Prebiotic Effect of Polysaccharides and Flavonoids from Passiflora foetida Fruits on the Human Intestinal Microbiota Associated with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyses of the Two Aqueous-Extracted Fractions
2.2. Dynamic Simulator of the Gastrointestinal Tract
2.3. Experimental Protocol Using the BFBL Gut Model
2.4. Microbiota Composition Analysis
2.5. Metabolite Production Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Compositions of PFP and PFF
3.2. The Obesity-Associated Microbiota Stabilized in the BFBL Gut Model
3.3. The Production of Metabolites During the Stabilization Period of Normal Weight and Obesity Associated Microbiota
3.4. Impact of PFP and PFF Feeding on the Obese Microbiota
3.5. Impacts of PFP and PFF Consumption on Metabolite Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineerig 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Ward, R.E.; Benninghoff, A.D.; Hintze, K.J. Food matrix and the microbiome: Considerations for preclinical chronic disease studies. Nutr. Res. 2020, 78, 1–10. [Google Scholar] [CrossRef]
- Hegazi, R.; Halpern, B. Looking beyond fat in obesity: The frequently overlooked importance of muscle mass. Rev. Endocr. Metab. Disord. 2025, 26, 165–182. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Li, Y.; Liu, W.; Ding, Z.; Ma, H.; Seeram, N.P.; Mu, Y.; Huang, X.; Li, L. Jamun (Eugenia jambolana Lam.) Fruit Extract Prevents Obesity by Modulating the Gut Microbiome in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801307. [Google Scholar] [CrossRef] [PubMed]
