Recent Advances on Seaweed-Derived Pigments for FoodApplication and Current Legal Framework
Abstract
1. Introduction
2. Materials and Methodology
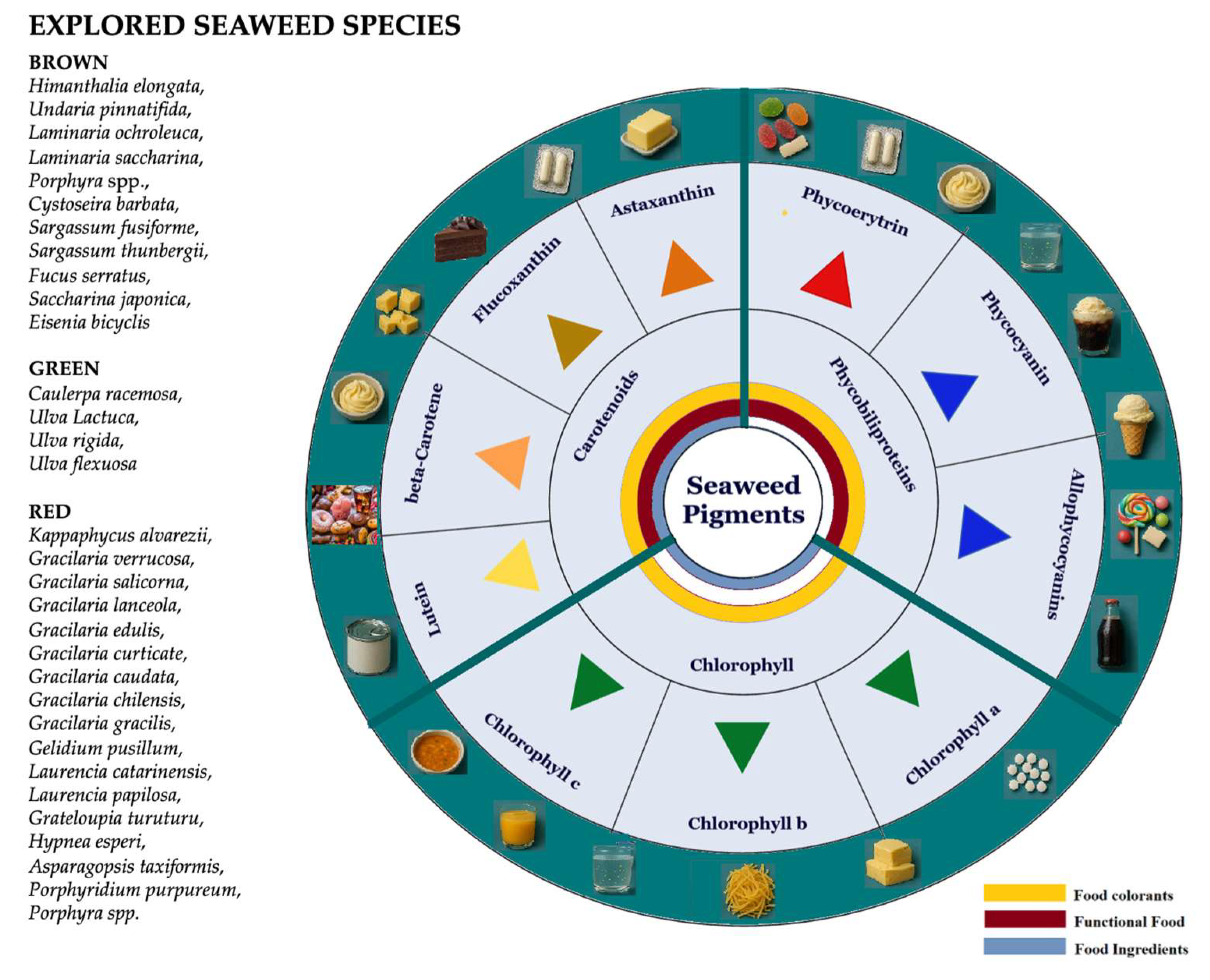
3. Seaweed-Derived Pigments
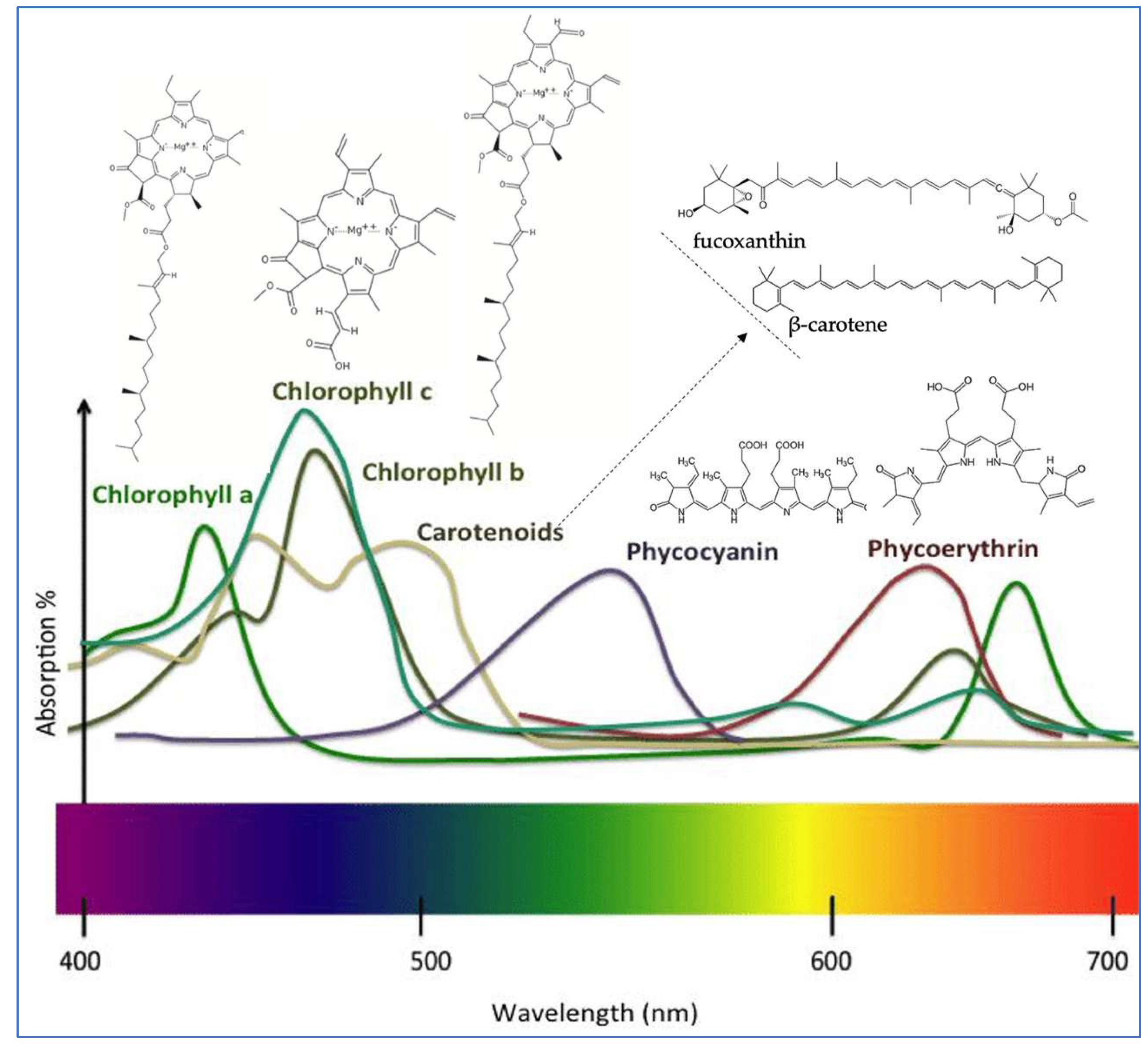
3.1. Seaweeds Chlorophylls
3.2. Seaweeds Carotenoids
3.3. Seaweeds Phycobiliproteins
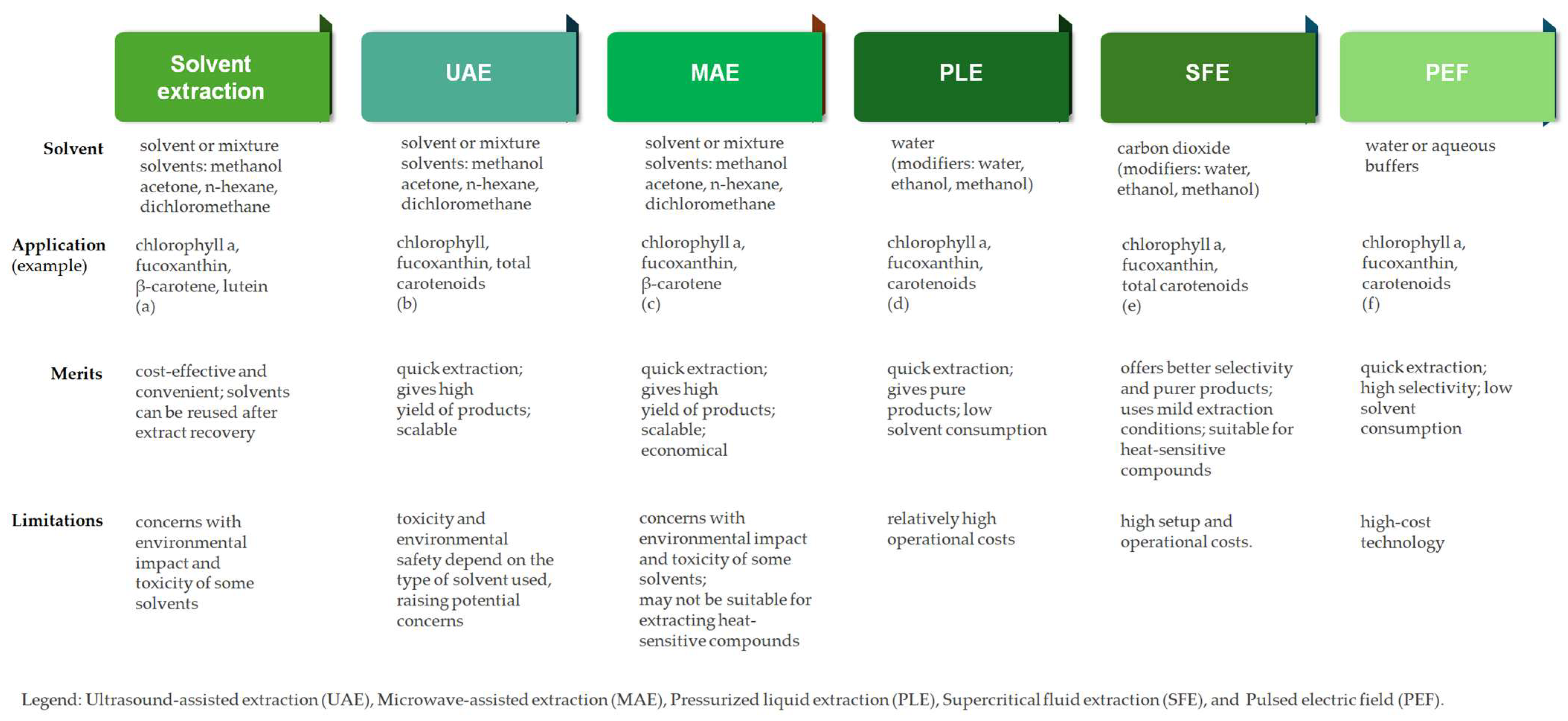
4. Novel Extraction and Purification Methods for Seaweed-Derived Pigments
4.1. Extraction Strategies
4.2. Purification Strategies
5. Stabilization Techniques to Prevent Seaweed-Derived Pigments Degradation
5.1. Stabilization of Chlorophylls
5.2. Stabilization of Carotenoids
5.3. Stabilization of Phycobiliproteins
6. Aquaculture Sustainable Production of Seaweed-Derived Pigments
7. Current Legal Framework
7.1. Chlorophylls as Food Additive
7.2. Carotenoids as Food Additive
7.3. Phycobiliproteins as Food Additives
8. Limitations and Perspectives of Seaweed-Derived Pigments Applications
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavan, A.; Pawar, J.; Kakde, U.; Venkatachalam, M.; Fouillaud, M.; Dufossé, L.; Deshmukh, S.K. Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring. Fermentation 2025, 11, 395. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Lun, J.; Wang, X.; Du, G.; Chen, J. Recent advances of natural pigments from algae. Food Prod. Process. Nutr. 2023, 5, 155. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Afraz, M.T.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.-A. Recent progress in natural seaweed pigments: Green extraction, health-promoting activities, techno-functional properties and role in intelligent food packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Liu, C.; Gao, J.; Jiang, H.; Sun, J.; Gao, X.; Mao, X. Value-added utilization technologies for seaweed processing waste in a circular economy: Developing a sustainable modern seaweed industry. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70027. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, H.S.; Kwon, M. Stability of Phycocyanin in Food and Beverage Applications: A Review. Trends Food Sci. Technol. 2023, 135, 101–112. [Google Scholar] [CrossRef]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Waagbø, R.; Lock, E.-J.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. Cooking effects on bioaccessibility of chlorophyll pigments of the main edible seaweeds. Food Chem. 2019, 295, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, P.; Lourenço-Lopes, C.; Silva, A.; Pereira, A.G.; Fraga-Corral, M.; Zhao, C.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Pigment composition of nine brown algae from the Iberian northwestern coastline: Influence of the extraction solvent. Mar. Drugs 2022, 20, 113. [Google Scholar] [CrossRef]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J.; Piwowar, A. Commercialization aspects of carotenoids. In Carotenoids: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–357. [Google Scholar]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of Chlorophyll Degradation and Color Loss in Heated Broccoli Juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, L 295, 1–177. [Google Scholar]
