Influence of Malting on Volatile Composition and Technological Properties of Polish Pea Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Malting Technology
2.3. Analysis of Malt Technological Properties—Congress Mashing
2.4. Analysis of Carbohydrates in Worts
2.5. Analysis of Volatile Comopounds in Pea Seeds and Pea Malts
2.6. Data Analysis
3. Results and Discussion
3.1. Technological Properties of Pea Malts
3.2. Concentration of Carbohydrates in Pea Worts
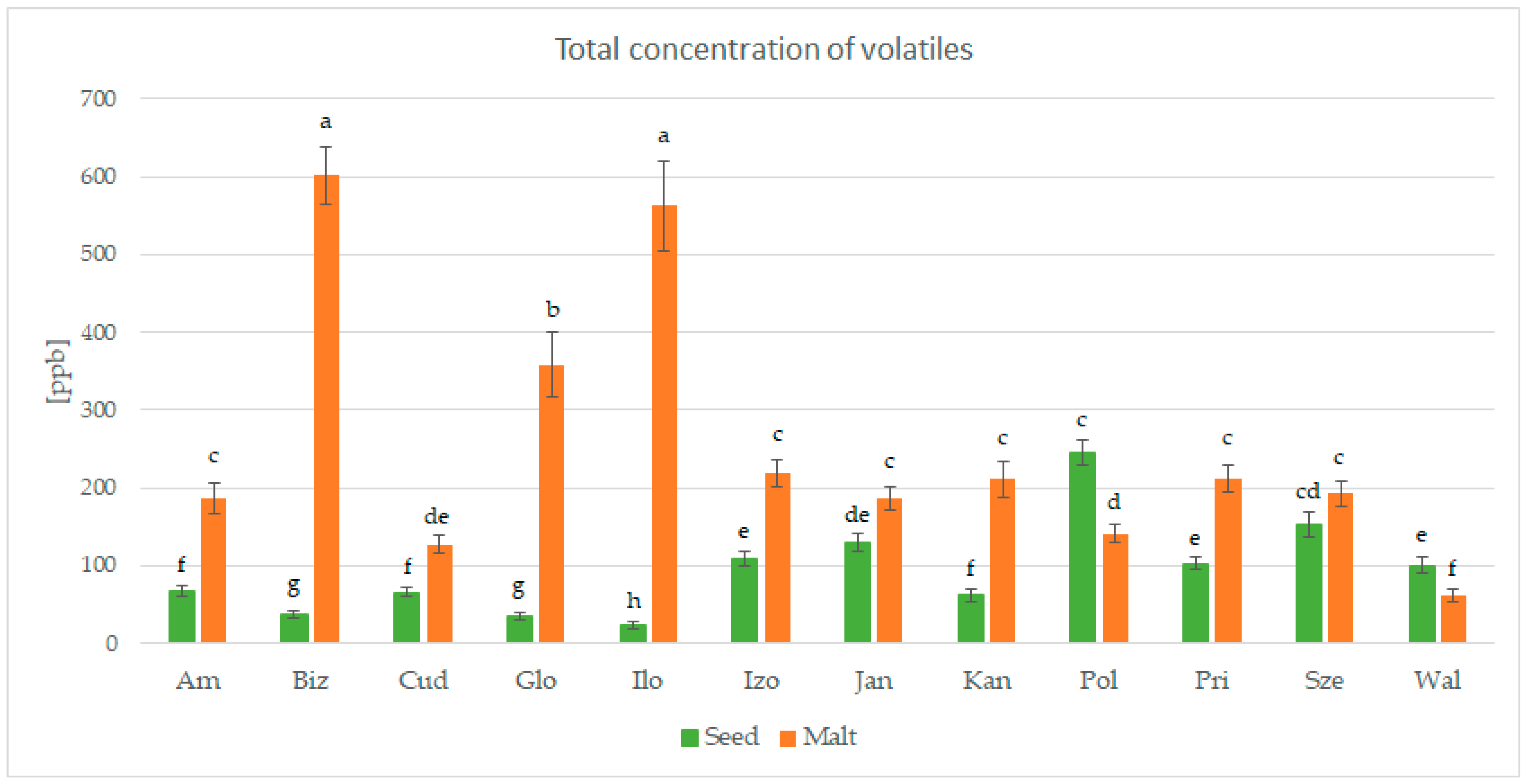
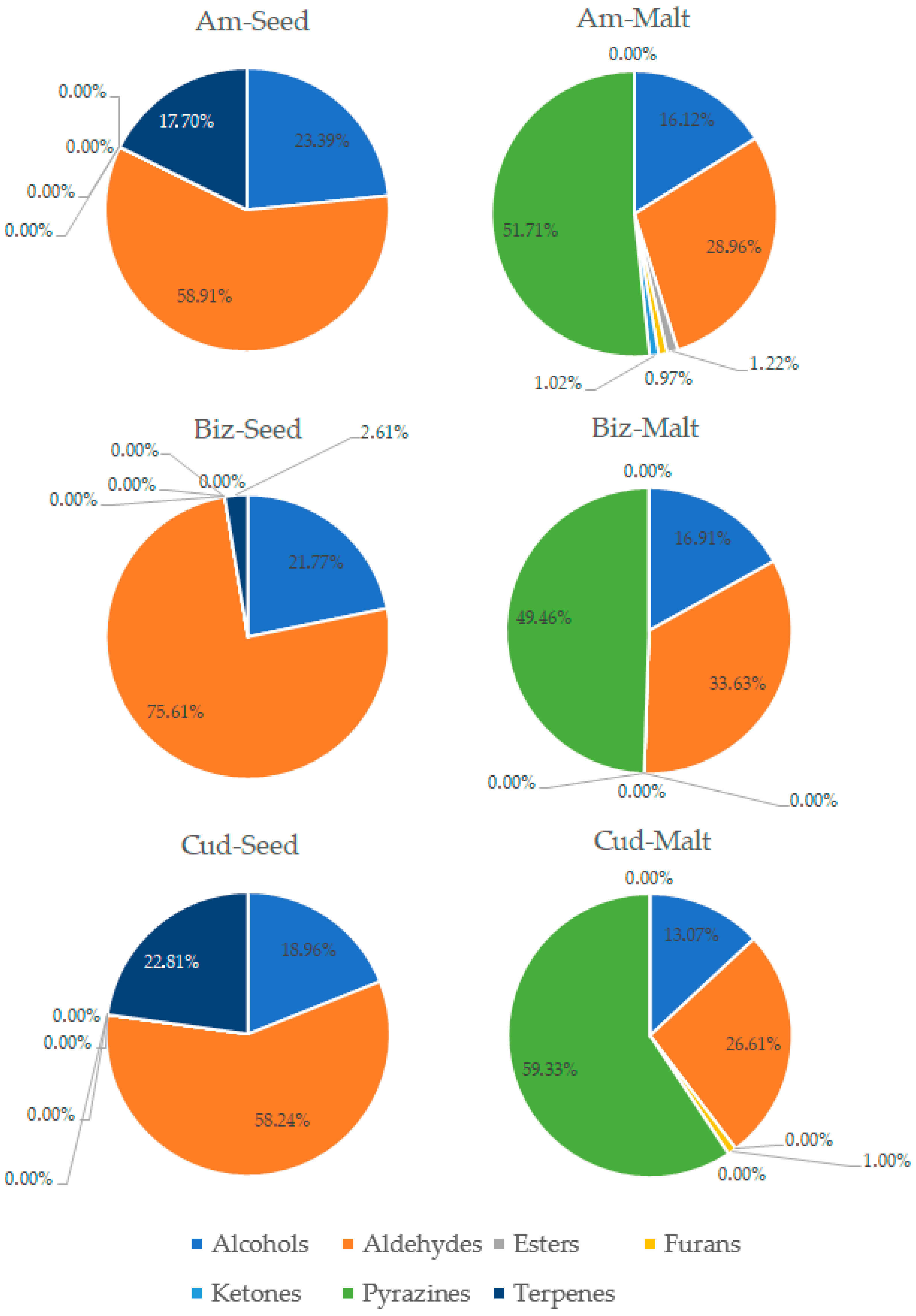
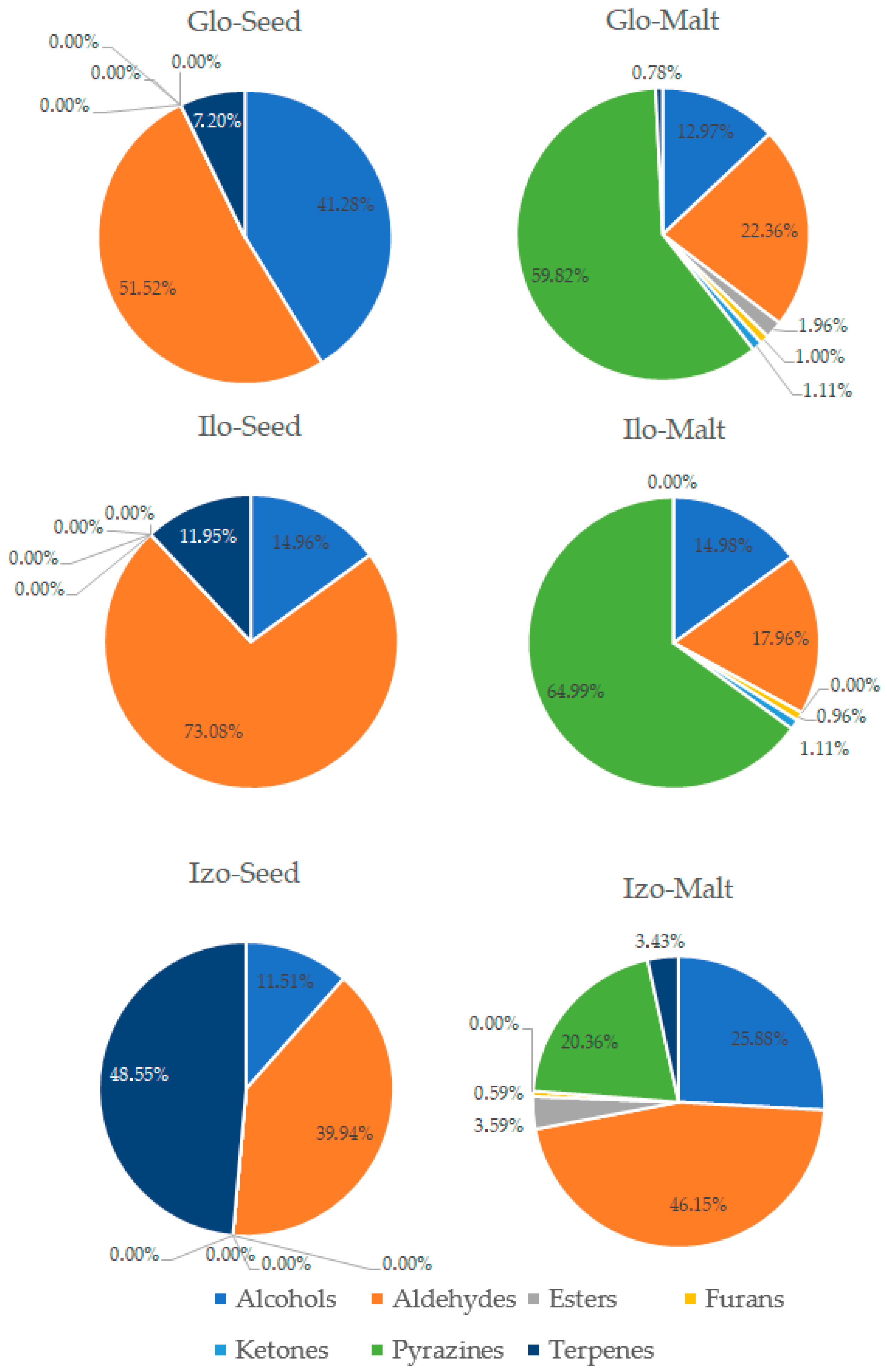
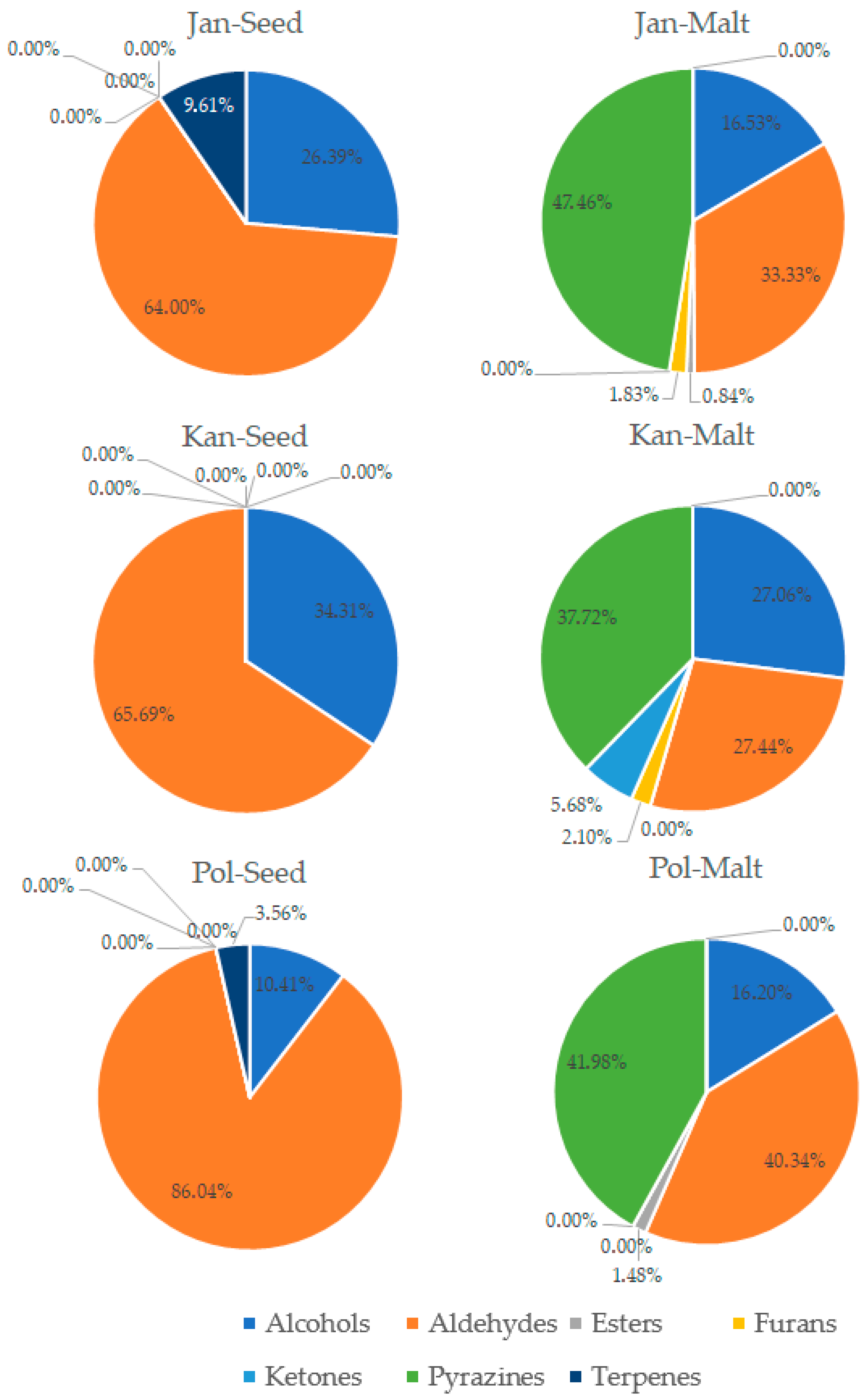
3.3. Analysis of Volatile Compounds in Pea Seeds and Pea Malts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bamforth, C. Grain to Glass: The Basics of Malting and Brewing. In Beer: Tap into the Art and Science of Brewing, 3rd ed.; Bamforth, C., Ed.; Oxford University Press, Inc.: New York, NY, USA, 2009; pp. 50–59. [Google Scholar]
- Kunze, W. Technology Brewing and Malting, 6th ed.; VLB: Berlin, Germany, 2019. [Google Scholar]
- Salamon, A.; Kowalska, H.; Ignaczak, A.; Marzec, A.; Kowalska, J.; Szafrańska, A. Characteristics of Oat and Buckwheat Malt Grains for Use in the Production of Fermented Foods. Foods 2023, 12, 3747. [Google Scholar] [CrossRef]
- Chawanda, E.T.; Manhokwe, S.; Jombo, T.Z.; Mugadza, D.T.; Njini, M.; Manjeru, P. Optimisation of Malting Parameters for Quinoa and Barley: Application of Response Surface Methodology. J. Food Qual. 2022, 2022, 5279177. [Google Scholar] [CrossRef]
- Gasiński, A.; Błażewicz, J.; Kawa-Rygielska, J.; Śniegowska, J.; Zarzecki, M. Analysis of Physicochemical Parameters of Congress Worts Prepared from Special Legume Seed Malts, Acquired with and without Use of Enzyme Preparations. Foods 2021, 10, 304. [Google Scholar] [CrossRef]
- Trummer, J.; Watson, H.; De Clippeleer, J.; Poreda, A. Brewing with 10% and 20% Malted Lentils—Trials on Laboratory and Pilot Scales. Appl. Sci. 2021, 11, 9817. [Google Scholar] [CrossRef]