- Agrinier, A.L.; Morissette, A.; Daoust, L.; Gignac, T.; Marois, J.; Varin, T.V.; Pilon, G.; Larose, R.; Gagnon, C.; Desjardins, Y.; et al. Camu-camu decreases hepatic steatosis and liver injury markers in overweight, hypertriglyceridemic individuals: A randomized crossover trial. Cell Rep. Med. 2024, 5, 101682. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Arunachalam, K.; Parimelazhagan, T. Antioxidant, analgesic, anti-inflammatory and antipyretic effects of polyphenols from Passiflora subpeltata leaves—A promising species of Passiflora. Ind. Crop Prod. 2014, 54, 272–280. [Google Scholar] [CrossRef]
- Han, X.; Song, Y.; Huang, R.; Zhu, M.; Li, M.; Requena, T.; Wang, H. Anti-inflammatory and gut microbiota modulation potentials of flavonoids extracted from Passiflora foetida Fruits. Foods 2023, 12, 2889. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, M.; Hao, H.; Deng, J.; Li, M.; Sun, Y.; Yang, R.; Wang, H.; Huang, R. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. Int. J. Biol. Macromol. 2019, 136, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Marcó, A.; Rubio, R.; Compañó, R.; Casals, I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002, 5, 1019–1026. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists International: Arlington, VA, USA, 2005. [Google Scholar]
- Zhang, X.; An, S.; Zhou, L.; Chen, C.; Yang, X. Structural Characterization and Anti-Gout Activity of a Novel Acidic Sanghuangporus vaninii Polysaccharide. Molecules 2025, 30, 3536. [Google Scholar] [CrossRef]
- Requena, T.; Song, Y.; Peláez, C.; Martínez-Cuesta, M.C. Modulation and metabolism of obesity-associated microbiota in a dynamic simulator of the human gut microbiota. LWT–Food Sci. Technol. 2021, 141, 110921. [Google Scholar] [CrossRef]
