Effect of Dry Roasting on the Physicochemical, Nutritional, and Techno-Functional Properties of Tri-Color Quinoa Flours
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Physicochemical Characteristics
Proximate Analysis, Energy, and Total Dietary Fiber (TDF) Content
Resistant and Digestible Starch
Fatty Acid (FA) Composition
Mineral Content
Anti-Nutritional Factors (ANFs)
- Phytic Acid Content
- Saponin Content
- Tannin Content
- Oxalate Content
Trypsin Inhibitor Activity (TIA)
Water Activity and Color Parameters
Thermal Properties
Secondary Structure Analysis
Microstructure and Elemental Analysis
2.2.3. Protein Quality
2.2.4. Techno-Functional Properties
Protein Solubility
Water Absorption and Oil Absorption Capacity (WAC and OAC)
Emulsifying Capacity and Stability (EC and ES)
Foaming Capacity and Stability (FC and FS)
Swelling Capacity (SC)
Least Gelation Concentration (LGC)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
3.1.1. Proximate Composition and Energy Content
3.1.2. TDF and Starch Content
3.1.3. Fatty Acid Composition
3.1.4. Mineral Content
3.1.5. Microstructure and Elemental Analysis
3.1.6. ANFs
3.1.7. Color and Water Activity
3.1.8. Thermal Properties
3.1.9. Secondary Structure Analysis
3.2. Protein Quality
3.2.1. Amino Acid Composition
3.2.2. AA Score, EAAI and p-BV
3.2.3. IVPD and IVPDCAAS
3.3. Techno-Functional Properties
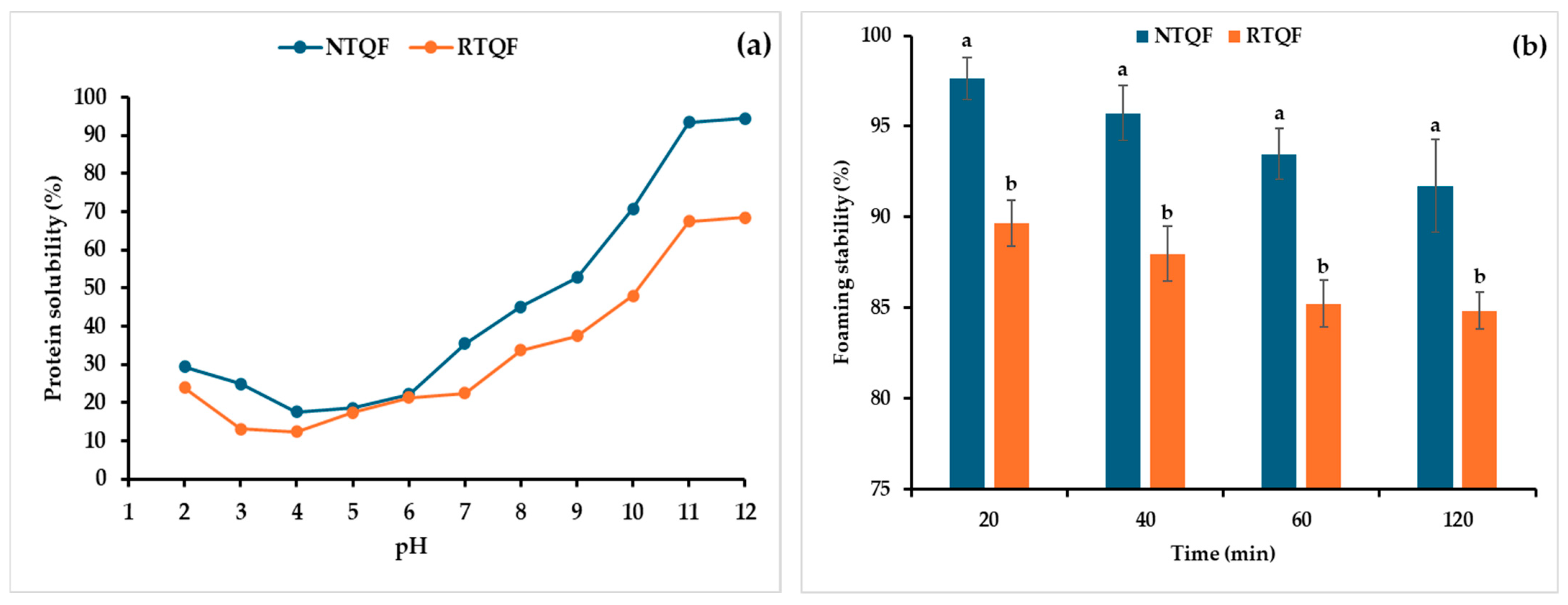
3.3.1. Protein Solubility
3.3.2. WAC and OAC
3.3.3. EC and ES
3.3.4. FC and FS
3.3.5. SC
3.3.6. LGC
3.4. Interactions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Beniwal, S.K.; Devi, A.; Sindhu, R. Effect of grain processing on nutritional and physico-chemical, functional and pasting properties of amaranth and quinoa flours. Indian J. Tradit. Knowl. 2019, 18, 500–507. [Google Scholar]
- Kheto, A.; Joseph, D.; Islam, M.; Dhua, S.; Das, R.; Kumar, Y.; Vashishth, R.; Sharanagat, V.S.; Kumar, K.; Nema, P.K. Microwave roasting induced structural, morphological, antioxidant, and functional attributes of Quinoa (Chenopodium quinoa Willd). J. Food Process. Preserv. 2022, 46, e16595. [Google Scholar] [CrossRef]
- Babiker, E.E.; Uslu, N.; Ghafoor, K.; AL-Juhaimi, F.; Özcan, M.M.; Ahmed, I.A.M. Variations in bioactive properties, fatty acid compositions, and phenolic compounds of quinoa grain and oils roasted in a pan. J. Food Process. Preserv. 2022, 46, e16161. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Sandberg, A.-S.; Carlsson, N.-G.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Effect of fermentation and dry roasting on the nutritional quality and sensory attributes of quinoa. Food Sci. Nutr. 2019, 7, 3902–3911. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Fidelis, M.; Ren, Y.; Stone, A.K.; Ai, Y.; Nickerson, M.T. The functional attributes of Peruvian (Kankolla and Blanca juli blend) and Northern quinoa (NQ94PT) flours and protein isolates, and their protein quality. Food Res. Int. 2020, 128, 108799. [Google Scholar] [CrossRef] [PubMed]