- Meena, R.S.; Lal, R. Legumes and Sustainable Use of Soils. In Legumes for Soil Health and Sustainable Management, 1st ed.; Meena, R.S., Das, A., Yadav, G.S., Lal, R., Eds.; Springer Nature: Singapore, 2018; pp. 1–32. [Google Scholar]
- Cheng, S.; Langrish, T.A.G. A Review of the Treatments to Reduce Anti-Nutritional Factors and Fluidized Bed Drying of Pulses. Foods 2025, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J.; Mikulski, D.; Kłosowski, G. Changes in the raffinose family oligosaccharides content in the lentil and common bean seeds during malting and mashing processes. Sci. Rep. 2022, 12, 17911. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J. Malting—A method for modifying volatile composition of black, brown and green lentil seeds. PLoS ONE 2023, 18, e0290616. [Google Scholar] [CrossRef]
- Gasiński, A.; Noguera-Artiaga, L.; Kawa-Rygielska, J. Influence of Malted Chickpea on the Composition of Volatiles in Hummus. Molecules 2025, 30, 1231. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J. Assessment of green lentil malt as a substrate for gluten-free beer brewing. Sci. Rep. 2024, 14, 504. [Google Scholar] [CrossRef]
- EBC—Analytica. 4.5.1 Extract of Malt: Congress Mash; Experimental Station for Variety Assessment, Chemical and Technology Laboratory: Nürnberg, Germany, 1998. [Google Scholar]
- Cai, G.; Li, X.; Zhang, C.; Zhang, M.; Lu, J. Dextrin as the main turbidity components in wort produced from major malting barley cultivars of Jiangsu province in China. J. Inst. Brew. 2016, 122, 543–546. [Google Scholar] [CrossRef]
- Barreiro, J.A.; Fernández, S.; Sandoval, A.J. Water sorption characteristics of six row barley malt (Hordeum vulgare). LWT-Food Sci. Technol. 2003, 36, 37–42. [Google Scholar] [CrossRef]
- Piornos, J.A.; Koussissi, E.; Balagiannis, D.P.; Brouwer, E.; Parker, J.K. Alcohol-free and low-alcohol beers: Aroma chemistry and sensory characteristics. Compr. Rev. Food Sci. Food Saf. 2023, 22, 233–259. [Google Scholar] [CrossRef]