- Barroso, E.; Cueva, C.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Development of human colonic microbiota in the computer-controlled dynamic SIMulator of the GastroIntestinal tract SIMGI. LWT–Food Sci. Technol. 2015, 61, 283–289. [Google Scholar] [CrossRef]
- Kondo, R.; Nedwell, D.B.; Purdy, K.J.; Silva, S.Q. Detection and Enumeration of Sulphate-Reducing Bacteria in Estuarine Sediments by Competitive PCR. Geomicrobiol. J. 2004, 21, 145–157. [Google Scholar] [CrossRef]
- Baldwin, J.; Collins, B.; Wolf, P.G.; Martinez, K.; Shen, W.; Chuang, C.C.; Zhong, W.; Cooney, P.; Cockrell, C.; Chang, E.; et al. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J. Nutr. Biochem. 2016, 27, 123–135. [Google Scholar] [CrossRef]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 2003, 55, 541–555. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microb. 2004, 70, 7220–7228. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R.; Poulsen, S.K.; Larsen, T.M.; Bahl, M.I. Microbial enterotypes, inferred by the Prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new nordic diet. Appl. Environ. Microb. 2014, 80, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.L.R.F.; Monteiro, M.; Adorno, M.A.T.; Sakamoto, I.K.; Sivieri, K. An exploratory study on the influence of orange juice on gut microbiota using a dynamic colonic model. Food Res. Int. 2016, 84, 160–169. [Google Scholar] [CrossRef]
- Werner, B.; Fujii, H. Prokinetic and antispasmodic constituent discovered in Perilla Frutescens—Development of a gut health ingredient. Planta Medica 2012, 78, CL61. [Google Scholar]
