Fatty Acid Profile, Lipid Quality Indices and Oxidative Stability of Snacks Consumed by Children Aged 6–24 Months in Rural Matiari, Sindh, Pakistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Design
2.3. Dietary Data Collection
2.4. Sampling and Categorization of Snack Foods
2.5. Fat and Fatty Acids Analysis
2.6. Trans Fat Analysis by FT-IR Spectroscopy
2.7. The Lipid Quality Indices
- Index of Atherogenicity (AI):AI = (C12:0 + (4 × C14:0) + C16:0)/(Σn-3 PUFA + Σn-6 PUFA + Σ MUFA)
- Index of Thrombogenicity (TI):TI = (C14:0 + C16:0 + C18:0)/((0.5 × C18:1) + (0.5 × other MUFA) + (0.5 × Σn-6 PUFA)+ (3 × Σn-3 PUFA) + Σn-3 PUFA/Σn-6PUFA)
- Hypocholesterolemic Fatty Acids (DFAs):DFA = UFA + C18:0
- Hypercholesterolemic Fatty Acids (OFAs):OFA = C12:0 + C14:0 + C16:0
- Hypocholesterolemic and Hypercholesterolemic Fatty Acids (H/H):H/H = (C18:1n-9 + C18:2n-6 + C18:3n-3)/(C12:0 + C14:0 + C16:0)
- f.
- Nutritional Index (NI)NI = ∑(Hypercholesterolemic Fatty Acids)/∑(Hypocholesterolemic Fatty Acids)
- Hypocholesterolemic Fatty Acids (h): C18:1 (oleic), C18:2 (linoleic), C18:3 (linolenic);
- Hypercholesterolemic Fatty Acids (H): C14:0 (myristic), C16:0 (palmitic).
2.8. Oxidative Stability of Fat Present in High-Fat Snacks:
3. Results
3.1. Energy and Fat Content of High-Fat Snacks:
3.2. Fatty Acid Composition of High-Fat Snacks
3.3. Lipid Quality Index Assessment
3.4. Oxidative Stability of Oil Extracted from Snacks
4. Conclusions
5. Limitations of Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, K.-H.; Plasser, E.; Proell, C.; Kanzler, S. Comprehensive studies on the trans fatty acid content of Austrian foods: Convenience products, fast food and fats. Food Chem. 2008, 108, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. The State of the World’s Children 2023: For Every Child, Vaccination. UNICEF Innocenti—Global Office of Research and Foresight, Florence, April 2023. Available online: https://www.unicef.org/reports/state-worlds-children-2023 (accessed on 27 August 2025).
- Black, R.E.; Victora, C.g.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef]
- Birch, L.L.; Ventura, A.K. Preventing childhood obesity: What works? Int. J. Obes. 2009, 33, S74–S81. [Google Scholar] [CrossRef]
- Paul, I.M.; Bartok, C.J.; Downs, D.S.; Stifter, C.A.; Ventura, A.K.; Birch, L.L. Opportunities for the primary prevention of obesity during infancy. Adv. Pediatr. 2009, 56, 107. [Google Scholar] [CrossRef]
- Bajwa, M.J.; Nadeem, M.A.; Khalid, N. Educating Parents on Children Daily Dietary Intakes Concentration of Heavy Metal in Biscuits and Rusks in Pakistan. Pak. Soc. Sci. Rev. 2023, 7, 1185–1199. [Google Scholar]
- Nestel, P.J.; Mori, T.A. Dairy foods: Is its cardiovascular risk profile changing? Curr. Atheroscler. Rep. 2022, 24, 33–40. [Google Scholar] [CrossRef]
- Nestel, P. Trans fatty acids: Are its cardiovascular risks fully appreciated? Clin. Ther. 2014, 36, 315–321. [Google Scholar] [CrossRef]
- Soomro, S.I.; Memon, N.; Bhanger, M.I.; Memon, S.; Memon, A.A. Mineral content of Pakistani foods: An update of food composition database of Pakistan through indirect method. J. Food Compos. Anal. 2016, 51, 45–54. [Google Scholar] [CrossRef]
- Clark, H.; Coll-Seck, A.M.; Banerjee, A.; Peterson, S.; Dalglish, S.L.; Ameratunga, S.; Balabanova, D.; Bhan, M.K.; Bhutta, Z.A.; Borrazzo, A.; et al. A future for the world’s children? A WHO–UNICEF–Lancet Commission. Lancet 2020, 395, 605–658. [Google Scholar] [CrossRef]
- Leonez, D.G.V.R.; Melhem, A.R.d.F.; Vieira, D.G.; de Mello, D.F.; Saldan, P.C. Complementary feeding indicators for children aged 6 to 23 months according to breastfeeding status. Rev. Paul. Pediatr. 2020, 39, e2019408. [Google Scholar] [CrossRef]
- Soomro, S.I.; Jamil, Z.; Memon, N.; Ahmed, S.; Umrani, F.; Choudhri, I.A.; Mohammed, S.; Qureshi, K.; Raza, G.; Jakhro, S.; et al. Nutrient dataset development via FAO/INFOODS approach for infant nutritional survey in rural Matiari, Pakistan. J. Food Compos. Anal. 2024, 133, 106471. [Google Scholar] [CrossRef]
- FAO/INFOODS. Guidelines for Food Matching; Version 1.2; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Puwastien, P.; Siong, T.E.; Kantasubrata, J.; Craven, G.; Feliciano, R.R.; Judprasong, K. Asean Manual of Nutrient Analysis; Regional Centre of ASEAN Network of Food Data System, Institute of Nutrition, Mahidol University: Salaya, Thailand, 2011. [Google Scholar]
- Sayre, D.A. INSDE ISO 14000: The Competitive Advantage of Environmental Management; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Paszczyk, B.; Łuczyńska, J. The comparison of fatty acid composition and lipid quality indices in hard cow, sheep, and goat cheeses. Foods 2020, 9, 1667. [Google Scholar] [CrossRef]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.D.; White, P.J. Adaptation of the AOCS official method for measuring hydroperoxides from small-scale oil samples. J. Am. Oil Chem. Soc. 2001, 78, 1267–1269. [Google Scholar] [CrossRef]
- AOCS. Method Cd 8–53; American Oil Chemists’ Society: Champaign, IL, USA, 1960. [Google Scholar]
- Saldivar, S. Snack Foods: Types and composition. In Encyclopedia of Food and Health; Elsevier Publications: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chester, M.A. Nomenclature of glycolipids (IUPAC recommendations 1997). Pure Appl. Chem. 1997, 69, 2475–2488. [Google Scholar] [CrossRef]
- Saini, D.; Barthwal, R.; Sharma, S.K.; Rawat, N. Chemistry of Food Fats, Oils, and Other Lipids; Springer: Cham, Switzerland, 2022; Available online: https://www.researchgate.net/publication/364176707 (accessed on 27 August 2025).