- Ivanov, K.; Petelkov, I.; Shopska, V.; Denkova, R.; Gochev, V.; Kostov, G. Investigation of mashing regimes for low-alcohol beer production. J. Inst. Brew. 2016, 122, 508–516. [Google Scholar] [CrossRef]
- Li, G.; Liu, F.; Kun-Farkas, G.; Kiss, Z. Technological factors influencing buffering capacity of wort. J. Am. Soc. Brew. Chem. 2015, 73, 236–239. [Google Scholar] [CrossRef]
- Sorokin, S.A.; Novoselov, A.G.; Kuznetsov, A.Y.; Baranov, I.V.; Rumiantceva, O.N.; Mironova, D.Y.; Kiliashov, A.A.; Kravtsova, E.V. Comprehensive studies of the physical and thermophysical properties of wort. BIO Web Conf. 2024, 103, 00037. [Google Scholar] [CrossRef]
- Juszczak, L.; Gałkowska, D.; Witczak, T.; Fortuna, T. Effect of Maltodextrins on the Rheological Properties of Potato Starch Pastes and Gels. Int. J. Food Sci. 2013, 2013, 869362. [Google Scholar] [CrossRef]
- Sadosky, P.; Schwarz, P.B.; Horsley, R.D. Effect of arabinoxylans, beta-glucans, and dextrins on the viscosity and membrane filterability of a beer model solution. J. Am. Soc. Brew. Chem. 2002, 60, 153–162. [Google Scholar]
- Bellut, K.; Arendt, E.K. Chance and challenge: Non-saccharomyces yeasts in nonalcoholic and low alcohol beer brewing–A review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Prado, R.; Gastl, M.; Becker, T. Aroma and color development during the production of specialty malts: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4816–4840. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.D.; Fitzgerald, N. Investigating the Potential of an In-Situ Method for Monitoring the Malting of Barley Using Solid Phase Microextraction with a Portable Gas Chromatography Mass Spectrometry Instrument. Beverages 2020, 6, 72. [Google Scholar] [CrossRef]
- Guido, L.F.; Ferreira, I.M. The Role of Malt on Beer Flavour Stability. Fermentation 2023, 9, 464. [Google Scholar] [CrossRef]