- Benmeziane-Derradji, F.; Djermoune-Arkoub, L.; Ayat, N.E.H.; Aoufi, D. Impact of roasting on the physicochemical, functional properties, antioxidant content and microstructure changes of Algerian lentil (Lens culinaris) flour. J. Food Meas. Charact. 2020, 14, 2840–2853. [Google Scholar] [CrossRef]
- Sharma, S.; Kataria, A.; Singh, B. Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). LWT 2022, 160, 113256. [Google Scholar] [CrossRef]
- Marie, A.; Gazzar, M.; El-Salam, R.A. Gluten-free cupcake enriched with treated black quinoa flour. Egypt. J. Food Sci. 2024, 52, 243–267. [Google Scholar] [CrossRef]
- Stone, A.K.; Parolia, S.; House, J.D.; Wang, N.; Nickerson, M.T. Effect of roasting pulse seeds at different tempering moisture on the flour functional properties and nutritional quality. Food Res. Int. 2021, 147, 110489. [Google Scholar] [CrossRef] [PubMed]
- Norouzian, A.; Sangatash, M.M.; Sahraiyan, B. Investigating the nutritional, technological and sensory properties of compact food bar containing raw and processed quinoa. J. Food Sci. Technol. 2024, 21, 153. [Google Scholar] [CrossRef]
- Badia-Olmos, C.; Laguna, L.; Haros, C.M.; Tárrega, A. Techno-Functional and rheological properties of alternative plant-based flours. Foods 2023, 12, 1411. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of AOAC International, 19th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Vujić, D.N.; Ačanski, M.M.; Bodroža-Solarov, M.I.; Hristov, N.S.; Krunić, M.N. Performance of GC-MS analysis for differentiation of various types of flour by creating dendrogram of liposoluble extract. Chem. Ind. Chem. Eng. Q. 2012, 18, 555–561. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. Elemental analysis of plant tissue by plasma emission spectroscopy: Collaborative study. J. Assoc. Off. Anal. Chem. 1985, 68, 499–505. [Google Scholar] [CrossRef]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, O.O.; Awodiran, S.T.; Ajayi, A.O.; Ogunmoyela, T.F. Effect of high-temperature, short-time cooking conditions on in vitro protein digestibility, enzyme inhibitor activity and amino acid profile of selected legume grains. Heliyon 2020, 6, e05419. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.; Zhao, Y.; Zheng, X.; Lu, J.; Liang, Y. Simultaneous HPLC Determination of amino acids in tea infusion coupled to pre-column derivatization with 2,4-dinitrofluorobenzene. Food Anal. Methods 2016, 9, 1307–1314. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition. FAO Epert Consultation, Food and Nutrition Paper 92; FAO: Rome, Italy, 2013. Available online: https://pubmed.ncbi.nlm.nih.gov/26369006 (accessed on 7 September 2025).