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Hunter, J.E. Dietary trans fatty acids: Review of recent human studies and food industry responses. Lipids 2006, 41, 967–992. [Google Scholar] [CrossRef]
- Geraldo, J.M.; Alfenas, R.D.C. Role of diet on chronic inflammation prevention and control: Current evidence. Arq. Bras. Endocrinol. Metabol. 2008, 52, 951–967. [Google Scholar] [CrossRef]
- Havlicekova, Z.; Jesenak, M.; Banovcin, P.; Kuchta, M. Beta-palmitate—A natural component of human milk in supplemental milk formulas. Nutr. J. 2015, 15, 28. [Google Scholar] [CrossRef]
- Lopes, C.; Aro, A.; Azevedo, A.; Ramos, E.; Barros, H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J. Am. Diet. Assoc. 2007, 107, 276–286. [Google Scholar] [CrossRef]
- Vardavas, C.; Yiannopoulos, S.; Kiriakakis, M.; Poulli, E.; Kafatos, A. Fatty acid and salt contents of snacks in the Cretan and Cypriot market: A child and adolescent dietary hazard. Food Chem. 2007, 101, 924–931. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [CrossRef] [PubMed]
- Joshee, K.; Abhang, T.; Kulkarni, R. Fatty acid profiling of 75 Indian snack samples highlights overall low trans fatty acid content with high polyunsaturated fatty acid content in some samples. PLoS ONE 2019, 14, e0225798. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.C.; Lopes, A.L.; Macedo, R.C.O.; Correa, C.S.; Ramis, T.R.; Ribeiro, J.L.; Reischak-Oliveira, A. Inflammatory markers, endothelial function and cardiovascular risk. J. Vasc. Bras. 2014, 13, 108–115. [Google Scholar] [CrossRef][Green Version]
- Aftab Kandhro, A.K.; Sherazi, S.T.H.; Mahesar, S.A.; Bhanger, M.I.; Talpur, M.Y.; Arain, S. Monitoring of fat content, free fatty acid and fatty acid profile including trans-fat in Pakistani biscuits. J. Am. Oil Chem. Soc. 2008, 85, 1057–1061. [Google Scholar] [CrossRef]
- Moss, J. Labeling of trans fatty acid content in food, regulations and limits—The FDA view. Atheroscler. Suppl. 2006, 7, 57–59. [Google Scholar] [CrossRef]
- Van Poppel, G.; van Erp-Baart, M.-A.; Leth, T.; Gevers, E.; Van Amelsvoort, J.; Lanzmann-Petithory, D.; Kafatos, A.; Aro, A. Trans fatty acids in foods in Europe: The TRANSFAIR study. J. Food Compos. Anal. 1998, 11, 112–136. [Google Scholar] [CrossRef]