- Gasiński, A.; Pytlarz, E.; Hamkało, O.; Kawa-Rygielska, J. Technological properties and composition of volatile compounds in winter wheat malts grown with addition of seed meals into soil. Sci. Rep. 2023, 13, 637. [Google Scholar] [CrossRef]
- Filipowska, W.; Jaskula-Goiris, B.; Ditrych, M.; Bustillo Trueba, P.; De Rouck, G.; Aerts, G.; Powell, C.; Cook, D.; De Cooman, L. On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: A review. J. Inst. Brew. 2021, 127, 107–126. [Google Scholar] [CrossRef]
- Svoboda, Z.; Mikulíková, R.; Běláková, S.; Benešová, K.; Marová, I.; Nesvadba, Z. Optimization of Modern Analytical SPME and SPDE Methods for Determination of Trans-2-nonenal in Barley, Malt and Beer. Chromatographia 2011, 73, 157–161. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Maillard Reaction Products in Different Types of Brewing Malt. J. Agric. Food Chem. 2020, 68, 14274–14285. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Granier, T.; Dkhil, H.; Haupt, T.; Ellis, G.; Natsch, A. Stability of limonene and monitoring of a hydroperoxide in fragranced products. Flavour Fragr. J. 2014, 29, 277–286. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kanzler, C.; Klaußnitzer, S.; Kroh, L.W.; Rohn, S. The Formation of Methyl Ketones during Lipid Oxidation at Elevated Temperatures. Molecules 2021, 26, 1104. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Guo, X.; Qiang, J.; Cao, Y.; Zhang, S.; Yu, X. Influence of triacylglycerol structure on the formation of lipid oxidation products in different vegetable oils during frying process. Food Chem. 2025, 464, 141783. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W. Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines. Molecules 2024, 29, 3597. [Google Scholar] [CrossRef]
- Xia, X.; Zhou, T.; Zhang, H.; Cui, H.; Zhang, F.; Hayat, K.; Zhang, X.; Ho, C.-T. Simultaneously Enhanced Formation of Pyrazines and Furans during Thermal Degradation of the Glycyl-l-Glutamine Amadori Compound by Selected Exogenous Amino Acids and Appropriate Elevated Temperatures. J. Agric. Food Chem. 2023, 71, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shen, H.; Yang, X.; Liu, T.; Yang, Y.; Zhou, X.; Zhao, P.; Guo, Y. Effect of free amino acids and peptide hydrolysates from sunflower seed protein on the formation of pyrazines under different heating conditions. RSC Adv. 2021, 11, 27772–27781. [Google Scholar] [CrossRef]