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Chang, L.; Gu, Z.; Bandillo, N.; Chen, B.; Rao, J. Fractionation, structural characteristics, functionality, aromatic profile, and in vitro digestibility of lentil (Lens culinaris) proteins. ACS Food Sci. Technol. 2023, 3, 478–488. [Google Scholar] [CrossRef]
- Sohaimy, S.; Mohamed, S.; Shehata, M.; Mehany, T.; Zaitoun, M. Compositional Analysis and Functional Characteristics of Quinoa Flour. Annu. Res. Rev. Biol. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Badia-Olmos, C.; Sánchez-García, J.; Laguna, L.; Zúñiga, E.; Mónika Haros, C.; Maria Andrés, A.; Tarrega, A. Flours from fermented lentil and quinoa grains as ingredients with new techno-functional properties. Food Res. Int. 2024, 177, 113915. [Google Scholar] [CrossRef]
- Manzanilla-Valdez, M.; Boesch, C.; Orfila, C.; Montaño, S.; Hernández-’Alvarez, A.-J. Unveiling the nutritional spectrum: A comprehensive analysis of protein quality and antinutritional factors in three varieties of quinoa (Chenopodium quinoa Wild). Food Chem. 2024, 24, 101814. [Google Scholar] [CrossRef]
- Abdelmegiud, M.H.; El-Soukkary, F.A.H.; EL-Naggar, E.A.; Abdelsalam, R.R. Physico-Chemical, functional and antioxidant properties of some flours types as gluten-free ingredients compared to wheat flour. Asian J. Appl. Chem. Res. 2021, 10, 21–30. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Özcan, M.M.; Uslu, N.; Mohammed, B.M.; Adiamo, O. The role of roasting on changes in bioactive properties, fatty acid and phenolic compound profiles in the free and bound fraction of black quinoa seeds. Int. J. Food Sci. Technol. 2024, 59, 807–815. [Google Scholar] [CrossRef]
- McCleary, B.V.; McLoughlin, C.; Charmier, L.M.J.; McGeough, P. Measurement of available carbohydrates, digestible, and resistant starch in food ingredients and products. Cereal Chem. 2020, 97, 114–137. [Google Scholar] [CrossRef]
- Miraji, K.F.; Linnemann, A.R.; Fogliano, V.; Laswai, H.S.; Capuano, E. Dry-heat processing at different conditions impact the nutritional composition and in vitro starch and protein digestibility of immature rice-based products. Food Funct. 2021, 12, 7527–7545. [Google Scholar] [CrossRef]
- Ejoh, N.; Onyeulo, C.G. Roasting’s Effects on Proximate and Amino Acid Content of Maize. Indones. J. Agric. Res. 2022, 5, 1–10. [Google Scholar] [CrossRef]
- Diaz-Valencia, Y.K.; Alca, J.J.; Calori-Domingues, M.A.; Zanabria-Galvez, S.J.; Da Cruz, S.H. Nutritional composition, total phenolic compounds and antioxidant activity of quinoa (Chenopodium quinoa Willd.) of different colours. Nov. Biotechnol. Chim. 2018, 17, 74–85. [Google Scholar] [CrossRef]
- Dong, J.; Huang, L.; Chen, W.; Zhu, Y.; Dun, B.; Shen, R. Effect of heat-moisture treatments on digestibility and physicochemical property of whole quinoa flour. Foods 2021, 10, 3042. [Google Scholar] [CrossRef] [PubMed]
- Babiker, E.E.; Uslu, N.; Juhaimi, F.; Ahmed, I.A.M.; Özcan, M.M.; Almusallam, I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT Food Sci. Technol. 2020, 139, 110537. [Google Scholar] [CrossRef]