- Lis, K.; Bartuzi, Z. Plant food dyes with antioxidant properties and allergies—Friend or enemy? Antioxidants 2023, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Dewi, E.N.; Purnamayati, L. The effect of natural dye from Caulerpa Sp. microcapsules on jelly drink quality. Food Res. 2023, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, P.S.; Pahalawattaarachchi, V.; Ranaweera, K.K.D.S. Seaweed extract as a natural food coloring agent in jelly desserts on chemical, microbial and sensory quality. Acad. Agric. J. 2016, 1, 65–69. [Google Scholar]
- Kumar, A.; Krishnamoorthy, E.; Devi, H.M.; Uchoi, D.; Tejpal, C.; Ninan, G.; Zynudheen, A. Influence of sea grapes (Caulerpa racemosa) supplementation on physical, functional, and anti-oxidant properties of semi-sweet biscuits. J. Appl. Phycol. 2018, 30, 1393–1403. [Google Scholar] [CrossRef]
- Amoriello, T.; Mellara, F.; Amoriello, M.; Ceccarelli, D.; Ciccoritti, R. Powdered seaweeds as a valuable ingredient for functional breads. Eur. Food Res. Technol. 2021, 247, 2431–2443. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Innovative nonthermal technologies: Chlorophyllin and visible light significantly reduce microbial load on Basil. Food Technol. Biotechnol. 2019, 57, 126. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Yahia, E.M.; de Jesus Ornelas-Paz, J.; Emanuelli, T.; Jacob-Lopes, E.; Zepka, L.Q.; Cervantes-Paz, B. Chemistry, stability, and biological actions of carotenoids. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health; Wiley: Hoboken, NJ, USA, 2017; p. 285. [Google Scholar]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.; García-Oliveira, P.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Cakmakci, S.; Tahmas-Kahyaoglu, D.; Erkaya, T.; Cebi, K.; Hayaloglu, A.A. β-Carotene contents and quality properties of set type yoghurt supplemented with carrot juice and sugar. J. Food Process Preserv. 2024, 38, 1155–1163. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of beta-carotene by complex coacervation using amaranth carboxymethyl starch and lactoferrin for application in gummy candies. Food Hydrocoll. 2023, 139, 108488. [Google Scholar] [CrossRef]
- Sellimi, S.; Benslima, A.; Ksouda, G.; Montero, V.B.; Hajji, M.; Nasri, M. Safer and healthier reduced nitrites Turkey meat sausages using lyophilized Cystoseirabarbata seaweed extract. J. Complement. Integr. Med. 2018, 15, 20170061. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, R.; Suda, M.; Sho, A.; Takahashi, T.; Sashima, T.; Abe, M.; Hosokawa, M.; Miyashita, K. Stability of fucoxanthin in dried Undaria pinnatifida (wakame) and baked products (scones) containing wakame powder. Food Sci. Technol. Res. 2012, 18, 687–693. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, K.; Oyamada, C.; Sato, A.; Fukushi, A.; Arakane, T.; Motoyama, M.; Yamazaki, M.; Mitsumoto, M. Effects of fucoxanthin addition to ground chicken breast meat on lipid and colour stability during chilled storage, before and after cooking. Asian-Australas. J. Anim. Sci. 2008, 21, 1067–1072. [Google Scholar] [CrossRef]