- Ma, Y.J.; Wu, J.H.; Li, X.; Xu, X.B.; Wang, Z.Y.; Wu, C.; Du, M.; Song, L. Effect of alkyl distribution in pyrazine on pyrazine flavor release in bovine serum albumin solution. RSC Adv. 2019, 9, 36951–36959. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Zamora, R. Amino Acid Degradations Produced by Lipid Oxidation Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1242–1252. [Google Scholar] [CrossRef]
- Wietstock, P.C.; Kunz, T.; Methner, F.J. Relevance of Oxygen for the Formation of Strecker Aldehydes during Beer Production and Storage. J. Agric. Food Chem. 2016, 64, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Bouckaert, C.; Van Lancker, F.; De Meulenaer, B.; De Kimpe, N. Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. J. Agric. Food Chem. 2011, 59, 11058–11062. [Google Scholar] [CrossRef]
- Matsui, K.; Sasahara, S.; Akakabe, Y.; Kajiwara, T. Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. Biosci. Biotechnol. Biochem. 2003, 67, 2280–2282. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, X.; Chen, Q.; Zhang, H.; Sun, H.; Zhang, W.-M.; Li, C. Oxidative stabilities of olive and camellia oils: Possible mechanism of aldehydes formation in oleic acid triglyceride at high temperature. LWT Food Sci. Technol. 2019, 118, 108858. [Google Scholar] [CrossRef]
- Villalobos Solis, M.I.; Patel, A.; Orsat, V.; Singh, J.; Lefsrud, M. Fatty acid profiling of the seed oils of some varieties of field peas (Pisum sativum) by RP-LC/ESI-MS/MS: Towards the development of an oilseed pea. Food Chem. 2013, 139, 986–993. [Google Scholar] [CrossRef]