- Roe, M.; Pinchen, H.; Church, S.; Elahi, S.; Walker, M.; Farron-Wilson, M.; Buttriss, J.; Finglas, P. Trans fatty acids in a range of UK processed foods. Food Chem. 2013, 140, 427–431. [Google Scholar] [CrossRef]
- Krettek, A.; Thorpenberg, S.; Bondjers, G. Trans Fatty Acids and Health: A Review of Health Hazards and Existing Legislation; EPRS: Brussels, Belgium, 2008. [Google Scholar]
- Assaf, R.R. Overview of local, state, and national government legislation restricting trans fats. Clin. Ther. 2014, 36, 328–332. [Google Scholar] [CrossRef]
- Maingrette, F.; Li, L.; Renier, G. C-reactive protein enhances macrophage lipoprotein lipase expression. J. Lipid Res. 2008, 49, 1926–1935. [Google Scholar] [CrossRef][Green Version]
- Pakistan Standard Quality Control Authority (PSQCA). Pakistan Standard Specification for Cooking Oil and Banaspati (PS:2858–2023); Standards Development Centre, Agriculture and Food Division: Karachi, Pakistan, 2023; Available online: https://psqca.com.pk/cs/newitems2021/agri/newharcompayaz2021/PS2858-2023forCookingOilBlended3rdRev.pdf (accessed on 27 August 2025).[Green Version]
- Codex Alimentarius Commission. Codex Standard for Named Vegetable Oils (CODEX-STAN 210–1999); FAO/WHO: Rome, Italy, 1999; Available online: https://www.fao.org/3/y2774e/y2774e03.htm (accessed on 27 August 2025).[Green Version]
- FAO/WHO. Fats and Oils in Human Nutrition: Report of a Joint Expert Consultation; FAO Food and Nutrition Paper No. 57; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar][Green Version]
- Sulieman, A.E.R.M.; El-Makhzangy, A.; Ramadan, M.F. Antiradical performance and physicochemical characteristics of vegetable oils upon frying of French fries: A preliminary comparative study. J. Food Lipids 2006, 13, 259–276. [Google Scholar] [CrossRef]
- Peng, G.-J.; Chang, M.-H.; Fang, M.; Liao, C.-D.; Tsai, C.-F.; Tseng, S.-H.; Kao, Y.-M.; Chou, H.-K.; Cheng, H.-F. Incidents of major food adulteration in Taiwan between 2011 and 2015. Food Control 2017, 72, 145–152. [Google Scholar] [CrossRef]
- Sebastian, A.; Ghazani, S.M.; Marangoni, A.G. Quality and safety of frying oils used in restaurants. Food Res. Int. 2014, 64, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, M.; Conforti, P.A.; Lupano, C.E. Lipid oxidation in biscuits: Comparison of different lipid extraction methods. J. Food Meas. Charact. 2015, 9, 104–109. [Google Scholar] [CrossRef]