- Zahrah, Z.; Amin, M.N.G.; Alamsjah, M.A. The effect of fucoxanthin as coloring agent on the quality of Shrimp Paste. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012079. [Google Scholar] [CrossRef]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pérez, J.P.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef] [PubMed]
- Chini Zittelli, G.; Lauceri, R.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2023, 22, 1733–1789. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Shanmugam, M. Isolation of phycoerythrin from Kappaphycusalvarezii: A potential natural colourant in ice cream. J. Appl. Phycol. 2020, 32, 4221–4233. [Google Scholar] [CrossRef]
- Wen, Y.; Shan, S.; Ye, F.; Liao, W.; Wu, X.; Chen, W.; Zhao, C. Prospects of phycoerythrin: Structural features, antioxidation and applications in food. Food Chem. 2025, 463, 141425. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Amorim, K.; Lage-Yusty, M.-A.; López-Hernández, J. Changes in bioactive compounds content and antioxidant activity of seaweed after cooking processing. CyTA-J. Food 2012, 10, 321–324. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Lopes, A.; Correia-Sá, L.; Vieira, M.; Delerue-Matos, C.; Soares, C.; Grosso, C. Sustainable Carotenoid Extraction from Macroalgae: Optimizing Microwave-Assisted Extraction Using Response Surface Methodology. Life 2024, 14, 1573. [Google Scholar] [CrossRef]
- Zhang, Y.; Hawboldt, K.; MacQuarrie, S.; Thomas, R. Recovery of alginate, fucoidan, phenolics, carotenoids, and hydrochar from beach-cast Ascophyllum nodosum using subcritical water extraction. J. Clean. Prod. 2025, 521, 146203. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.-J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Castejón, N.; Thorarinsdottir, K.A.; Einarsdóttir, R.; Kristbergsson, K.; Marteinsdóttir, G. Exploring the potential of icelandic seaweeds extracts produced by aqueous pulsed electric fields-assisted extraction for cosmetic applications. Mar. Drugs 2021, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Jimenez-Lopez, C.; Fraga, M.; Lourenco-Lopes, C.; Garcia-Oliveira, P.; Lorenzo, J.M.; Simal-Gandara, J. Extraction, properties, and applications of bioactive compounds obtained from microalgae. Curr. Pharm. Des. 2020, 26, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidiumpusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsí, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Marine Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Takasu, S.; Goto, M. Extraction of fucoxanthin isomers from the edible brown seaweed Undaria pinnatifida using supercritical CO2: Effects of extraction conditions on isomerization and recovery of fucoxanthin. J. Oleo Sci. 2022, 71, 1097–1106. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yang, C.H.; Huang, K.S.; Shaw, J.F. Chlorophyllides: Preparation, purification, and application. Biomolecules 2021, 11, 115. [Google Scholar] [CrossRef]
- Lumbessy, S.Y.; Junaidi, M.; Diniarti, N.; Setyowati, D.N.; Mukhlis, A.; Tambaru, R. Identification of chlorophyll pigment on Gracilaria Salicornia seaweed. IOP Conf. Ser. Earth Environ. Sci. 2021, 681, 012017. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, D.; Kateja, N. Recent developments in chromatographic purification of biopharmaceuticals. Biotechnol. Lett. 2018, 40, 895–905. [Google Scholar] [CrossRef]
