Current Status and Future Prospects on Nanodelivery Systems Targeting the Small Intestine for Absorption of Bioactive Substances
Abstract
1. Introduction
2. Adsorption Barriers
2.1. pH and Enzymes in the Stomach and Small Intestine
2.2. Mucus
2.3. Intestinal Epithelial Cells
3. Passive Targeted Nanodelivery Systems
3.1. pH-Sensitive Nanodelivery Systems
3.2. Enzyme-Responsive Nanodelivery Systems
3.3. Mucoadhesive Nanodelivery Systems
3.4. Mucus-Penetrating Nanodelivery Systems
3.5. Composite Nanodelivery Systems
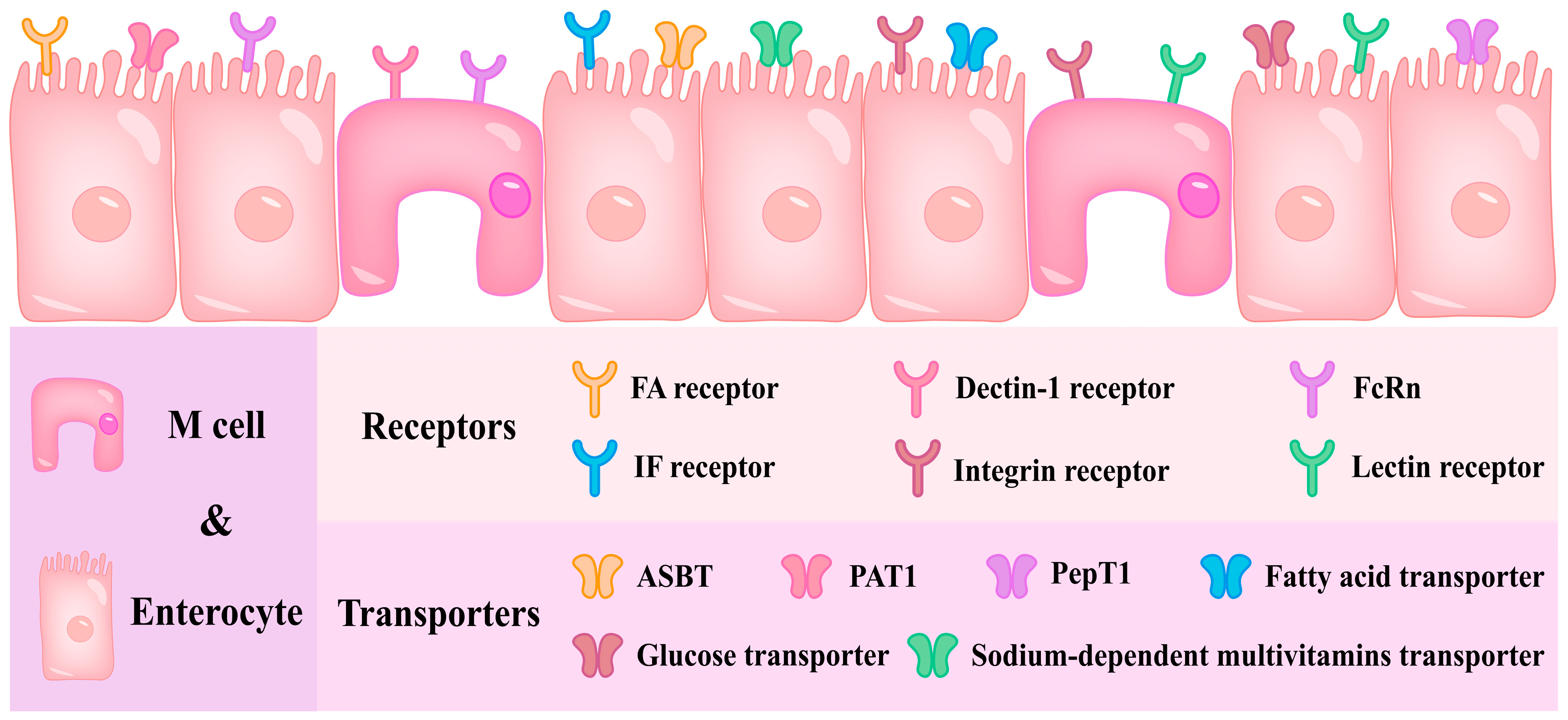
4. Active Targeted Nanodelivery Systems
4.1. Single Ligand–Receptor-Mediated Nanodelivery Systems
4.2. Single Ligand–Transporter-Mediated Nanodelivery Systems
4.3. Multiple Ligand–Receptor/Transporter-Mediated Nanodelivery Systems
5. Forms and Efficiencies of Passive/Active Targeted Nanodelivery Systems Entering Systemic Circulation
6. Clinical Trials
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Chen, G.; Zhang, H.; Shang, L.; Zhao, Y. Bioinspired oral delivery devices. Nat. Rev. Bioeng. 2023, 1, 208–225. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zheng, Z.; Langer, R.; Jaklenec, A. Advanced oral delivery systems for nutraceuticals. Adv. Healthc. Mater. 2025, 2500271. [Google Scholar] [CrossRef] [PubMed]
- Venkidasamy, B.; Shelar, A.; Dhanapal, A.R.; Nile, A.S.; Patil, R.; Zhang, Y.; Kuksal, K.; Nile, S.H. Emerging biopolymer nanocarriers for controlled and protective delivery of food bioactive compounds–current status and future perspective. Food Hydrocoll. 2025, 160, 110769. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Anal, A.K.; Boonlao, N.; Ruktanonchai, U.R. Emulsion systems stabilized with biopolymers to enhance oral bioaccessibility and bioavailability of lipophilic bioactive compounds. Curr. Opin. Food Sci. 2023, 50, 101001. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Q.; Dong, Z.; Meng, T.; Hu, F.; Wang, J.; Yuan, H. Nanocarriers transport across the gastrointestinal barriers: The contribution to oral bioavailability via blood circulation and lymphatic pathway. Adv. Drug Deliv. Rev. 2023, 203, 115130. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Pan, Y.; Yang, H.; Han, J.; Liu, J.; Liu, W. Specific surface modification of liposomes for gut targeting of food bioactive agents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3685–3706. [Google Scholar] [CrossRef]
- Peng, X.; Li, D.; Liu, Y. Construction of efficient carotenoid delivery vehicles based on the intestinal epithelial transport pathway: Current applications and future trends. Trends Food Sci. Technol. 2024, 147, 104473. [Google Scholar] [CrossRef]
- Jia, L.; Wang, W.; Zhao, H.; Ding, X.; Zheng, M.; Cai, D.; Wang, Y.; Wang, Z.; Liu, H. Innovative nano delivery systems for astaxanthin: Enhancing stability, bioavailability, and targeted therapeutic applications. J. Agric. Food Chem. 2025, 73, 3286–3304. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 381–406. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, Y.; Pu, F.; Yang, C.; Xiao, X.; Du, H.; He, J.; Lu, S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chem. 2024, 430, 137115. [Google Scholar] [CrossRef] [PubMed]
- Premathilaka, R.; Rashidinejad, A.; Golding, M.; Singh, J. Oral delivery of hydrophobic flavonoids and their incorporation into functional foods: Opportunities and challenges. Food Hydrocoll. 2022, 128, 107567. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.-H.; Yang, X.; Hu, J.-Q.; Zhu, Y.-Z.; Yan, P.-Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhao, D.; Liu, X.; Liu, B.; Li, Y. The stomach, small intestine, and colon-specific gastrointestinal tract delivery systems for bioactive nutrients. Adv. Colloid Interface Sci. 2025, 341, 103503. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhang, Z.; Deng, B.; Liu, J.; Wang, L.; Liu, H.; Koo, S.; Chen, S.; Li, Y.; Yaremenko, A.V.; et al. Oral drug delivery platforms for biomedical applications. Mater. Today 2023, 62, 296–326. [Google Scholar] [CrossRef]
- Suzuki, K.; Kim, K.S.; Bae, Y.H. Long-term oral administration of Exendin-4 to control type 2 diabetes in a rat model. J. Control. Release 2019, 294, 259–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, X.; Nie, D.; Liu, C.; Gan, Y. Ligand-modified nanocarriers for oral drug delivery: Challenges, rational design, and applications. J. Control. Release 2022, 352, 813–832. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Wang, Y.; Ashaolu, T.J.; Sharma, M.; Rawal, S.; Patel, K.; Askari, G.; Javanmard, S.H.; Rostamabadi, H. Biopolymer nanovehicles for oral delivery of natural anticancer agents. Adv. Funct. Mater. 2023, 33, 2209419. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral delivery of biologics for precision medicine. Adv. Mater. 2020, 32, 1901935. [Google Scholar] [CrossRef]
- Ren, A.; Hu, J.; Qin, C.; Xia, N.; Yu, M.; Xu, X.; Yang, H.; Han, M.; Zhang, L.; Ma, L. Oral administration microrobots for drug delivery. Bioact. Mater. 2024, 39, 163–190. [Google Scholar] [CrossRef]
- Chen, C.; Beloqui, A.; Xu, Y. Oral nanomedicine biointeractions in the gastrointestinal tract in health and disease. Adv. Drug Deliv. Rev. 2023, 203, 115117. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, H.; Song, B.; Shi, Y.; Cao, Z. Recent advances in oral insulin delivery technologies. J. Control. Release 2024, 366, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bajka, B.H.; Rigby, N.M.; Cross, K.L.; Macierzanka, A.; Mackie, A.R. The influence of small intestinal mucus structure on particle transport ex vivo. Colloids Surf. B Biointerfaces 2015, 135, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Huang, Y. Nanoscale technology of mucoadhesive interactions. Adv. Drug Deliv. Rev. 2004, 56, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Duffy, C.V.; David, L.; Crouzier, T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 2015, 20, 51–59. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, S.; Guo, Y.; Han, Y.; Ma, C.; Li, R.; Yang, X. Classification and design strategies of polysaccharide-based nano-nutrient delivery systems for enhanced bioactivity and targeted delivery: A review. Int. J. Biol. Macromol. 2024, 256, 128440. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Z.; Liu, K.; Lu, Y.; Wu, W.; Qi, J.; Chen, Z. Targeting strategies of oral nano-delivery systems for treating inflammatory bowel disease. Int. J. Pharm. 2021, 600, 120461. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Louisthelmy, R.; Maisel, K. Mechanisms of nanoparticle transport across intestinal tissue: An oral delivery perspective. ACS Nano 2023, 17, 13044–13061. [Google Scholar] [CrossRef]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Tran, H.; ElSayed, M.E.H. Progress and limitations of oral peptide delivery as a potentially transformative therapy. Expert Opin. Drug Deliv. 2022, 19, 163–178. [Google Scholar] [CrossRef]
