Synergistic Effects of Dysmorphococcus globosus on Selenium Enrichment and Astaxanthin Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Purification of Microalgae
2.3. Identification of Microalgae
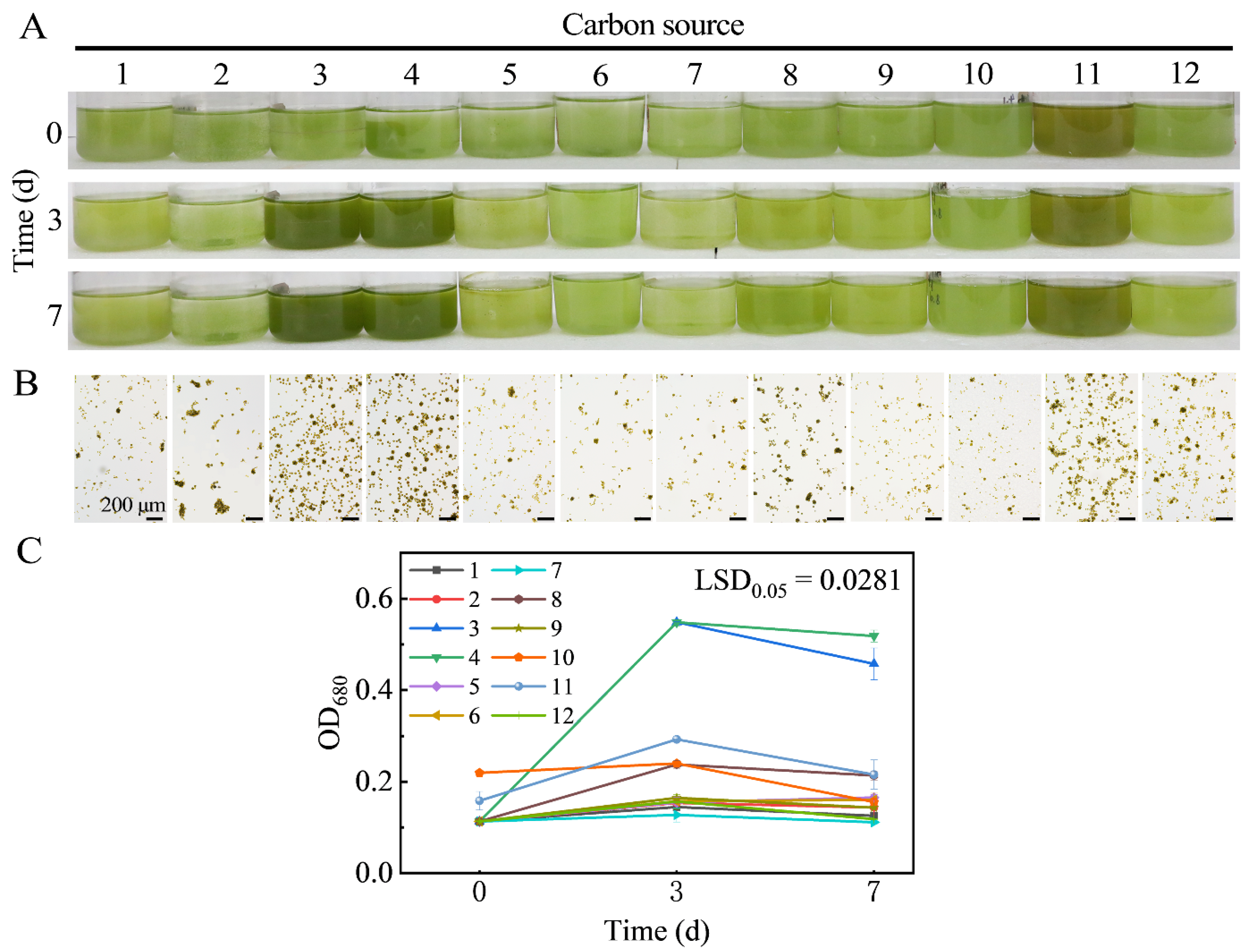
2.4. Optimization of Carbon Source in Culture Medium
2.5. Screening of Selenium-Tolerant Microalgal Strains
2.6. Establishment of a Selenium-Enriched Microalgae Culture System
2.7. Synergistic Accumulation Effects of Selenium and Astaxanthin
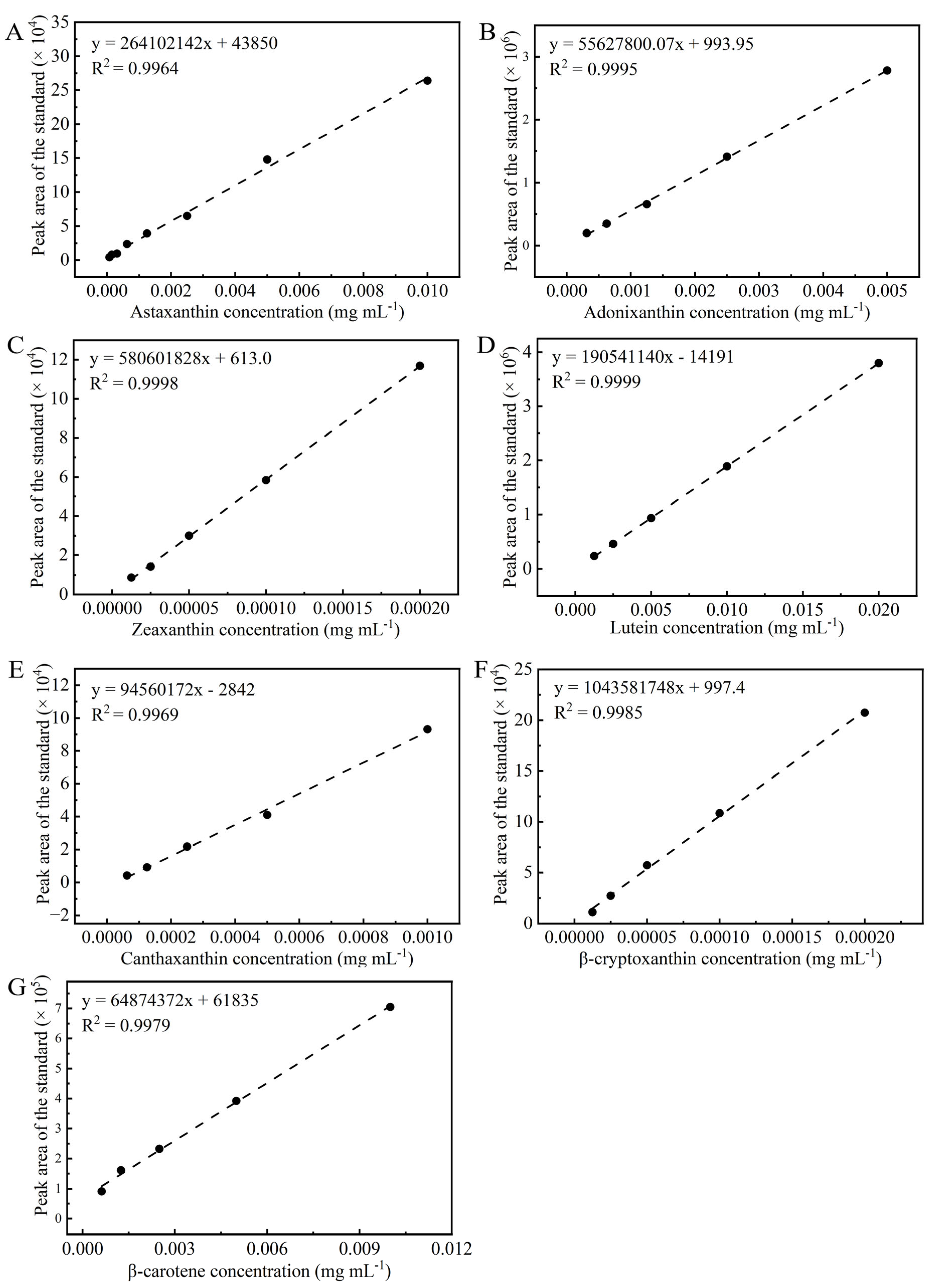
2.7.1. Detection of Pigments Using High-Performance Liquid Chromatography
2.7.2. Detection of Selenium Content and Morphology
2.8. Data Processing
3. Results and Discussion
3.1. Isolation, Purification, and Identification of Microalgae
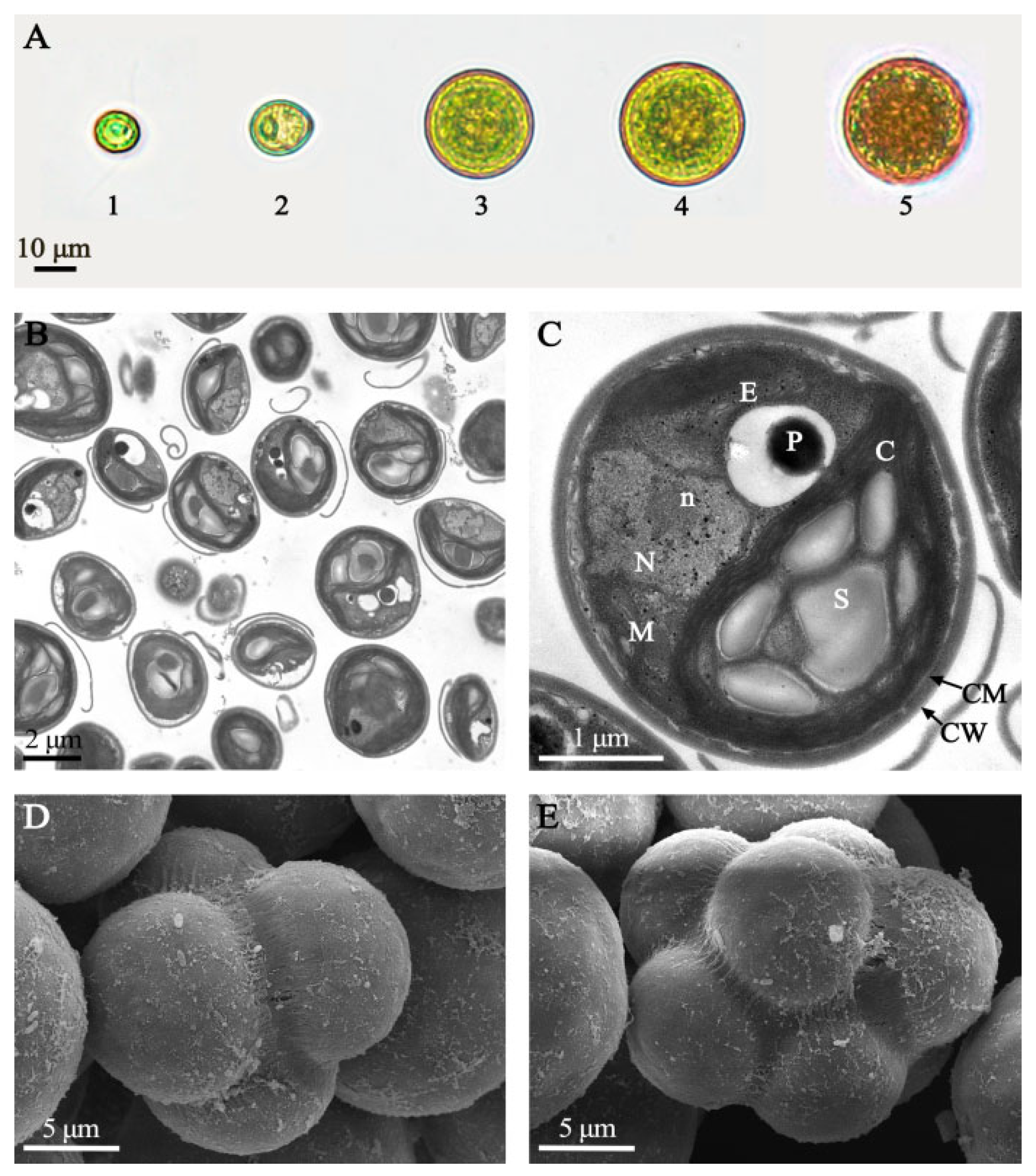
3.1.1. Morphological Characterization of Microalgae
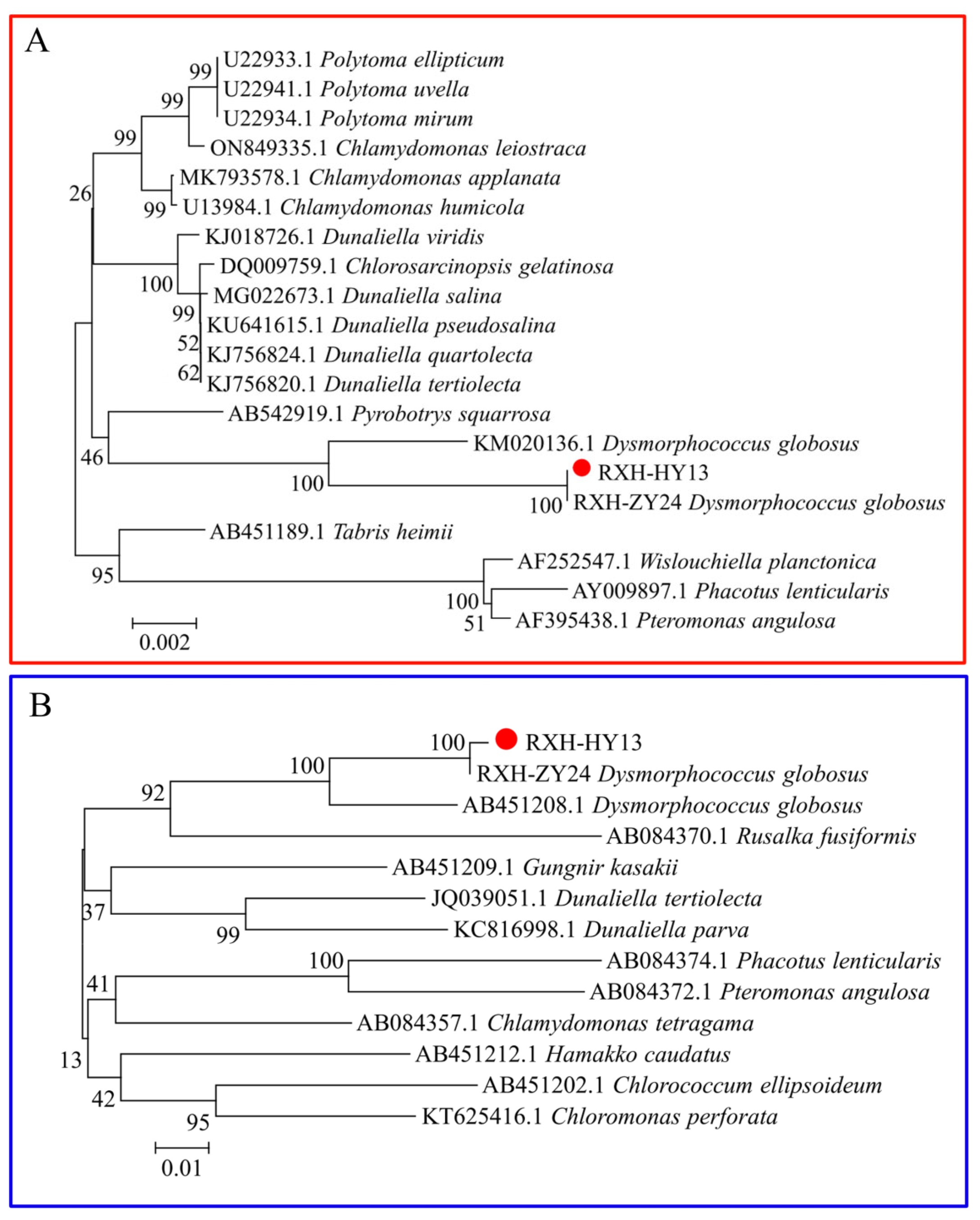
3.1.2. Molecular Biological Characterization of Microalgae
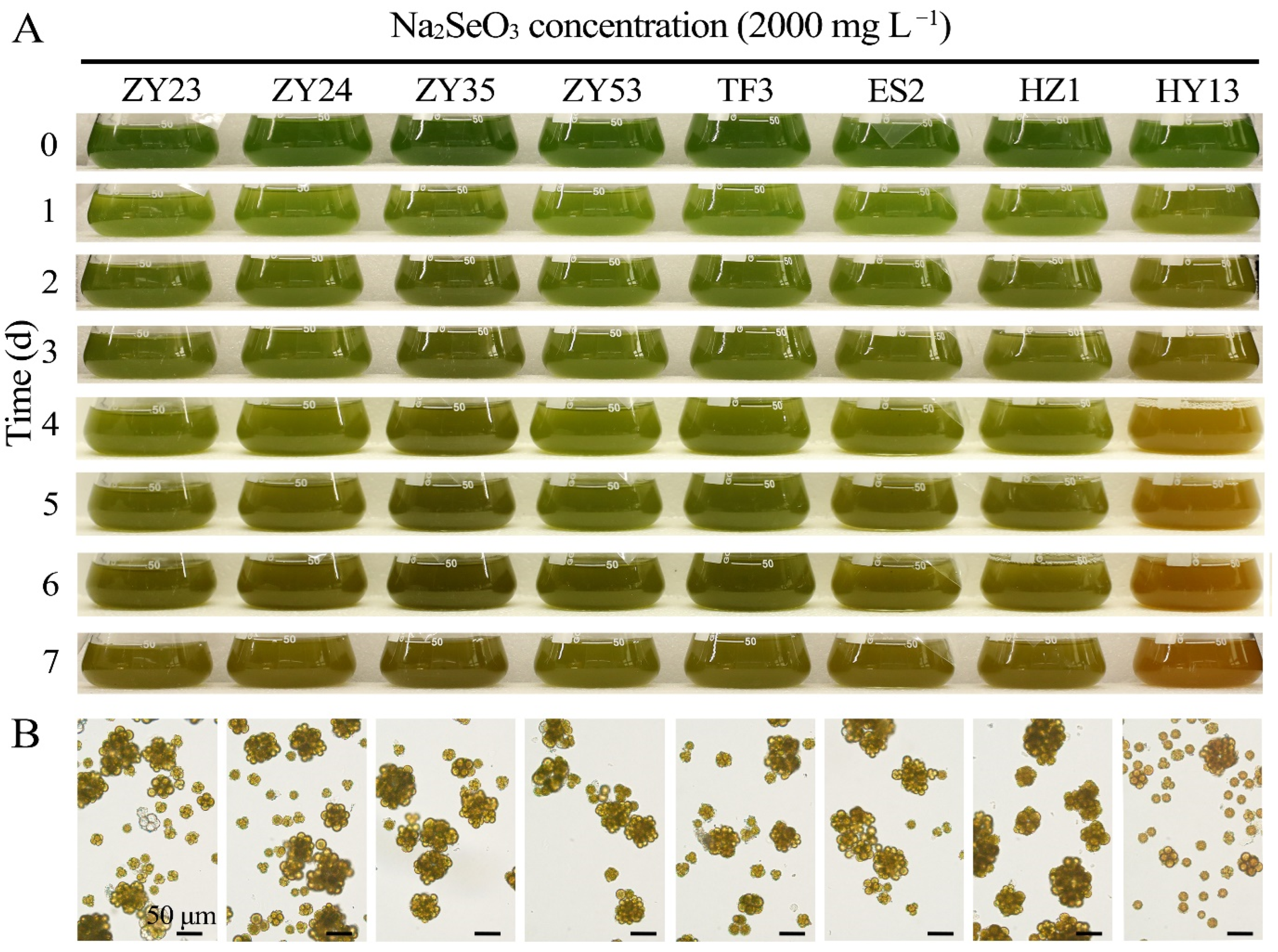
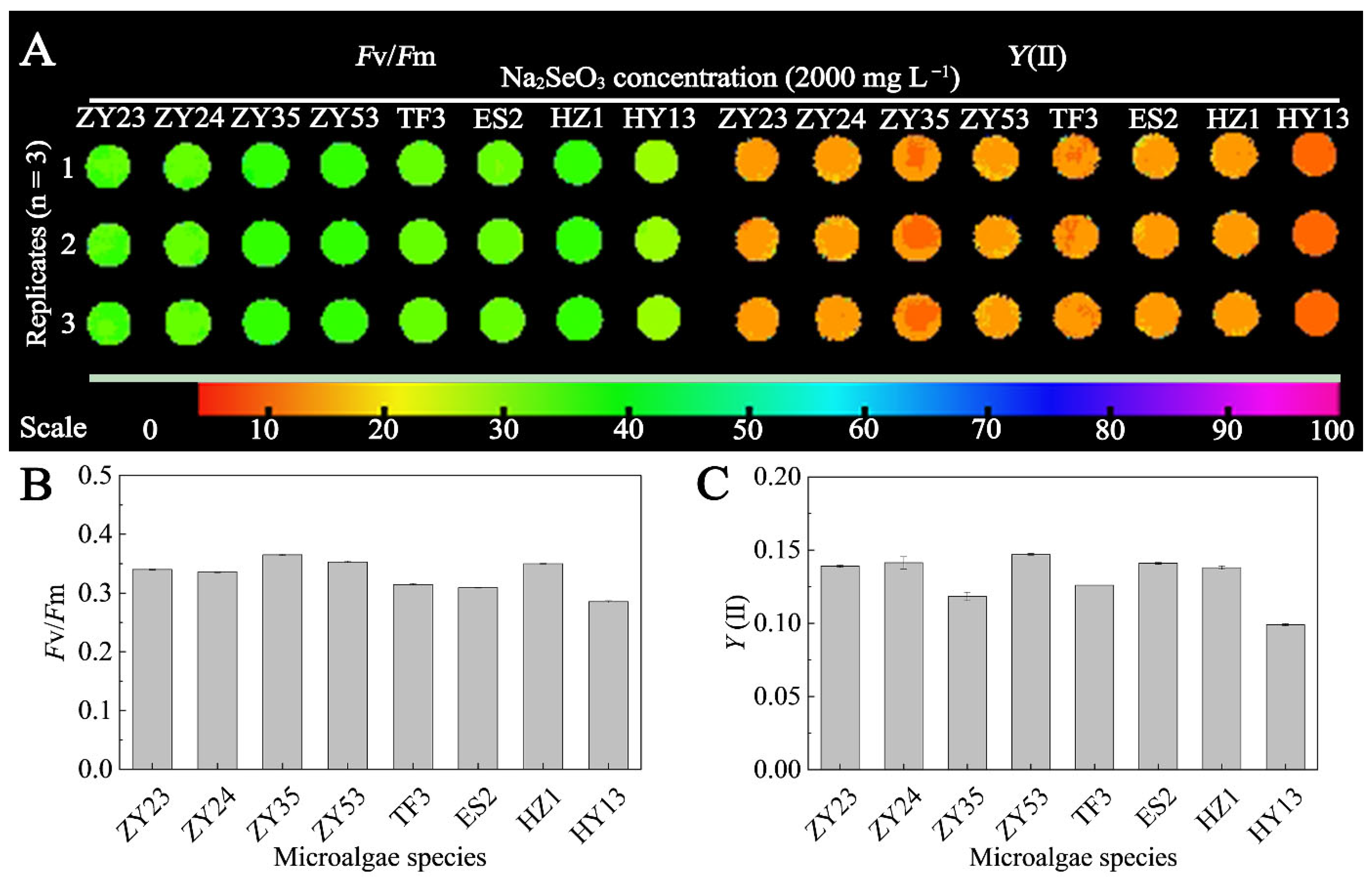
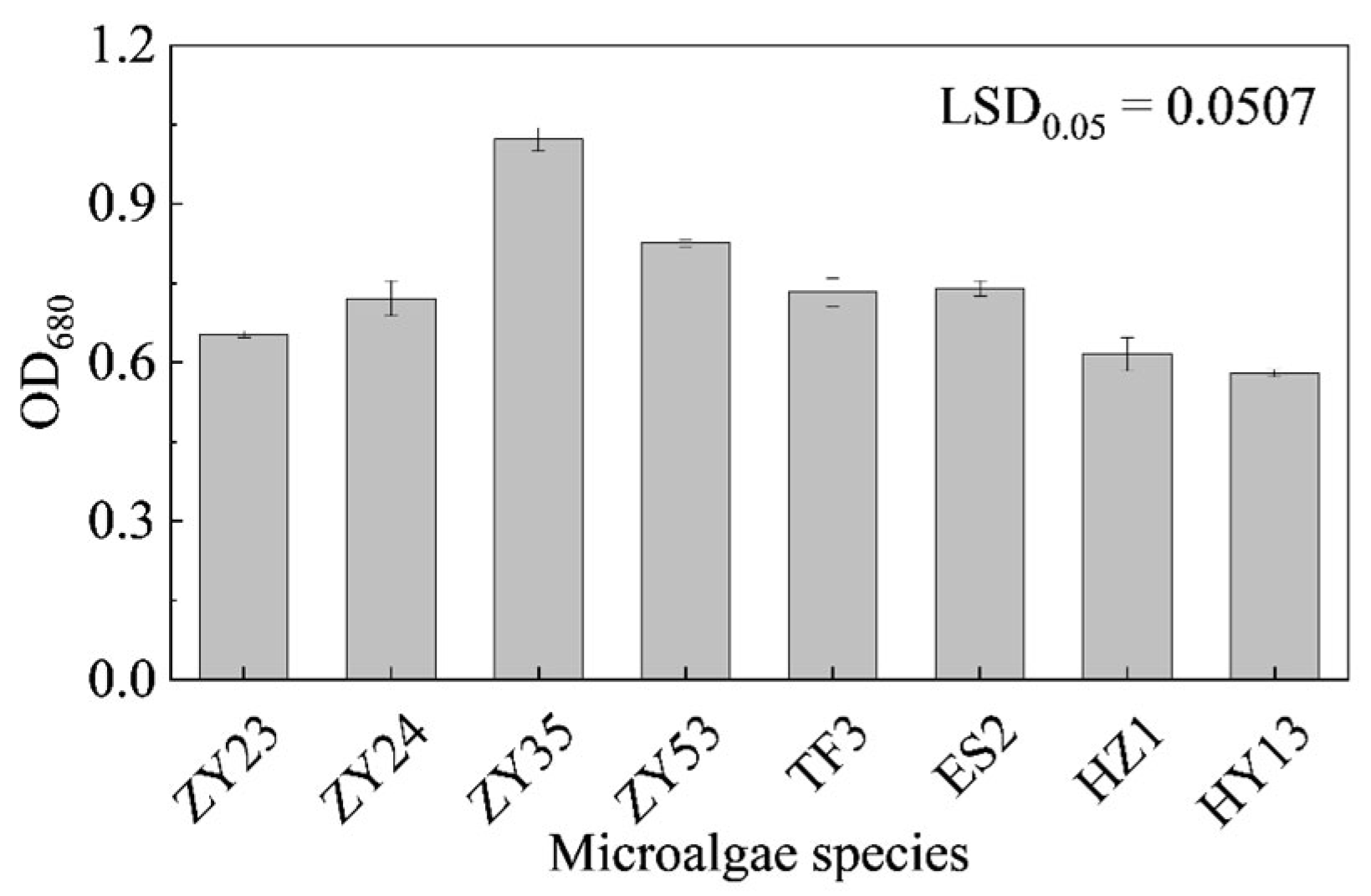
3.2. Comparison of Selenium Tolerance of D. globosus from Different Habitat Sources
3.3. Effects of Different Concentrations of Na2SeO3 on Astaxanthin and Selenium Enrichment of HY13
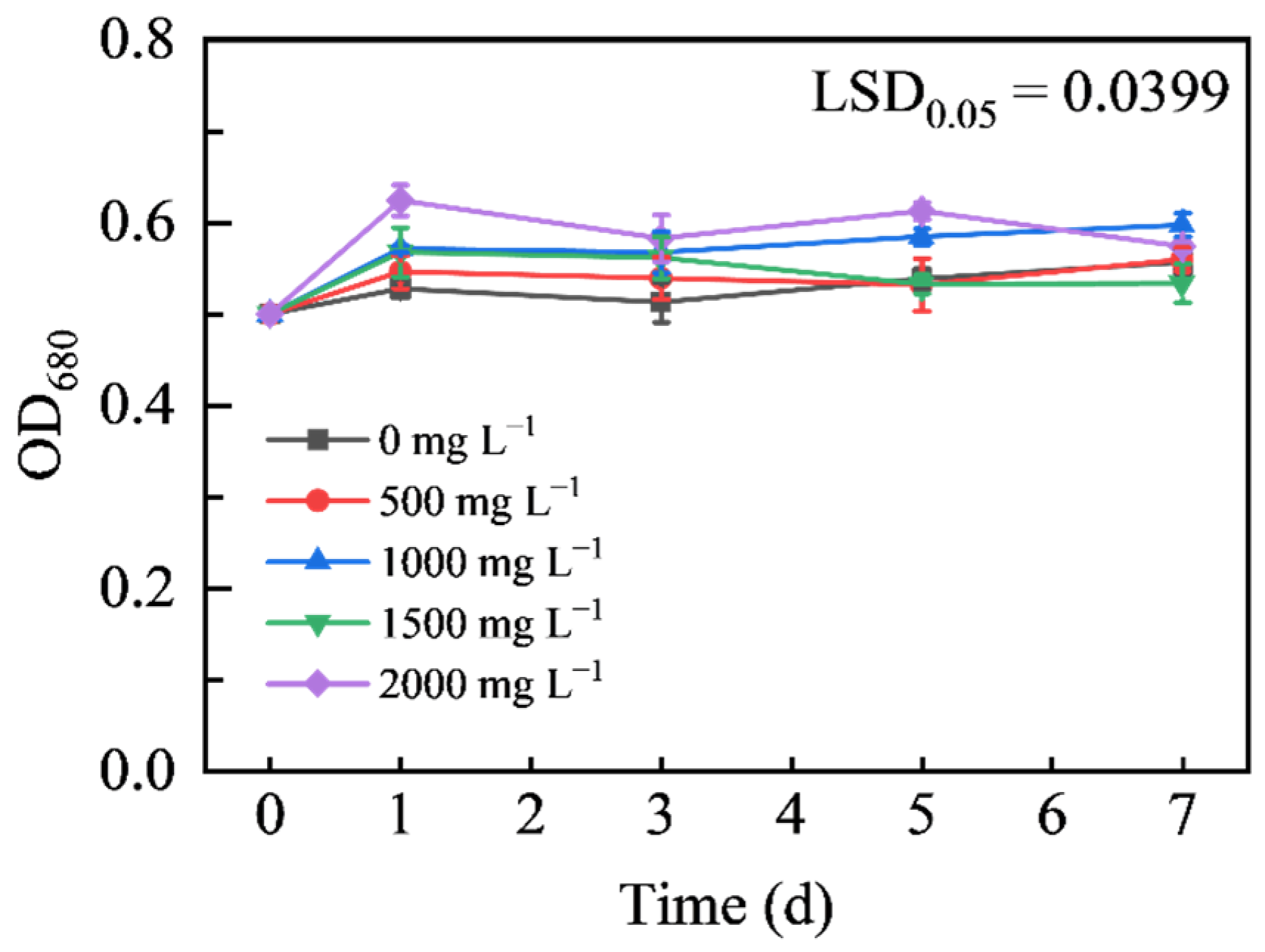
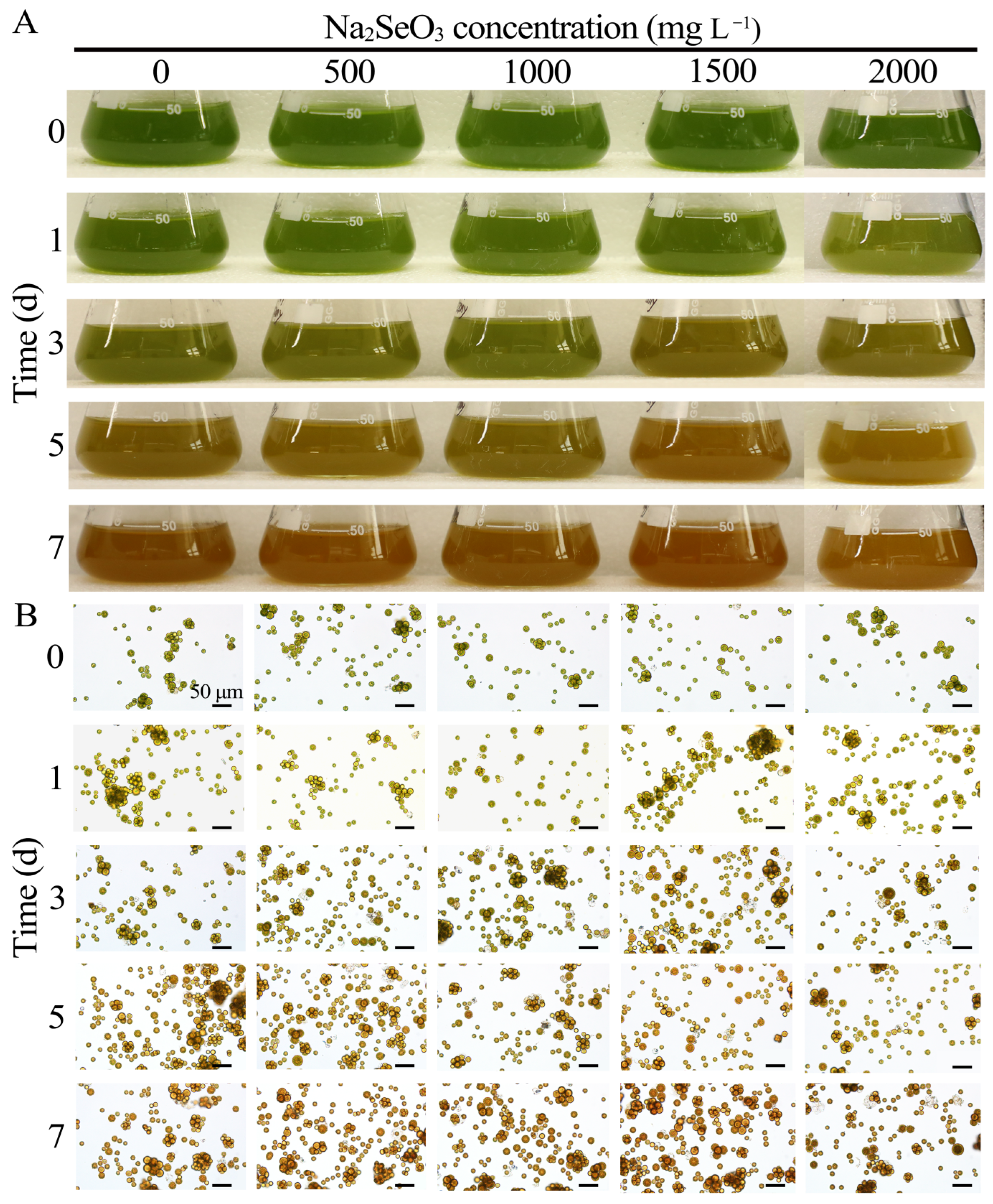
3.3.1. Effects of Different Concentrations of Na2SeO3 Treatment on the Growth of HY13