- Cazon, P.; Silva, A.S. Natural pigments from food wastes: New approaches for the extraction and encapsulation. Curr. Opin. Green Sustain. Chem. 2024, 47, 100929. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Pippolini, C.; Marabottini, R.; Esti, M. Impact of Storage on the Color of a Green Isotonic Beverage With β-Cyclodextrin/Chlorophyll Complexes. Int. J. Food Sci. 2025, 2025, 6273830. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of carotenoids as food colorants via formation of cyclodextrin inclusion complexes: A review. Polysaccharides 2021, 2, 454–476. [Google Scholar] [CrossRef]
- Lelis, C.A.; Galvan, D.; Conte-Junior, C.A. Nanocarriers for β-carotene based on milk protein. Food Bioprocess Technol. 2023, 16, 43–67. [Google Scholar] [CrossRef]
- Tolve, R.; Bianchi, F.; Lomuscio, E.; Sportiello, L.; Simonato, B. Current advantages in the application of microencapsulation in functional bread development. Foods 2022, 12, 96. [Google Scholar] [CrossRef]
- Nowruzi, B.; Konur, O.; Anvar, S.A.A. The stability of the phycobiliproteins in the adverse environmental conditions relevant to the food storage. Food Bioprocess Technol. 2022, 15, 2646–2663. [Google Scholar] [CrossRef]
- Kang, Y.R.; Lee, Y.K.; Kim, Y.J.; Chang, Y.H. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum Arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a noble technique for the application of bioactive compounds in the food industry: A comprehensive review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Emon, D.D.; Islam, M.S.; Mazumder, M.A.R.; Aziz, M.G.; Rahman, M.S. Recent applications of microencapsulation techniques for delivery of functional ingredient in food products: A comprehensive review. Food Chem. Adv. 2025, 6, 100923. [Google Scholar] [CrossRef]
- Mardani, M.; Siahtiri, S.; Besati, M.; Baghani, M.; Baniassadi, M.; Nejad, A.M. Microencapsulation of natural products using spray drying; an overview. J. Microencapsul. 2024, 41, 649–678. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liang, Z.P.; Chang, X.Y.; Li, F.T.; Wang, X.Q.; Lian, X.J. Progress of microencapsulated phycocyanin in food and pharma industries: A review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef]
- Indrawati, R.; Sukowijoyo, H.; Wijayanti, R.D.E.; Limantara, L. Encapsulation of brown seaweed pigment by freeze drying: Characterization and its stability during storage. Procedia Chem. 2015, 14, 353–360. [Google Scholar] [CrossRef]
- Ledari, S.A.; Milani, J.M.; Shahidi, S.A.; Golkar, A. Fabrication and optimization of ultra-long stable microencapsulated chlorophyll using combinations of wall material via response surface methodology. Heliyon 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Priamo, W.L.; de Cezaro, A.M.; Ferreira, S.R.; Oliveira, J.V. Precipitation and encapsulation of β-carotene in PHBV using carbon dioxide as anti-solvent. J. Supercrit. Fluids 2010, 54, 103–109. [Google Scholar] [CrossRef]
- Xia, F.; Hu, D.; Jin, H.; Zhao, Y.; Liang, J. Preparation of lutein proliposomes by supercritical anti-solvent technique. Food Hydrocoll. 2012, 26, 456–463. [Google Scholar] [CrossRef]