- Song, J.G.; Lee, S.H.; Han, H.-K. Development of an M cell targeted nanocomposite system for effective oral protein delivery: Preparation, in vitro and in vivo characterization. J. Nanobiotechnol. 2021, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, J.; Brettner, F.E.B.; Gier, S.; Vogel-Kindgen, S.; Windbergs, M. Unlocking the potential of microfold cells for enhanced permeation of nanocarriers in oral drug delivery. Eur. J. Pharm. Biopharm. 2024, 202, 114408. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Fukuyama, S. NALT- versus PEYER’S-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-B.; Lin, Y.-J.; Chen, K.-H.; Luo, P.-K.; Chuang, S.-H.; Yu, Y.-T.; Tai, H.-M.; Chen, C.-T.; Lin, K.-J.; Sung, H.-W. Engineering nano- and microparticles as oral delivery vehicles to promote intestinal lymphatic drug transport. Adv. Mater. 2021, 33, 2104139. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, D.; Wu, C.; Zhao, W. Intestinal absorption of bioactive oligopeptides: Paracellular transport and tight junction modulation. Food Funct. 2024, 15, 6274–6288. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, Y.; Cao, Y.; Sun, C.; Lu, W.; Fang, Y. Advances in the interaction between food-derived nanoparticles and the intestinal barrier. J. Agric. Food Chem. 2024, 72, 3291–3301. [Google Scholar] [CrossRef]
- Ray, S.; Moonshi, S.S.; Ta, H.T. “Therapies through gut:” Targeted drug delivery for non-gastrointestinal diseases by oral administration. Adv. Healthc. Mater. 2025, 14, 2403162. [Google Scholar] [CrossRef] [PubMed]
- Zaiki, Y.; Lim, L.Y.; Wong, T.W. Critical material designs for mucus- and mucosa-penetrating oral insulin nanoparticle development. Int. Mater. Rev. 2023, 68, 121–139. [Google Scholar] [CrossRef]
- He, Y.; Cheng, M.; Yang, R.; Li, H.; Lu, Z.; Jin, Y.; Feng, J.; Tu, L. Research progress on the mechanism of nanoparticles crossing the intestinal epithelial cell membrane. Pharmaceutics 2023, 15, 1816. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef]
- Schouten, P.J.C.; Soto-Aguilar, D.; Aldalbahi, A.; Ahamad, T.; Alzahly, S.; Fogliano, V. Design of sporopollenin-based functional ingredients for gastrointestinal tract targeted delivery. Curr. Opin. Food Sci. 2022, 44, 100809. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Mu, T. Materials and structure of polysaccharide-based delivery carriers for oral insulin: A review. Carbohydr. Polym. 2024, 323, 121364. [Google Scholar] [CrossRef]
- Prata, A.S.; Nascimento, R.F.; Grosso, C.R.F. Designing polymeric interactions toward smart particles. Curr. Opin. Food Sci. 2022, 46, 100867. [Google Scholar] [CrossRef]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in oral drug delivery systems: Challenges and opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Deng, L.; Dong, H.; Dong, A.; Zhang, J. A strategy for oral chemotherapy via dual pH-sensitive polyelectrolyte complex nanoparticles to achieve gastric survivability, intestinal permeability, hemodynamic stability and intracellular activity. Eur. J. Pharm. Biopharm. 2015, 97, 107–117. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Amiri, H.; Guillemin, G.J.; Gupta, V.K.; Rajaei, A.; Yang, Y.; Peng, W.; Pan, J.; Aghbashlo, M.; et al. Current and emerging applications of saccharide-modified chitosan: A critical review. Biotechnol. Adv. 2023, 66, 108172. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, C.; Liu, L.; Zhao, L.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Pan, B.; Tang, X. Progress and prospects of polysaccharide-based nanocarriers for oral delivery of proteins/peptides. Carbohydr. Polym. 2023, 312, 120838. [Google Scholar] [CrossRef]
- Diep, E.; Schiffman, J.D. Ethanol-free cross-linking of alginate nanofibers enables controlled release into a simulated gastrointestinal tract model. Biomacromolecules 2023, 24, 2908–2917. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kitts, D.D.; Dolati, D.; Pratap-Singh, A.; Singh, A. Enhancing resveratrol bioavailability and intestinal uptake using an oil-based blueberry extract formulated with Chitosan/PEG containing nanoparticles. Food Hydrocoll. 2024, 156, 110373. [Google Scholar] [CrossRef]
- Xie, C.; Huang, M.; Ying, R.; Wu, X.; Hayat, K.; Shaughnessy, L.K.; Tan, C. Olive pectin-chitosan nanocomplexes for improving stability and bioavailability of blueberry anthocyanins. Food Chem. 2023, 417, 135798. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Tie, S.; Hou, S.; Wang, H.; Song, Y.; Rai, R.; Tan, M. Facile synthesis of nano-nanocarriers from chitosan and pectin with improved stability and biocompatibility for anthocyanins delivery: An in vitro and in vivo study. Food Hydrocoll. 2020, 109, 106114. [Google Scholar] [CrossRef]
