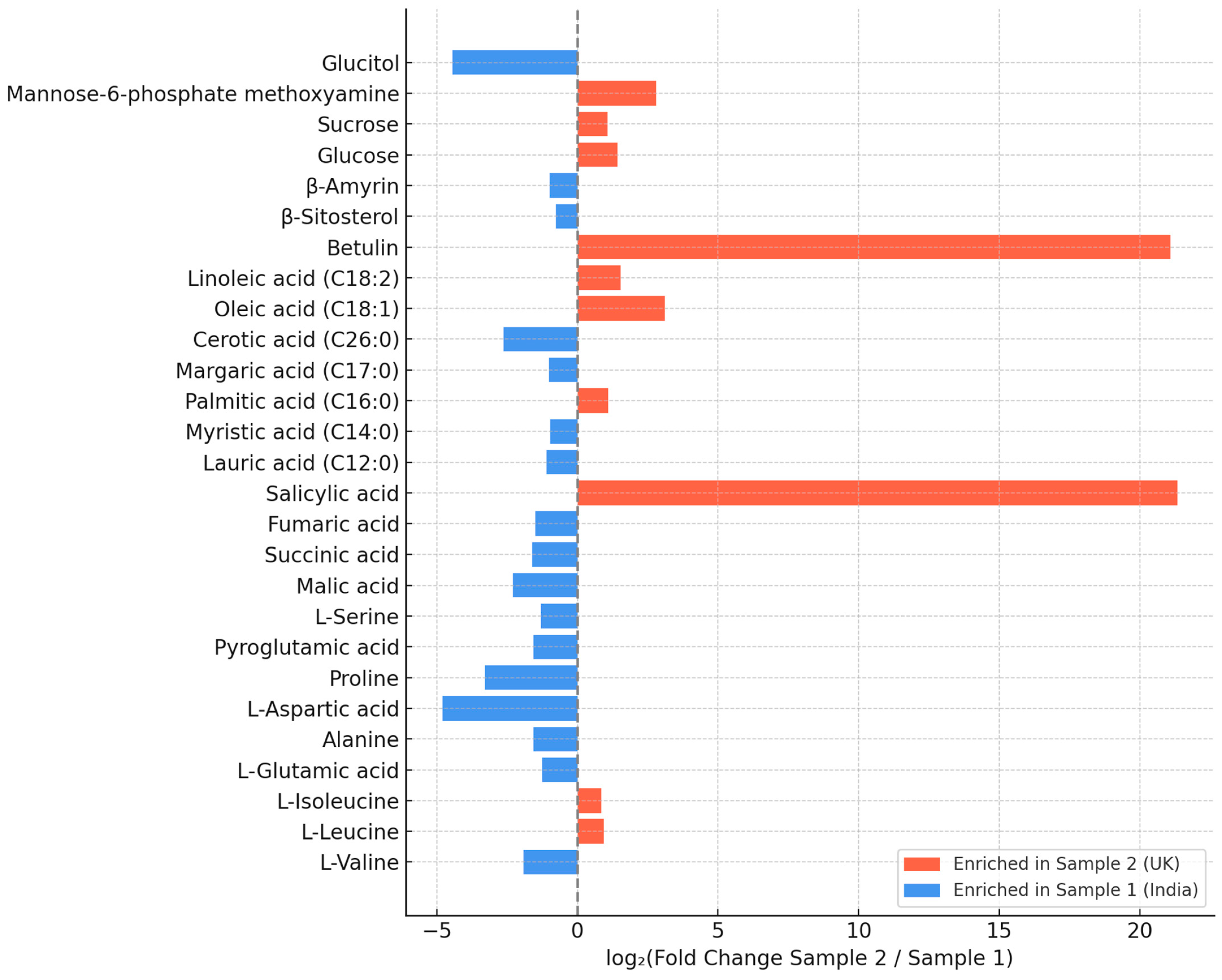
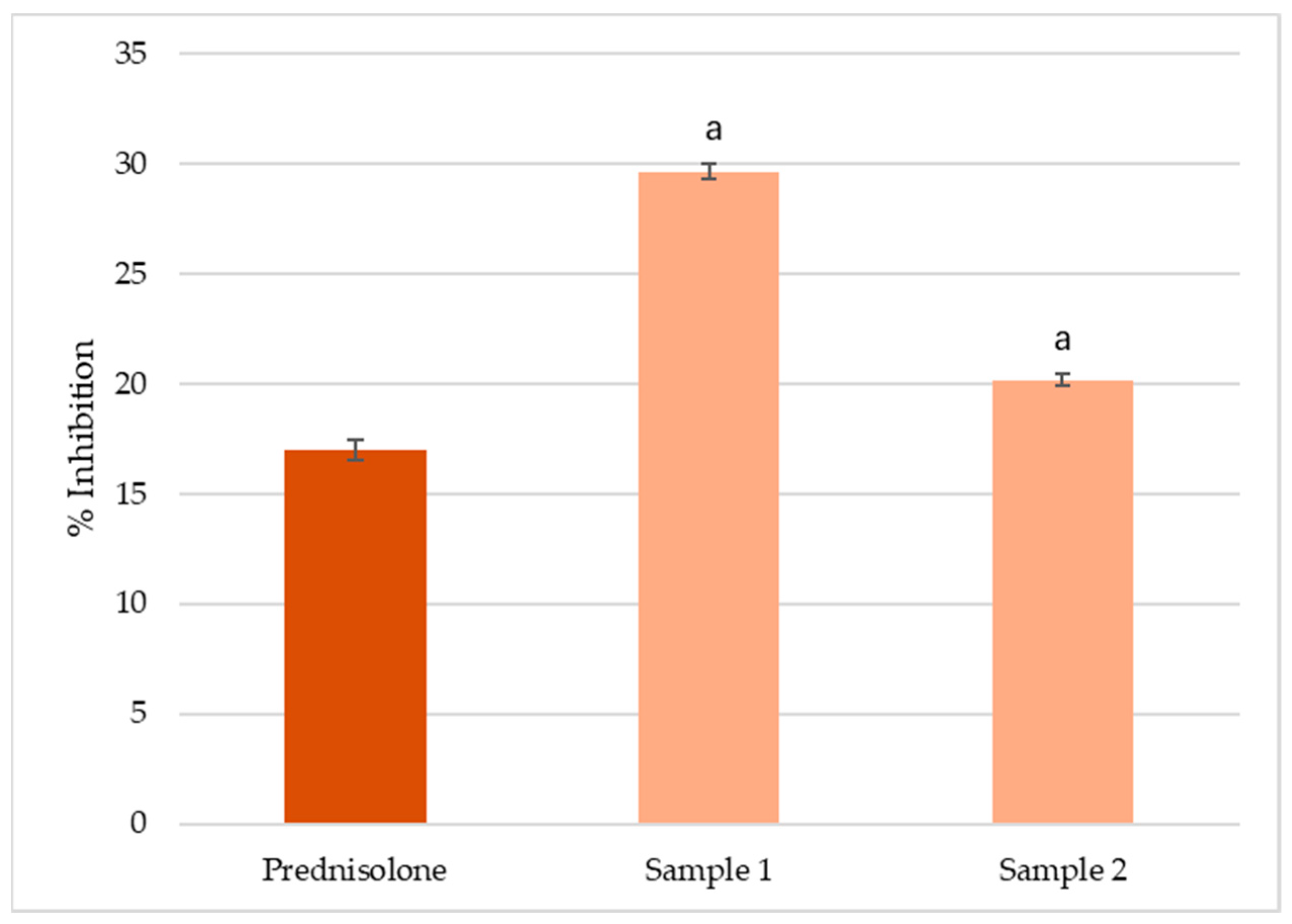
| Parameters | Sample 1 (India) | Sample 2 (UK) |
|---|---|---|
| D[3,2] (µm) | 38.00 ± 0.68 a | 39.95 ± 0.67 a |
| D[4,3] (µm) | 70.20 ± 0.83 b | 72.54 ± 0.87 a |
| Dv10 (µm) | 19.20 ± 0.12 a | 21.20 ± 0.17 a |
| Dv50 (µm) | 60.40 ± 0.53 b | 65.40 ± 0.62 a |
| Dv90 (µm) | 136.00 ± 0.46 b | 140.00 ± 0.43 a |
| Pharmacological Agents | TCR, mN | ASC, mN | FSMC, n/min | p |
|---|---|---|---|---|
| Basal spontaneous contractile activity | 2.07 ± 0.05 | 1.53 ± 0.17 | 5.00 ± 0.30 | - |
| ACh | 5.16 ± 0.05 a | 2.27 ± 0.20 a | 4.95 ± 0.03 | 0.01 |
| Sample 2 | 4.17 ± 0.10 a | 2.15 ± 0.09 a | 4.88 ± 0.17 | 0.01 |
| Sample 1 | 3.50 ± 0.13 a | 1.98 ± 0.05 a | 5.03 ± 0.09 | 0.01 |
| Sample 2 + ACh | 4.97 ± 0.10 | 1.96 ± 0.09 | 4.87 ± 0.11 | 0.06 |
| Sample 1 + ACh | 5.07 ± 0.12 | 2.07 ± 0.05 | 4.89 ± 0.22 | 0.06 |
| ACh + Sample 2 | 3.49 ± 0.18 b | 0.60 ± 0.01 b | 5.10 ± 0.21 | 0.04 |
| ACh + Sample 1 | 2.71 ± 0.09 b | 0.43 ± 0.07 b | 5.06 ± 0.02 | 0.03 |
| Atropine | 2.01 ± 0.05 | 1.99 ± 0.05 | 4.94 ± 0.04 | 0.06 |