- Duan, R.; Huang, K.; Guan, X.; Li, S.; Xia, J.; Shen, M.; Sun, Z.; Yu, Z. Tectorigenin ameliorated high-fat diet-induced nonalcoholic fatty liver disease through anti-inflammation and modulating gut microbiota in mice. Food Chem. Toxicol. 2022, 164, 112948. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Duque, A.; Saad, S.M.I.; Sivieri, K. Gut microbiome approaches to treat obesity in humans. Appl. Microbiol. Biotechnol. 2019, 103, 1081–1094. [Google Scholar] [CrossRef]
- Frederik, L.M.; Wange, L.M.; Vendelbo, L.M.; Anni, L.; Christian, M.; Michaelsen, K.F.; Iain, B.M.; Rask, L.T. Intestinal Enterococcus abundance correlates inversely with excessive weight gain and increased plasma leptin in breastfed infants. FEMS Microbiol. Ecol. 2020, 96, fiaa066. [Google Scholar]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Ignacio, A.; Fernandes, M.R.; Rodrigues, V.A.; Groppo, F.C.; Cardoso, A.L.; Avila-Campos, M.J.; Nakano, V. Correlation between body mass index and faecal microbiota from children. Clin. Microbiol. Infect. 2016, 22, 258.e1–258.e8. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Castaneda-Marquez, A.C.; Diaz-Benitez, C.E.; Bahena-Roman, M.; Campuzano-Benitez, G.E.; Galvan-Portillo, M.; Campuzano-Rincon, J.C.; Lagunas-Martinez, A.; Bermudez-Morales, V.H.; Orbe-Orihuela, Y.C.; Peralta-Romero, J.; et al. Lactobacillus paracasei as a protective factor of obesity induced by an unhealthy diet in children. Obes. Res. Clin. Pract. 2020, 14, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Joerg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 44, 817–829. [Google Scholar] [CrossRef]