- Shen, R.; Yang, X.; Lin, D. PH sensitive double-layered emulsions stabilized by bacterial cellulose nanofibers/soy protein isolate/chitosan complex enhanced the bioaccessibility of curcumin: In vitro study. Food Chem. 2023, 402, 134262. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wang, Y.; Zhang, F.; Wei, Y.; Li, N.; Xu, Y. Preparation, stability, and antibacterial activity of carboxymethylated Anemarrhena asphodeloides polysaccharide-chitosan nanoparticles loaded curcumin. Int. J. Biol. Macromol. 2024, 264, 130787. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Q.; Qiu, C.; Wang, J.; Jin, Z.; Hu, Y. Encapsulation and controlled delivery of curcumin by self-assembled cyclodextrin succinate/chitosan nanoparticles. Food Hydrocoll. 2024, 157, 110465. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Wang, D.; Xu, Y.; Wang, C.-Y. Effects of coating layers chitosan/pectin on lipid stability and in vitro digestion of astaxanthin-loaded multilayer emulsions. LWT 2023, 173, 114282. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Zhou, L.; Gao, J.; Zheng, H.; Lin, H.; Zhu, G.; Qin, X.; Cao, W. Carboxymethyl cellulose and carboxymethyl chitosan-based composite nanogel as a stable delivery vehicle for oyster peptides: Characterization, absorption and transport mechanism. Food Chem. 2024, 442, 138464. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; He, Y.; Fan, D.; Wu, Z. Preparation of pH-sensitive chitosan-deoxycholic acid-sodium alginate nanoparticles loaded with ginsenoside Rb(1) and its controlled release mechanism. Int. J. Biol. Macromol. 2023, 234, 123736. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Dong, Y.; Wang, W.; Wang, J.; Wu, Z.; Wang, W.; He, Y.; Bao, G. Preparation of pH-sensitive carboxymethyl chitosan nanoparticles loaded with ginsenoside Rb1 and evaluation of drug release in vitro. Int. J. Biol. Macromol. 2024, 267, 131487. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Jung, H.-S.; Chang, P.-S. Stimuli-responsive polymer-complexed liposome nanocarrier provides controlled release of biomolecules. Food Hydrocoll. 2022, 125, 107397. [Google Scholar] [CrossRef]
- Liu, K.; Huang, R.L.; Zha, X.Q.; Li, Q.M.; Pan, L.H.; Luo, J.P. Encapsulation and sustained release of curcumin by a composite hydrogel of lotus root amylopectin and chitosan. Carbohydr. Polym. 2020, 232, 115810. [Google Scholar] [CrossRef]
- Martins, J.P.; D’Auria, R.; Liu, D.; Fontana, F.; Ferreira, M.P.A.; Correia, A.; Kemell, M.; Moslova, K.; Mäkilä, E.; Salonen, J.; et al. Engineered multifunctional albumin-decorated porous silicon nanoparticles for FcRn translocation of insulin. Small 2018, 14, 1800462. [Google Scholar] [CrossRef]
- Dai, J.; Nagai, T.; Wang, X.; Zhang, T.; Meng, M.; Zhang, Q. pH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int. J. Pharm. 2004, 280, 229–240. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Tatham, L.M.; Owen, A.; Rannard, S.; McDonald, T.O. Layer by layer self-assembly for coating a nanosuspension to modify drug release and stability for oral delivery. Food Hydrocoll. 2023, 144, 108908. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, C.; Liu, K.; Wang, Z.; Zhang, X. Enzyme-responsive polymeric supra-amphiphiles formed by the complexation of chitosan and ATP. Langmuir 2012, 28, 14562–14566. [Google Scholar] [CrossRef]
- Leichner, C.; Jelkmann, M.; Prüfert, F.; Laffleur, F.; Bernkop-Schnürch, A. Intestinal enzyme delivery: Chitosan/tripolyphosphate nanoparticles providing a targeted release behind the mucus gel barrier. Eur. J. Pharm. Biopharm. 2019, 144, 125–131. [Google Scholar] [CrossRef]
- Huang, J.; Shu, Q.; Wang, L.; Wu, H.; Wang, A.Y.; Mao, H. Layer-by-layer assembled milk protein coated magnetic nanoparticle enabled oral drug delivery with high stability in stomach and enzyme-responsive release in small intestine. Biomaterials 2015, 39, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, Y.; Huang, J.; Liu, B.; Li, X.; Tian, J.; de Vries, R.; Li, B.; Li, Y. Optimizing anthocyanin oral delivery: Effects of food biomacromolecule types on nanocarrier performance for enhanced bioavailability. Food Chem. 2024, 454, 139682. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Qiu, C.; Long, J.; Jin, Z. Encapsulation of polyphenols in protein-based nanoparticles: Preparation, properties, and applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 11341–11355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, W.; Jiang, J.; Khan, M.A.; Wusigale; Liang, L. A comprehensive review of protein-based carriers with simple structures for the co-encapsulation of bioactive agents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2017–2042. [Google Scholar] [CrossRef]
- Zeng, Z.; Deng, S.; Liu, Y.; Li, C.; Fang, Z.; Hu, B.; Chen, H.; Wang, C.; Chen, S.; Wu, W.; et al. Targeting transportation of curcumin by soybean lipophilic protein nano emulsion: Improving its bioaccessibility and regulating intestinal microorganisms in mice. Food Hydrocoll. 2023, 142, 108781. [Google Scholar] [CrossRef]
- Wang, L.; Jia, W.; Yang, Q.; Cai, H.; Zhao, X. Casein nanoparticles as oral delivery carriers for improved bioavailability and hypoglycemic activity of apigenin. Food Hydrocoll. 2024, 146, 109194. [Google Scholar] [CrossRef]
- Chang, R.; Liu, B.; Wang, Q.; Zhang, J.; Yuan, F.; Zhang, H.; Chen, S.; Liang, S.; Li, Y. The encapsulation of lycopene with α-lactalbumin nanotubes to enhance their anti-oxidant activity, viscosity and colloidal stability in dairy drink. Food Hydrocoll. 2022, 131, 107792. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Jia, F.; Jiang, N.; Wang, C.; Sun, R.; Ma, Y. Construction of quaternary ammonium chitosan-coated protein nanoparticles as novel delivery system for curcumin: Characterization, stability, antioxidant activity and bio-accessibility. Food Chem. 2024, 455, 139923. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, C.; Xiao, Y.; Zhang, J.; Yu, J.; Li, X.; Hamid, N.; Sun, A. Enhanced stability and bioavailability of mulberry anthocyanins through the development of sodium caseinate-konjac glucomannan nanoparticles. Food Chem. 2024, 439, 138150. [Google Scholar] [CrossRef]
- He, J.-R.; Zhu, J.-J.; Yin, S.-W.; Yang, X.-Q. Bioaccessibility and intracellular antioxidant activity of phloretin embodied by gliadin/sodium carboxymethyl cellulose nanoparticles. Food Hydrocoll. 2022, 122, 107076. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Fan, S.; Ma, J.; Yan, Y.; Wang, M.; Tang, X.; Lv, P.; Zhang, Y. Targeted delivery of food functional ingredients in precise nutrition: Design strategy and application of nutritional intervention. Crit. Rev. Food Sci. Nutr. 2023, 64, 7854–7877. [Google Scholar] [CrossRef]
- Xiang, C.; Gao, J.; Ye, H.; Ren, G.; Ma, X.; Xie, H.; Fang, S.; Lei, Q.; Fang, W. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Wen, J.; Sui, Y.; Shi, J.; Xiong, T.; Cai, F.; Mei, X. Nanoemulsions base on the Rice bran albumin-sweet potato leaf polyphenol-dextran complexes: Interaction mechanisms, stability and Astaxanthin release behaviour. Food Chem. 2025, 475, 143276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, X.; Luo, X.; Ding, J.; Fan, F.; Li, P.; Shen, X.; Fang, Y. Encapsulation of selenium-containing peptides in xanthan gum-lysozyme nanoparticles as a powerful gastrointestinal delivery system. Food Res. Int. 2022, 156, 111351. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef]