| Atropine + ACh | 1.96 ± 0.11 c | 1.88 ± 0.03 c | 4.87 ± 0.26 | 0.02 |
| Atropine + Sample 2 | 2.60 ± 0.41 b | 2.07 ± 0.05 | 5.00 ± 0.15 | 0.01 |
| Atropine + Sample 1 | 2.88 ± 0.03 b | 1.87 ± 0.05 | 4.90 ± 0.06 | 0.05 |
| Verapamil | 1.11 ± 0.04 a | 0.75 ± 0.02 a | 5.09 ± 0.06 | 0.02 |
| Verapamil + Sample 2 | 2.37 ± 0.10 b | 2.00 ± 0.11 | 4.98 ± 0.12 | 0.04 |
| Verapamil + Sample 1 | 2.90 ± 0.08 b | 1.94 ± 0.06 | 4.92 ± 0.21 | 0.03 |
| Sample 2 + Verapamil | 0.23 ± 0.03 d | 0.14 ± 0.02 d | 5.05 ± 0.04 | 0.03 |
| Sample 1 + Verapamil | 0.56 ± 0.07 d | 0.34 ± 0.03 d | 4.88 ± 0.19 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova, N.; Gerasimova, A.; Todorova, M.; Pencheva, M.; Dincheva, I.; Batovska, D.; Gledacheva, V.; Slavchev, V.; Stefanova, I.; Nikolova, S.; et al. Metabolite Signatures and Particle Size as Determinants of Anti-Inflammatory and Gastrointestinal Smooth Muscle Modulation by Chlorella vulgaris. Foods 2025, 14, 3319. https://doi.org/10.3390/foods14193319