- Bozdemir, S.; Güneşer, O.; Yilmaz, E. Properties and stability of deep-fat fried chickpea products. Grasas Aceites 2015, 66, e065. [Google Scholar] [CrossRef]
- Lopez-Varela, S.; Sánchez-Muniz, F.J.; Garrido-Polonio, C.; Arroyo, R.; Cuesta, C. Relationship between chemical and physical indexes and column and HPSE chromatography methods for evaluating frying oil. Z. Für Ernährungswissenschaft 1995, 34, 308–313. [Google Scholar] [CrossRef]
- López-Sobaler, A.M.; Aparicio, A.; Rubio, J.; Marcos, V.; Sanchidrián, R.; Santos, S.; Pérez-Farinós, N.; Dal-Re, M.Á.; Villar-Villalba, C.; Yusta-Boyo, M.J.; et al. Adequacy of usual macronutrient intake and macronutrient distribution in children and adolescents in Spain: A National Dietary Survey on the Child and Adolescent Population, ENALIA 2013–2014. Eur. J. Nutr. 2019, 58, 705–719. [Google Scholar] [CrossRef]
- Totani, N.; Yasaki, N.; Doi, R.; Hasegawa, E. Active Cooling of Oil after Deep-frying. J. Oleo Sci. 2017, 66, 1095–1100. [Google Scholar] [CrossRef]
- Jacobsen, C. Understanding and reducing oxidative flavour deterioration in foods. In Oxidation in Foods and Beverages and Antioxidant Applications; Elsevier: Amsterdam, The Netherlands, 2010; pp. 122–142. [Google Scholar]
- Esfarjani, F.; Khoshtinat, K.; Zargaraan, A.; Mohammadi-Nasrabadi, F.; Salmani, Y.; Saghafi, Z.; Hosseini, H.; Bahmaei, M. Evaluating the rancidity and quality of discarded oils in fast food restaurants. Food Sci. Nutr. 2019, 7, 2302–2311. [Google Scholar] [CrossRef]
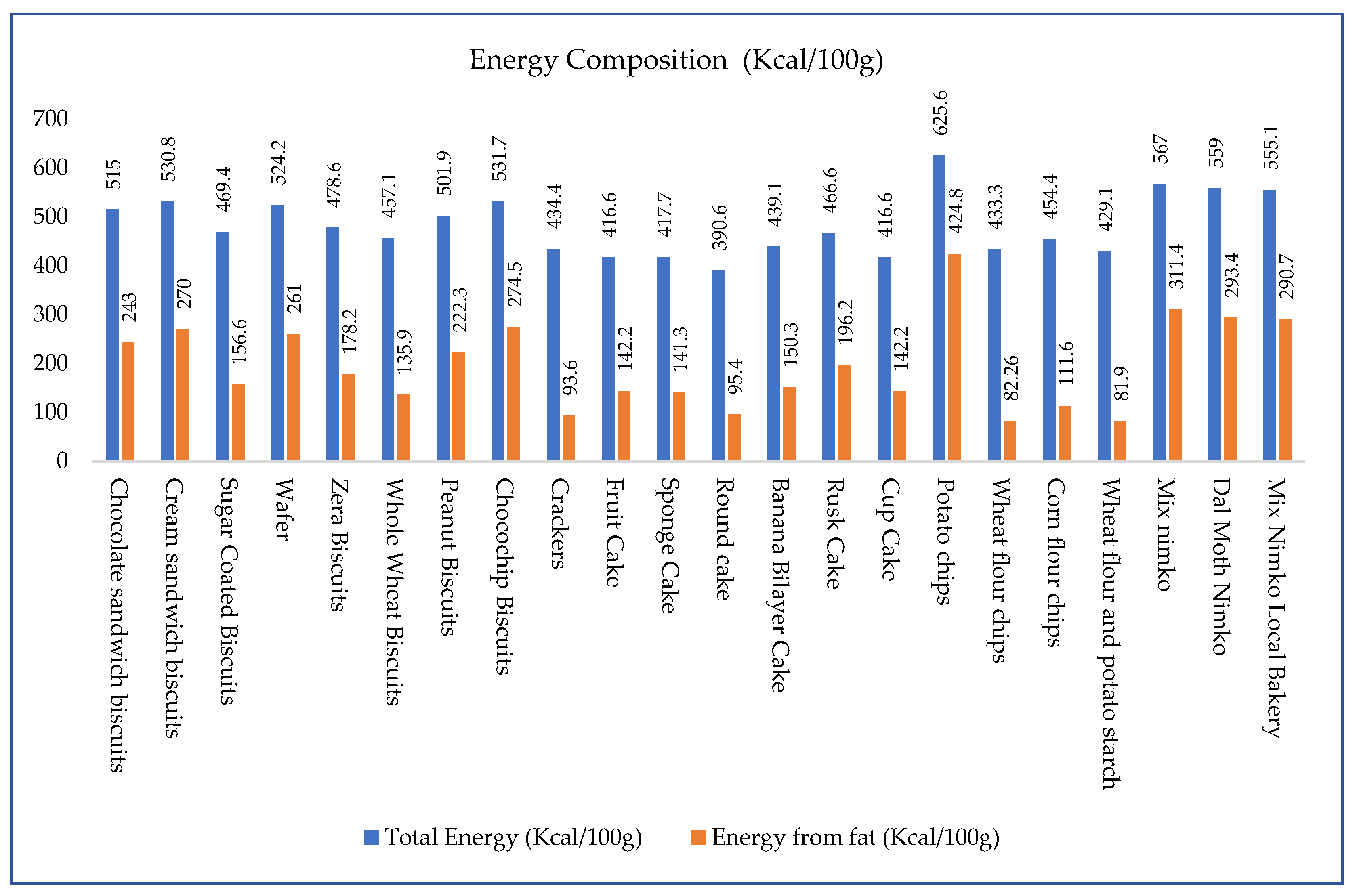

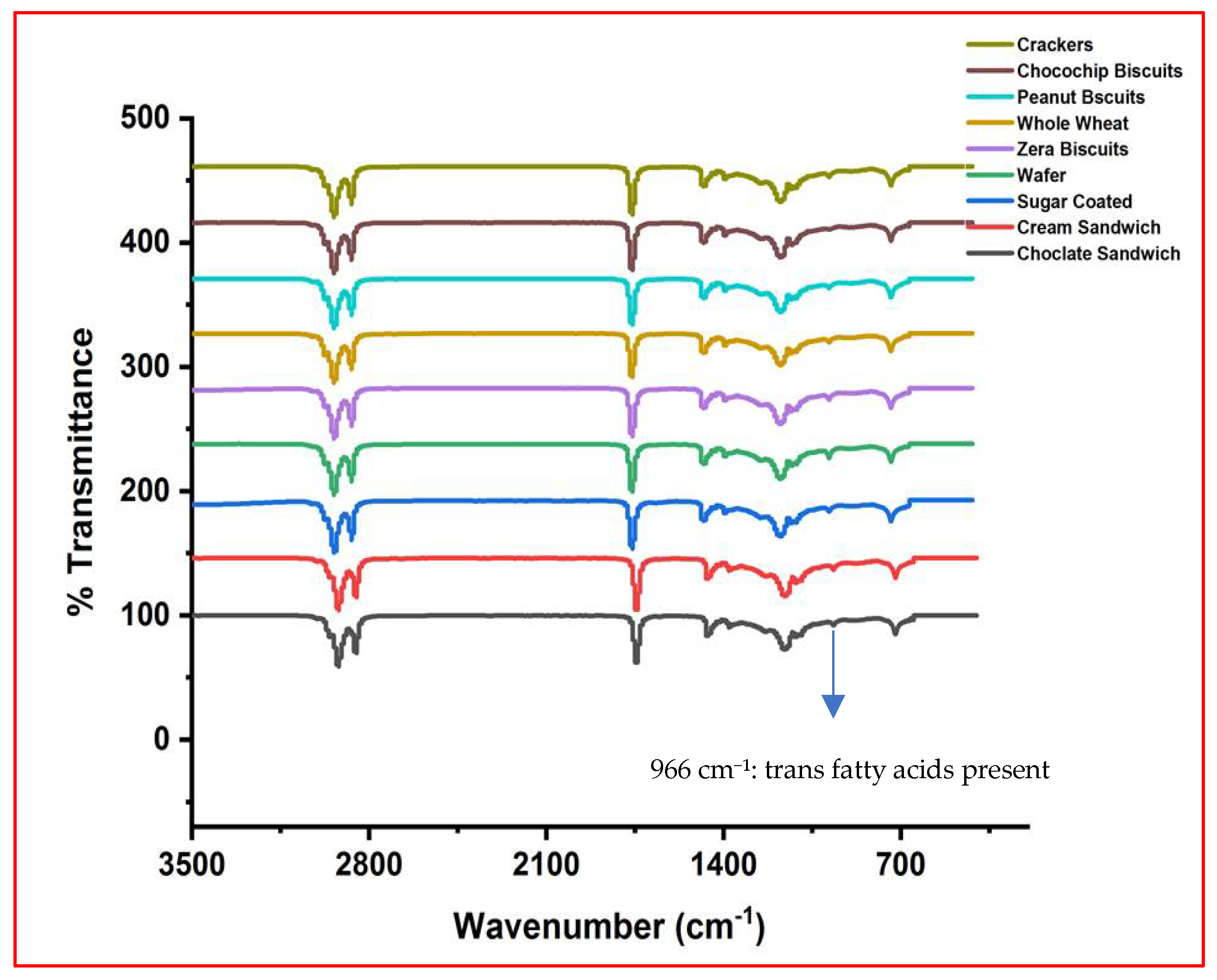


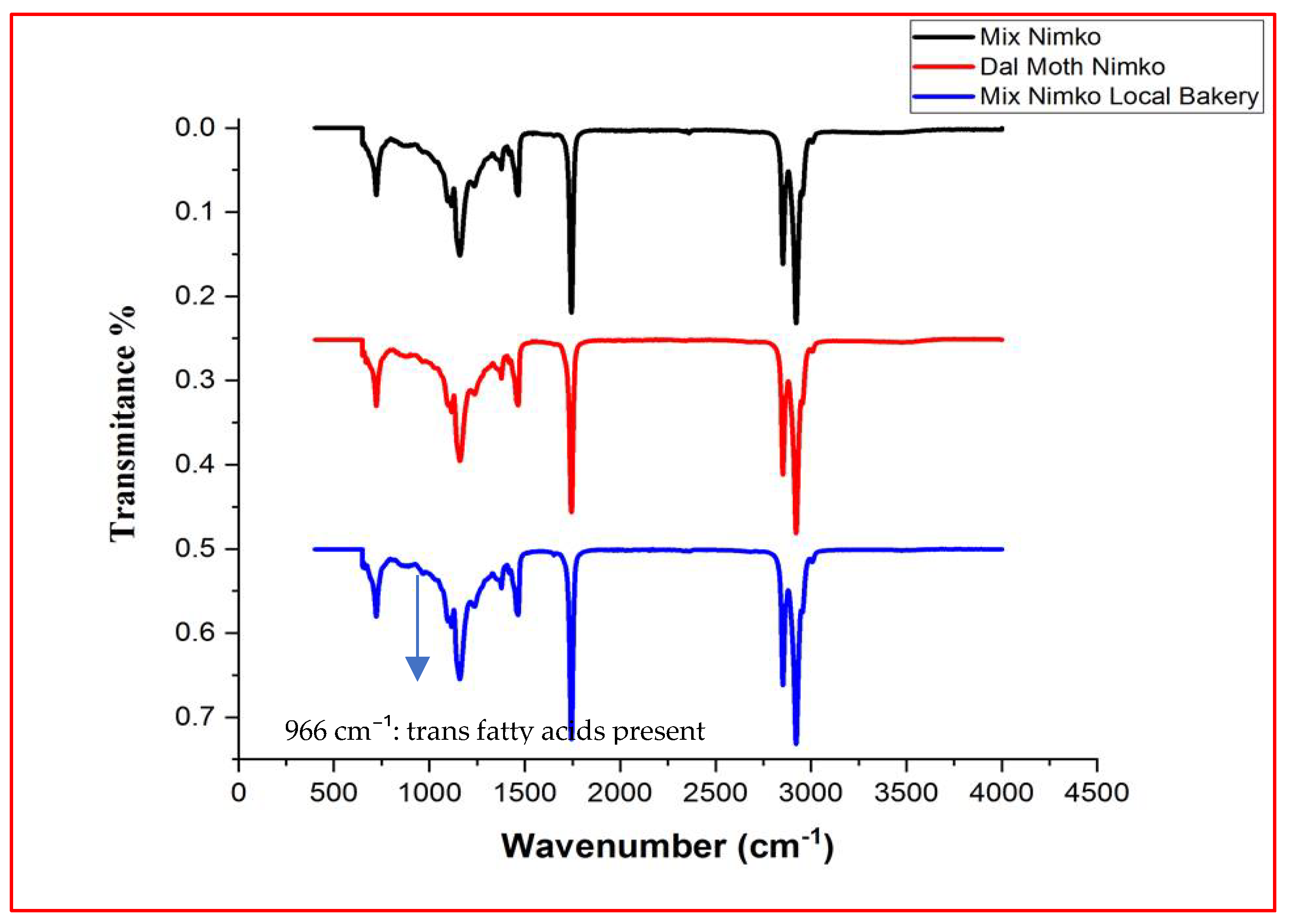
| Samples | Lauric Acid C12:0 | Myristic Acid C14:0 | Palmitic Acid C16:0 | Stearic Acid C18:0 | Arachidic Acid C20:0 | Palmitoleic Acid C16:1 (n-9) | Oleic Acid C18:1 (n-9) | Linoleic Acid C18:2n 9c,12c | ΣSFA | ΣUFA | ΣMUFA | ΣPUFA | UFA/SFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chocolate Sandwich Biscuits | _ | 0.18 ± 0.01 | 9.76 ± 0.1 | 1.55 ± 0 | 0.09 ± 0 | _ | 12.26 ± 0.1 | 1.97 ± 0 | 11.58 | 14.23 | 12.26 | 1.97 | 1.23 |
| Cream Sandwich Biscuits | 0.49 ± 0 | 0.4 ± 0.01 | 10.76 ± 0.9 | 1.66 ± 0.4 | 0.1 ± 0.1 | _ | 13.34 ± 0.4 | 1.96 ± 0 | 13.41 | 15.30 | 13.34 | 1.96 | 1.14 |
| Sugar-Coated Biscuits | _ | 0.12 ± 0 | 6.5 ± 2.2 | 1.35 ± 2.7 | 0.07 ± 0 | _ | 7.5 ± 3.6 | 1.11 ± 1.3 | 8.04 | 8.61 | 7.50 | 1.11 | 1.07 |
