Supplementation with Probiotic Camel Milk Powder Improves Serum Glucose and Cholesterol as Well as the Related Cytokines in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Randomization, Blinding, and Intervention Protocol
2.2. Camel Milk Powder and Probiotic Powder Preparation
2.3. Blood Sample Collection and Measurements
2.4. Cytokines and Hormones Assays
2.5. Fecal Sample Collection and Gut Microbiota Analysis
2.6. Fecal Metabolomics Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Study Population
3.2. Changes in Glycemic Indices and Serum Insulin
3.3. Changes in Lipid Profile and Cardiovascular Risk
3.4. Changes in Inflammatory Cytokines
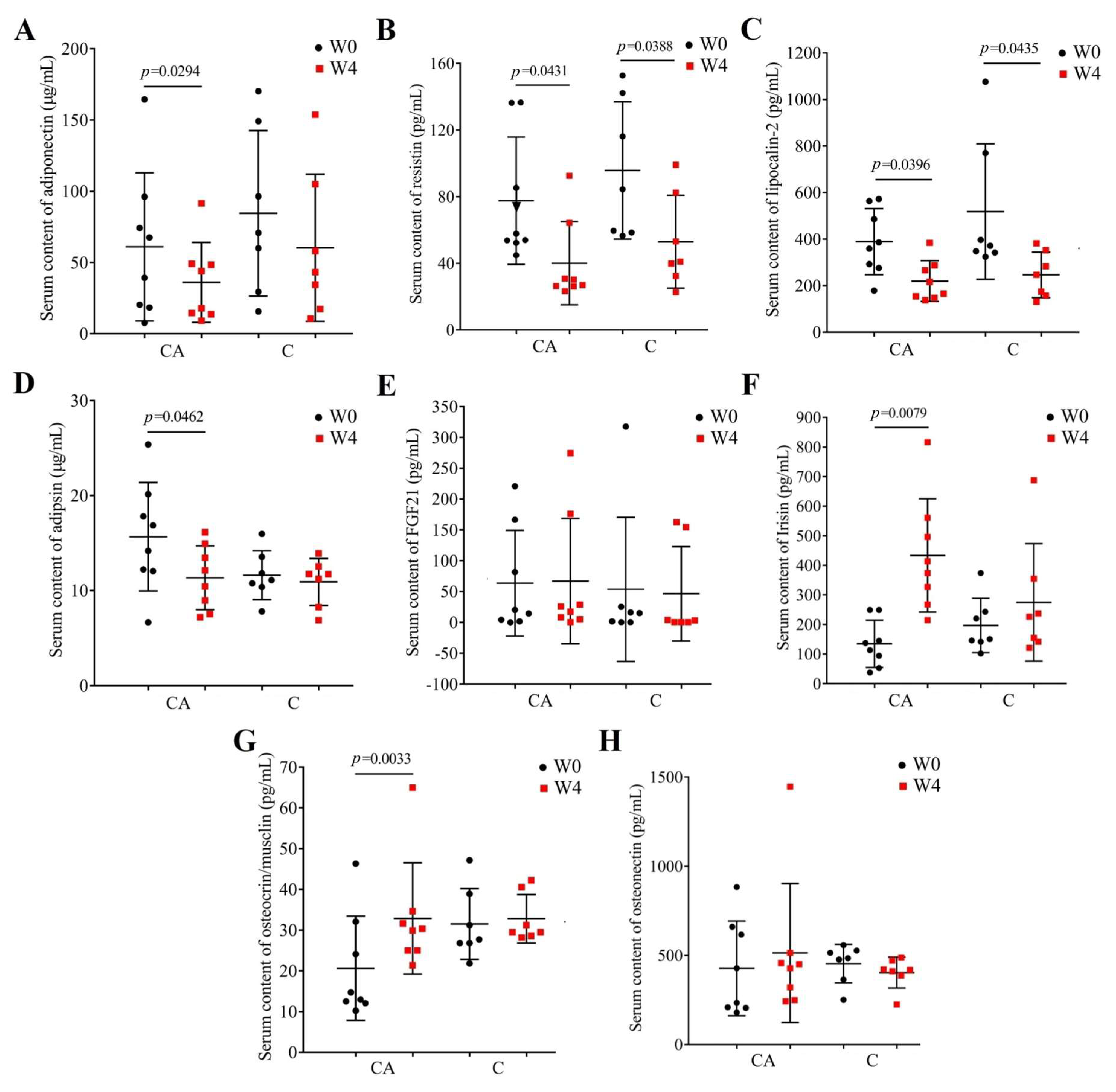
3.5. Changes in Adipokines and Myokines Profile
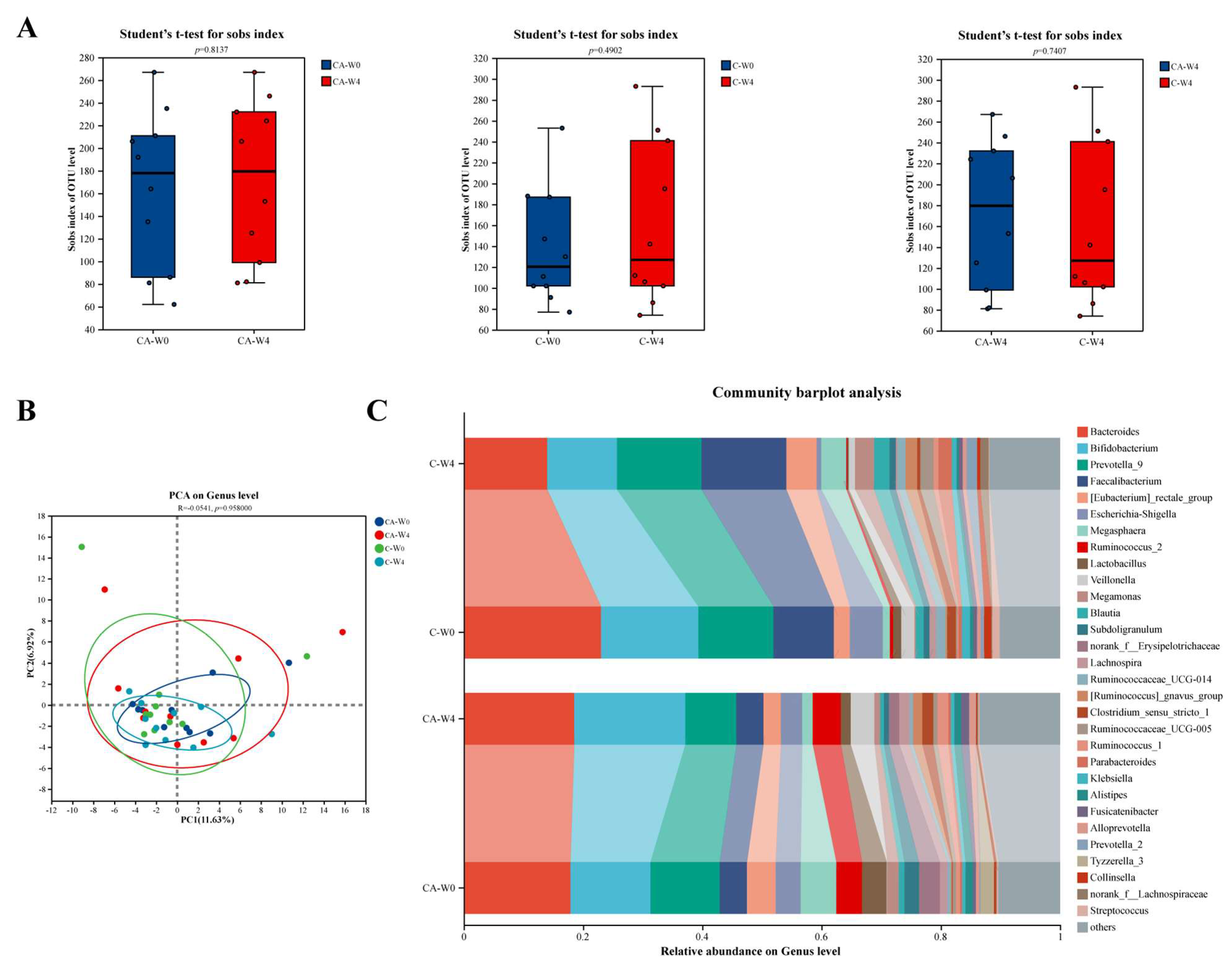
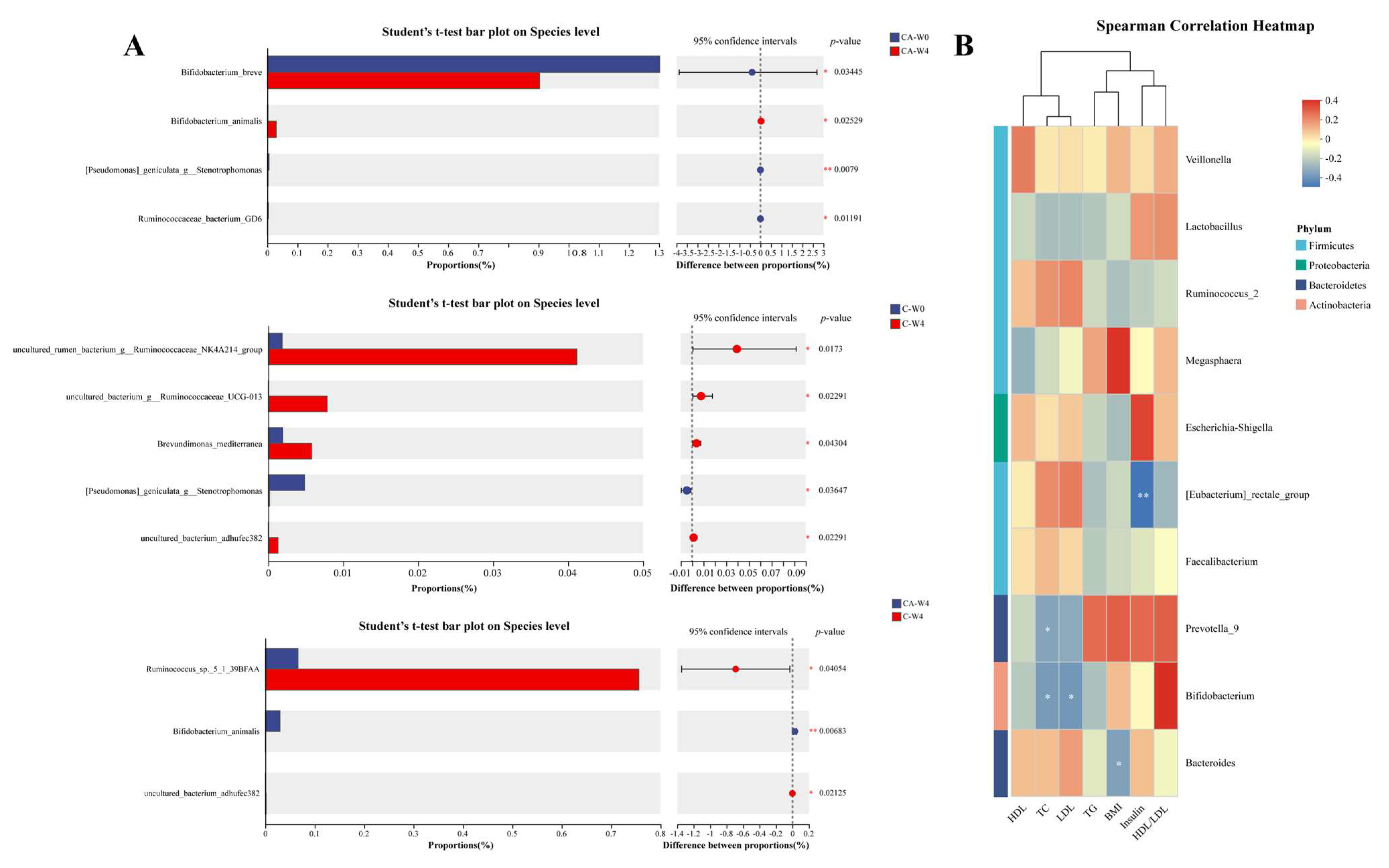
3.6. Changes in Gut Microbiota
3.7. Changes in Fecal Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBA6 | Bifidobacterium animalis A6 |
| T2DM | Type 2 diabetes mellitus |
| W0 | The beginning of the study |
| W4 | The end of the study |
| CA | Camel milk supplemented with BBA6 |
| C | Camel milk |
| TC | Total cholesterol |
| TG | Total triglyceride |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte chemotactic protein-1 |
| FGF-21 | Fibroblast growth factor-21 |
| OTUs | Operational taxonomic units |
| PLS-DA | Squares-discriminant analysis |
| VIP | Variable importance in projection |
References
- Ballan, R.; Saad, S.M.I. Characteristics of the gut microbiota and potential effects of probiotic supplements in individuals with type 2 diabetes mellitus. Foods 2021, 10, 2528. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, J.; Lin, Y.; You, Y.; Huang, W.; Zhan, J. Evaluation of hypoglycemic polyphenolic compounds in blueberry extract: Functional effects and mechanisms. Antioxidants 2024, 13, 1490. [Google Scholar] [CrossRef]
- Esfandiar, Z.; Hosseini-Esfahani, F.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. The association of dietary polyphenol intake with the risk of type 2 diabetes: Tehran lipid and glucose study. Diabetes Metab. Syndr. Obes. 2020, 13, 1643–1652. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhan, L.; Xu, C.; Sun, L.; Jiang, H.; Sun, C.; Li, X. LC-Q-TOF-MS characterization of polyphenols from white bayberry fruit and its antidiabetic effect in kk-ay mice. ACS Omega 2020, 5, 17839–17849. [Google Scholar] [CrossRef]
- Nwakiban Atchan, A.P.; Shivashankara, S.T.; Piazza, S.; Tchamgoue, A.D.; Beretta, G.; Dell’Agli, M.; Magni, P.; Agbor, G.A.; Kuiaté, J.-R.; Manjappara, U.V. Polyphenol-rich extracts of xylopia and aframomum species show metabolic benefits by lowering hepatic lipid accumulation in diet-induced obese mice. ACS Omega 2022, 7, 11914–11928. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Marette, A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr. Rev. 2018, 76, 16–28. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.A.; Palakkott, A.R.; Ashraf, A.; Iratni, R. The molecular basis of the anti-diabetic properties of camel milk. Diabetes Res. Clin. Pract. 2018, 146, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Mejía, E.; Batista, K.A.; Fernández, J.J.A.; Fernandes, K.F. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res. Int. 2019, 121, 238–246. [Google Scholar] [CrossRef]