- Vermeiren, J.; Van den Abbeele, P.; Laukens, D.; Vigsnaes, L.K.; De Vos, M.; Boon, N.; Van de Wiele, T. Decreased colonization of fecal Clostridium coccoides/Eubacterium rectale species from ulcerative colitis patients in an in vitro dynamic gut model with mucin environment. FEMS Microbiol. Ecol. 2012, 79, 685–696. [Google Scholar] [CrossRef]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, O.; Takahashi, T.; Asahara, T.; Nomoto, K.; Onodera, H. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig. Dis. Sci. 2013, 58, 1717–1726. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Din, A.U.; Hassan, A.; Wang, Y.; Wang, G. Beneficial effects of Enterococcus faecalis in hypercholesterolemic mice on cholesterol transportation and gut microbiota. Appl. Microbiol. Biotechnol. 2019, 103, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tian, Y.; Yu, J.; Zhang, R.; Zhang, X.; Guo, P. The pandanus tectorius fruit extract (PTF) modulates the gut microbiota and exerts anti-hyperlipidaemic effects. Phytomedicine 2019, 58, 152863. [Google Scholar] [CrossRef]
- Cani, P.D. Is colonic propionate delivery a novel solution to improve metabolism and inflammation in overweight or obese subjects? Gut 2019, 68, 1352–1353. [Google Scholar] [CrossRef]

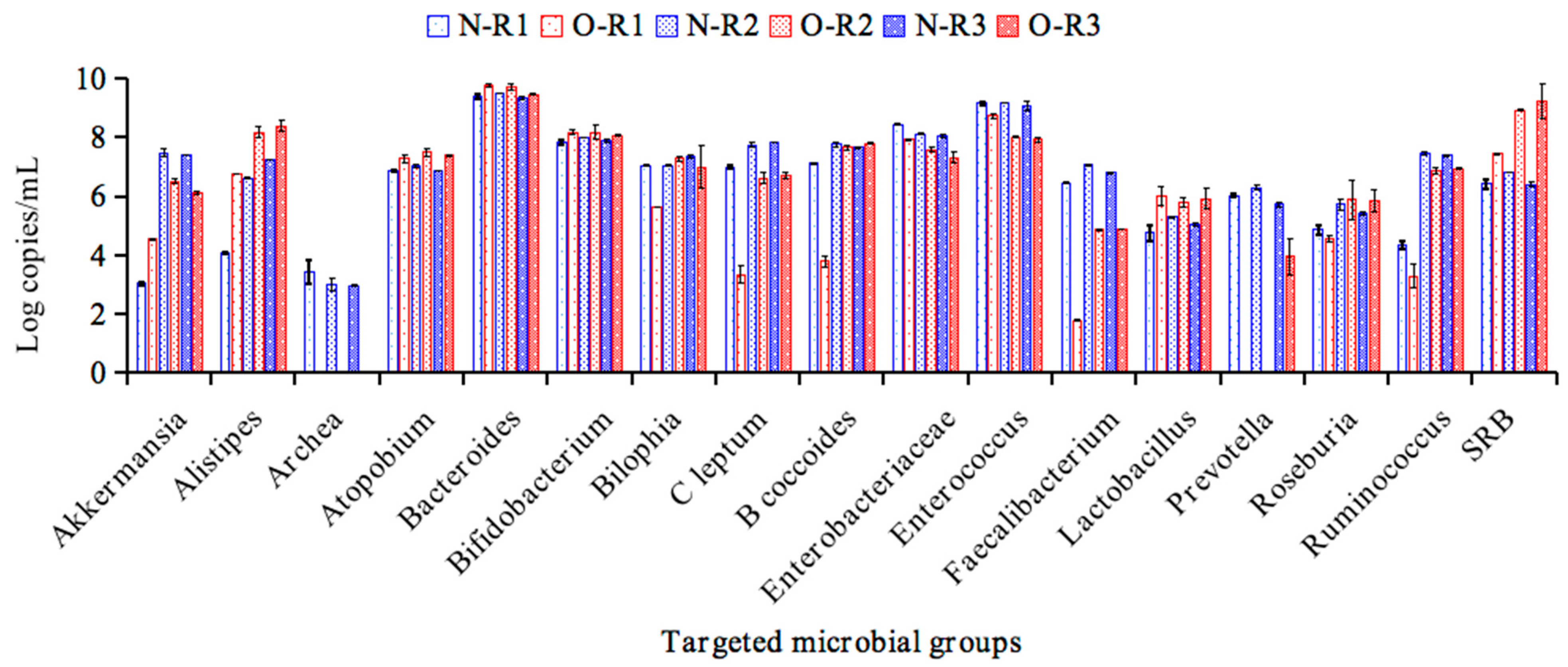

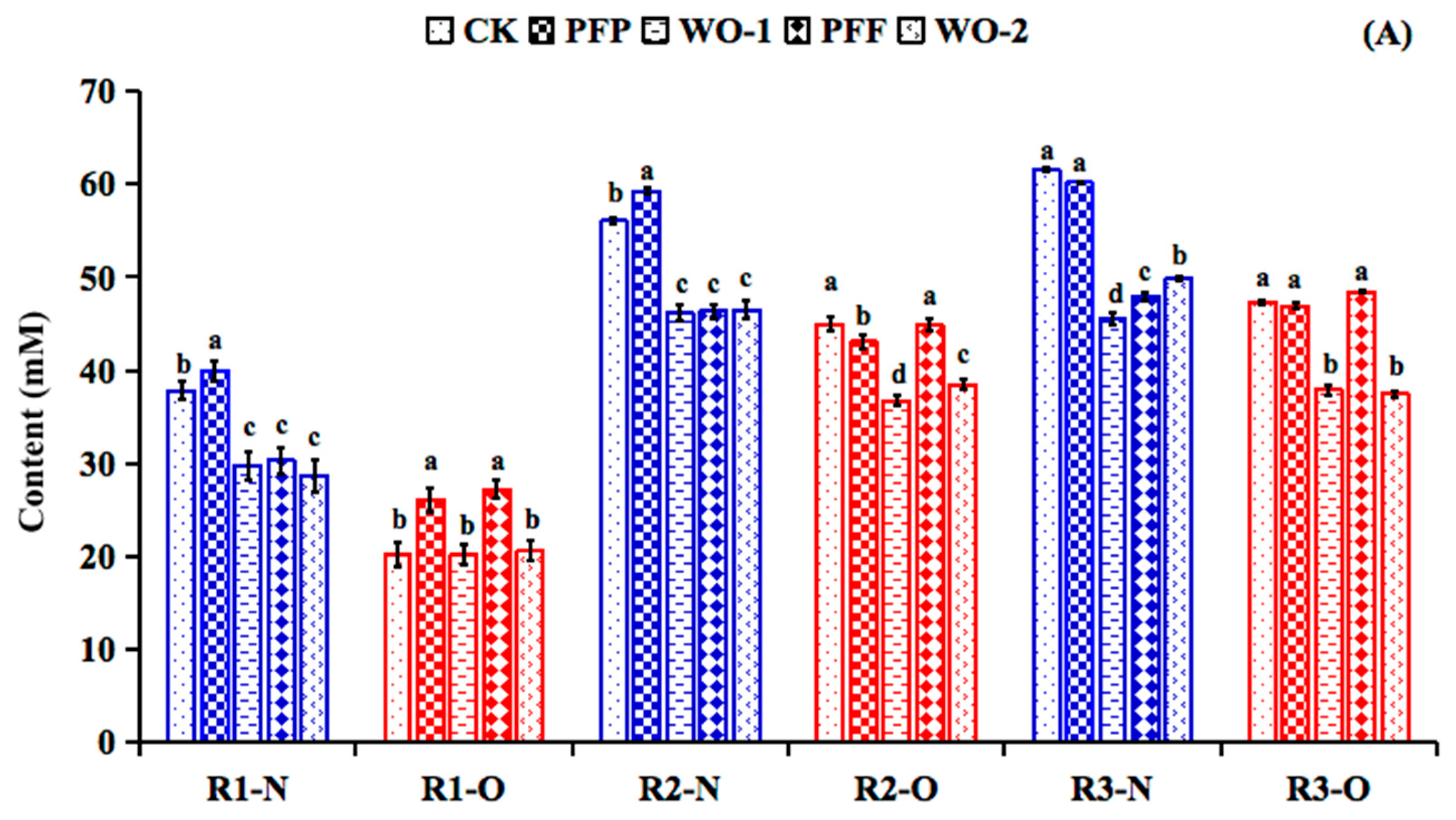
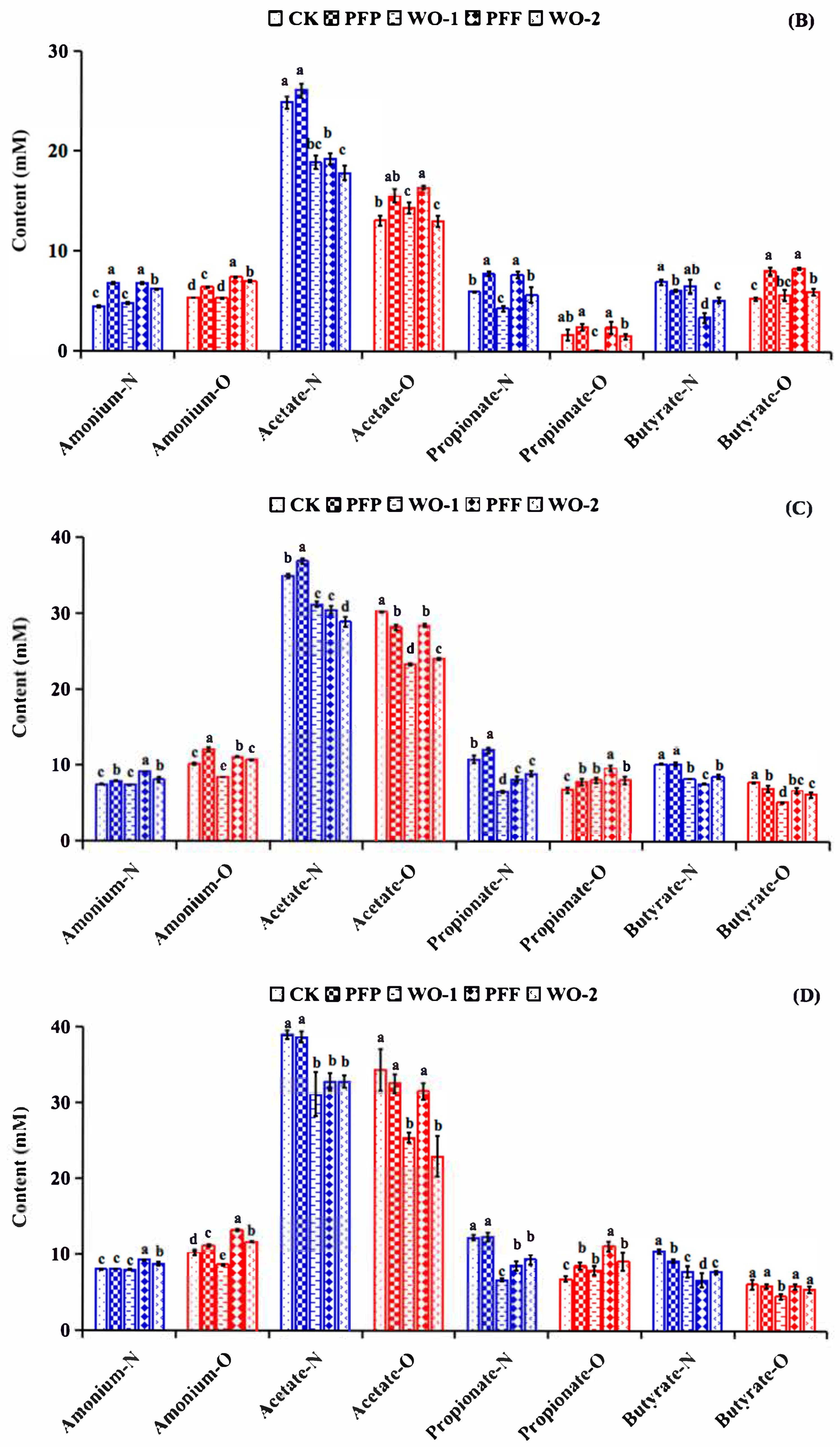
| Bacterial Group | Regions | CK | PFP | WO-1 | PFF | WO-2 |
|---|---|---|---|---|---|---|
| Akkermansia | R1-N | 3.04 ± 0.05 d | 3.09 ± 0.09 d | 4.25 ± 0.07 c | 4.42 ± 0.06 b | 4.70 ± 0.13 a |
| R1-O | 4.54 ± 0.02 a | 3.99 ± 0.09 c | 4.33 ± 0.25 ab | 4.09 ± 0.04 bc | 3.86 ± 0.06 c | |
| R2-N | 7.48 ± 0.13 b | 7.77 ± 0.13 b | 7.45 ± 0.17 b | 8.22 ± 0.13 a | 8.43 ± 0.06 a | |
| R2-O | 6.50 ± 0.07 bc | 6.05 ± 0.55 c | 6.93 ± 0.05 b | 7.64 ± 0.20 a | 7.77 ± 0.14 a | |