- Urango, A.C.M.; Meireles, M.A.A.; Silva, E.K. Maillard conjugates produced from proteins and prebiotic dietary fibers: Technological properties, health benefits and challenges. Trends Food Sci. Technol. 2024, 147, 104438. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Liu, R.; Su, W.; Song, Y.; Tan, M. Preparation of lutein nanoparticles by glycosylation of saccharides and casein for protecting retinal pigment epithelial cells. J. Agric. Food Chem. 2024, 72, 6347–6359. [Google Scholar] [CrossRef]
- Hussain, A.; Hussain, M.; Ashraf, W.; Karim, A.; Muhammad Aqeel, S.; Khan, A.; Hussain, A.; Khan, S.; Lianfu, Z. Preparation, characterization and functional evaluation of soy protein isolate-peach gum conjugates prepared by wet heating Maillard reaction. Food Res. Int. 2024, 192, 114681. [Google Scholar] [CrossRef]
- Zhong, L.; Tian, J.; Hu, Q.; Zhao, L.; Zhan, Q.; Zhao, M. Mitochondria-targeted nanoparticles based on glycated oat protein for enhanced curcumin bioavailability and antioxidant activity. Food Biosci. 2024, 60, 104386. [Google Scholar] [CrossRef]
- Zhong, L.; Xu, J.; Hu, Q.; Zhan, Q.; Ma, N.; Zhao, M.; Zhao, L. Improved bioavailability and antioxidation of β-carotene-loaded biopolymeric nanoparticles stabilized by glycosylated oat protein isolate. Int. J. Biol. Macromol. 2024, 263, 130298. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bai, X.; Liu, X.; Liu, F. Enhanced functional properties of pea protein isolate microgel particles modified with sodium alginate: Mixtures and conjugates. Food Chem. 2024, 441, 138358. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Xie, Y.; Li, T.; Wang, Y.; Zhang, X.; Xia, B.; Huang, J.; Wang, S.; Dong, W. Ultra-stable pickering emulsion stabilized by anisotropic pea protein isolate-fucoidan conjugate particles through Maillard reaction. Int. J. Biol. Macromol. 2024, 264, 130589. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Shao, J.; Yue, X.; Li, M. Maillard reaction-based conjugates as carrier strategies for delivery of bioactive compounds: A review. Curr. Opin. Food Sci. 2025, 61, 101260. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, H.; McClements, D.J. Application of static in vitro digestion models for assessing the bioaccessibility of hydrophobic bioactives: A review. Trends Food Sci. Technol. 2022, 122, 314–327. [Google Scholar] [CrossRef]
- Butnarasu, C.; Petrini, P.; Bracotti, F.; Visai, L.; Guagliano, G.; Fiorio Pla, A.; Sansone, E.; Petrillo, S.; Visentin, S. Mucosomes: Intrinsically mucoadhesive glycosylated mucin nanoparticles as multi-drug delivery platform. Adv. Healthc. Mater. 2022, 11, 2200340. [Google Scholar] [CrossRef]
- Spleis, H.; Sandmeier, M.; Claus, V.; Bernkop-Schnürch, A. Surface design of nanocarriers: Key to more efficient oral drug delivery systems. Adv. Colloid Interface Sci. 2023, 313, 102848. [Google Scholar] [CrossRef]
- del Castillo-Santaella, T.; Aguilera-Garrido, A.; Galisteo-González, F.; Gálvez-Ruiz, M.J.; Molina-Bolívar, J.A.; Maldonado-Valderrama, J. Hyaluronic acid and human/bovine serum albumin shelled nanocapsules: Interaction with mucins and in vitro digestibility of interfacial films. Food Chem. 2022, 383, 132330. [Google Scholar] [CrossRef]
- Ho, D.-K.; Frisch, S.; Biehl, A.; Terriac, E.; De Rossi, C.; Schwarzkopf, K.; Lautenschläger, F.; Loretz, B.; Murgia, X.; Lehr, C.-M. Farnesylated glycol chitosan as a platform for drug delivery: Synthesis, characterization, and investigation of mucus–particle interactions. Biomacromolecules 2018, 19, 3489–3501. [Google Scholar] [CrossRef]
- Hua, Y.; Wei, Z.; Xue, C. Chitosan and its composites-based delivery systems: Advances and applications in food science and nutrition sector. Crit. Rev. Food Sci. Nutr. 2021, 63, 4579–4598. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Rimsueb, N.; Jantimaporn, A.; Janyaphisan, T.; Woraprayote, W.; Visessanguan, W.; Ruktanonchai, U.R. Cationic liposome of hen egg white lysozyme for enhanced its stability, activity and accessibility in gastro-intestinal tract. Food Biosci. 2023, 53, 102470. [Google Scholar] [CrossRef]
- Andretto, V.; Taurino, G.; Guerriero, G.; Guérin, H.; Lainé, E.; Bianchi, M.G.; Agusti, G.; Briançon, S.; Bussolati, O.; Clayer-Montembault, A.; et al. Nanoemulsions Embedded in Alginate Beads as Bioadhesive Nanocomposites for Intestinal Delivery of the Anti-Inflammatory Drug Tofacitinib. Biomacromolecules 2023, 24, 2892–2907. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Garrido, A.; Molina-Bolívar, J.A.; Gálvez-Ruiz, M.J.; Galisteo-González, F. Mucoadhesive properties of liquid lipid nanocapsules enhanced by hyaluronic acid. J. Mol. Liq. 2019, 296, 111965. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Kanika; Ahmad, A.; Kumar, A.; Rahul; Mishra, R.K.; Ali, N.; Navik, U.; Parvez, S.; Khan, R. Leveraging thiol-functionalized biomucoadhesive hybrid nanoliposome for local therapy of ulcerative colitis. Biomaterials 2025, 312, 122747. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and current trends in the synthesis of novel polymers with enhanced mucoadhesive properties. Macromol. Biosci. 2019, 19, 1900194. [Google Scholar] [CrossRef]
- Fang, L.; Wang, L.; Yao, Y.; Zhang, J.; Wu, X.; Li, X.; Wang, H.; Zhang, X.; Gong, X.; Chang, J. Micro- and nano-carrier systems: The non-invasive and painless local administration strategies for disease therapy in mucosal tissues. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 153–171. [Google Scholar] [CrossRef]