- Eliasson, B.; Allansson Kjölhede, E.; Salö, S.; Fabrin Nielsen, N.; Eeg-Olofsson, K. Associations between hba1c and glucose time in range using continuous glucose monitoring in type 1 diabetes: Cross-sectional population-based study. Diabetes Ther. 2024, 15, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, F.Q.; Tang, P.; Gao, T.H.; Yang, C.X.; Tan, L.; Yue, P.; Hua, Y.N.; Liu, S.J.; Guo, J.L. Regulation of the intestinal flora: A potential mechanism of natural medicines in the treatment of type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 151, 113091. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and Streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, 12, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- Koay, K.-P.; Tsai, B.C.-K.; Kuo, C.-H.; Kuo, W.-W.; Luk, H.-N.; Day, C.H.; Chen, R.-J.; Chen, M.Y.-C.; Padma, V.V.; Huang, C.-Y. Hyperglycemia-induced cardiac damage is alleviated by heat-inactivated Lactobacillus reuteri gmnl-263 via activation of the IGF1r survival pathway. Probiotics Antimicrob. Proteins 2021, 13, 1044–1053. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Chen, Y.; Cao, Z.; Liu, C.; Bao, R.; Wang, Y.; Huang, S.; Pan, S.; Qin, L.; et al. Akkermansia muciniphila supplementation in patients with overweight/obese type 2 diabetes: Efficacy depends on its baseline levels in the gut. Cell Metab. 2025, 37, 592–605.e6. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Li, G.; Feng, H.; Mao, X.-L.; Deng, Y.-J.; Wang, X.-B.; Zhang, Q.; Guo, Y.; Xiao, S.-M. The effects of probiotics supplementation on glycaemic control among adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomised clinical trials. J. Transl. Med. 2023, 21, 442. [Google Scholar] [CrossRef]
- Zikou, E.; Dovrolis, N.; Dimosthenopoulos, C.; Gazouli, M.; Makrilakis, K. The effect of probiotic supplements on metabolic parameters of people with type 2 diabetes in Greece—A randomized, double-blind, placebo-controlled study. Nutrients 2023, 15, 4663. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, A.; Babajafari, S.; Mazloomi, S.M.; Shams, M. The effects of soymilk plus probiotics supplementation on cardiovascular risk factors in patients with type 2 diabetes mellitus: A randomized clinical trial. BMC Endocr. Disord. 2023, 23, 36. [Google Scholar] [CrossRef]
- Gu, Y.; Li, X.; Chen, H.; Sun, Y.; Yang, L.; Ma, Y.; Yong Chan, E.C. Antidiabetic effects of multi-species probiotic and its fermented milk in mice via restoring gut microbiota and intestinal barrier. Food Biosci. 2022, 47, 101619. [Google Scholar] [CrossRef]