| Wafer | _ | 0.22 ± 0 | 8.98 ± 3.8 | 1.33 ± 0.3 | 0.09 ± 0 | _ | 16.47 ± 4.2 | 0.6 ± 0.1 | 10.62 | 17.07 | 16.47 | 0.60 | 1.61 |
| Zera Biscuits | _ | 0.13 ± 0 | 6.91 ± 0.5 | 1.15 ± 0.4 | 0.09 ± 0.1 | _ | 9.16 ± 0 | 1.47 ± 0.1 | 8.28 | 10.63 | 9.16 | 1.47 | 1.28 |
| Whole Wheat Biscuits | _ | 0.1 ± 0 | 5.3 ± 0.2 | 0.82 ± 0.1 | 0.06 ± 0 | _ | 7.03 ± 0.4 | 1.13 ± 0.1 | 6.28 | 8.16 | 7.03 | 1.13 | 1.30 |
| Peanut Biscuits | _ | 0.14 ± 0 | 7.91 ± 0 | 1.2 ± 0 | 0.16 ± 0 | _ | 11.5 ± 0 | 2.46 ± 0 | 9.41 | 13.96 | 11.50 | 2.46 | 1.48 |
| Chocochip Biscuits | 2.27 ± 0 | 1.25 ± 0.1 | 11.55 ± 0.9 | 2.01 ± 0.3 | _ | _ | 10.18 ± 0.3 | 1.86 ± 0.1 | 17.08 | 12.04 | 10.18 | 1.86 | 0.70 |
| Crackers | _ | 0.07 ± 0.1 | 3.91 ± 0.3 | 0.59 ± 0.7 | 0.04 ± 0.1 | _ | 4.63 ± 0.2 | 0.7 ± 0.3 | 4.61 | 5.33 | 4.63 | 0.70 | 1.16 |
| Fruit Cake | _ | 0.11 ± 0 | 5.57 ± 0.7 | 0.82 ± 0.3 | 0.05 ± 0 | _ | 6.93 ± 0.4 | 1.63 ± 0.1 | 6.55 | 8.56 | 6.93 | 1.63 | 1.31 |
| Sponge Cake | _ | 0.12 ± 0.1 | 5.79 ± 3.2 | 1.08 ± 0.6 | 0.06 ± 0 | 0.06 ± 0 | 6.74 ± 4.6 | 1.15 ± 0.7 | 7.05 | 7.95 | 6.80 | 1.15 | 1.13 |
| Round Cake | _ | 0.07 ± _- | 0.69 ± 0.22 | 0.29 ± 0.1 | 0.06 ± 0 | 0.06 ± 0.1 | 7.29 ± 1.1 | 1.74 ± 0.6 | 1.11 | 9.09 | 7.35 | 1.74 | 8.19 |
| Banana Bilayer Cake | _ | 0.11 ± 0.3 | 6.13 ± 0.6 | 2.09 ± 2.8 | _ | _ | 6.02 ± 3.6 | 1.61 ± 0.1 | 8.33 | 7.63 | 6.02 | 1.61 | 0.92 |
| Rusk Cake | _ | 0.17 ± 0 | 7.79 ± 0.3 | 1.31 ± 0.8 | _ | _ | 9.63 ± 1.3 | 1.51 ± 0.2 | 9.27 | 11.14 | 9.63 | 1.51 | 1.20 |
| Cup Cake | _ | 0.05 ± 0 | 3.04 ± 0 | 0.82 ± 0 | 0.06 ± 0 | _ | 7.1 ± 0 | 4.05 ± 0 | 3.97 | 11.15 | 7.10 | 4.05 | 2.81 |
| Potato Chips | _ | 0.32 ± 0 | 16.61 ± 0.1 | 2.26 ± 0.1 | 0.3 ± 0 | _ | 21.07 ± 0.3 | 4.56 ± 0.5 | 19.49 | 25.63 | 21.07 | 4.56 | 1.32 |
| Wheat Flour Chips | 0.03 ± 0 | 0.07 ± 0 | 3.15 ± 0 | 0.46 ± 0 | 0.05 ± 0 | _ | 4.98 ± 0 | _ | 3.76 | 4.98 | 4.98 | 0.00 | 1.32 |
| Corn Flour Chips | _ | 0.08 ± 0.1 | 4.46 ± 0.3 | 0.59 ± 0.1 | 0.06 ± 0.1 | _ | 5.56 ± 1.5 | 1.09 ± 1.5 | 5.19 | 6.65 | 5.56 | 1.09 | 1.28 |
| Wheat Flour and Potato Starch | _ | 0.06 ± 0 | 3.06 ± 0 | 0.46 ± 0 | 0.06 ± 0 | _ | 5.08 ± 0 | _ | 3.64 | 5.08 | 5.08 | 0.00 | 1.40 |
| Mix Nimko | _ | 0.23 ± 0 | 12.44 ± 0.3 | 1.62 ± 0.1 | 0.13 ± 0 | _ | 15.31 ± 0.2 | 3.41 ± 0.2 | 14.42 | 18.72 | 15.31 | 3.41 | 1.30 |