- Laffleur, F.; Netsomboon, K.; Bernkop-Schnürch, A.; Westmeier, D.; Stauber, R.H.; Docter, D. Comprehensive mucoadhesive study of anionic polymers and their derivate. Eur. Polym. J. 2017, 93, 314–322. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Laffleur, F.; Bernkop-Schnürch, A. Per-thiolated cyclodextrins: Nanosized drug carriers providing a prolonged gastrointestinal residence time. Carbohydr. Polym. 2023, 300, 120275. [Google Scholar] [CrossRef] [PubMed]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; McClements, D.J.; Liu, X.; Liu, F. Overcoming biopotency barriers: Advanced oral delivery strategies for enhancing the efficacy of bioactive food ingredients. Adv. Sci. 2024, 11, 2401172. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, S.; Wang, L.; Zhang, Y.; Chen, H.; Fu, Z.; Zhang, M.; Luo, H.; Liu, J. Reinforcement of the intestinal mucosal barrier via mucus-penetrating PEGylated bacteria. Nat. Biomed. Eng. 2024, 8, 823–841. [Google Scholar] [CrossRef]
- Ibrahim, M.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; Ishida, T. Investigation of anti-PEG antibody response to PEG-containing cosmetic products in mice. J. Control. Release 2023, 354, 260–267. [Google Scholar] [CrossRef]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.N.; Lai, S.K. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal. Chem. 2016, 88, 11804–11812. [Google Scholar] [CrossRef]
- Chen, B.-M.; Su, Y.-C.; Chang, C.-J.; Burnouf, P.-A.; Chuang, K.-H.; Chen, C.-H.; Cheng, T.-L.; Chen, Y.-T.; Wu, J.-Y.; Roffler, S.R. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal. Chem. 2016, 88, 10661–10666. [Google Scholar] [CrossRef]
- Henry, C.E.; Wang, Y.-Y.; Yang, Q.; Hoang, T.; Chattopadhyay, S.; Hoen, T.; Ensign, L.M.; Nunn, K.L.; Schroeder, H.; McCallen, J.; et al. Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus. Acta Biomater. 2016, 43, 61–70. [Google Scholar] [CrossRef]
- Chen, B.-M.; Chen, E.; Lin, Y.-C.; Tran, T.T.M.; Turjeman, K.; Yang, S.-H.; Cheng, T.-L.; Barenholz, Y.; Roffler, S.R. Liposomes with low levels of grafted poly(ethylene glycol) remain susceptible to destabilization by anti-poly(ethylene glycol) antibodies. ACS Nano 2024, 18, 22122–22138. [Google Scholar] [CrossRef]
- Stengel, D.; Demirel, B.H.; Knoll, P.; Truszkowska, M.; Laffleur, F.; Bernkop-Schnürch, A. PEG vs. zwitterions: How these surface decorations determine cellular uptake of lipid-based nanocarriers. J. Colloid Interface Sci. 2023, 647, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R.K. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2022, 299, 102563. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, L.; Sun, R.; Luo, B.; Zhou, Y.; Zhang, Y.; Liang, Y.; Xiao, B.; Wang, C. Pulmonary delivery of mucus-traversing PF127-modified silk fibroin nanoparticles loading with quercetin for lung cancer therapy. Asian J. Pharm. Sci. 2023, 18, 100833. [Google Scholar] [CrossRef] [PubMed]
- Zierden, H.C.; Josyula, A.; Shapiro, R.L.; Hsueh, H.T.; Hanes, J.; Ensign, L.M. Avoiding a sticky situation: Bypassing the mucus barrier for improved local drug delivery. Trends Mol. Med. 2021, 27, 436–450. [Google Scholar] [CrossRef]
- Popov, A.; Enlow, E.; Bourassa, J.; Chen, H. Mucus-penetrating nanoparticles made with “mucoadhesive” poly(vinyl alcohol). Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1863–1871. [Google Scholar] [CrossRef]
- Olmsted, S.S.; Padgett, J.L.; Yudin, A.I.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 2001, 81, 1930–1937. [Google Scholar] [CrossRef]
- Rao, R.; Liu, X.; Li, Y.; Tan, X.; Zhou, H.; Bai, X.; Yang, X.; Liu, W. Bioinspired zwitterionic polyphosphoester modified porous silicon nanoparticles for efficient oral insulin delivery. Biomater. Sci. 2021, 9, 685–699. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Zheng, J.; Ratner, B.D.; Jiang, S. Protein adsorption on oligo(ethylene glycol)-terminated alkanethiolate self-assembled monolayers: The molecular basis for nonfouling behavior. J. Phys. Chem. B 2005, 109, 2934–2941. [Google Scholar] [CrossRef]
- Shan, W.; Zhu, X.; Tao, W.; Cui, Y.; Liu, M.; Wu, L.; Li, L.; Zheng, Y.; Huang, Y. Enhanced oral delivery of protein drugs using zwitterion-functionalized nanoparticles to overcome both the diffusion and absorption barriers. ACS Appl. Mater. Interfaces 2016, 8, 25444–25453. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Anderson, C.M.H. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011, 164, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Z.; Zhao, D.; Li, D.; He, C.; Chen, X. A pH-triggered self-unpacking capsule containing zwitterionic hydrogel-coated MOF nanoparticles for efficient oral exendin-4 delivery. Adv. Mater. 2021, 33, e2102044. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhong, Y.; Fan, W.; Xiang, J.; Wang, G.; Zhou, Q.; Wang, J.; Geng, Y.; Sun, R.; Zhang, Z.; et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion–drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 2021, 5, 1019–1037. [Google Scholar] [CrossRef]
- Fan, W.; Wei, Q.; Xiang, J.; Tang, Y.; Zhou, Q.; Geng, Y.; Liu, Y.; Sun, R.; Xu, L.; Wang, G.; et al. Mucus penetrating and cell-binding polyzwitterionic micelles as potent oral nanomedicine for cancer drug delivery. Adv. Mater. 2022, 34, 2109189. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Wang, H.; Bao, S.; Lang, L.; Cui, C.; Song, H.; Yang, J.; Liu, W. The unprecedented biodegradable polyzwitterion: A removal-free patch for accelerating infected diabetic wound healing. Adv. Mater. 2024, 36, 2404297. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Chen, D.; Wang, Z.; Wang, H.; Bao, S.; Zhang, P.; Yang, J.; Liu, W. Low hysteresis zwitterionic supramolecular polymer ion-conductive elastomers with anti-freezing properties, high stretchability, and self-adhesion for flexible electronic devices. Mater. Horiz. 2024, 11, 2628–2642. [Google Scholar] [CrossRef]
- Yuan, D.; Li, Q.; Zhang, Q.; Zhou, F.; Zhao, Q.; Zhao, M. Enhanced curcumin transportation across epithelial barrier by mucus-permeable soy protein nanoparticles-mediated dual transcytosis pathways. Food Chem. 2024, 437, 137771. [Google Scholar] [CrossRef]
- Yuan, D.; Niu, Z.; Zheng, W.; Zhao, Q.; Zhou, F.; Zhao, M. Mind the particle rigidity: Blooms the bioavailability via rapidly crossing the mucus layer and alters the intracellular fate of curcumin. ACS Nano 2024, 18, 27026–27041. [Google Scholar] [CrossRef]
- Wang, S.; Meng, S.; Zhou, X.; Gao, Z.; Piao, M.G. pH-Responsive and mucoadhesive nanoparticles for enhanced oral Insulin delivery: The effect of hyaluronic acid with different molecular weights. Pharmaceutics 2023, 15, 820. [Google Scholar] [CrossRef]