| R3-N | 7.39 ± 0.01 c | 7.87 ± 0.19 b | 7.73 ± 0.03 b | 8.31 ± 0.01 a | 8.35 ± 0.02 a | |
| R3-O | 6.12 ± 0.03 c | 6.25 ± 0.07 c | 6.55 ± 0.14 b | 7.62 ± 0.19 a | 7.61 ± 0.07 a | |
| Alistipes | R1-N | 4.07 ± 0.03 b | 5.01 ± 0.03 a | 3.54 ± 0.05 c | 3.91 ± 0.24 b | 3.47 ± 0.02 c |
| R1-O | 6.76 ± 0.01 a | 5.85 ± 0.47 b | 5.17 ± 0.09 b | 5.54 ± 0.08 b | 5.56 ± 0.33 b | |
| R2-N | 6.62 ± 0.03 c | 7.60 ± 0.21 b | 7.96 ± 0.04a | 7.79 ± 0.08 b | 7.92 ± 0.02 a | |
| R2-O | 8.17 ± 0.18 a | 7.53 ± 0.09 b | 7.77 ± 0.11 b | 7.54 ± 0.15 b | 7.79 ± 0.13 b | |
| R3-N | 7.23 ± 0.01 d | 7.66 ± 0.02 b | 7.94 ± 0.02a | 7.50 ± 0.04 c | 7.67 ± 0.11 b | |
| R3-O | 8.39 ± 0.13 a | 7.47 ± 0.21 b | 8.25 ± 0.20 a | 7.43 ± 0.18 b | 8.12 ± 0.15 a | |
| Archaea | R1-N | 3.44 ± 0.39 ab | 2.98 ± 0.19 bc | 3.64 ± 0.05 a | 2.50 ± 0.21 c | 3.46 ± 0.24 ab |
| R1-O | ND c | 2.16 ± 0.58 b | 1.94 ± 0.44 b | 2.49 ± 0.38 b | 3.72 ± 0.18 a | |
| R2-N | 2.99 ± 0.21 | 3.36 ± 0.14 | 2.85 ± 0.50 | 2.86 ± 0.48 | 3.40 ± 0.24 | |
| R2-O | ND d | 1.57 ± 0.09 c | 2.26 ± 0.29 b | 2.15 ± 0.25 b | 3.26 ± 0.41 a | |
| R3-N | 2.97 ± 0.01 bc | 3.77 ± 0.35 a | 2.92 ± 0.16 c | 2.60 ± 0.05 c | 3.36 ± 0.08 b | |
| R3-O | ND c | 1.15 ± 0.17 b | 1.37 ± 0.62 b | 1.85 ± 0.25 ab | 2.42 ± 0.35 a | |
| Atopobium | R1-N | 6.87 ± 0.03 d | 7.59 ± 0.13 a | 7.36 ± 0.03 b | 7.06 ± 0.14 c | 7.31 ± 0.02 b |
| R1-O | 7.28 ± 0.30 | 7.36 ± 0.07 | 7.66 ± 0.15 | 7.53 ± 0.04 | 7.48 ± 0.07 | |
| R2-N | 7.00 ± 0.06 | 7.60 ± 0.09 | 7.12 ± 0.04 | 7.20 ± 0.20 | 7.18 ± 0.02 | |
| R2-O | 7.49 ± 0.21 b | 7.60 ± 0.09 b | 7.65 ± 0.01 a | 7.43 ± 0.02 b | 7.49 ± 0.01 ab | |
| R3-N | 6.85 ± 0.01 c | 7.30 ± 0.05 a | 7.03 ± 0.01 b | 7.05 ± 0.02 b | 7.09 ± 0.05 b | |