Panova N, Gerasimova A, Todorova M, Pencheva M, Dincheva I, Batovska D, Gledacheva V, Slavchev V, Stefanova I, Nikolova S, et al. Metabolite Signatures and Particle Size as Determinants of Anti-Inflammatory and Gastrointestinal Smooth Muscle Modulation by Chlorella vulgaris. Foods. 2025; 14(19):3319. https://doi.org/10.3390/foods14193319
Chicago/Turabian StylePanova, Natalina, Anelia Gerasimova, Mina Todorova, Mina Pencheva, Ivayla Dincheva, Daniela Batovska, Vera Gledacheva, Valeri Slavchev, Iliyana Stefanova, Stoyanka Nikolova, and et al. 2025. "Metabolite Signatures and Particle Size as Determinants of Anti-Inflammatory and Gastrointestinal Smooth Muscle Modulation by Chlorella vulgaris" Foods 14, no. 19: 3319. https://doi.org/10.3390/foods14193319
APA StylePanova, N., Gerasimova, A., Todorova, M., Pencheva, M., Dincheva, I., Batovska, D., Gledacheva, V., Slavchev, V., Stefanova, I., Nikolova, S., Mincheva, I., Szechyńska-Hebda, M., & Nikolova, K. (2025). Metabolite Signatures and Particle Size as Determinants of Anti-Inflammatory and Gastrointestinal Smooth Muscle Modulation by Chlorella vulgaris. Foods, 14(19), 3319. https://doi.org/10.3390/foods14193319