- Deveci, G.; Çelik, E.; Ağagündüz, D.; Bartkiene, E.; Rocha, J.M.F.; Özogul, F. Certain fermented foods and their possible health effects with a focus on bioactive compounds and microorganisms. Fermentation 2023, 9, 923. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yilmaz, B.; Cemali, Ö.; Šimat, V.; Akkus, G.; Kulawik, P.; Ozogul, F. Impact of dairy food products on type 2 diabetes: Gut-pancreas axis for lower glucose level. Trends Food Sci. Technol. 2024, 153, 104741. [Google Scholar] [CrossRef]
- Khan, M.Z.; Xiao, J.; Ma, Y.; Ma, J.; Liu, S.; Khan, A.; Khan, J.M.; Cao, Z. Research Development on Anti-Microbial and Antioxidant Properties of Camel Milk and Its Role as an Anti-Cancer and Anti-Hepatitis Agent. Antioxidants 2021, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Wattoo, F.H.; Wattoo, M.H.S.; Gulfraz, M.; Masud, T.; Shah, I.; Ali, S.; Alavi, S.E. Camel milk as an alternative treatment regimen for diabetes therapy. Food Sci. Nutr. 2021, 9, 1347–1356. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Jain, S.; Shah, S.; Chopra, A.; Agarwal, V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.P.; Tantia, P.; Jain, S.; Agrawal, R.; Agrawal, V. Camel milk: A possible boon for type 1 diabetic patients. Cell Mol. Biol. 2013, 59, 99–107. [Google Scholar]
- Agrawal, R.P.; Saran, S.; Sharma, P.; Gupta, R.P.; Kochar, D.K.; Sahani, M.S. Effect of camel milk on residual beta-cell function in recent onset type 1 diabetes. Diabetes Res. Clin. Pract. 2007, 77, 494–495. [Google Scholar] [CrossRef]
- Abdalla, K.; Fadlalla, A. Effects of sudanese dromedary’s camel raw milk on insulin doses and carbohydrate metabolism in type 1 diabetic patients. J. Biomol. Res. Ther. 2018, 7, 24994. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, F.; Zhang, M.; Fang, B.; Zhao, L.; Dong, L.; Zhou, X.; Ge, S. Hypoglycemic effect of camel milk powder in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Food Sci. Nutr. 2021, 9, 4461–4472. [Google Scholar] [CrossRef]
- Dou, Z.; Liu, C.; Feng, X.; Xie, Y.; Yue, H.; Dong, J.; Zhao, Z.; Chen, G.; Yang, J. Camel whey protein (CWP) ameliorates liver injury in type 2 diabetes mellitus rats and insulin resistance (IR) in HepG2 cells via activation of the PI3K/Akt signaling pathway. Food Funct. 2022, 13, 255–269. [Google Scholar] [CrossRef]
- Sboui, A.; Atig, C.; Khabir, A.; Hammadi, M.; Khorchani, T. Camel milk used as an adjuvant therapy to treat type 2 diabetic patients: Effects on blood glucose, Hba1c, cholesterol, and TG levels. J. Chem. 2022, 2022, 5860162. [Google Scholar] [CrossRef]
- Alhaj, O.A.; Altooq, N.J.; Alenezi, A.F.; Janahi, A.I.; Janahi, M.I.; Humood, A.M.; AlRasheed, M.M.; Bragazzi, N.L.; Jahrami, H.A.; Faye, B. Camel milk composition by breed, season, publication year, and country: A global systematic review, meta-analysis, and meta-regression. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2520–2559. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, X.; Wang, X.; Wang, X.; Chen, L.; Guo, H.; Zhang, M.; Li, Y. Bifidobacterium animalis subsp. lactis A6 Alleviates Obesity Associated with Promoting Mitochondrial Biogenesis and Function of Adipose Tissue in Mice. Molecules 2020, 25, 1490. [Google Scholar] [CrossRef]