- Hatamian, M.; Noshad, M.; Abdanan-Mehdizadeh, S.; Barzegar, H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS J. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Tenyang, N.; Ponka, R.; Tiencheu, B.; Tonfack Djikeng, F.; Womeni, H.M. Effect of boiling and oven roasting on some physicochemical properties of sunflower seeds produced in Far North, Cameroon. Food Sci. Nutr. 2022, 10, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, I.A.; Al Juhaimi, F.Y.; Osman, M.A.; Al Maiman, S.A.; Hassan, A.B.; Alqah, H.A.S.; Babiker, E.E.; Ghafoor, K. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT 2020, 131, 109825. [Google Scholar] [CrossRef]
- Nhara, R.B.; Pisa, C.; Chigede, N.; Gwazani, R.; Muteveri, M.; Murimoga, L.; Ruzengwe, F.M.; Nhara, R.B.; Pisa, C.; Chigede, N.; et al. Processing of millets. IntechOpen 2024, 9, 8–9. [Google Scholar] [CrossRef]
- Dhliwayo, T.; Chopera, P.; Matsungo, T.M.; Chidewe, C.; Mukanganyama, S.; Nyakudya, E.; Mtambanengwe, F.; Mapfumo, P.; Nyanga, L.K. Effect of germination and roasting on the proximate, mineral and anti-nutritional factors in finger millet (Eleucine coracana), cowpeas. Afr. J. Food Agric. Nutr. Dev. 2023, 23, 24346–24362. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ali, A.; Ain, H.B.U.; Kausar, S.; Khalil, A.A.; Aadil, R.M.; Zeng, X.A. Bioaccessibility mechanisms, fortification strategies, processing impact on bioavailability, and therapeutic potentials of minerals in cereals. Futur. Foods 2024, 10, 100425. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Arfat, Y.A. Functional, rheological, microstructural and antioxidant properties of quinoa flour in dispersions as influenced by particle size. Food Res. Int. 2019, 116, 302–311. [Google Scholar] [CrossRef]
- Ponessa, G.I.; Such, P.; González, J.A.; Mercado, M.I.; Buedo, S.E.; González, D.A.; Lalla, E.; Freemantle, J.; Daly, M.G. Tolerance of high mountain quinoa to simulated extraplanetary conditions. Changes in surface mineral concentration, seed viability and early growth. Acta Astronaut. 2022, 195, 502–512. [Google Scholar] [CrossRef]
- Goszkiewicz, A.; Kołodziejczyk, E.; Ratajczyk, F. Comparison of microwave and convection method of roasting sunflower seeds and its effect on sensory quality, texture and physicochemical characteristics. Food Struct. 2020, 25, 100144. [Google Scholar] [CrossRef]
- Huynh, N.K.; Nguyen, D.H.M.; Nguyen, H.V.H. Effects of processing on oxalate contents in plant foods: A review. J. Food Compos. Anal. 2022, 112, 104685. [Google Scholar] [CrossRef]
- Tavano, O.L.; Amistá, M.J.M.; Del Ciello, G.; Rodrigues, M.C.M.; Bono Nishida, A.M.; Valadares, L.A.; Siqueira, B.M.; Gomes, R.A.S.; Parolini, M.T.; Silva Junior, S.I. Isolation and evaluation of quinoa (Chenopodium quinoa Willd.) protein fractions. A nutritional and bio-functional approach to the globulin fraction. Curr. Res. Food Sci. 2022, 5, 1028–1037. [Google Scholar] [CrossRef]