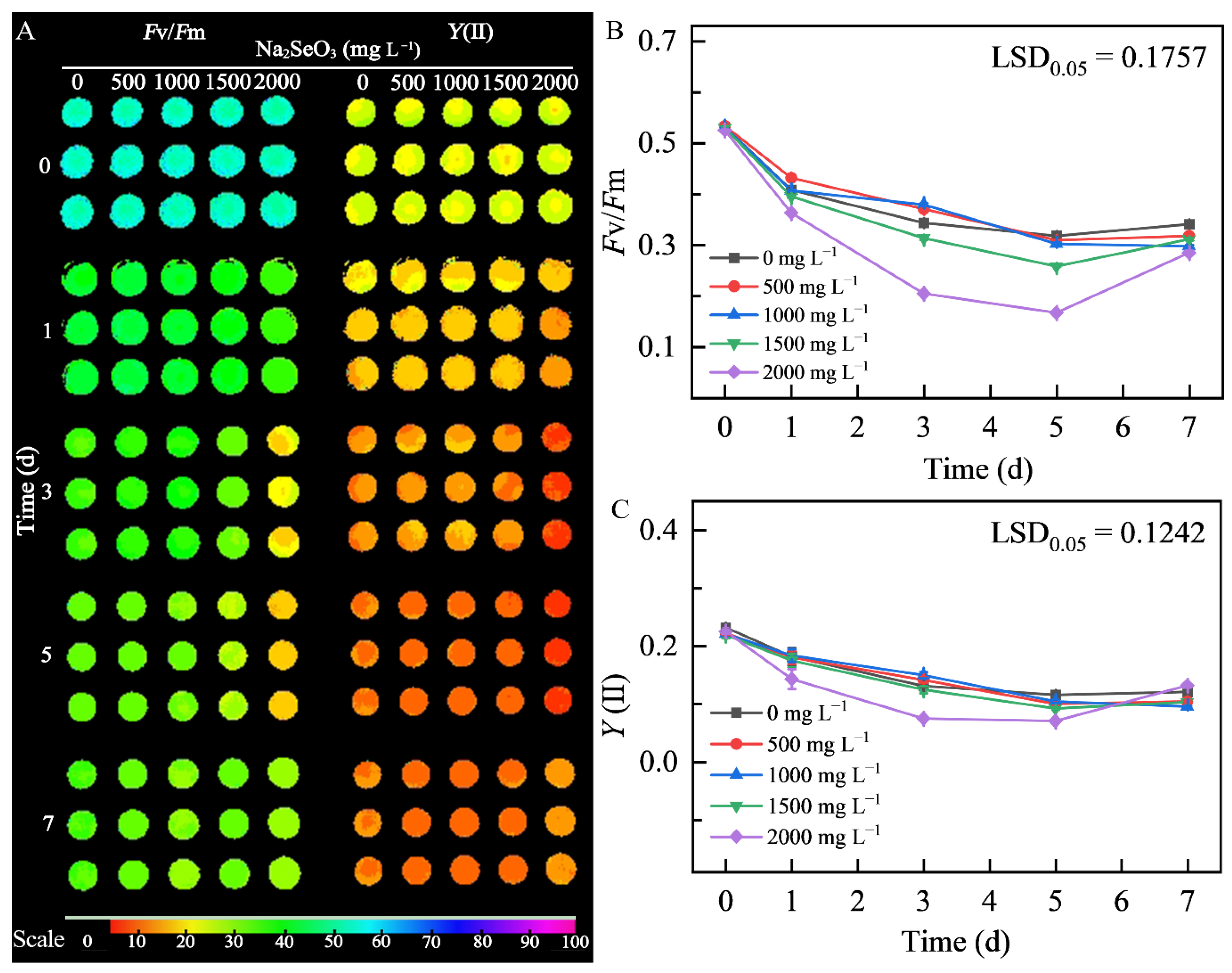
3.3.2. Effects of Different Concentrations of Na2SeO3 Treatment on Chlorophyll Fluorescence of HY13
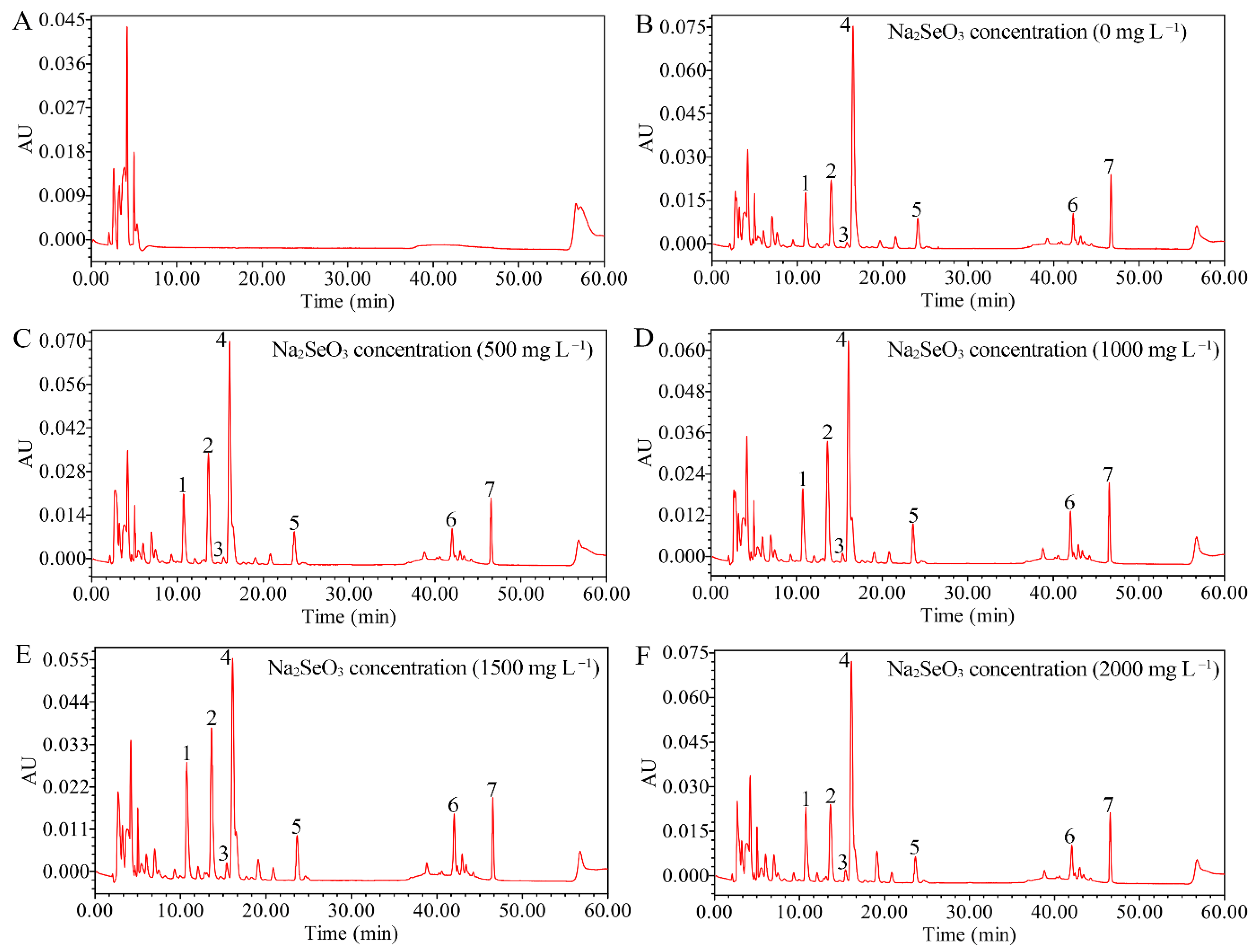
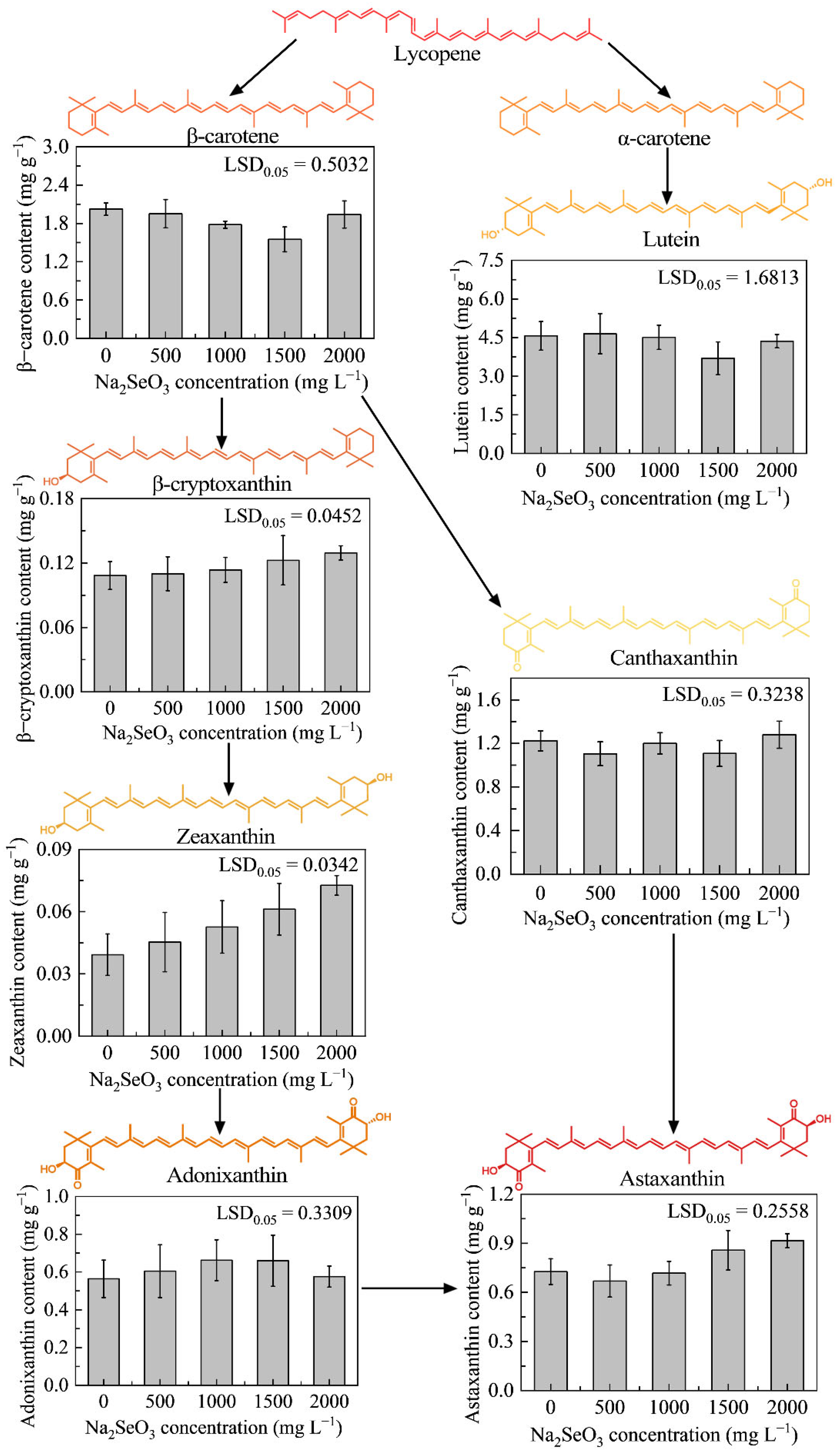
3.3.3. Effects of Different Concentrations of Na2SeO3 on Carotenoid Accumulation in HY13
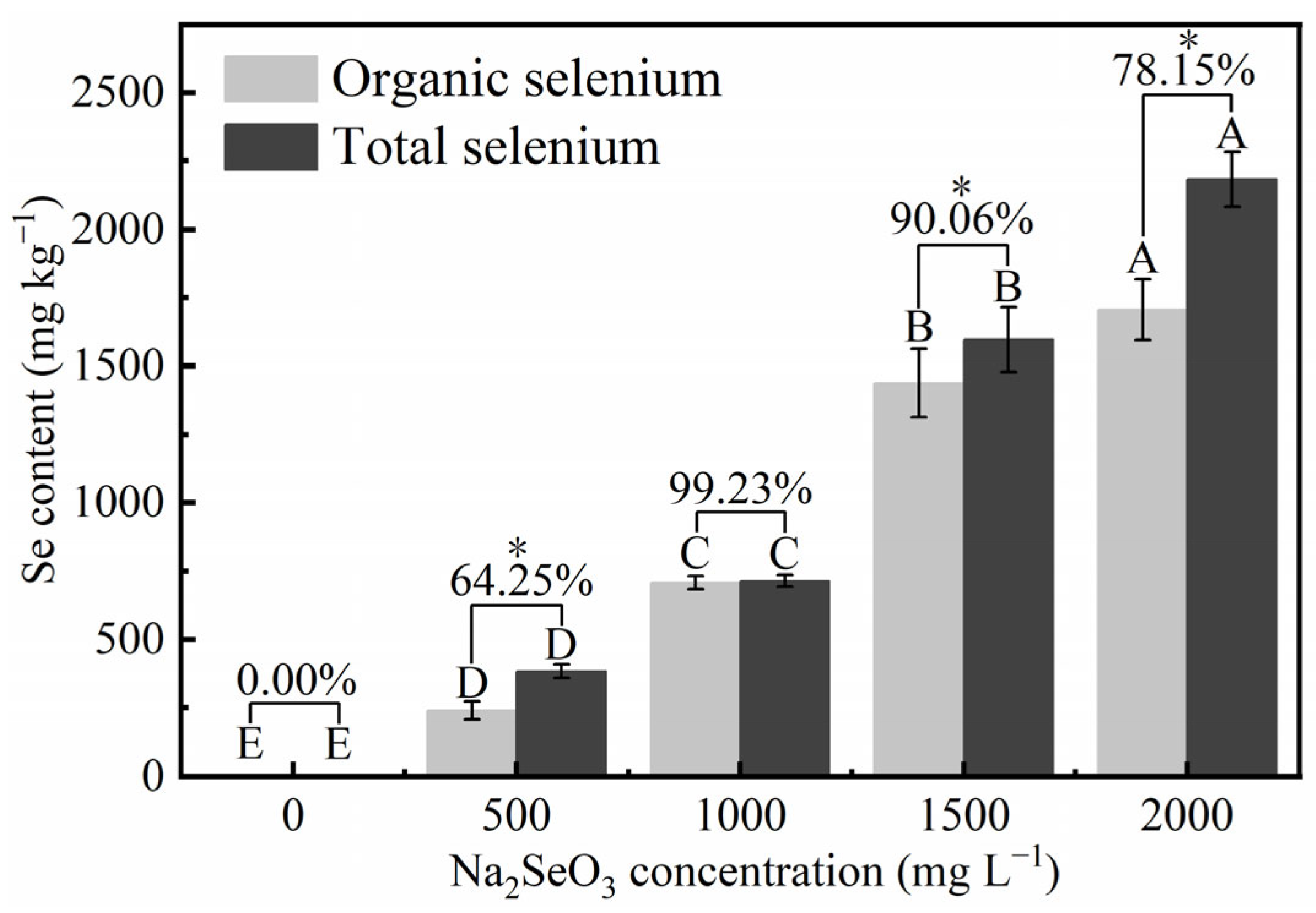
3.3.4. Effects of Different Concentrations of Na2SeO3 Treatment on the Organic Selenium Content of HY13
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | Dry weight |
| HPLC | High-performance liquid chromatography |
| LSD | Least significant difference |
| LC50 | Half-lethal concentration |
| OD | Optical density |
| PSII | Photosystem II |
Appendix A
References
- World Farmers’ Organisation. WFO Policy on Sustainable Global Food Security and Nutrition; World Farmers’ Organisation: Rome, Italy, 2024. [Google Scholar]
- Xie, X.; Zhan, J.; Ren, M. Microalgae: A solution for food security and multiplanetary farming. Food Front. 2025, 6, 1242–1251. [Google Scholar] [CrossRef]
- Lei, X.G.; Combs, G.F., Jr.; Sunde, R.A.; Caton, J.S.; Arthington, J.D.; Vatamaniuk, M.Z. Dietary selenium across species. Annu. Rev. Nutr. 2022, 42, 337–375. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Sun, R.; Wang, C.; Wang, J. Study on selenium assimilation and transformation in radish sprouts cultivated using maillard reaction products. Foods 2024, 13, 2761. [Google Scholar] [CrossRef]