- Huo, Y.; Zhao, G.; Li, J.; Wang, R.; Ren, F.; Li, Y.; Wang, X. Bifidobacterium animalis subsp. lactis A6 Enhances Fatty Acid β-Oxidation of Adipose Tissue to Ameliorate the Development of Obesity in Mice. Nutrients 2022, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Tian, L.; Lu, C.; Teng, D.; Teng, W.; Li, J. Association between blood glucose indicators and metabolic diseases in the Chinese population: A national cross-sectional study. Chin. Med. J. 2025, 138, 2159–2169. [Google Scholar] [CrossRef]
- Simundic, A.M.; Cornes, M.; Grankvist, K.; Lippi, G.; Nybo, M. Standardization of collection requirements for fasting samples: For the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin. Chim. Acta 2014, 432, 33–37. [Google Scholar] [CrossRef]
- Trifunovic, D.; Stankovic, S.; Sobic-Saranovic, D.; Marinkovic, J.; Petrovic, M.; Orlic, D.; Beleslin, B.; Banovic, M.; Vujisic-Tesic, B.; Petrovic, M.; et al. Acute insulin resistance in ST-segment elevation myocardial infarction in non-diabetic patients is associated with incomplete myocardial reperfusion and impaired coronary microcirculatory function. Cardiovasc. Diabetol. 2014, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Takanohashi, A.; Prust, M.; Wang, J.; Gordish-Dressman, H.; Bloom, M.; Rice, G.I.; Schmidt, J.L.; Crow, Y.J.; Lebon, P.; Kuijpers, T.W.; et al. Elevation of proinflammatory cytokines in patients with Aicardi-Goutières syndrome. Neurology 2013, 80, 997–1002. [Google Scholar] [CrossRef]
- Ahlquist, D.A.; Sargent, D.J.; Loprinzi, C.L.; Levin, T.R.; Rex, D.K.; Ahnen, D.J.; Knigge, K.; Lance, M.P.; Burgart, L.J.; Hamilton, S.R.; et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann. Intern. Med. 2008, 149, 441–450. [Google Scholar] [CrossRef]
- Gautam, A. Gautam, A., Ed.; Phenol-chloroform DNA isolation method. In DNA and RNA Isolation Techniques for Non-Experts; Springer International Publishing: Cham, Switzerland, 2022; pp. 33–39. [Google Scholar]
- Wang, H.; Ainiwaer, A.; Song, Y.; Qin, L.; Peng, A.; Bao, H.; Qin, H. Perturbed gut microbiome and fecal and serum metabolomes are associated with chronic kidney disease severity. Microbiome 2023, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Sharma, P.; Gafoorunissa, S.J.; Ibrahim, S.A.; Shah, B.; Shukla, D.K.; Kaur, T. Effect of camel milk on glucose metabolism in adults with normal glucose tolerance and type 2 diabetes in Raica community: A crossover study. Acta Biomed. 2011, 82, 181–186. [Google Scholar]
- Mohamad, R.H.; Zekry, Z.K.; Al-Mehdar, H.A.; Salama, O.; El-Shaieb, S.E.; El-Basmy, A.A.; Al-said, M.G.; Sharawy, S.M. Camel milk as an adjuvant therapy for the treatment of type 1 diabetes: Verification of a traditional ethnomedical practice. J. Med. Food 2009, 12, 461–465. [Google Scholar] [CrossRef]
- Agrawal, P.; Swami, S.; Beniwal, R.; Kochar, D.; Sahani, M.; Tuteja, F.; Ghouri, S. Effect of camel milk on glycemic control, risk factors and diabetes quality of life in type-1 diabetes: A randomised prospective controlled study. J. Camel Pract. Res. 2003, 10, 1048–1052. [Google Scholar]