- Koriyama, T.; Teranaka, K.; Kumagai, M. Impact of roasting on functional properties of hard-to-cook beans under adverse storage conditions. Foods 2025, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.L.J.; Santos, N.C.; Ferreira, I.L.S.; Pedro, M.S.; Morais, J.R.F.; de Oliveira, A.P.; Ribeiro, V.H.A.; Silva, V.M.A.; de Lima, T.L.B.; de Moraes, M.S.; et al. Impact of treatment with superheated steam on the structural, thermal, and functional characteristics of quinoa starch. Starch Stärke 2023, 75, 2300013. [Google Scholar] [CrossRef]
- Tekgül Barut, Y.; Çalışkan Koç, G.; Rayman Ergün, A.; Bozkır, H.; Pandiselvam, R. Effect of different roasting methods on the proximate by composition, flow properties, amino acid compositions, colour, texture, and sensory profile of the chickpeas. Int. J. Food Sci. Technol. 2023, 58, 482–492. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, X.; Zhang, Z.; Yang, F.; Wei, Y.; Zhang, Z.; Yang, F. Heat treatment of quinoa (Chenopodium quinoa Willd.) albumin: Effect on structural, functional, and in vitro digestion properties. Front. Nutr. 2022, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Minj, J.; Murphy, K.M.; Solverson, P.M.; Rust, B.M.; Carbonero, F. Impact of quinoa and food processing on gastrointestinal health: A narrative review. Crit. Rev. Food Sci. Nutr. 2024, 1–14. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.L.; Wang, J.; Zou, L.; Zhao, X.; Wang, D.; Zhao, Y.; Ge, Z.; Zhang, L.; Qin, P. Evaluation of the nutritional, in vitro protein digestive and bioactive characteristics of a quinoa-based protein beverage. Food Chem. X 2025, 28, 102609. [Google Scholar] [CrossRef]
- Venlet, N.V.; Hettinga, K.A.; Schebesta, H.; Bernaz, N. Perspective: A Legal and Nutritional Perspective on the Introduction of Quinoa-Based Infant and Follow-on Formula in the EU. Adv. Nutr. 2021, 12, 1100. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Effect of malting and roasting of chickpea on functional and nutritional qualities of its protein fractions. Int. J. Food Sci. Technol. 2022, 57, 3990–4000. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Ribéreau, S.; Mondor, M.; Cuevas-Rodríguez, E.O.; Arcand, Y.; Hernández-Alvarez, Á.J. Impact of processing on the in vitro protein quality, bioactive compounds, and antioxidant potential of 10 selected pulses. Legum. Sci. 2021, 2, e88. [Google Scholar] [CrossRef]
- Ghumman, A.; Mudgal, S.; Singh, N.; Ranjan, B.; Kaur, A.; Rana, J.C. Physicochemical, functional and structural characteristics of grains, flour and protein isolates of Indian quinoa lines. Food Res. Int. 2021, 140, 109982. [Google Scholar] [CrossRef]
- Nabubuya, A.; Mugabi, R.; Kaggwa, A.; Ainebyona, P.; Nalugya, R. Influence of roasting on the proximate, functional and sensory properties of jackfruit seeds and amaranth grain composite complementary flours. Tanzania J. Sci. 2022, 48, 156–169. [Google Scholar] [CrossRef]
- Byarugaba, R.; Nabubuya, A.; Muyonga, J.; Mwakha, A. Effects of roasting conditions on the proximate composition and functional properties of common bean (Phaseolus vulgaris) flours. Tanzan. J. Sci. 2023, 49, 546–558. [Google Scholar] [CrossRef]