- Fan, B.; Liu, L.; Zheng, Y.; Xing, Y.; Shen, W.; Li, Q.; Wang, R.; Liang, G. Novel pH-responsive and mucoadhesive chitosan-based nanoparticles for oral delivery of low molecular weight heparin with enhanced bioavailability and anticoagulant effect. J. Drug Deliv. Sci. Technol. 2022, 78, 103955. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Z.; Ma, D.; Yin, T.H.; Zhao, K. Palmitic acid- and cysteine-functionalized nanoparticles overcome mucus and epithelial barrier for oral delivery of drug. Bioeng. Transl. Med. 2023, 8, e10510. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, H.; Zhang, Y.; Wu, P.; He, B.; Dai, W.; Zhang, H.; Zhang, Q.; Zhao, R.; Wang, X. Thiolated nanoparticles overcome the mucus barrier and epithelial barrier for oral delivery of insulin. Mol. Pharm. 2020, 17, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, J.; Shi, J.; Gu, X.; Chen, H.; Wang, X.; Wang, L.; Wang, P.; Hou, X.; He, Y.; et al. Functional polymeric core–shell hybrid nanoparticles overcome intestinal barriers and inhibit breast cancer metastasis. Chem. Eng. J. 2022, 427, 131742. [Google Scholar] [CrossRef]
- Pang, H.; Huang, X.; Xu, Z.P.; Chen, C.; Han, F.Y. Progress in oral insulin delivery by PLGA nanoparticles for the management of diabetes. Drug Discov. Today 2023, 28, 103393. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Liu, M.; Shan, W.; Zhang, Z.; Huang, Y. Biomimetic viruslike and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers for oral insulin delivery. ACS Appl. Mater. Interfaces 2018, 10, 9916–9928. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yu, M.; Wang, A.; Qiu, Y.; Fan, W.; Hovgaard, L.; Yang, M.; Li, Y.; Wang, R.; et al. The upregulated intestinal folate transporters direct the uptake of ligand-modified nanoparticles for enhanced oral insulin delivery. Acta Pharm. Sin. B 2022, 12, 1460–1472. [Google Scholar] [CrossRef]
- Han, R.; He, H.; Lu, Y.; Lu, H.; Shen, S.; Wu, W. Oral targeted drug delivery to post-gastrointestinal sites. J. Control. Release 2024, 370, 256–276. [Google Scholar] [CrossRef]
- Kumar, R.; Islam, T.; Nurunnabi, M. Mucoadhesive carriers for oral drug delivery. J. Control. Release 2022, 351, 504–559. [Google Scholar] [CrossRef]
- Kim, K.S.; Na, K.; Bae, Y.H. Nanoparticle oral absorption and its clinical translational potential. J. Control. Release 2023, 360, 149–162. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Xu, H.; Yang, X.; Liu, N.; Xu, Y.; Ma, R.; Zhai, J.; Ma, Y.; Guan, S. Aptamer-modified M cell targeting liposomes for oral delivery of macromolecules. Colloids Surf. B Biointerfaces 2023, 222, 113109. [Google Scholar] [CrossRef]
- Pinto, S.; Hosseini, M.; Buckley, S.T.; Yin, W.; Garousi, J.; Gräslund, T.; van Ijzendoorn, S.; Santos, H.A.; Sarmento, B. Nanoparticles targeting the intestinal Fc receptor enhance intestinal cellular trafficking of semaglutide. J. Control. Release 2024, 366, 621–636. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Huang, X.; Joseph, J.; Chen, W.; Liu, C.; Zhou, J.; Kong, N.; Joshi, N.; Du, J.; et al. Glucose-responsive oral insulin delivery platform for one treatment a day in diabetes. Matter 2021, 4, 3269–3285. [Google Scholar] [CrossRef]
- Liu, A.; He, M.; Liu, C.; Ye, Z.; Tan, C.-P.; Liu, Y.; Gong, J.; Lei, J.; He, Y.; Zhu, S.; et al. Prevention of hypercholesterolemia with “liposomes in microspheres” composite carriers: A promising approach for intestinal-targeted oral delivery of astaxanthin. J. Agric. Food Chem. 2024, 72, 6118–6132. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, G.; Chen, S.; Luo, S.; Zhang, J. Biomimetic and bioinspired strategies for oral drug delivery. Biomater. Sci. 2020, 8, 1020–1044. [Google Scholar] [CrossRef] [PubMed]
- Fowler, R.; Vllasaliu, D.; Trillo, F.F.; Garnett, M.; Alexander, C.; Horsley, H.; Smith, B.; Whitcombe, I.; Eaton, M.; Stolnik, S. Nanoparticle transport in epithelial cells: Pathway switching through bioconjugation. Small 2013, 9, 3282–3294. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.-m.; Zhu, B.; Yang, L.; et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017, 15, 18. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Xu, J.; Zhang, J.; Xu, S.; Zhang, Q.; Huang, J.; Peng, J.; Xu, H.; Du, Q.; et al. Fabrication of a polysaccharide-protein/protein complex stabilized oral nanoemulsion to facilitate the therapeutic effects of 1,8-cineole on atherosclerosis. ACS Nano 2023, 17, 9090–9109. [Google Scholar] [CrossRef]
- Liu, R.; Fei, S.; Zhang, X.; Hua, Z.; Tan, M. Layer-by-layer oral-deliverable nanoparticles targeted microfold cells to promote lutein absorption in alleviating dry eye disease. Chem. Eng. J. 2024, 479, 147590. [Google Scholar] [CrossRef]
- He, J.; Ding, R.; Tao, Y.; Zhao, Z.; Yuan, R.; Zhang, H.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Folic acid-modified reverse micelle-lipid nanocapsules overcome intestinal barriers and improve the oral delivery of peptides. Drug Deliv. 2023, 30, 2181744. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Fei, S.; Tan, M. Orally deliverable lutein nanoparticles as robust ROS scavenger for dry eye disease by targeting Peyer’s patches and mitochondria of ocular cell. Chem. Eng. J. 2024, 494, 153024. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liang, J.; Zhang, M.; Li, Z.; Wang, Z.; Dang, B.; Feng, N. Mucosal transfer of wheat germ agglutinin modified lipid–polymer hybrid nanoparticles for oral delivery of oridonin. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, Y.; Chi, C.; Wang, T.; Chen, L.; Li, X. Starch nanocarriers for enhanced M-cell transport and oral delivery of bioactive proteins. ACS Appl. Nano Mater. 2023, 6, 4793–4802. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Asghar, S.; Wu, Y.; Chen, Z.; Jin, X.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 7897–7911. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Z.; Peng, J.; Sun, R.; Zhang, L.; Chen, Y.; Pan, D.; Huang, J.; Gong, Z.; Chen, Y.; et al. Fabrication of a protein-dextran conjugates formed oral nanoemulsion and its application to deliver the essential oil from Alpinia zerumbet Fructus. Int. J. Biol. Macromol. 2023, 249, 125918. [Google Scholar] [CrossRef]