- Agrawal, R.; Beniwal, R.; Sharma, S.; Kochar, D.; Tuteja, F.; Ghorui, S. Effect of raw camel milk in type 1 diabetic patients: 1 year randomised study. J. Camel Pract. Res. 2005, 12, 27–35. [Google Scholar]
- Agrawal, R.P.; Beniwal, R.; Kochar, D.K.; Tuteja, F.C.; Ghorui, S.K.; Sahani, M.S.; Sharma, S. Camel milk as an adjunct to insulin therapy improves long-term glycemic control and reduction in doses of insulin in patients with type-1 diabetes a 1 year randomized controlled trial. Diabetes Res. Clin. Pract. 2005, 68, 176–177. [Google Scholar] [CrossRef]
- Borén, J.; Öörni, K.; Catapano, A.L. The link between diabetes and cardiovascular disease. Atherosclerosis 2024, 394, 117607. [Google Scholar] [CrossRef]
- Magbola, S. Camel milk improvement of antioxidant and lipid profile in hypercholesterolemic rabbits. J. Cell Anim. Biol. 2018, 12, 1–4. [Google Scholar] [CrossRef][Green Version]
- Meena, S.; Rajput, Y.S.; Sharma, R.; Singh, R. Effect of goat and camel milk vis a vis cow milk on cholesterol homeostasis in hypercholesterolemic rats. Small Rumin. Res. 2019, 171, 8–12. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Niasari Naslaji, A.; Mirmiran, P.; Zraif Yeganeh, M.; Hedayati, M.; Azizi, F.; Moosavi Movahedi, A. Effect of camel milk on blood sugar and lipid profile of patients with type 2 diabetes: A pilot clinical trial. Int. J. Endocrinol. Metab. 2015, 13, e21160. [Google Scholar] [CrossRef]
- Manaer, T.; Yu, L.; Nabi, X.-H.; Dilidaxi, D.; Liu, L.; Sailike, J. The beneficial effects of the composite probiotics from camel milk on glucose and lipid metabolism, liver and renal function and gut microbiota in db/db mice. BMC Complement. Med. Ther. 2021, 21, 127. [Google Scholar] [CrossRef]
- Manaer, T.; Sailike, J.; Sun, X.; Yeerjiang, B.; Nabi, X. Therapeutic effects of composite probiotics derived from fermented camel milk on metabolic dysregulation and intestinal barrier integrity in type 2 diabetes rats. Front. Pharmacol. 2025, 15, 1520158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, H.; Zhang, L.; Liu, Z.; Huang, Y.; Liu, Q.; Jin, L.; Zhu, M.; Zhang, L. Inflammation in diabetes complications: Molecular mechanisms and therapeutic interventions. MedComm 2024, 5, e516. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of cmel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Badr, G.; Ramadan, N.K.; Sayed, L.H.; Badr, B.M.; Omar, H.M.; Selamoglu, Z. Why whey? Camel whey protein as a new dietary approach to the management of free radicals and for the treatment of different health disorders. Iran. J. Basic Med. Sci. 2017, 20, 338–349. [Google Scholar] [CrossRef]