- Anuhya; Dobhal, N. Evaluation of physical characteristics and impact of germination on functional properties of quinoa (Chenopodium quinoa Willd.). Asian J. Dairy Food Res. 2024, 43. [Google Scholar] [CrossRef]
- Ozolina, K.; Sarenkova, I.; Muizniece-brasava, S. Estimation of roasted and raw faba bean and lentil flour functional properties. Food Nutr. J. 2024, 9, 1–8. [Google Scholar] [CrossRef]

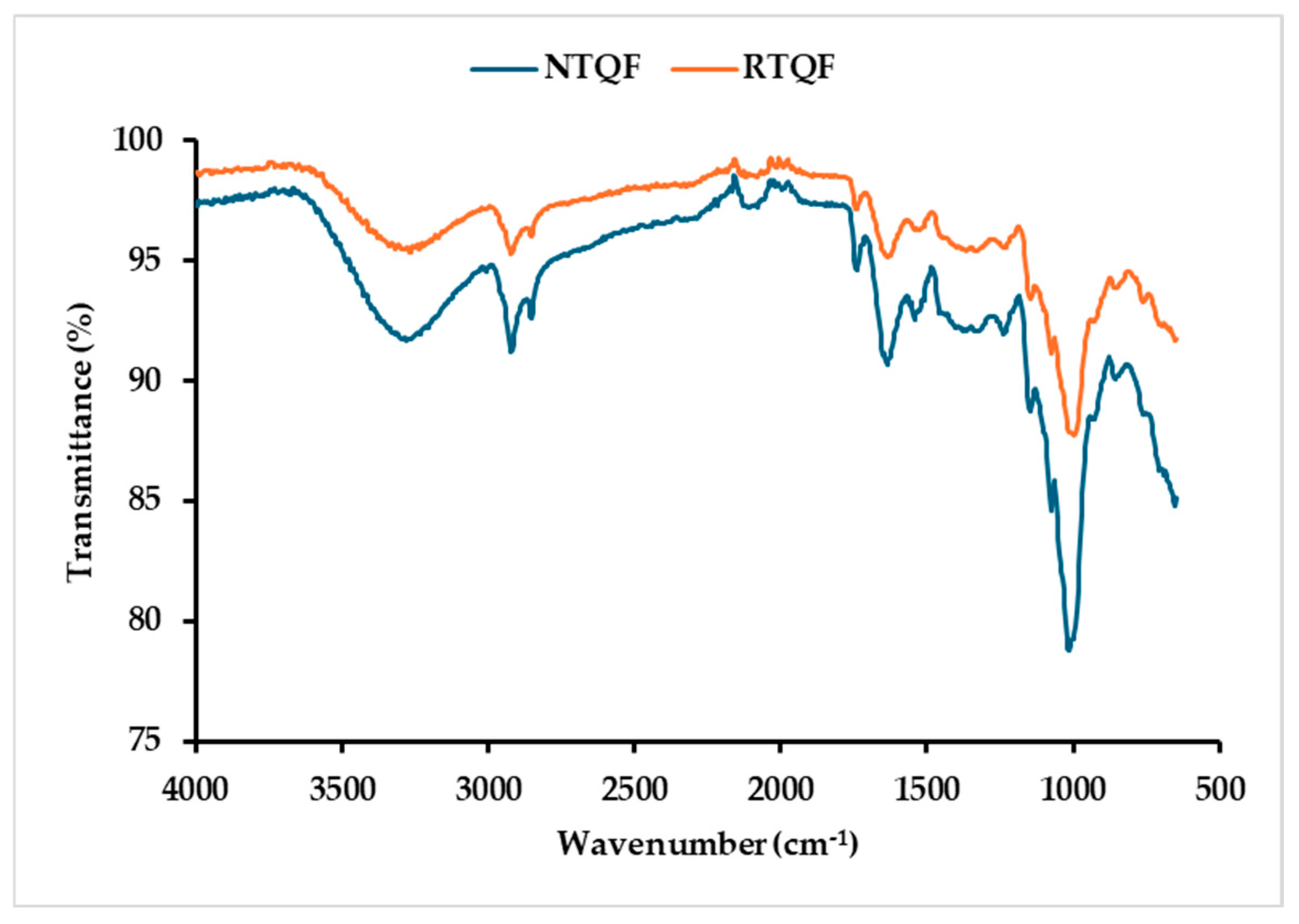

| Parameter | NTQF | RTQF |
|---|---|---|
| Physico-Chemical | ||
| Moisture (%) | 7.99 ± 0.10 a | 4.97 ± 0.05 b |
| Ash (%) | 2.45 ± 0.08 a | 2.54 ± 0.05 a |
| Lipid (%) | 5.59 ± 0.03 a | 5.80 ± 0.15 a |
| Protein (%) | 14.97 ± 0.25 a | 15.10 ± 0.10 a |
| Carbohydrate (%) | 69.01 ± 0.30 b | 71.59 ± 0.25 a |
| Energy (kcal/100 g) | 386.22 ± 0.06 b | 398.97 ± 0.81a |
| Digestible starch (%) | 5.36 ± 0.52 a | 5.48 ± 0.16 a |
| Resistant starch (%) | 1.48 ± 0.14 a | 1.63 ± 0.13 a |
| Total starch (%) | 6.84 ± 0.40 a | 7.11 ± 0.13 a |
| Total dietary fiber (%) | 17.31 ± 0.88 a | 15.04 ± 0.04 b |
| Phytic acid (mg/g) | 2.94 ± 0.10 a | 1.88 ± 0.13 b |
| Oxalate (mg/g) | 5.42 ± 0.33 a | 3.86 ± 0.04 b |
| Saponin (mg/g) | 13.64 ± 0.77 a | 13.36 ± 1.0 a |
| Tannin (mg/g) | 1.35 ± 0.08 a | 1.25 ± 0.02 a |
| TIA (TIU/mg) | 2.93 ± 0.72 a | 2.68 ± 0.57 a |
| Water activity | 0.23 ± 0.00 a | 0.17 ± 0.01 b |
| L* | 68.20 ± 0.26 a | 67.77 ± 0.15 a |
| a* | 7.44 ± 0.21 b | 7.93 ± 0.15 a |
| b* | 31.37 ± 0.12 b | 32.40 ± 0.35 a |
| ΔE* | - | 1.26 ± 0.22 |
| Minerals (mg/kg) | ||
| Ca | 544.50 ± 0.14 a | 495.60 ± 0.14 b |
| K | 10,800.50 ± 0.71 a | 10,250.50 ± 0.71 b |
| Mg | 1993.50 ± 0.71 a | 1891.50 ± 0.71 b |
| Na | 13.07 ± 0.02 b | 13.65 ± 0.01 a |
| P | 4087 ± 0.54 a | 3740 ± 0.85 b |
| S | 1783 ± 0.35 a | 1701.50 ± 0.71 b |
| Cu | 5.68 ± 0.01 a | 5.52 ± 0.01 b |
| Fe | 110.25 ± 0.35 a | 60.38 ± 0.01 b |
| Mn | 26.31 ± 0.04 a | 22.70 ± 0.01 b |
| Zn | 26.26 ± 0.01 a | 25.93 ± 0.01 b |
| Mo | <0.16 | <0.16 |
| Al | 69.61 ± 0.02 a | 11.52 ± 0.01 b |
| B | 10.72 ± 0.01 a | 9.10 ± 0.01 b |
| Thermal Properties | ||
| Ton (°C) | 172.05 ± 1.84 b | 180.73 ± 2.37 a |
| Tp (°C) | 172.19 ± 1.36 b | 181.36 ± 2.66 a |
| Te (°C) | 175.87 ± 0.83 b | 184.76 ± 2.94 a |
| ΔH (J/g) | 152.54 ± 12.30 a | 92.32 ± 8.72 b |
| Fatty Acids (%) | NTQF | RTQF |
|---|---|---|
| Myristic acid (C14:0) | 0.15 ± 0.03 b | 0.21 ± 0.01 a |
| Palmitic acid (C16:0) | 8.15 ± 0.63 b | 9.93 ± 0.16 a |
| Stearic acid (C18:0) | 3.10 ± 0.40 b | 4.04 ± 0.02 a |
| Oleic acid (C18:1) | 40.30 ± 5.19 b | 54.19 ± 1.09 a |
| Linoleic acid (C18:2n-6) | 26.18 ± 2.42 a | 22.81 ± 0.55 a |
| Arachidic acid (C20:0) | 0.50 ± 0.04 a | 0.49 ± 0.01 a |
| Linolenic acid (C18:3n-3) | 3.25 ± 0.46 a | 2.29 ± 0.17 b |
| Docosanoic acid (C22:0) | 0.88 ± 0.05 a | 0.92 ± 0.02 a |
| Erucic acid (C22:1ω9) | 0.38 ± 0.09 a | 0.18 ± 0.02 b |
| Arachidonic (C20:4n-6) | 0.05 ± 0.03 a | 0.02 ± 0.01 b |
| SFA | 16.11 ± 1.19 a | 17.03 ± 0.04 a |
| MUFA | 44.29 ± 4.40 b | 56.74 ± 1.15 a |
| PUFA | 39.60 ± 4.99 a | 26.23 ± 1.18 b |
| Amino Acid (g/100 g) | NTQF | RTQF |
|---|---|---|
| Essential Amino Acids (EAA) | ||