- de Paz, E.; Martin, A.; Duarte, C.M.; Cocero, M.J. Formulation of β-carotene with poly-(ε-caprolactones) by PGSS process. Powder Technol. 2022, 217, 77–83. [Google Scholar] [CrossRef]
- de Paz, E.; Martín, Á.; Cocero, M.J. Formulation of β-carotene with soybean lecithin by PGSS (Particles from Gas Saturated Solutions)-drying. J. Supercrit. Fluids 2012, 72, 125–133. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitation. J Supercrit Fluids 2023, 83, 97–103. [Google Scholar] [CrossRef]
- Moraes, M.; Carvalho, J.M.P.; Silva, C.R.; Cho, S.; Sola, M.R.; Pinho, S.C. Liposomes encapsulating beta-carotene produced by the proliposomes method: Characterisation and shelf life of powders and phospholipid vesicles. Int. J. Food Sci. Technol. 2013, 48, 274–282. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wang, F.; Xia, G.; Liu, H.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X.; et al. Isolation of fucoxanthin from Sargassum thunbergii and preparation of microcapsules based on palm stearin solid lipid core. Front. Mater. Sci. 2017, 11, 66–74. [Google Scholar] [CrossRef]
- Handå, A.; Forbord, S.; Wang, X.; Broch, O.J.; Dahle, S.W.; Størseth, T.R.; Reitan, K.I.; Olsen, Y.; Skjermo, J. Seasonal-and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 2013, 414, 191–201. [Google Scholar] [CrossRef]
- Felaco, L.; Olvera-Novoa, M.A.; Robledo, D. Multitrophic integration of the tropical red seaweed Solieria filiformis with sea cucumbers and fish. Aquaculture 2020, 527, 735475. [Google Scholar] [CrossRef]
- Stedt, K.; Trigo, J.P.; Steinhagen, S.; Nylund, G.M.; Forghani, B.; Pavia, H.; Undeland, I. Cultivation of seaweeds in food production process waters: Evaluation of growth and crude protein content. Algal Res. 2022, 63, 102647. [Google Scholar] [CrossRef]
- Rugiu, L.; Hargrave, M.S.; Enge, S.; Sterner, M.; Nylund, G.M.; Pavia, H. Kelp in IMTAs: Small variations in inorganic nitrogen concentrations drive different physiological responses of Saccharina latissima. J. Appl. Phycol. 2021, 33, 1021–1034. [Google Scholar] [CrossRef]
- Abreu, M.H.; Varela, D.A.; Henríquez, L.; Villarroel, A.; Yarish, C.; Sousa-Pinto, I.; Buschmann, A.H. Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis C.J. Bird, J. McLachlan & E.C. Oliveira: Productivity and physiological performance. Aquaculture 2009, 293, 211–220. [Google Scholar] [CrossRef]
- Boderskov, T.; Schmedes, P.S.; Bruhn, A.; Rasmussen, M.B.; Nielsen, M.M.; Pedersen, M.F. The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. J. Appl. Phycol. 2016, 28, 1153–1165. [Google Scholar] [CrossRef]
- Syamsuddin, R.; Azis, H.Y. Comparative study on the growth, carotenoid, fibre and mineral content of the seaweed Caulerpa lentillifera cultivated indoors and in the sea. IOP Conf. Ser. Earth Environ. Sci. 2019, 370, 012019. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EC) No.1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union 2008, L354, 16–33. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj (accessed on 19 July 2025).
- European Commission. Commission Regulation (EU) No1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, L295, 1–177. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011R1129 (accessed on 22 July 2025).