| Dal Moth Nimko | _ | 0.22 ± 0 | 11.91 ± 0.2 | 1.59 ± 0 | 0.18 ± 0.2 | _ | 14.74 ± 1.5 | 2.72 ± 1.4 | 13.90 | 17.46 | 14.74 | 2.72 | 1.26 |
| Mix Nimko Local Bakery | _ | 0.22 ± 0 | 11.3 ± 0.8 | 1.64 ± 0.3 | 0.13 ± 0 | _ | 17.54 ± 1.2 | _ | 13.29 | 17.54 | 17.54 | 0.00 | 1.32 |
| Samples | AI | TI | h/H | NI | DFA | OFA | Lipid Quality Interpretation with Breast Milk |
|---|---|---|---|---|---|---|---|
| Chocolate Sandwich Biscuits | 0.7 | 0.81 | 0.2 | 1.24 | 15.8 | 9.94 | AI/TI high, h/H very low → Worse than breast milk; poor lipid balance for children |
| Cream Sandwich Biscuits | 0.76 | 0.84 | 0.17 | 1.19 | 17 | 11.7 | High AI/TI, very low h/H → Poor for pediatric intake |
| Sugar-Coated Biscuits | 0.77 | 0.92 | 0.17 | 1.08 | 9.96 | 6.62 | High AI/TI, low h/H → Suboptimal for children; DFA slightly lower than breast milk |
| Wafer | 0.54 | 0.62 | 0.07 | 1.62 | 18.4 | 9.2 | Moderate AI/TI, very low h/H → Worse than breast milk; not ideal for children |
| Zera Biscuits | 0.66 | 0.77 | 0.21 | 1.3 | 11.8 | 7.04 | AI/TI above breast milk, h/H low → Needs improvement |
| Whole Wheat Biscuits | 0.66 | 0.76 | 0.21 | 1.31 | 8.98 | 5.4 | AI/TI high, h/H low → Suboptimal fat quality for children |
| Peanut Biscuits | 0.58 | 0.66 | 0.3 | 1.51 | 15.2 | 8.05 | Closer to breast milk h/H, DFA moderate → Acceptable for children |
| Chocochip Biscuits | 1.25 | 1.23 | 0.12 | 0.81 | 14.1 | 15.1 | Very high AI/TI, very low h/H → Poor fat profile; highly unsuitable |
| Crackers | 0.75 | 0.86 | 0.18 | 1.17 | 5.92 | 3.98 | AI/TI high, h/H very low → Poor lipid quality vs. breast milk |
| Fruit Cake | 0.66 | 0.76 | 0.29 | 1.32 | 9.38 | 5.68 | AI/TI high, h/H low → Moderate to poor relative to breast milk |
| Sponge Cake | 0.75 | 0.89 | 0.2 | 1.13 | 9.03 | 5.91 | High AI/TI, low h/H → Poor lipid quality for children |
| Round Cake | 0.08 | 0.11 | 2.53 | 9.19 | 9.38 | 0.76 | AI/TI much lower, h/H excellent → Exceeds breast milk quality; very healthy for children |
| Banana Bilayer Cake | 0.82 | 1.09 | 0.26 | 0.92 | 9.72 | 6.24 | AI/TI high, h/H low → Poor relative to breast milk |
| Rusk Cake | 0.71 | 0.83 | 0.19 | 1.2 | 12.5 | 7.96 | High AI/TI, low h/H → Suboptimal for children |
| Cup Cake | 0.28 | 0.35 | 1.31 | 2.86 | 12 | 3.09 | AI/TI slightly lower, h/H moderate → Acceptable, closer to breast milk profile |