- Du, Y.; Tian, C.; Wang, M.; Huang, D.; Wei, W.; Liu, Y.; Li, L.; Sun, B.; Kou, L.; Kan, Q.; et al. Dipeptide-modified nanoparticles to facilitate oral docetaxel delivery: New insights into PepT1-mediated targeting strategy. Drug Deliv. 2018, 25, 1403–1413. [Google Scholar] [CrossRef]
- Fang, H.; Chen, L.; Deng, Z.; Gao, Y.; Yang, Y.; Chen, Q.; Liu, Z. In situ polymerization of zwitterions on therapeutic proteins to enable their effective oral delivery. ACS Nano 2023, 17, 1128–1143. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Li, D.; Lin, S.; Chen, H.; Wu, W.; Zhang, W. Linolenic acid conjugated chitosan micelles for improving the oral absorption of doxorubicin via fatty acid transporter. Carbohydr. Polym. 2023, 300, 120233. [Google Scholar] [CrossRef]
- Cui, Z.; Qin, L.; Guo, S.; Cheng, H.; Zhang, X.; Guan, J.; Mao, S. Design of biotin decorated enterocyte targeting muco-inert nanocomplexes for enhanced oral insulin delivery. Carbohydr. Polym. 2021, 261, 117873. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Bai, Y.; Wang, L.; Liu, X.; Zhou, R.; Li, L.; Wu, R.; Zhang, Z.; Zhu, X.; Huang, Y. Promoting apical-to-basolateral unidirectional transport of nanoformulations by manipulating the nutrient-absorption pathway. J. Control. Release 2020, 323, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Y.; Luo, Y. Mechanisms of carotenoid intestinal absorption and the regulation of dietary lipids: Lipid transporter-mediated transintestinal epithelial pathways. Crit. Rev. Food Sci. Nutr. 2024, 64, 1791–1816. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Peng, X.; Liu, Y.; Li, D. The pro-absorptive effect of glycosylated zein-fatty acid complexes on fucoxanthin via the lipid transporter protein delivery pathway. Food Chem. 2024, 446, 138892. [Google Scholar] [CrossRef]
- Zhou, X.H.; Po, A.L.W. Peptide and protein drugs: II. Non-parenteral routes of delivery. Int. J. Pharm. 1991, 75, 117–130. [Google Scholar] [CrossRef]
- Han, X.; Lu, Y.; Xie, J.; Zhang, E.; Zhu, H.; Du, H.; Wang, K.; Song, B.; Yang, C.; Shi, Y.; et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat. Nanotechnol. 2020, 15, 605–614. [Google Scholar] [CrossRef]
- Fan, W.; Xia, D.; Zhu, Q.; Li, X.; He, S.; Zhu, C.; Guo, S.; Hovgaard, L.; Yang, M.; Gan, Y. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials 2018, 151, 13–23. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Liu, Y.; Duan, H.; Chen, L.; Zhang, X.; Jin, M.; Cui, M.; Quan, X.; Pan, L.; et al. Versatile flexible micelles integrating mucosal penetration and intestinal targeting for effectively oral delivery of paclitaxel. Acta Pharm. Sin. B 2023, 13, 3425–3443. [Google Scholar] [CrossRef]
- Lei, T.; Yang, Z.; Jiang, C.; Wang, X.; Yang, W.; Yang, X.; Xie, R.; Tong, F.; Xia, X.; Huang, Q.; et al. Mannose-integrated nanoparticle hitchhike glucose transporter 1 recycling to overcome various barriers of oral delivery for Alzheimer’s disease therapy. ACS Nano 2024, 18, 3234–3250. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, L.; Jiang, Q.; Sun, Y.; Zhao, D.; Sun, M.; He, Z.; Sun, J.; Wang, Y. Intestinal OCTN2- and MCT1-targeted drug delivery to improve oral bioavailability. Asian J. Pharm. Sci. 2020, 15, 158–172. [Google Scholar] [CrossRef]
- Xi, Z.; Ahmad, E.; Zhang, W.; Li, J.; Wang, A.; Faridoon; Wang, N.; Zhu, C.; Huang, W.; Xu, L.; et al. Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. J. Control. Release 2022, 342, 1–13. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C.; Han, F.; Liu, Y.; Hani, U.E.; Zhong, Y.; Huang, D.; Chen, W.; Qian, H. Oral delivery of berberine by liver-targeted zwitterionic nanoparticles to overcome multi-intestinal barriers and extend insulin treatment duration. Chem. Eng. J. 2024, 485, 150129. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Lu, Y.; Quan, H.; Wang, Y.; Song, S.; Guo, H. Advanced oral drug delivery systems for gastrointestinal targeted delivery: The design principles and foundations. J. Nanobiotechnol. 2025, 23, 400. [Google Scholar] [CrossRef]




| Bioactive Substances | Main Material | Model | Main Results | References |
|---|---|---|---|---|
| Anthocyanin | Modified starch | In vitro and vivo | The bioavailability of anthocyanin in nanoemulsions in mice was about 2 times that of free anthocyanin. | [74] |
| Curcumin | Soybean lipophilic protein | In vitro and vivo | The nanoemulsion increased the bioaccessibility of curcumin from 46.33% of free curcumin to 76.14%. | [77] |
| Zein, sodium caseinate and quaternary ammonium chitosan | In vitro and vivo | The bioaccessibility of curcumin in free curcumin, uncoated zein–sodium caseinate nanoparticles and positively charged quaternary ammonium chitosan-coated protein nanoparticles is 10.8%, 23.7% and 45.2%, respectively. | [80] | |
| Apigenin | Casein | In vitro and vivo | The bioavailability of apigenin in nanoparticles in rats was 3.0 times higher than that in free group. | [78] |
| Mulberry anthocyanins | Caseinate and konjac glucomannan | In vitro | After continuous digestion in simulated intestinal juice for 3 h, the cumulative release of nanoparticles was 85.07%, which was higher than 74.30% of free anthocyanins. | [81] |
| Phloretin | Gliadin and sodium carboxymethyl cellulose | In vitro | The bioaccessibility of phloretin in nanoparticles (55%) was more than twice that of free phloretin (23%) after digestion with simulated gastrointestinal juice. | [82] |
| Types | Advantages | Disadvantages |
|---|---|---|
| pH-sensitive | This type can solve the premature release of bioactive substances in the gastrointestinal tract and their insufficient accumulation in the target tissue (the small intestine) through the pH difference between the small intestine and stomach. | This type focuses primarily on the impact of chemical barriers corresponding to pH on the delivery system, largely ignoring the effect of other gastrointestinal barriers. |
| Enzyme-responsive | This type releases bioactive substances in the small intestine after enzyme-related high specific response. | Similarly to pH-sensitive nanodelivery systems, this type also fails to account for the obstruction posed by additional gastrointestinal barriers, particularly physical barriers governing the penetration and absorption mechanisms of bioactive substances. |