- U.S. Food and Drug Administration. Code of Federal Regulations, Title 21, Part 73, Section 125: Sodium Copper Chlorophyllin; 2002. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-73/subpart-A/section-73.125 (accessed on 19 July 2025).
- Food and Drug Administration. Listing of color additives exempt from certification; Sodium copper chlorophyllin. Fed. Regist. 2002, 67, 35429–35431. Available online: https://www.federalregister.gov/documents/2002/05/20/02-12544/listing-of-color-additives-exempt-from-certification-sodium-copper-chlorophyllin (accessed on 19 July 2025).
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). Scientific Opinion on the re-evaluation of mixed carotenes (E 160a (i)) and β-carotene (E 160a (ii)) as food additives. EFSA J. 2012, 10, 2593. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the re-evaluation of β-carotene (E160a(ii)) as a food additive. EFSA J. 2016, 14, 4434. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). 21 CFR 73.95—Beta-Carotene; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.ecfr.gov/current/title-21/section-73.95 (accessed on 19 July 2025).
- U. S. Food and Drug Administration (FDA). 21 CFR 184.1245—Beta-Carotene as GRAS Nutrient; β-Carotene Can Be Used in Dietary Supplements and Fortified Foods; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.ecfr.gov/current/title-21/section-184.1245 (accessed on 19 July 2025).
- U.S. Food and Drug Administration. 21 CFR § 73.530—Spirulina Extract. Electronic Code of Federal Regulations. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-73/subpart-A/section-73.530 (accessed on 19 July 2025).
- European Food Safety Authority (EFSA). Scientific Opinion on Re-Evaluation of Copper Complexes of Chlorophylls (E 141(i)) and Chlorophyllins (E 141(ii)) as Food Additives. EFSA J. 2015, 13, 4151. [Google Scholar] [CrossRef]
- European Commission. Consolidated version of Commission Regulation (EU) No 231/2012 as of 27 April 2025. Off. J. Eur. Union 2025, L127. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02012R0231-20250427 (accessed on 22 July 2025).
- FAO/WHO. Compendium of Food Additive Specifications: Joint FAO/WHO Expert Committee on Food Additives (JECFA); Food and Agriculture Organization: Rome, Italy, 2021; Available online: https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/detail/en/c/453/ (accessed on 22 July 2025).
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin. Off. J. Eur. Union. 2005, L70, 1–16. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32005R0396 (accessed on 22 July 2025).
- U.S. Food and Drug Administration (FDA). Code of Federal Regulations (CFR) Title 21, Part 73—Listing of Color Additives Exempt from Certification; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-73 (accessed on 19 July 2025).
- U.S. Food and Drug Administration (FDA). Code of Federal Regulations (CFR) Title 21, Part 170—Food Additives; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-170 (accessed on 22 July 2025).
- U.S. Food and Drug Administration (FDA). 21 CFR 70.25—Labeling Requirements for Color Additives; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.ecfr.gov/current/title-21/section-70.25 (accessed on 22 July 2025).