- Gao, P.; Bao, Y.; Wang, S.; Lei, L.; Wang, B.; Xiao, L.; Cheng, K.; Wang, Y.; Zhang, S.; Dong, L. Mechanism of palmitoleic acid oxidation into volatile compounds during heating. Flavour Fragr. J. 2023, 38, 95–107. [Google Scholar] [CrossRef]
- Behrends, T.; Schmid, J.; Blank, L.M.; Buhler, B. Biotransformation of Fatty Acids to Fatty Aldehydes. WO2012025629A1, 1 March 2012. [Google Scholar]
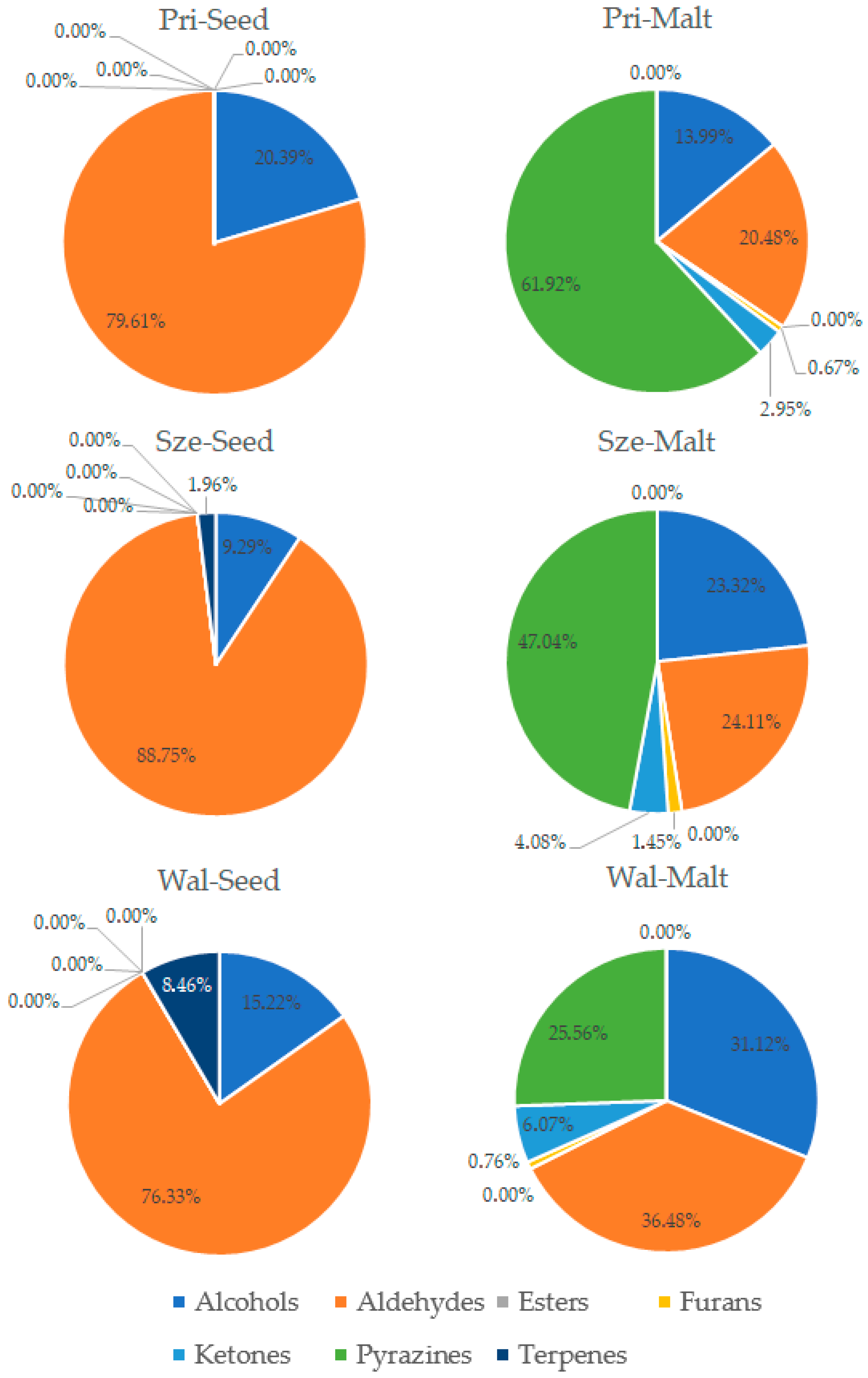
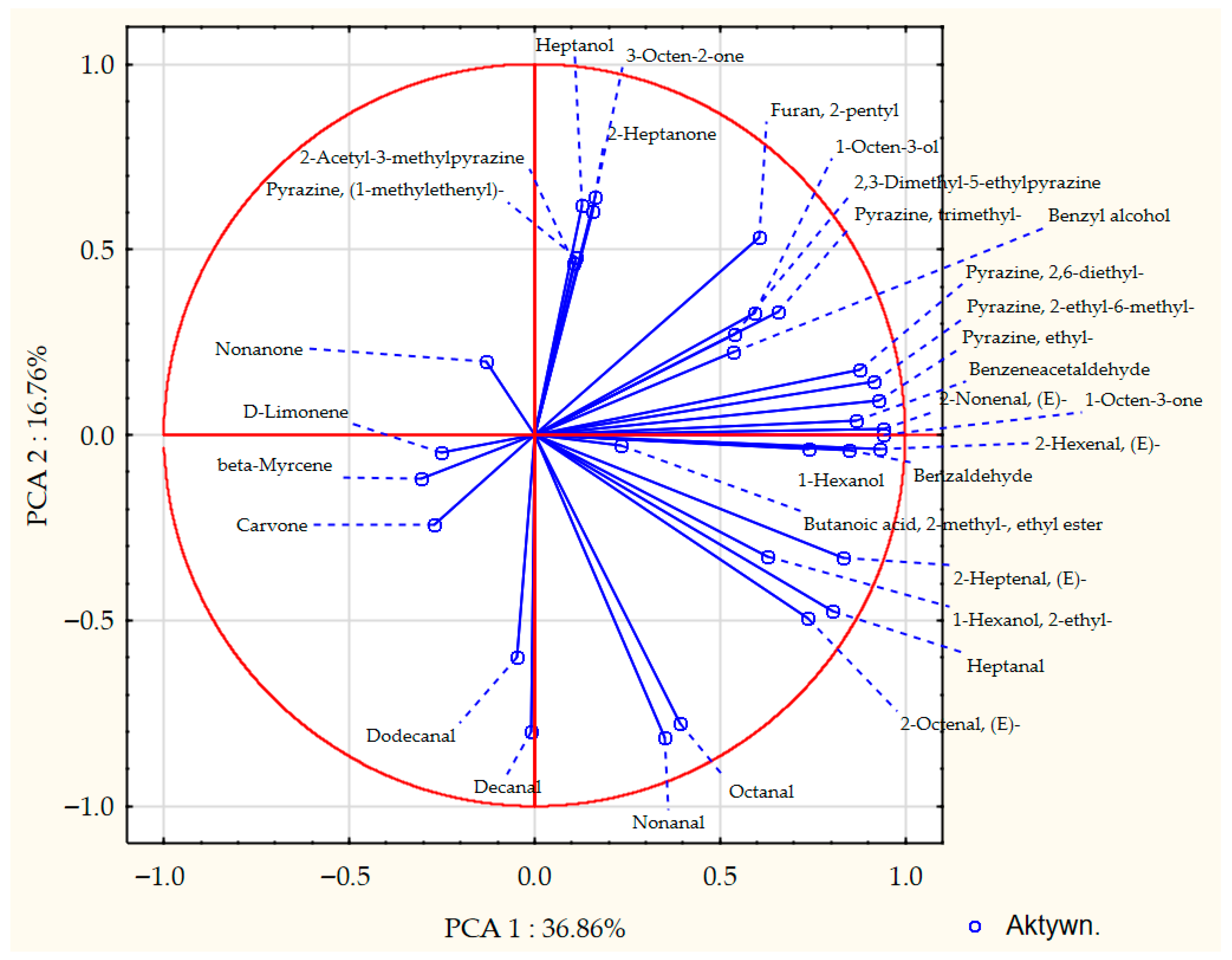
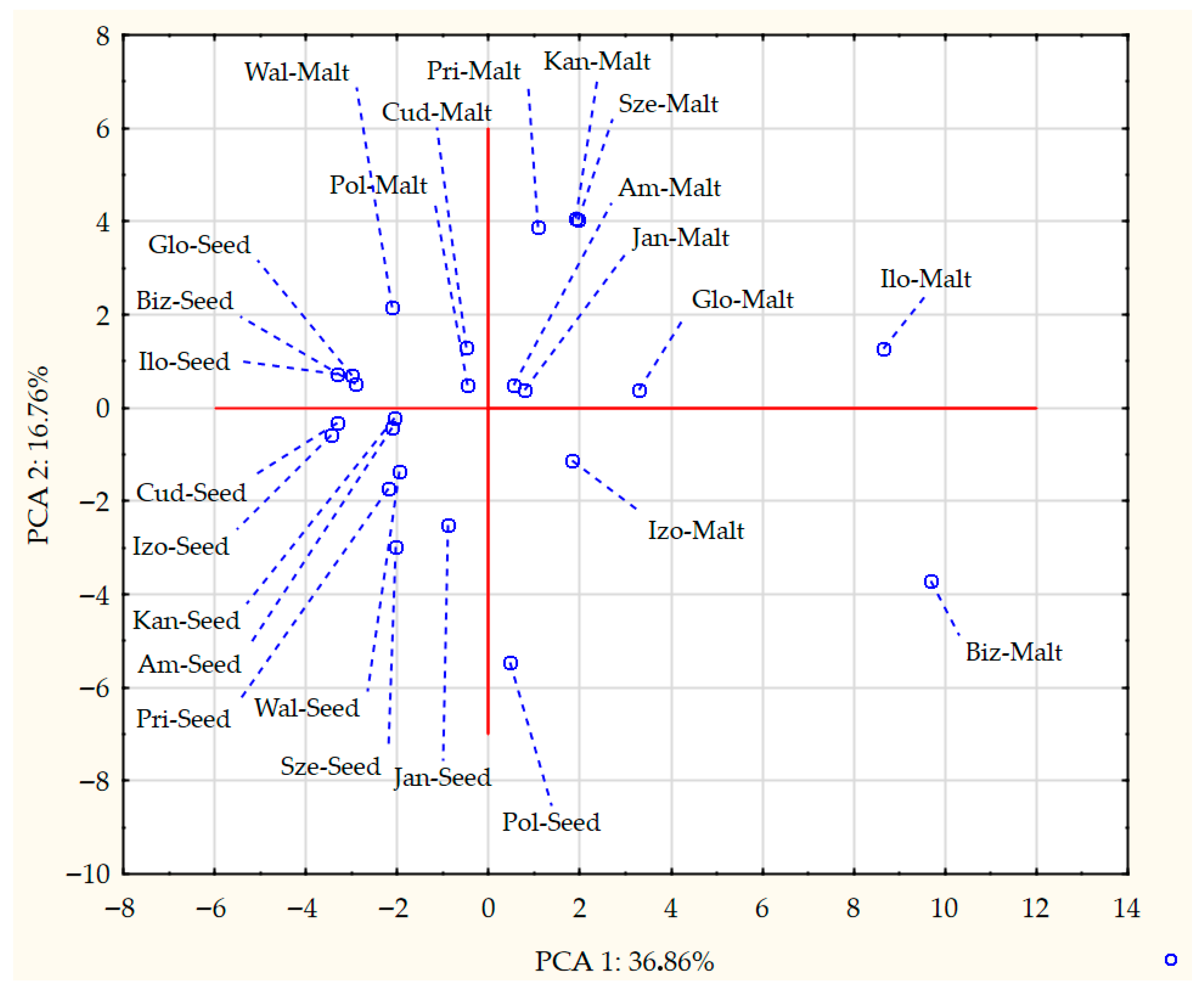
| Sample 1 | Saccharification Time [min] | Filtration Time [min] | Wort Volume [cm3] | Wort Extract [%w/w] | Wort pH | Wort Viscosity [mPa·s] |
|---|---|---|---|---|---|---|
| Am | X | 16 ± 1 i | 205 ± 5 c | 3.60 ± 0.08 j | 6.05 ± 0.02 g | 1.679 ± 0.030 c |
| Biz | X | 21 ± 1 h | 200 cd | 4.13 ± 0.05 h | 6.06 ± 0.01 g | 1.555 ± 0.064 de |
| Cud | X | 48 ± 2 d | 195 ± 5 d | 3.93 ± 0.05 i | 6.09 ± 0.03 ef | 1.660 ± 0.072 c |
| Glo | X | 16 ± 1 i | 205 c | 3.97 ± 0.05 i | 6.20 ± 0.03 c | 1.466 ± 0.042 gh |
| Ilo | X | 23 ± 2 h | 195 ± 5 d | 3.17 ± 0.05 l | 6.26 ± 0.02 ab | 1.525 ± 0.019 e |
| Izo | X | 30 ± 3 g | 195 ± 5 d | 3.27 ± 0.05 k | 5.89 ± 0.03 hi | 1.867 ± 0.027 a |
| Jan | X | 18 ± 1 i | 207.5 ± 2.5 bc | 3.90 ± 0.08 i | 6.28 ± 0.01 a | 1.503 ± 0.004 f |
| Kan | X | 23 ± 1 h | 207.5 ± 2.5 bc | 3.67 ± 0.05 j | 6.19 ± 0.01 c | 1.383 ± 0.056 i |
| Pol | X | 31 ± 2 g | 205 ± 5 c | 4.10 ± 0.08 h | 6.13 ± 0.02 e | 1.484 ± 0.008 g |
| Pri | X | 120 a | 127.5 ± 2.5 g | 3.90 ± 0.08 i | 6.29 ± 0.02 a | 1.657 ± 0.031 c |
| Sze | X | 57 ± 1 c | 175 ± 5 e | 3.17 ± 0.05 l | 6.23 ± 0.02 bc | 1.686 ± 0.023 c |
| Wal | X | 29 ± 1 g | 205 ± 5 c | 2.90 ± 0.08 m | 5.98 ± 0.03 h | 1.560 ± 0.053 e |
| Am-A | X | 17 ± 1 i | 215 ± 5 b | 5.57 ± 0.05 cd | 6.09 ± 0.02 f | 1.485 ± 0.008 g |
| Biz-A | X | 28 ± 2 g | 202.5 ± 2.5 cd | 5.70 ± 0.08 c | 6.11 ± 0.02 e | 1.484 ± 0.009 g |
| Cud-A | X | 38 ± 1 e | 200 ± 5 cd | 5.33 ± 0.05 e | 6.03 ± 0.02 g | 1.600 ± 0.049 d |
| Glo-A | 50a | 17 ± 1 i | 210 ± 5 bc | 5.47 ± 0.05 d | 6.20 ± 0.02 c | 1.533 ± 0.041 ef |