| Mucoadhesive | This type could typically prolong the intestinal residence time by adhering to the intestinal mucus, thereby improving the delivery efficiency. | This type may adhere non-specifically to surfaces to which they are not intended to adhere (e.g., gastric mucosa and intestinal contents). In addition, if they trapped in loose mucus layer may be removed with the renewal of mucus, reducing the absorption of bioactive substances by the human body. |
| Mucus-penetrating | Compared with mucoadhesive nanodelivery systems, this type may be a more promising strategy to increase the absorption of bioactive substances. Because they are more likely to reach the epithelial cells and subsequently be absorbed. | The gastrointestinal stability of this type needs to be ensured, particularly their structural integrity maintenance during digestion, a critical prerequisite for ensuring mucus-penetrating capability. |
| Composite | Compared with a single type of delivery system, composite nanodelivery systems that consider multiple barriers have been shown to be more advantageous in the delivery of bioactive substances. | This type still ignores the last barrier to overcome the absorption to a certain extent, namely, the intestinal epithelial cell layer. |
| Bioactive Substances | Main Material | Receptors | Model | Main Results | References |
|---|---|---|---|---|---|
| Exenatide | DSPE–PEG–FA, LabrafacWL1349, Span80 | FA receptor | In vitro and vivo | The bioavailability of the targeted nanocomplex (7.53%) was 1.28 and 2.04 times that of the non-targeted and free exenatide groups, respectively. | [161] |
| Lutein | Phycocyanin, triphenylphosphonium-modified chitosan, phycocyanin, 3-boronobenzoic acid-modified yeast β-glucan yeast | Dectin-1 receptor | In vitro and vivo | The concentration of lutein in the targeted nanoparticles in the blood and eyeball of rats was 2.54 and 1.82 times higher than that of free lutein, respectively. | [162] |
| Oridonin | DSPE–PEG2000, LIPOID S100, PLGA, WGA–DOPE | Lectin receptor | In vitro and vivo | The bioavailability of the targeted nanosystem was 9.09 and 1.96 times higher than that of the suspension and non-targeted nanosystem, respectively. | [163] |
| Ovalbumin | Cationic quaternary ammonium corn starch, carboxymethyl corn starch, GRGDS peptide | Integrin α5β1 | In vitro | Compared with the unmodified nanocapsules, the targeted peptide-modified nanocapsules exhibited significantly better ability to target M cells and transport efficiency. | [164] |
| Insulin | P(GA–co–GAPBAPE), DSPE–PEG–Mal, Fc fragment of Immunoglobulin G | FcRn | In vitro and vivo | The continuous hypoglycaemic ability of targeted nanoparticles was up to 16 h, which was 4 times longer than that of free insulin | [154] |
| VB12–chitosan conjugate, calcium chloride, ammonium phosphate | IF receptor | In vitro and vivo | The bioavailability of VB12-modified nanoparticles (26.91%) was 4.3 times that of unmodified nanoparticles. | [165] |
| Bioactive Substances | Main Material | Receptors | Model | Main Results | References |
|---|---|---|---|---|---|
| Curcumin | Taurocholic acid–polyethylene glycol 100–monostearate | ASBT | In vitro and vivo | The highest bioavailability of the targeted nanolipids was 15.21 and 65.00 times that of the non-targeted and free curcumin groups, respectively. | [166] |
| EOFAZ | Bovine serum albumin–dextran sulphate conjugate, sodium deoxycholate | ASBT | In vitro and vivo | The bioavailability of deoxycholic acid-targeted modified nanoemulsion and untargeted modified nanoemulsion was 3.83 and 2.65 times that of the free group, respectively. | [167] |
| Docetaxel | Dipeptide modified polyoxyethylene stearate, PLGA | PepT1 | In vitro and vivo | The bioavailability of targeted nanoparticles was 4.39 and 1.95 times higher than that of docetaxel solution and unmodified solution and unmodified, respectively. | [168] |
| Insulin, immunoglobin G | Polymer polymerised from three monomers (cationic, anionic, and zwitterionic ones) | PAT1 | In vitro and vivo | The bioavailability of free insulin, polymer/insulin and polymer/immunoglobin G nanocomplexes encapsulated in enteric capsules was 1.3%, 16.9% and 12.5%, respectively. | [169] |
| Doxorubicin | Linolenic acid conjugated chitosan | Fatty acid transporter 4 | In vitro and vivo | The bioavailability of targeted nanomicelles in rats was 1.66 times higher than that of free doxorubicin. | [170] |
| Insulin | Biotin grafted chitosan, hyaluronic acid | Sodium-dependent multivitamins transporter | In vitro and vivo | Free insulin did not reduce blood glucose, and the highest hypoglycaemic ability of targeted nanocomposites was 1.92 times that of non-targeted nanocomposites. | [171] |
| DSPE–PEG–Fru, PLGA, DSPE–PEG | Glucose transporter 2 | In vitro and vivo | The bioavailability of fructose- targeted nanoparticles was 2.35 and 3.78 times that of non-targeted and free groups, respectively. | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Su, M.; Zhang, Y.; Feng, Q.; Liu, Y.; Zeng, Z.; Zhang, Q.; Fang, Z.; Li, S.; Chen, H. Current Status and Future Prospects on Nanodelivery Systems Targeting the Small Intestine for Absorption of Bioactive Substances. Foods 2025, 14, 3234. https://doi.org/10.3390/foods14183234
Zhang H, Su M, Zhang Y, Feng Q, Liu Y, Zeng Z, Zhang Q, Fang Z, Li S, Chen H. Current Status and Future Prospects on Nanodelivery Systems Targeting the Small Intestine for Absorption of Bioactive Substances. Foods. 2025; 14(18):3234. https://doi.org/10.3390/foods14183234
Chicago/Turabian StyleZhang, Hong, Mengjie Su, Yu Zhang, Qiuxia Feng, Yuntao Liu, Zhen Zeng, Qing Zhang, Zhengfeng Fang, Shanshan Li, and Hong Chen. 2025. "Current Status and Future Prospects on Nanodelivery Systems Targeting the Small Intestine for Absorption of Bioactive Substances" Foods 14, no. 18: 3234. https://doi.org/10.3390/foods14183234
APA StyleZhang, H., Su, M., Zhang, Y., Feng, Q., Liu, Y., Zeng, Z., Zhang, Q., Fang, Z., Li, S., & Chen, H. (2025). Current Status and Future Prospects on Nanodelivery Systems Targeting the Small Intestine for Absorption of Bioactive Substances. Foods, 14(18), 3234. https://doi.org/10.3390/foods14183234