- Fernández, M.; Alonso, R.; Martínez, C. Increasing Demand for Natural Colors in Food Products: Trends and Challenges. Curr. Opin. Food Sci. 2023, 48, 120–130. [Google Scholar] [CrossRef]
- Ashaolu, T.J. The powerful phycobiliproteins-phycocyanin and phycoerythrin: Pleiotropic applications and biofunctional uses. Algal Res. 2024, 82, 103636. [Google Scholar] [CrossRef]
- Gupta, A.K.; Banerjee, P.; Roy, S. Enhancing the Stability of Phycoerythrin for Use as a Food Additive. Int. J. Food Sci. Technol. 2022, 57, 3421–3433. [Google Scholar] [CrossRef]
- Salido, M.; Soto, M.; Seoane, S. Seaweed: Nutritional and gastronomic perspective. A review. Algal Res. 2024, 77, 10335. [Google Scholar] [CrossRef]
- Parente, G.D.L.; de Melo, B.D.N.; de Souza, J.A.; da Conceição, M.M.; Ubbink, J.; Braga, A.L.M. Fortification of traditional tapioca “pancakes” from the Brazilian northeast with microencapsulated carrot carotenoid. LWT 2021, 152, 112301. [Google Scholar] [CrossRef]
- Czubaszek, A.; Czaja, A.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kucharska, A.Z. Quality of bread enriched with microencapsulated anthocyanin extracts during in vitro simulated digestion. J. Cereal Sci. 2023, 113, 103724. [Google Scholar] [CrossRef]
- Shamshad, A.; Butt, M.S.; Nayik, G.A.; Al Obaid, S.; Ansari, M.J.; Karabagias, I.K.; Sarwar, N.; Ramniwas, S. Effect of storage on physicochemical attributes of ice cream enriched with microencapsulated anthocyanins from black carrot. Food Sci. Nutr. 2023, 11, 3976–3988. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.; Condurache, N.N.; Stănciuc, N.; Andronoiu, D.G.; Aprodu, I.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Râpeanu, G. Advanced composites based on sea buckthorn carotenoids for mayonnaise enrichment. Polymers 2022, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.S.; Patel, B.K.; Campanella, O.H.; Rodrigues, C.E.D.C.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich extract from Guaraná Peels and study of microparticle functionality through incorporation into an oatmeal paste. Foods 2023, 12, 1170. [Google Scholar] [CrossRef] [PubMed]
- Bisht, B.; Begum, J.S.; Dmitriev, A.A.; Kurbatova, A.; Singh, N.; Nishinari, K.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Unlocking the potential of future version 3D food products with next generation microalgae blue protein integration: A review. Trends Food Sci. Technol. 2024, 147, 104471. [Google Scholar] [CrossRef]
- Alasibi, S.; Kazir, M.; Israel, Á.; Livney, Y.D. Algal protein-based 3D-printed fish-analogs as a new approach for sustainable seafood. CRFS 2024, 9, 100905. [Google Scholar] [CrossRef]
- Duarah, R.; Hazarika, P.; Boruah, P.; Lakshmi, D.S.; Hazarika, S. Macroalgal (seaweed) derived bioplastic as a sustainable food packaging material–A critical review. Int. J. Biol. Macromol. 2025, 323, 147094. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Bin Abu Sofian, A.D.A.; Lim, H.R.; Chew, K.W.; Show, P.L. Advancing 3D printing through integration of machine learning with algae-based biopolymers. ChemBioEng Rev. 2024, 11, 406–425. [Google Scholar] [CrossRef]
- Lin, Q.; Zhong, L.; Zeng, M.; Kraithong, S.; Xia, X.; Kuang, W.; Wang, Q.; Huang, R. Seaweed polysaccharides as potential Prebiotics: Rationale, factors, Prebiotic activity manifestations, gut health mechanisms and extraintestinal impacts. Trends Food Sci. Technol. 2025, 163, 105202. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine algae: A treasure trove of bioactive anti-inflammatory compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef]
| Pigment Class | Food Matrix | Major Instability Factors | Molecular Interactions | Stabilization Strategies | Ref. |
|---|---|---|---|---|---|
| Chlorophylls | Acidic beverages, fruit juices | Acid-induced Mg2+ loss; light/heat degradation | Proton substitution destabilizes porphyrin; weak protection from proteins/lipids | pH adjustment; use of chlorophyllin; encapsulation | [53] |
| Carotenoids (fucoxanthin, β-carotene, astaxanthin) | Dairy emulsions, dressings, bakery products | Heat- and light-induced isomerization; oxidative cleavage | Partition into lipid droplets; binding to hydrophobic protein sites reduces oxidation | Nano and microencapsulation (gum arabic, maltodextrin) | [54] |
| Phycobiliproteins (phycocyanin, phycoerythrin) | Beverages, gels, confectionery | Denaturation at pH < 5; thermal unfolding (>55 °C); photo-oxidation | Electrostatic interactions with proteins and polysaccharides can partially stabilized | Encapsulation in alginate/pectin beads; coacervation with proteins | [57] |
| Pigments | Source | Stabilization Method | Encapsulation Agents | Food Product | Ref |