- Badr, G.; Ramadan, N.K.; Abdel-Tawab, H.S.; Ahmed, S.F.; Mahmoud, M.H. Camel whey protein protects lymphocytes from apoptosis via the PI3K-AKT, NF-κB, ATF-3, and HSP-70 signaling pathways in heat-stressed male mice. Biochem. Cell Biol. 2018, 96, 407–416. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Badr, G.; El Shinnawy, N.A. Camel whey protein improves lymphocyte function and protects against diabetes in the offspring of diabetic mouse dams. Int. J. Immunopathol. Pharmacol. 2016, 29, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, myokines, and hepatokines: Crosstalk and metabolic repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef]
- Chen, Z.T.; Weng, Z.X.; Lin, J.D.; Meng, Z.-X. Myokines: Metabolic regulation in obesity and type 2 diabetes. Life Metab. 2024, 3, loae006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, R.-W.; Kunutsor, S.K.; Chowdhury, R.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Plasma adiponectin levels and type 2 diabetes risk: A nested case-control study in a chinese population and an updated meta-analysis. Sci. Rep. 2018, 8, 406. [Google Scholar] [CrossRef]
- Gómez-Banoy, N.; Guseh, J.S.; Li, G.; Rubio-Navarro, A.; Chen, T.; Poirier, B.; Putzel, G.; Rosselot, C.; Pabón, M.A.; Camporez, J.P.; et al. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat. Med. 2019, 25, 1739–1747. [Google Scholar] [CrossRef]
- Munhoz, A.C.; Serna, J.D.C.; Vilas-Boas, E.A.; Caldeira da Silva, C.C.; Santos, T.G.; Mosele, F.C.; Felisbino, S.L.; Martins, V.R.; Kowaltowski, A.J. Adiponectin reverses β-Cell damage and impaired insulin secretion induced by obesity. Aging Cell 2023, 22, e13827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xiao, Y.; Li, R.; Hong, S.; Li, S.; Wang, L.; Zeng, R.; Liao, K. Quantitative analysis of secretome from adipocytes regulated by insulin. Acta Biochim. Biophys. Sin. 2009, 41, 910–921. [Google Scholar] [CrossRef]
- Auguet, T.; Quintero, Y.; Terra, X.; Martínez, S.; Lucas, A.; Pellitero, S.; Aguilar, C.; Hernández, M.; Del Castillo, D.; Richart, C. Upregulation of Lipocalin 2 in Adipose Tissues of Severely Obese Women: Positive Relationship with Proinflammatory Cytokines. Obesity 2011, 19, 2295–2300. [Google Scholar] [CrossRef]
- Wang, W.; Ye, S.; Qian, L.; Xing, Y.; Ren, A.; Chen, C.; Li, S.; Xu, J.; Liu, Q.; Dong, L.; et al. Elevated serum lipocalin 2 levels are associated with indexes of both glucose and bone metabolism in type 2 diabetes mellitus. Endokrynol. Pol. 2018, 69, 276–282. [Google Scholar] [CrossRef]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, L.; Hu, Y.; Li, Q.; An, C.; Yu, X.; Shu, L.; Chen, A.; Niu, C.; Zhou, L.; et al. Resistin induces hypertension and insulin resistance in mice via a TLR4-dependent pathway. Sci. Rep. 2016, 6, 22193. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Kim, J.; Kim, B.-K.; Seo, H.J.; Kim, J.-Y.; Lee, J.-E.; Lee, J.; You, J.; Jin, S.; Kwon, Y.-W.; et al. Resistin regulates inflammation and insulin resistance in humans via the endocannabinoid system. Research 2024, 7, 0326. [Google Scholar] [CrossRef]
- Oh, K.-J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.-H. Metabolic adaptation in obesity and type ii diabetes: Myokines, adipokines and hepatokines. Int. J. Mol. Sci. 2016, 18, 8. [Google Scholar] [CrossRef]