- Behera, P.; Sahu, H.B. Effective removal of selenium from aqueous solution using iron-modified dolochar: A comprehensive study and machine learning predictive analysis. Environ. Res. 2024, 263, 120003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhan, S.; Han, F.; Liu, Y.; Wu, H.; Huang, Z. The possible mechanism of physiological adaptation to the low-Se diet and its health risk in the traditional endemic areas of Keshan diseases. Biol. Trace Elem. Res. 2022, 200, 2069–2083. [Google Scholar]
- Wu, H.; Chen, Z.; Wang, O.; Jiang, T.; Huang, J.; Wang, J.; Lin, J. Kashin–beck disease: A risk factor for sarcopenia and its interaction with selenium. Nutrients 2024, 16, 4343. [Google Scholar] [CrossRef]
- Etteieb, S.; Magdouli, S.; Zolfaghari, M.; Brar, S. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020, 698, 134339. [Google Scholar]
- Ragini, R.; Arumugam, M. In vivo studies on bioavailability, toxicity, and antioxidant defense of organic selenium–enriched microalga biomass in Wistar rats. J. Appl. Phycol. 2023, 35, 1699–1713. [Google Scholar]
- Zhang, Y.; Lian, Q.; Zhao, J.; He, Y.; Dai, H.; Liu, X.; Zhang, W.; Bi, J. Volatile substances, quality and non–targeted metabolomics analysis of commercially available selenium-enriched rice. Molecules 2024, 29, 5703. [Google Scholar]