| Histidine (His) | 1.66 ± 0.03 a | 1.55 ± 0.02 b |
| Isoleucine (IIe) | 7.45 ± 0.35 a | 7.39 ± 0.04 a |
| Leucine (Leu) | 6.78 ± 0.10 a | 6.24 ± 0.08 b |
| Lysine (Lys) | 4.08 ± 0.11 a | 4.24 ± 0.13 a |
| Methionine (Met) | 3.35 ± 0.01 a | 2.04 ± 0.04 a |
| Phenylalanine (Phe) | 2.62 ± 0.03 a | 1.79 ± 0.01 b |
| Threonine (Thr) | 2.37 ± 0.04 a | 2.41 ± 0.02 a |
| Tryptophan (Trp) | 0.71 ± 0.03 a | 0.66 ± 0.00 b |
| Valine (Val) | 3.60 ± 0.08 b | 4.26 ± 0.05 a |
| Non-Essential Amino Acids (NEAA) | ||
| Alanine (Ala) | 1.05 ± 0.06 a | 1.05 ± 0.16 a |
| Arginine (Arg) | 1.66 ± 0.53 a | 1.98 ± 0.52 a |
| Aspartic acid (Asx) | 5.07 ± 0.26 a | 3.17 ± 0.46 b |
| Cysteine (Cys) | 0.18 ± 0.00 a | 0.20 ± 0.00 a |
| Glutamic acid (Glx) | 2.51 ± 0.20 a | 1.83 ± 0.00 b |
| Glycine (Gly) | 1.31 ± 0.11 a | 1.12 ± 0.24 a |
| Proline (Pro) | 1.57 ± 0.01 a | 1.38 ± 0.01 b |
| Serine (Ser) | 8.16 ± 0.48 a | 3.78 ± 0.03 b |
| Tyrosine (Tyr) | 1.39 ± 0.02 b | 2.17 ± 0.03 a |
| EAA | 32.63 ± 0.49 a | 30.59 ± 0.31 b |
| NEAA | 22.91 ± 0.65 a | 16.67 ± 0.46 b |
| Total amino acids (TAA) | 55.55 ± 0.15 a | 47.26 ± 0.77 b |
| E/T ratio (%) | 58.75 ± 0.01 b | 64.72 ± 0.00 a |
| Aromatic amino acids (AAA) | 4.73 ± 0.02 a | 4.63 ± 0.04 a |
| Branched chain amino acids (BCAA) | 17.84 ± 0.52 a | 17.90 ± 0.09 a |
| Fischer’s ratio | 3.77 ± 0.10 b | 3.87 ± 0.02 a |
| Hydrophobic amino acids (BAA) | 26.88 ± 0.36 a | 25.93 ± 0.55 b |
| Hydrophilic amino acids (PAA) | 27.15 ± 0.52 b | 24.81 ± 0.31 a |
| Sulfur-containing amino acids (SAA) | 3.54 ± 0.01 a | 2.24 ± 0.04 b |
| IVPD | 76.30 ± 2.84 a | 79.41 ± 1.81 a |
| Amino Acid (mg/g Protein) | Reference AA Profile 1 | NTQF | RTQF | |||
|---|---|---|---|---|---|---|
| 0.5 y (Infants) | 1–2 y (Preschoolers) | 11–14 y (Adolescents) | >18 y (Adults) | |||
| His | 20 | 18 | 16 | 15 | 16.56 ± 0.30 a | 15.48 ± 0.15 b |
| Ile | 32 | 31 | 30 | 30 | 74.52 ± 3.47 a | 73.93 ± 0.36 a |
| Leu | 66 | 63 | 60 | 59 | 67.81 ± 0.97 a | 62.44 ± 0.77 b |
| Lys | 57 | 52 | 48 | 45 | 40.85 ± 1.06 a | 42.45 ± 1.33 a |
| SAA | 28 | 26 | 23 | 22 | 35.36 ± 0.06 a | 22.37 ± 0.44 b |
| AAA | 52 | 46 | 41 | 38 | 40.14 ± 0.44 a | 39.65 ± 0.40 a |
| Thr | 31 | 27 | 25 | 23 | 23.67 ± 0.40 a | 24.06 ± 0.17 a |
| Trp | 8.5 | 7.4 | 6.5 | 6.0 | 7.13 ± 0.28 a | 6.63 ± 0.01 b |
| Val | 43 | 42 | 40 | 39 | 36.04 ± 0.81 b | 42.60 ± 0.47 a |
| Animo Acid Score | ||||||
| His | - | - | - | - | 0.83 | 0.77 |
| Ile | - | - | - | - | 2.33 | 2.31 |
| Leu | - | - | - | - | 1.03 | 0.95 |
| Lys | - | - | - | - | 0.72 | 0.74 |
| Met + Cys | - | - | - | - | 1.31 | 0.83 |
| Phe + Tyr | - | - | - | - | 0.77 | 0.76 |
| Thr | - | - | - | - | 0.76 | 0.78 |
| Trp | - | - | - | - | 0.84 | 0.78 |
| Val | - | - | - | - | 0.84 | 0.99 |
| EAAI (Infants) | - | - | - | - | 0.99 ± 0.01 a | 0.93 ± 0.01 b |
| p-BV (Infants) | - | - | - | - | 0.96 ± 0.01 a | 0.89 ± 0.01 b |
| IVPDCAAS (Infants) | - | - | - | - | 0.55 ± 0.01 b | 0.59 ± 0.02 a |
| His | - | - | - | - | 0.92 | 0.86 |
| Ile | - | - | - | - | 2.40 | 2.38 |
| Leu | - | - | - | - | 1.08 | 0.99 |