| Potato Chips | 0.66 | 0.75 | 0.27 | 1.34 | 27.9 | 16.9 | AI/TI high, OFA very high → Energy-dense but worse than breast milk; moderate DHA/PUFA not accounted for |
| Wheat Flour Chips | 0.65 | 0.74 | 0 | 1.35 | 5.44 | 3.25 | AI/TI high, h/H very low → Poor fat quality vs. breast milk |
| Corn Flour Chips | 0.68 | 0.77 | 0.24 | 1.29 | 7.24 | 4.54 | Moderate AI/TI, h/H low → Below breast milk standards |
| Wheat + Potato Starch Chips | 0.61 | 0.71 | 0 | 1.42 | 5.54 | 3.12 | AI/TI high, h/H very low → Poor lipid profile for pediatric intake |
| Mix Nimko | 0.68 | 0.76 | 0.27 | 1.31 | 20.3 | 12.7 | AI/TI slightly above breast milk, h/H lower → Average nutritional quality |
| Dal Moth Nimko | 0.69 | 0.79 | 0.22 | 1.27 | 19.1 | 12.1 | AI/TI above breast milk, h/H low → Borderline acceptable fat quality |
| Local Bakery Mix Nimko | 0.66 | 0.75 | 0 | 1.33 | 19.2 | 11.5 | AI/TI high, h/H very low → Needs improvement for children |
| Samples | p-Anisidine Value (Arbitrary Unit) | Peroxide Value (mEq/kg) | TOTOX (Arbitrary Unit) | Conjugated Diene | Conjugated Triene | FFA (%) |
|---|---|---|---|---|---|---|
| Chocolate sandwich biscuits | 28.9 ± 3.3 | 0.26 ± 0.1 | 29.4 ± 3.1 | 4.0 ± 1.2 | 1.3 ± 0.1 | 0.22 ± 0.03 |
| Cream sandwich biscuits | 11.2 ± 2.9 | 0.4 ± 0.2 | 11.9 ± 2.8 | 8.3 ± 0.4 | 1.1 ± 0.2 | 0.27 ± 0.03 |
| Sugar-coated biscuits | 18.4 ± 1.7 | 0.5 ± 0.1 | 19.4 ± 2.2 | 5.9 ± 1.2 | 0.9 ± 0.1 | 0.28 ± 0.02 |
| Wafer | 6.2 ± 0.6 | 0.5 ± 0.1 | 7.2 ± 0.61 | 10.5 ± 3.7 | 0.9 ± 0.2 | 0.13 ± 0.03 |
| Zera biscuits | 12.9 ± 0.5 | 0.4 ± 0.2 | 13.8 ± 0.87 | 5.6 ± 0.1 | 1.13 ± 0.1 | 0.15 ± 0.02 |
| Whole wheat biscuits | 23.4 ± 2.9 | 1.2 ± 0.1 | 25.9 ± 1.7 | 3.4 ± 1.9 | 0.9 ± 0.2 | 0.21 ± 0.07 |
| Peanut biscuits | 10.4 ± 1.5 | 1.7 ± 0.2 | 13.7 ± 1.8 | 14.8 ± 0.7 | 1.3 ± 0.1 | 0.25 ± 0.02 |
| Chocochip biscuits | 9.7 ± 0.6 | 0.3 ± 0.1 | 10.4 ± 0.7 | 6.8 ± 2.3 | 1.1 ± 0.2 | 0.30 ± 0.03 |
| Crackers | 11.9 ± 4.0 | 0.3 ± 0.1 | 12.5 ± 3.8 | 6.5 ± 0.2 | 0.70 ± 0.01 | 0.17 ± 0.03 |
| Fruit cake | 13.5 ± 1.5 | 0.9 ± 0.2 | 15.3 ± 1.9 | 4.6 ± 1.0 | 3.8 ± 0.04 | 1.11 ± 0.2 |
| Sponge cake | 6.0 ± 1.7 | 1.6 ± 0.2 | 9.2 ± 1.9 | 6.7 ± 0.6 | 0.95 ± 0.1 | 0.17 ± 0.05 |
| Round cake | 18.4 ± 3.6 | 0.4 ± 0.2 | 19.3 ± 3.4 | 2.4 ± 0.5 | 0.85 ± 0.04 | 0.37 ± 0.12 |