- Li, Y.; Xiao, Y.; Hao, J.; Fan, S.; Dong, R.; Zeng, H.; Liu, C.; Han, Y. Effects of selenate and selenite on selenium accumulation and speciation in lettuce. Plant Physiol. Biochem. 2022, 192, 162–171. [Google Scholar] [CrossRef]
- Somagattu, P.; Chinnannan, K.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Selenium dynamics in plants: Uptake, transport, toxicity, and sustainable management strategies. Sci. Total Environ. 2024, 949, 175033. [Google Scholar] [CrossRef] [PubMed]
- Keskinen, R.; Räty, M.; Yli-Halla, M. Selenium fractions in selenate-fertilized field soils of Finland. Nutr. Cycl. Agroecosystems 2011, 91, 17–29. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, Y.; Dai, L.; Wang, L.; Zhou, G.; Liang, T. Selenium spatial distribution and bioavailability of soil-plant systems in China: A comprehensive review. Environ. Geochem. Health 2024, 46, 341. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Li, Y.; Shi, C.; Zhang, Y.; Wang, P.; Zhang, H.; Wang, R.; Zhang, W.; Wen, P. Revealing the impact of organic selenium-enriched Lactiplantibacillus plantarum NML21 on yogurt quality through volatile flavor compounds and untargeted metabolomics. Food Chem. 2025, 474, 143223. [Google Scholar] [CrossRef]
- González-Salitre, L.; Román-Gutiérrez, A.; Contreras-López, E.; Bautista-Ávila, M.; Rodríguez-Serrano, G.; González-Olivares, L. Promising use of selenized yeast to develop new enriched food: Human health implications. Food Rev. Int. 2021, 39, 1594–1611. [Google Scholar] [CrossRef]