| Ilo-A | X | 35 ± 1 f | 240 ± 10 a | 6.27 ± 0.05 b | 6.27 ± 0.02 ab | 1.590 ± 0.023 d |
| Izo-A | X | 46 ± 2 d | 195 ± 5 d | 5.00 ± 0.03 f | 5.89 ± 0.01 i | 1.750 ± 0.079 bc |
| Jan-A | 50a | 16 ± 1 i | 205 ± 5 c | 5.37 ± 0.05 de | 6.26 ± 0.02 ab | 1.465 ± 0.004 h |
| Kan-A | X | 21 ± 1 h | 205 ± 5 c | 5.60 ± 0.03 cd | 6.16 ± 0.02 d | 1.469 ± 0.008 h |
| Pol-A | X | 29 ± 1 g | 210 b | 5.57 ± 0.07 cd | 6.11 ± 0.02 e | 1.608 ± 0.041 d |
| Pri-A | 50a | 28 ± 1 g | 230 ± 10 a | 6.57 ± 0.07 a | 6.25 ± 0.01 b | 1.507 ± 0.020 f |
| Sze-A | X | 93 ± 3 b | 160 ± 10 e | 6.27 ± 0.05 b | 5.97 ± 0.02 h | 1.812 ± 0.005 b |
| Wal-A | X | 30 ± 2 g | 200 cd | 4.87 ± 0.05 g | 5.93 ± 0.03 hi | 1.810 ± 0.034 b |
| Sample | Glucose Concentration [mg/dm3] | Maltose Concentration [mg/dm3] | Maltotriose Concentration [mg/dm3] | Dextrin Concentration [mg/dm3] |
|---|---|---|---|---|
| Am | 2.0224 ± 0.1096 f | 8.5777 ± 0.5284 ef | 0.3898 ± 0.0461 i | 1.1021 ± 0.1100 g |
| Biz | 1.5674 ± 0.1178 fg | 6.4593 ± 0.5116 g | 0.9856 ± 0.1248 g | 0.9646 ± 0.1062 gh |
| Cud | 1.4249 ± 0.1103 g | 5.400 ± 0.5116 h | 0.3642 ± 0.0311 i | 0.9011 ± 0.1118 h |
| Glo | 1.9063 ± 0.1273 f | 8.1664 ± 0.5093 f | 1.3423 ± 0.0252 f | 1.3834 ± 0.2074 f |
| Ilo | 1.2816 ± 0.1198 h | 5.4738 ± 0.6421 h | 0.7322 ± 0.1002 h | 0.8933 ± 0.0944 h |
| Izo | 1.0458 ± 0.1028 i | 4.3094 ± 0.6356 i | 0.3252 ± 0.0344 i | 0.8660 ± 0.0885 h |
| Jan | 1.7162 ± 0.1011 f | 7.7152 ± 0.5106 f | 0.9146 ± 0.1116 g | 1.2423 ± 0.2112 f |
| Kan | 1.4632 ± 0.1054 g | 5.8443 ± 0.6146 gh | 1.3518 ± 0.0206 f | 1.2034 ± 0.1857 fg |
| Pol | 1.7749 ± 0.1279 f | 7.2586 ± 0.4328 fg | 0.7253 ± 0.1124 h | 1.0349 ± 0.1621 gh |
| Pri | 1.3335 ± 0.1054 h | 5.5951 ± 0.7023 h | 0.6986 ± 0.1214 h | 0.9375 ± 0.1018 gh |
| Sze | 1.3126 ± 0.1099 h | 5.2802 ± 0.6062 hi | 0.6683 ± 0.0621 h | 0.8335 ± 0.0983 h |
| Wal | 1.1256 ± 0.1136 i | 4.1868 ± 0.6172 i | 0.3161 ± 0.0383 i | 1.0394 ± 0.1416 gh |
| Am-A | 4.1447 ± 0.1121 b | 13.1858 ± 0.3311 b | 5.2627 ± 0.3137 c | 10.8356 ± 0.3441 c |