| Lys | - | - | - | - | 0.79 | 0.82 |
| Met + Cys | - | - | - | - | 1.41 | 0.89 |
| Phe + Tyr | - | - | - | - | 0.87 | 0.86 |
| Thr | - | - | - | - | 0.88 | 0.89 |
| Trp | - | - | - | - | 1.02 | 0.95 |
| Val | - | - | - | - | 0.88 | 1.04 |
| EAAI (Preschool children) | - | - | - | - | 1.08 ± 0.01 a | 1.01 ± 0.01 a |
| p-BV (Preschool children) | - | - | - | - | 1.06 ± 0.01 a | 0.99 ± 0.01 b |
| IVPDCAAS (Preschool children) | - | - | - | - | 0.60 ± 0.02 a | 0.65 ± 0.02 a |
| His | - | - | - | - | 1.03 | 0.97 |
| Ile | - | - | - | - | 2.48 | 2.46 |
| Leu | - | - | - | - | 1.13 | 1.04 |
| Lys | - | - | - | - | 0.85 | 0.88 |
| Met + Cys | - | - | - | - | 1.54 | 0.97 |
| Phe + Tyr | - | - | - | - | 0.98 | 0.97 |
| Thr | - | - | - | - | 0.95 | 0.96 |
| Trp | - | - | - | - | 1.10 | 1.02 |
| Met + Cys | - | - | - | - | 0.90 | 1.07 |
| EAAI (Adolescents) | - | - | - | - | 1.17 ± 0.01 a | 1.10 ± 0.01 b |
| p-BV (Adolescents) | - | - | - | - | 1.16 ± 0.01 a | 1.08 ± 0.01 b |
| IVPDCAAS (Adolescents) | - | - | - | - | 0.65 ± 0.02 b | 0.70 ± 0.02 a |
| His | - | - | - | - | 1.10 | 1.03 |
| Ile | - | - | - | - | 2.48 | 2.46 |
| Leu | - | - | - | - | 1.15 | 1.06 |
| Lys | - | - | - | - | 0.91 | 0.94 |
| Met + Cys | - | - | - | - | 1.61 | 1.02 |
| Phe + Tyr | - | - | - | - | 1.06 | 1.04 |
| Thr | - | - | - | - | 1.03 | 1.05 |
| Trp | - | - | - | - | 1.19 | 1.11 |
| Val | - | - | - | - | 0.92 | 1.09 |
| EAAI (Adults) | - | - | - | - | 1.24 ± 0.01 a | 1.16 ± 0.01 b |
| p-BV (Adults) | - | - | - | - | 1.23 ± 0.01 a | 1.15 ± 0.01 b |
| IVPDCAAS (Adults) | - | - | - | - | 0.69 ± 0.02 b | 0.75 ± 0.02 a |
| Parameter | NTQF | RTQF |
|---|---|---|
| WAC (g/g) | 2.02 ± 0.08 b | 2.51 ± 0.13 a |
| OAC (g/g) | 1.81 ± 0.02 b | 1.86 ± 0.02 a |
| EC (%) | 84.77 ± 0.95 a | 44.44 ± 3.38 b |
| ES (%) | 59.89 ± 0.94 a | 33.79 ± 3.51 b |
| FC (%) | 37.67 ± 2.52 a | 24.50 ± 1.80 b |
| SC (%) | 3.72 ± 0.21 a | 3.06 ± 0.11 b |
| LGC—Flour Concentration (%, w/v) | ||
| 2% | - | - |
| 4% | - | - |
| 6% | - | - |
| 8% | - | - |
| 10% | + | - |
| 12% | + | + |
| 14% | ++ | + |
| 16% | +++ | ++ |
| 18% | +++ | +++ |
| 20% | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukunzi, Y.; Aryee, A.N.A. Effect of Dry Roasting on the Physicochemical, Nutritional, and Techno-Functional Properties of Tri-Color Quinoa Flours. Foods 2025, 14, 3237. https://doi.org/10.3390/foods14183237
Mukunzi Y, Aryee ANA. Effect of Dry Roasting on the Physicochemical, Nutritional, and Techno-Functional Properties of Tri-Color Quinoa Flours. Foods. 2025; 14(18):3237. https://doi.org/10.3390/foods14183237
Chicago/Turabian StyleMukunzi, Yvette, and Alberta N. A. Aryee. 2025. "Effect of Dry Roasting on the Physicochemical, Nutritional, and Techno-Functional Properties of Tri-Color Quinoa Flours" Foods 14, no. 18: 3237. https://doi.org/10.3390/foods14183237
APA StyleMukunzi, Y., & Aryee, A. N. A. (2025). Effect of Dry Roasting on the Physicochemical, Nutritional, and Techno-Functional Properties of Tri-Color Quinoa Flours. Foods, 14(18), 3237. https://doi.org/10.3390/foods14183237