- Luo, L.; Hou, X.; Yi, D.; Deng, G.; Wang, Z.; Peng, M. Selenium-enriched microorganisms: Metabolism, production, and applications. Microorganisms 2025, 13, 1849. [Google Scholar] [CrossRef]
- Hoyos, B.S.; Hernandez-Tenorio, F.; Miranda, A.M.; Villanueva-Mejía, D.F.; Sáez, A.A. Systematic analysis of genes related to selenium bioaccumulation in microalgae: A review. Biology 2023, 12, 703. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, Y.; Zhang, Y. Asgard archaeal selenoproteome reveals a roadmap for the archaea-to-eukaryote transition of selenocysteine incorporation machinery. ISME J. 2024, 18, wrae111. [Google Scholar] [CrossRef]
- Xie, X.; Jaleel, A.; Zhan, J.; Ren, M. Microalgae: Towards human health from urban areas to space missions. Front. Plant Sci. 2024, 15, 1419157. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin protection by carotenoid pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Bandyopadhyay, T.K.; Vanitha, K.; Bobby, M.N.; Nath Tiwari, O.; Bhunia, B.; Muthuraj, M. Astaxanthin from microalgae: A review on structure, biosynthesis, production strategies and application. Food Res. Int. 2024, 176, 113841. [Google Scholar]