| Biz-A | 4.5375 ± 0.1147 a | 14.8139 ± 0.4084 a | 4.8229 ± 0.5025 cd | 9.7117 ± 0.4052 d |
| Cud-A | 3.7745 ± 0.1033 d | 11.2798 ± 0.4206 cd | 4.1326 ± 0.3223 e | 10.9304 ± 0.4140 c |
| Glo-A | 4.3621 ± 0.1645 ab | 14.6471 ± 0.3363 a | 4.7837 ± 0.3279 d | 9.7221 ± 0.4570 d |
| Ilo-A | 3.8318 ± 0.1045 cd | 10.6672 ± 0.3123 d | 6.8807 ± 0.4169 b | 6.3376 ± 0.4453 e |
| Izo-A | 3.1349 ± 0.1103 e | 9.0223 ± 0.6127 e | 4.7699 ± 0.4127 d | 13.8059 ± 0.3281 a |
| Jan-A | 4.0424 ± 0.1282 bc | 14.1430 ± 0.5642 ab | 4.5585 ± 0.3827 de | 9.0408 ± 0.5386 d |
| Kan-A | 4.0086 ± 0.1099 bc | 13.5496 ± 0.4710 b | 4.9639 ± 0.4081 cd | 10.7546 ± 0.3158 c |
| Pol-A | 4.1205 ± 0.1211 bc | 11.9284 ± 0.4096 c | 7.8676 ± 0.4297 a | 6.8969 ± 0.3033 e |
| Pri-A | 3.9144 ± 0.1190 c | 13.1605 ± 0.3208 b | 5.3336 ± 0.4119 c | 12.0555 ± 0.4771 b |
| Sze-A | 3.6933 ± 0.1152 d | 9.8168 ± 0.6212 de | 7.1031 ± 0.5347 ab | 6.6166 ± 0.4030 e |
| Wal-A | 3.1894 ± 0.1139 e | 9.0664 ± 0.5334 e | 5.0445 ± 0.4330 c | 13.8469 ± 0.2080 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasiński, A.; Pietrzak, W.; Śniegowska, J.; Kawa-Rygielska, J. Influence of Malting on Volatile Composition and Technological Properties of Polish Pea Varieties. Foods 2025, 14, 3224. https://doi.org/10.3390/foods14183224
Gasiński A, Pietrzak W, Śniegowska J, Kawa-Rygielska J. Influence of Malting on Volatile Composition and Technological Properties of Polish Pea Varieties. Foods. 2025; 14(18):3224. https://doi.org/10.3390/foods14183224
Chicago/Turabian StyleGasiński, Alan, Witold Pietrzak, Joanna Śniegowska, and Joanna Kawa-Rygielska. 2025. "Influence of Malting on Volatile Composition and Technological Properties of Polish Pea Varieties" Foods 14, no. 18: 3224. https://doi.org/10.3390/foods14183224
APA StyleGasiński, A., Pietrzak, W., Śniegowska, J., & Kawa-Rygielska, J. (2025). Influence of Malting on Volatile Composition and Technological Properties of Polish Pea Varieties. Foods, 14(18), 3224. https://doi.org/10.3390/foods14183224