| Banana bilayer cake | 23.9 ± 1.1 | 0.4 ± 0.2 | 24.8 ± 0.7 | 10.5 ± 0.3 | 1.9 ± 0.01 | 1.02 ± 0.17 |
| Rusk cake | 18.4 ± 0.8 | 0.5 ± 0.1 | 19.4 ± 0.9 | 6.1 ± 1.9 | 0.9 ± 0.1 | 2.89 ± 0.04 |
| Cup cake | 40.7 ± 1.2 | 0.5 ± 0.1 | 41.7 ± 1.4 | 10.2 ± 3.4 | 3.8 ± 0.1 | 1.03 ± 0.30 |
| Potato chips | 13.0 ± 0.7 | 3.8 ± 0.3 | 20.6 ± 1.4 | 6.5 ± 3.0 | 1.67 ± 0.2 | 0.26 ± 0.03 |
| Wheat flour chips | 12.2 ± 2.1 | 1.3 ± 0.2 | 14.8 ± 2.1 | 5.8 ± 3.2 | 0.85 ± 0.02 | 0.58 ± 0.07 |
| Corn flour chips | 17.5 ± 4.6 | 1.4 ± 0.2 | 20.3 ± 4.2 | 9.9 ± 4.3 | 0.7 ± 0.1 | 0.62 ± 0.05 |
| Wheat flour and potato starch | 30.1 ± 0.1 | 4.1 ± 0.4 | 38.4 ± 0.9 | 13.7 ± 0.3 | 2.2 ± 0.1 | 0.58 ± 0.1 |
| Mix nimko | 7.3 ± 0.1 | 3.3 ± 0.6 | 13.9 ± 1.1 | 7.60 ± 0.32 | 2.04 ± 0.04 | 0.99 ± 0.26 |
| Dal moth nimko | 3.6 ± 3.4 | 3.4 ± 0.6 | 10.3 ± 3.2 | 5.05 ± 0.65 | 2.04 ± 0.10 | 2.27 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chohan, S.; Soomro, S.I.; Mahesar, S.A.; Ahmed, S.; Umrani, F.; Iqbal, N.T.; Iqbal, J.; Sadiq, K.; Qureshi, A.K.; Ali, A.; et al. Fatty Acid Profile, Lipid Quality Indices and Oxidative Stability of Snacks Consumed by Children Aged 6–24 Months in Rural Matiari, Sindh, Pakistan. Foods 2025, 14, 3302. https://doi.org/10.3390/foods14193302
Chohan S, Soomro SI, Mahesar SA, Ahmed S, Umrani F, Iqbal NT, Iqbal J, Sadiq K, Qureshi AK, Ali A, et al. Fatty Acid Profile, Lipid Quality Indices and Oxidative Stability of Snacks Consumed by Children Aged 6–24 Months in Rural Matiari, Sindh, Pakistan. Foods. 2025; 14(19):3302. https://doi.org/10.3390/foods14193302
Chicago/Turabian StyleChohan, Shazia, Sanam I. Soomro, Sarfaraz Ahmed Mahesar, Sheraz Ahmed, Fayaz Umrani, Najeeha T. Iqbal, Junaid Iqbal, Kamran Sadiq, Abdul Khalique Qureshi, Asad Ali, and et al. 2025. "Fatty Acid Profile, Lipid Quality Indices and Oxidative Stability of Snacks Consumed by Children Aged 6–24 Months in Rural Matiari, Sindh, Pakistan" Foods 14, no. 19: 3302. https://doi.org/10.3390/foods14193302
APA StyleChohan, S., Soomro, S. I., Mahesar, S. A., Ahmed, S., Umrani, F., Iqbal, N. T., Iqbal, J., Sadiq, K., Qureshi, A. K., Ali, A., & Memon, N. (2025). Fatty Acid Profile, Lipid Quality Indices and Oxidative Stability of Snacks Consumed by Children Aged 6–24 Months in Rural Matiari, Sindh, Pakistan. Foods, 14(19), 3302. https://doi.org/10.3390/foods14193302