- Zhang, J.; Liu, M.; Han, T.; Luo, L.; Zhang, Y.; Yuan, G.; Fang, X.; Han, F.; Chen, X.; Wang, Y. Advance toward function, production, and delivery of natural astaxanthin: A promising candidate for food ingredients with future perspectives. Food Chem. 2025, 463, 141428. [Google Scholar] [CrossRef]
- Xie, X.; Zhong, M.; Huang, X.; Yuan, X.; Mahna, N.; Mussagy, C.U.; Ren, M. Astaxanthin biosynthesis for functional food development and space missions. Crit. Rev. Biotechnol. 2024, 45, 923–937. [Google Scholar] [CrossRef]
- Ramarui, K.; Zhong, J.; Li, Y. Proteomic and phosphoproteomic analysis of a Haematococcus pluvialis (Chlorophyceae) mutant with a higher heterotrophic cell division rate reveals altered pathways involved in cell proliferation and nutrient partitioning. J. Phycol. 2024, 60, 1173–1189. [Google Scholar] [CrossRef]
- Zohir, W.F.; Kapase, V.U.; Kumar, S. Identification and characterization of a new microalga Dysmorphococcus globosus–HI from the Himalayan region as a potential source of natural astaxanthin. Biology 2022, 11, 884. [Google Scholar] [CrossRef]
- Hadziavdic, K.; Lekang, K.; Lanzen, A.; Jonassen, I.; Thompson, E.M.; Troedsson, C. Characterization of the 18S rRNA gene for designing universal eukaryote specific primers. PLoS ONE 2014, 9, e87624. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Naruya, S.; Masatoshi, N. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Geoffroy, L.; Gilbin, R.; Simon, O.; Floriani, M.; Adam, C.; Pradines, C.; Cournac, L.; Garnier-Laplace, J. Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol. 2007, 83, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of new microalgae-based sourdough “crostini”: Functional effects of Arthrospira platensis (spirulina) addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products through the Addition of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Administration U.S.F. A. D. GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices (accessed on 3 September 2025).
- GB/T31520—2015; General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China, S.A. O. T. P. S. R. O. C. Determination of Astaxanthin in Haematococcus—High Performance Liquid Chromatography Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China; Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- Grujić, V.J.; Todorović, B.; Kranvogl, R.; Ciringer, T.; Ambrožič-Dolinšek, J. Diversity and content of carotenoids and other pigments in the transition from the green to the red stage of Haematococcus pluvialis microalgae identified by HPLC-DAD and LC-QTOF-MS. Plants 2022, 11, 1026. [Google Scholar] [CrossRef]
- GB 5009.93—2017; National Health and Family Planning Commission of the People’s Republic of China, C.F. A. D. A. Food Safety National Standard—Determination of Selenium in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China; China Food and Drug Administration: Beijing, China, 2017.