- Paoletti, I.; Coccurello, R. Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. Int. J. Mol. Sci. 2024, 25, 13480. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Watanabe-Takano, H.; Ochi, H.; Chiba, A.; Matsuo, A.; Kanai, Y.; Fukuhara, S.; Ito, N.; Sako, K.; Miyazaki, T.; Tainaka, K.; et al. Mechanical load regulates bone growth via periosteal Osteocrin. Cell Rep. 2021, 36, 109380. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Yuan, X.P.; Yuan, Y.P.; Song, P.; Kong, C.Y.; Teng, T.; Hu, M.; Xu, S.C.; Ma, Z.G.; et al. Osteocrin, a novel myokine, prevents diabetic cardiomyopathy via restoring proteasomal activity. Cell Death Dis. 2021, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Thangaleela, S.; Khongtan, S.; Bharathi, M.; Kesika, P.; Saelee, M.; Choeisoongnern, T.; et al. Influence of Bifidobacterium breve on the Glycaemic Control, Lipid Profile and Microbiome of Type 2 Diabetic Subjects: A Preliminary Randomized Clinical Trial. Pharmaceuticals 2023, 16, 695. [Google Scholar] [CrossRef]
- Que, Y.; Cao, M.; He, J.; Zhang, Q.; Chen, Q.; Yan, C.; Lin, A.; Yang, L.; Wu, Z.; Zhu, D.; et al. Gut Bacterial Characteristics of Patients with Type 2 Diabetes Mellitus and the Application Potential. Front. Immunol. 2021, 12, 722206. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Ma, Q.; Tian, B.; Nie, C.; Chen, Z.; Li, J. Health beneficial effects of resistant starch on diabetes and obesity via regulation of gut microbiota: A review. Food Funct. 2020, 11, 5749–5767. [Google Scholar] [CrossRef]
- Pan, X.; Liu, P.; Zhang, Y.J.; Zhang, H.K.; Wei, H.; Jiang, J.Y.; Hui, Y.; Shang, E.X.; Li, W.W.; Wang, Y.; et al. Carboxymethyl chitosan-TK resistant starch complex ameliorates type 2 diabetes by regulating the gut microbiota. Int. J. Biol. Macromol. 2023, 253, 126930. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Liu, Y.; Liu, H.; Yu, H.; Wang, H.; Xia, Z. Effects of N-acetylcysteine on nicotinamide dinucleotide phosphate oxidase activation and antioxidant status in heart, lung, liver and kidney in streptozotocin-induced diabetic rats. Yonsei Med. J. 2012, 53, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Tuell, D.; Ford, G.; Los, E.; Stone, W. The Role of Glutathione and Its Precursors in Type 2 Diabetes. Antioxidants 2024, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kato, E.; Machikawa, T.; Kimura, S.; Katayama, S.; Kawabata, J. Hydroxylamine enhances glucose uptake in C2C12 skeletal muscle cells through the activation of insulin receptor substrate 1. Biochem. Biophys. Res. Commun. 2014, 445, 6–9. [Google Scholar] [CrossRef][Green Version]
- Thomas, D.D.; Stockman, M.C.; Yu, L.; Meshulam, T.; McCarthy, A.C.; Ionson, A.; Burritt, N.; Deeney, J.; Cabral, H.; Corkey, B.; et al. Effects of medium chain triglycerides supplementation on insulin sensitivity and beta cell function: A feasibility study. PLoS ONE 2019, 14, e0226200. [Google Scholar] [CrossRef]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Grapov, D.; Fahrmann, J.; Hwang, J.; Poudel, A.; Jo, J.; Periwal, V.; Fiehn, O.; Hara, M. Diabetes Associated Metabolomic Perturbations in NOD Mice. Metabolomics 2015, 11, 425–437. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Yun, J.; Zhang, G.; Zhang, Y.; Zhao, M.; Zabed, H.M.; Ravikumar, Y.; Qi, X. d-Arabitol Ameliorates Obesity and Metabolic Disorders via the Gut Microbiota–SCFAs–WAT Browning Axis. J. Agric. Food Chem. 2023, 71, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Willis, J.; Gearry, R.; Skidmore, P.; Fleming, E.; Frampton, C.; Carr, A. Inadequate Vitamin C Status in Prediabetes and Type 2 Diabetes Mellitus: Associations with Glycaemic Control, Obesity, and Smoking. Nutrients 2017, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Kositsawat, J.; Freeman, V.L. Vitamin C and A1c relationship in the National Health and Nutrition Examination Survey (NHANES) 2003–2006. J. Am. Coll. Nutr. 2011, 30, 477–483. [Google Scholar] [CrossRef]
- Dang, S.; Jain, A.; Dhanda, G.; Bhattacharya, N.; Bhattacharya, A.; Senapati, S. One carbon metabolism and its implication in health and immune functions. Cell Biochem. Funct. 2024, 42, e3926. [Google Scholar] [CrossRef] [PubMed]
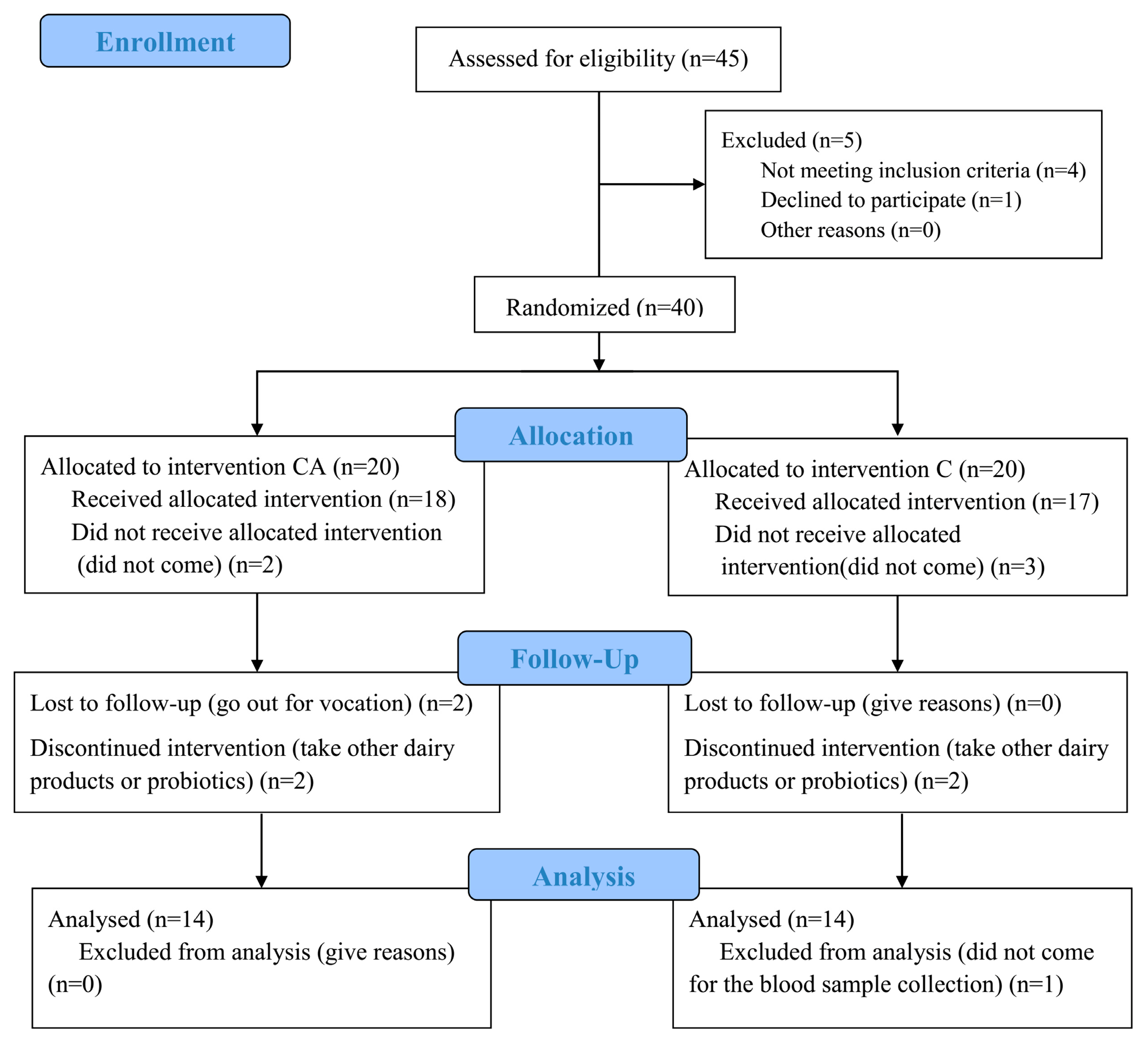
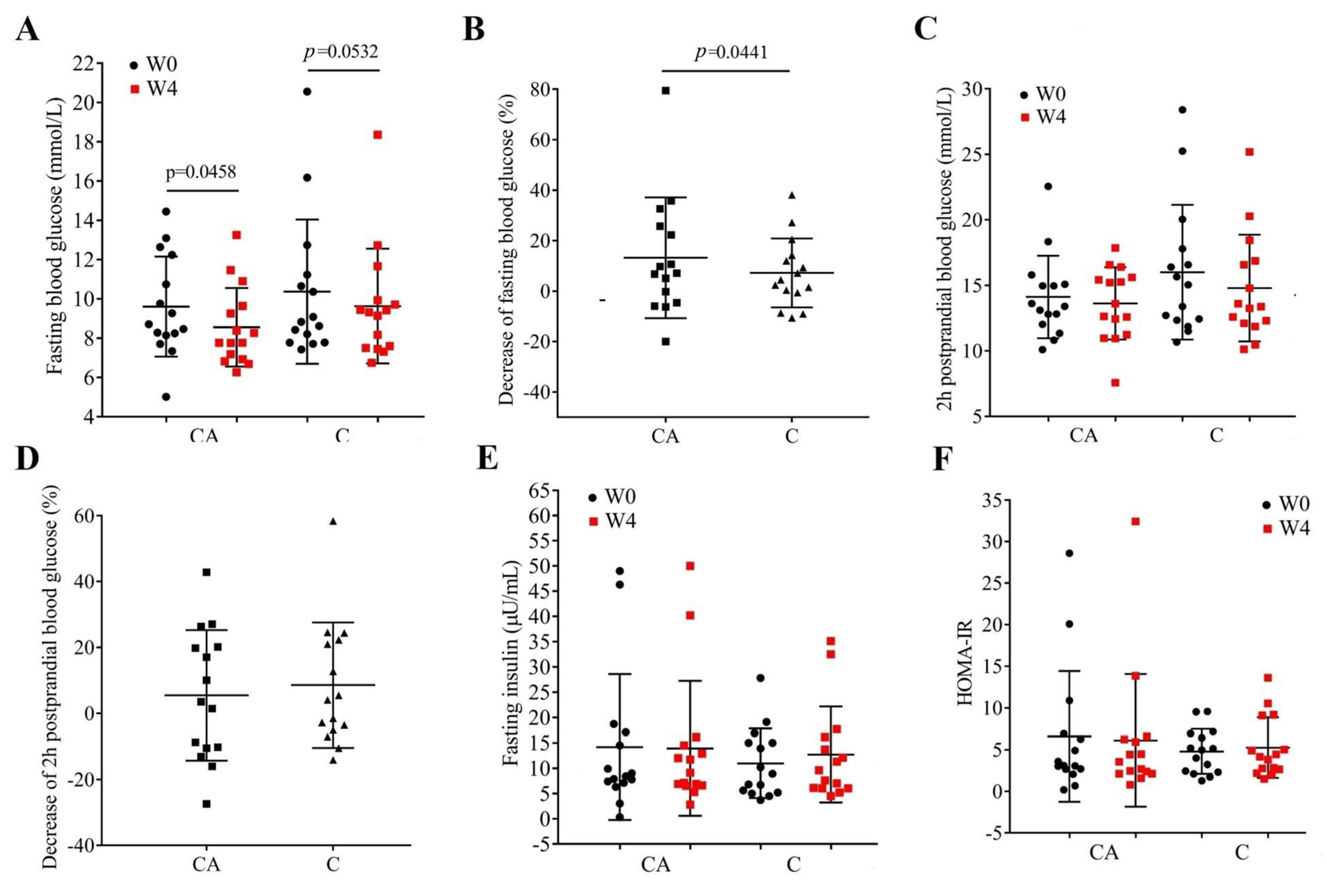
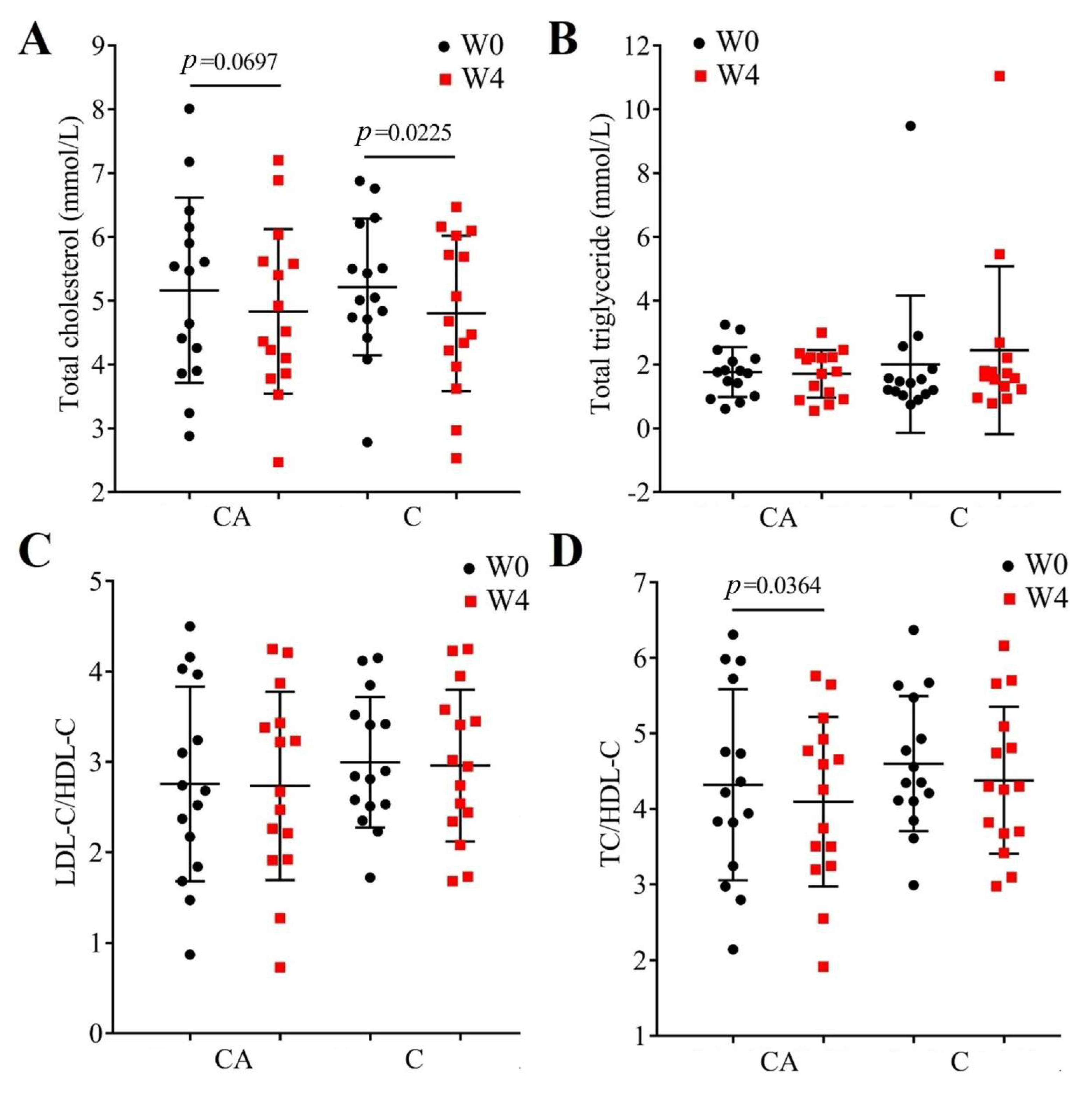
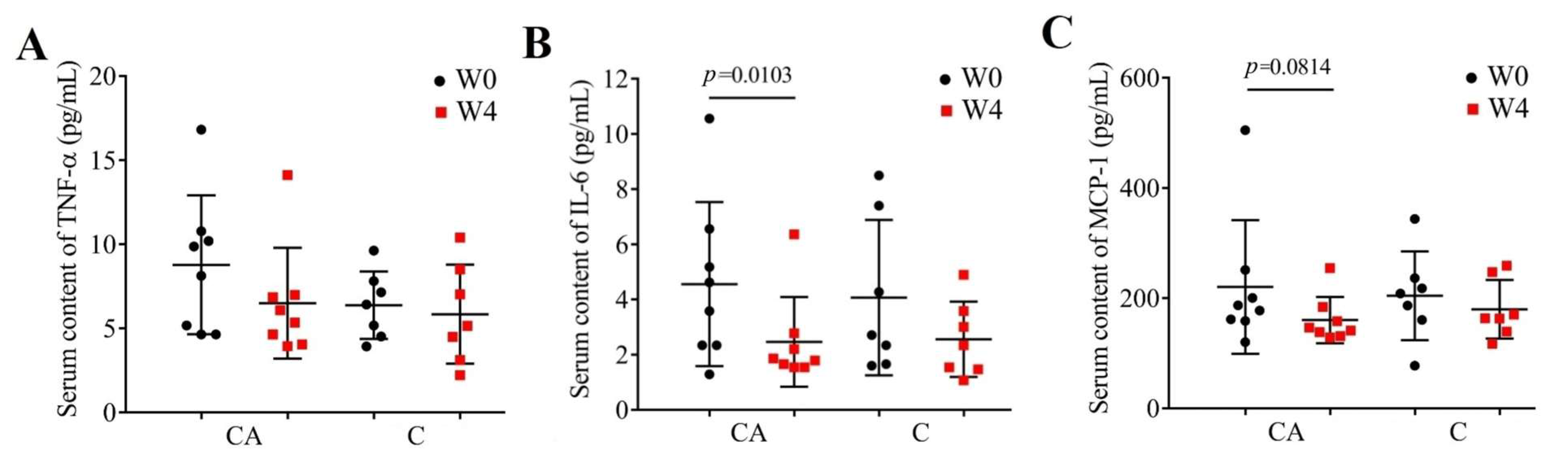

| Parameters | CA | C |
|---|---|---|
| Number (female/male) | 14 (10/4) | 14 (8/6) |
| Age (years) | 58.36 ± 6.25 | 57.29 ± 7.57 |
| BMI (kg/m2) | 26.56 ± 4.40 | 26.56 ± 4.40 |
| Fasting blood glucose (mmol/L) | 9.39 ± 2.50 | 10.48 ± 3.78 |
| 2 h postprandial blood glucose (mmol/L) | 14.05 ± 3.26 | 16.19 ± 5.28 |
| Insulin (μU/mL) | 11.69 ± 11.12 | 11.01 ± 7.11 |
| TG (mmol/L) | 5.20 ± 1.50 | 5.10 ± 1.00 |
| TC (mmol/L) | 1.77 ± 0.81 | 1.14 ± 0.14 |
| HDL-C (mmol/L) | 1.24 ± 0.26 | 1.22 ± 0.26 |
| LDL-C (mmol/L) | 3.27 ± 1.25 | 3.33 ± 0.86 |
| TC/HDL-C | 4.29 ± 1.31 | 4.47 ± 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, M.; Wang, R.; Ge, S.; Fang, B. Supplementation with Probiotic Camel Milk Powder Improves Serum Glucose and Cholesterol as Well as the Related Cytokines in Patients with Type 2 Diabetes Mellitus. Foods 2025, 14, 3318. https://doi.org/10.3390/foods14193318
Liu Y, Zhang M, Wang R, Ge S, Fang B. Supplementation with Probiotic Camel Milk Powder Improves Serum Glucose and Cholesterol as Well as the Related Cytokines in Patients with Type 2 Diabetes Mellitus. Foods. 2025; 14(19):3318. https://doi.org/10.3390/foods14193318
Chicago/Turabian StyleLiu, Yue, Ming Zhang, Ran Wang, Shaoyang Ge, and Bing Fang. 2025. "Supplementation with Probiotic Camel Milk Powder Improves Serum Glucose and Cholesterol as Well as the Related Cytokines in Patients with Type 2 Diabetes Mellitus" Foods 14, no. 19: 3318. https://doi.org/10.3390/foods14193318
APA StyleLiu, Y., Zhang, M., Wang, R., Ge, S., & Fang, B. (2025). Supplementation with Probiotic Camel Milk Powder Improves Serum Glucose and Cholesterol as Well as the Related Cytokines in Patients with Type 2 Diabetes Mellitus. Foods, 14(19), 3318. https://doi.org/10.3390/foods14193318