|---|---|---|---|---|---|
| Chlorophyll c, trans-fucoxanthincis-fucoxanthin, zeaxanthin, pheophytin a, E-carotene | Sargassum sp. | Microencapsulation by freezedrying | Maltodextrin, tween–80 | - | [65] |
| Chlorophylls a and b | Caulerpa sp. | Microencapsulation by freezedrying | Gelatin, gum Arabic, tween-80 | Jelly drinks | [17] |
| Chlorophylls a, b, c, d | Ulva intestinalis | Microencapsulation by freezedrying and by spray drying | Maltodextrin and whey protein isolate | - | [66] |
| β-Carotene | Sigma–Aldrich (Burlington, MA, USA) | Microencapsulation by supercritical micronization (SEDS) | Copolymer poly(3-hydroxybutirate-co-hydroxyvalerate) | - | [67] |
| Lutein | Shanghai Winherb Medical Co., Ltd. (Shanghai, China) | Proliposomes by supercritical micronization (SAS 9.4) | Hydrogenated soya phosphatidylcholine | [68] | |
| β-Carotene | Vitatene SA (Leon, Spain) | Microencapsulation by supercritical micronization (PGSS drying) | Poly-(ε-caprolactones) | - | [69] |
| β-Carotene | Vitatene SA | Microencapsulation by supercritical micronization (PGSS drying) | Soybean lecithin | - | [70] |
| Lycopene | Wako (Monza, Italy) | Microencapsulation by supercritical micronization (SEDS) | β-cyclodextrin | - | [71] |
| β-Carotene | Sigma-Aldrich | Proliposomes by spray drying | Phospholipon 90H and sucrose for liposome formation; liposomes stabilized with xanthan gum (thickening agent) | - | [72] |
| β-Carotene | MP Biomedicals (Irvine, CA, USA) | Microencapsulation by spray drying. Microspheres with chitosan–alginate beads | Maltodextrin; Chitosan; alginate | Pudding and yogurt | [27] |
| β-Carotene | Sigma-Aldrich | Microencapsulation by complex coacervation | Amaranth carboxymethyl starch and lactoferrin | Gummy candies | [28] |
| Fucoxanthin | Sargassum thunbergii | Microencapsulation by complex coacervation | Palm stearin, fish gelatin–gum arabic complex | - | [73] |
| Fucoxanthin | Undaria pinnatifida | Microencapsulation by spray drying | Different materials were tested: Hydroxypropyl-β-cyclodextrin, maltodextrin, gum arabic, whey protein isolate, isolated pea protein, and gelatin | - | [59] |
| Phycoerythrin | Kappaphycusalvarezii | Microencapsulation by freezedrying | Kappa–carrageenan and guar gum | Ice cream | [35] |
| Europe Union | United States (FDA) | |
|---|---|---|
| Chlorophylls | ||
| Regulation | Regulation (EC) No 1333/2008 [81,82] | CFR 73.125 [83,84] |
| Additive code | E140 (chlorophylls); E141 (copper complexes of chlorophylls and chlorophyllins) | Chlorophyllin copper complex |
| Status | Approved as colorants | Limited approved uses |
| Uses | Confectionery, ice cream and dessert, beverages, sauces and dressings | Citrus-based dry beverage mixes |
| Permitted levels | Regulation (EU) No 1129/2011 according to food categories [82] | Maximum concentration of 0.2% in the dry mix |
| Carotenoids | ||
| Regulation | Regulation (EC) No 1333/2008 [85,86] | 21 CFR Part 73 and GRAS [87,88] |
| Additive code | E160a–f, E161b–g | |
| Status | Approved as colorants | Permitted carotenoids: β-Carotene, Lutein |
| Uses | Margarine, cheese, snacks, beverages and desserts, bakery and confectionery | |
| Permitted levels | Regulation (EU) No 1129/2011according to food categories [82] | |
| Phycobiliproteins | ||
| Regulation | Regulation (EC) No 1333/2008 [86] Regulation EU No 1129/2011 [82] | CFR § 73.530 (Spirulina extract) [89] |
| Additive code | Not approved as additive | |
| Status | Allowed as coloring foodstuff | Approved (Spirulina extract) |
| Uses | Beverages, desserts, candies, chewing gum, ice cream | Beverages, desserts, candies, chewing gum, ice cream |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, E.F.; Gomes, L.R.; Grosso, C.; Delerue-Matos, C. Recent Advances on Seaweed-Derived Pigments for FoodApplication and Current Legal Framework. Foods 2025, 14, 3265. https://doi.org/10.3390/foods14183265
Vieira EF, Gomes LR, Grosso C, Delerue-Matos C. Recent Advances on Seaweed-Derived Pigments for FoodApplication and Current Legal Framework. Foods. 2025; 14(18):3265. https://doi.org/10.3390/foods14183265
Chicago/Turabian StyleVieira, Elsa F., Lígia Rebelo Gomes, Clara Grosso, and Cristina Delerue-Matos. 2025. "Recent Advances on Seaweed-Derived Pigments for FoodApplication and Current Legal Framework" Foods 14, no. 18: 3265. https://doi.org/10.3390/foods14183265
APA StyleVieira, E. F., Gomes, L. R., Grosso, C., & Delerue-Matos, C. (2025). Recent Advances on Seaweed-Derived Pigments for FoodApplication and Current Legal Framework. Foods, 14(18), 3265. https://doi.org/10.3390/foods14183265