- Zhang, Z.; Zhang, Y.; Hua, Y.; Chen, G.; Fu, P.; Liu, J. Heterotrophic selenium incorporation into Chlorella vulgaris K-01: Selenium tolerance, assimilation, and removal through microalgal cells. Foods 2024, 13, 405. [Google Scholar] [CrossRef]
- Ballesteros, I.; Terán, P.; Guamán-Burneo, C.; González, N.; Cruz, A.; Castillejo, P. DNA barcoding approach to characterize microalgae isolated from freshwater systems in Ecuador. Neotrop. Biodivers. 2021, 7, 170–183. [Google Scholar] [CrossRef]
- Drop, B.; Webber-Birungi, M.; Fusetti, F.; Kouril, R.; Redding, K.E.; Boekema, E.J.; Croce, R. Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J. Biol. Chem. 2011, 286, 44878–44887. [Google Scholar] [CrossRef]
- Li, W.; Ma, L.; Ye, Y.; Tang, Q.; Shen, Y.; Zou, Z.; Zhou, H.; Liang, C.; Wang, G. Selenium absorption, translocation and biotransformation in pak choi (Brassica chinensis L.) after foliar application of selenium nanoparticles. Food Chem. 2025, 463, 141439. [Google Scholar]
- Singh, P.; Singh, S.; Maurya, P.; Mohanta, A.; Dubey, H.; Khadim, S.R.; Singh, A.K.; Pandey, A.K.; Singh, A.K.; Asthana, R.K. Bioaccumulation of selenium in halotolerant microalga Dunaliella salina and its impact on photosynthesis, reactive oxygen species, antioxidative enzymes, and neutral lipids. Mar. Pollut. Bull. 2023, 190, 114842. [Google Scholar] [CrossRef]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit. Rev. Food Sci. Nutr. 2020, 60, 391–405. [Google Scholar]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Aizpuru, A.; González-Sánchez, A. Traditional and new trend strategies to enhance pigment contents in microalgae. World J. Microbiol. Biotechnol. 2024, 40, 272. [Google Scholar] [CrossRef]
- Shang, C.; Pang, B.; Zhang, J.; Yu, L.; Gan, S.; Li, Y.; Wu, H. Identification of interacting proteins of transcription factor DpAP2 related to carotenoid biosynthesis from marine microalga Dunaliella parva. Front. Mar. Sci. 2022, 9, 907065. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, X.; Yu, X.; Li, S.; Wang, K.; Wei, L.; Li, R.; Qin, S. Advancements of astaxanthin production in Haematococcus pluvialis: Update insight and way forward. Biotechnol. Adv. 2025, 79, 108519. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Teng, Y.; Yu, X.; Zhao, Y. Gamma-aminobutyric acid as a regulator of astaxanthin production in Haematococcus lacustris under salinity: Exploring physiology, signaling, autophagy, and multi-omics landscape. Bioresour. Technol. 2024, 413, 131466. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yang, S.; Liang, Q.; Gu, Z.; Wang, Y.; Mou, H.; Sun, H. Selecting a preculture strategy for improving biomass and astaxanthin productivity of Chromochloris zofingiensis. Appl. Microbiol. Biotechnol. 2024, 108, 117. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Piwowarek, K.; Brzezicka, K. Equilibrium modeling of selenium binding from aqueous solutions by Candida utilis ATCC 9950 yeasts. 3 Biotech 2018, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Kot, A.M.; Kolotylo, V. Bioaccumulation of selenium and production of carotenoids by the yeast Rhodotorula mucilaginosa. Biocatal. Agric. Biotechnol. 2023, 53, 102903. [Google Scholar] [CrossRef]
- Sęk, W.; Kot, A.M.; Kieliszek, M. The impact of selenium on the physiological activity of yeast cells Saccharomyces cerevisiae ATCC 7090 and Rhodotorula glutinis CCY 20-2-26. Front. Biosci. (Landmark Ed) 2025, 30, 26692. [Google Scholar] [CrossRef]
- Pires, R.; Costa, M.; Silva, J.; Pedras, B.; Concórdio-Reis, P.; Lapa, N.; Ventura, M. Se-enrichment of Chlorella vulgaris grown under different trophic states for food supplementation. Algal Res. 2022, 68, 102876. [Google Scholar] [CrossRef]
- Jiang, P.; Meng, J.; Zhang, L.; Huang, L.; Wei, L.; Bai, Y.; Liu, X.; Li, S. Purification and anti-inflammatory effect of selenium-containing protein fraction from selenium-enriched Spirulina platensis. Food Biosci. 2022, 45, 101469. [Google Scholar] [CrossRef]
- Vriens, B.; Behra, R.; Voegelin, A.; Zupanic, A.; Winkel, L.H. Selenium Uptake and Methylation by the Microalga Chlamydomonas reinhardtii. Environ. Sci. Technol. 2016, 50, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Tao, M.; Li, J.; Hu, Z. Effects of selenite on green microalga Haematococcus pluvialis: Bioaccumulation of selenium and enhancement of astaxanthin production. Aquat. Toxicol. 2017, 183, 21–27. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Vilchez, C.; Torronteras, R.; Vigara, J.; Gomez-Jacinto, V.; Janzer, N.; Gomez-Ariza, J.L.; Marova, I.; Garbayo, I. Effect of selenate on viability and selenomethionine accumulation of Chlorella sorokiniana grown in batch culture. Sci. World J. 2014, 2014, 401265. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Guo, S.-Y.; Li, L. Bioeffects of selenite on the growth of Spirulina platensis and its biotransformation. Bioresour. Technol. 2003, 89, 171–176. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Study on newly isolated Dysmorphococcus strains from reunion island as potential sources of high-value carotenoids. Foods 2024, 13, 3922. [Google Scholar] [CrossRef]
- Zan, Z.; Huang, X.; Hussain, Z.; Zhong, M.; Hou, C.; Ren, M.; Xie, X. Effects of culture medium enrichment with zinc on astaxanthin accumulation in a new strain of the microalga Dysmorphococcus globosus. Plants 2024, 13, 3338. [Google Scholar] [CrossRef]
- Ning, N.; Yuan, X.Y.; Dong, S.Q.; Wen, Y.Y.; Gao, Z.P.; Guo, M.J.; Guo, P.Y. Increasing selenium and yellow pigment concentrations in Foxtail Millet (Setaria italica L.) grain with foliar application of selenite. Biol. Trace Elem. Res. 2016, 170, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Mendes, N.A.C.; Silva, V.M.; Vicente, E.F.; dos Reis, A.R. Selenium increases photosynthetic pigments, flavonoid biosynthesis, nodulation, and growth of soybean plants (Glycine max L.). J. Soil Sci. Plant Nutr. 2023, 23, 1397–1407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, M.; Huang, X.; Zhang, X.; Hussain, Z.; Zan, Z.; Wang, Q.; Xie, X.; Ren, M. Synergistic Effects of Dysmorphococcus globosus on Selenium Enrichment and Astaxanthin Accumulation. Foods 2025, 14, 3249. https://doi.org/10.3390/foods14183249
Zhong M, Huang X, Zhang X, Hussain Z, Zan Z, Wang Q, Xie X, Ren M. Synergistic Effects of Dysmorphococcus globosus on Selenium Enrichment and Astaxanthin Accumulation. Foods. 2025; 14(18):3249. https://doi.org/10.3390/foods14183249
Chicago/Turabian StyleZhong, Moyu, Xinxin Huang, Xinyue Zhang, Zahid Hussain, Zhaohui Zan, Qi Wang, Xiulan Xie, and Maozhi Ren. 2025. "Synergistic Effects of Dysmorphococcus globosus on Selenium Enrichment and Astaxanthin Accumulation" Foods 14, no. 18: 3249. https://doi.org/10.3390/foods14183249
APA StyleZhong, M., Huang, X., Zhang, X., Hussain, Z., Zan, Z., Wang, Q., Xie, X., & Ren, M. (2025). Synergistic Effects of Dysmorphococcus globosus on Selenium Enrichment and Astaxanthin Accumulation. Foods, 14(18), 3249. https://doi.org/10.3390/foods14183249