| R3-O | 7.39 ± 0.03 b | 7.39 ± 0.03 b | 7.80 ± 0.12 a | 7.28 ± 0.13 b | 7.66 ± 0.29 ab | |
| B. coccoides | R1-N | 7.12 ± 0.02 d | 7.98 ± 0.02 a | 7.67 ± 0.13 b | 7.42 ± 0.14 c | 7.93 ± 0.12 a |
| R1-O | 3.81 ± 0.17 | 4.22 ± 0.15 | 4.03 ± 0.25 | 3.75 ± 0.24 | 4.10 ± 0.20 | |
| R2-N | 7.76 ± 0.09 b | 8.02 ± 0.12 a | 7.97 ± 0.02 a | 7.79 ± 0.12 ab | 7.98 ± 0.01 a | |
| R2-O | 7.65 ± 0.07 b | 7.95 ± 0.02 a | 7.76 ± 0.12 b | 7.61 ± 0.07 b | 7.63 ± 0.01 b | |
| R3-N | 7.66 ± 0.02 b | 7.99 ± 0.18 a | 7.89 ± 0.01 a | 7.64 ± 0.07 b | 7.86 ± 0.03 a | |
| R3-O | 7.80 ± 0.01 a | 7.56 ± 0.07 ab | 7.48 ± 0.23 b | 7.52 ± 0.07 ab | 7.18 ± 0.09 c | |
| Bacteroides | R1-N | 9.39 ± 0.06 b | 9.79 ± 0.10 a | 9.81 ± 0.06 a | 9.54 ± 0.16 b | 9.80 ± 0.03 a |
| R1-O | 9.77 ± 0.06 ab | 9.60 ± 0.04 bc | 9.88 ± 0.03 a | 9.51 ± 0.15 c | 9.70 ± 0.10 abc | |
| R2-N | 9.50 ± 0.01 | 9.63 ± 0.22 | 9.68 ± 0.01 | 9.60 ± 0.19 | 9.67 ± 0.02 | |
| R2-O | 9.71 ± 0.10 ab | 9.55 ± 0.10 bc | 9.84 ± 0.03 a | 9.42 ± 0.10 c | 9.71 ± 0.12 ab | |
| R3-N | 9.34 ± 0.03 | 9.63 ± 0.24 | 9.56 ± 0.01 | 9.59 ± 0.11 | 9.56 ± 0.03 | |
| R3-O | 9.47 ± 0.04 ab | 9.14 ± 0.04 c | 9.60 ± 0.02 a | 9.14 ± 0.20 c | 9.30 ± 0.05 bc | |
| Bifidobacterium | R1-N | 7.81 ± 0.08 c | 8.31 ± 0.12 a | 8.32 ± 0.05 a | 7.72 ± 0.05 d | 8.09 ± 0.06 b |
| R1-O | 8.17 ± 0.08 ab | 8.20 ± 0.06 ab | 8.34 ± 0.18 a | 8.22 ± 0.04 ab | 8.01 ± 0.25 b | |
| R2-N | 7.99 ± 0.01 | 8.11 ± 0.15 | 8.21 ± 0.01 | 7.84 ± 0.61 | 8.07 ± 0.06 | |
| R2-O | 8.18 ± 0.26 | 8.37 ± 0.20 | 8.24 ± 0.24 | 8.10 ± 0.14 | 8.04 ± 0.01 | |
| R3-N | 7.88 ± 0.03 b | 8.23 ± 0.23 a | 8.00 ± 0.15 ab | 8.12 ± 0.07 ab | 8.02 ± 0.05 ab | |
| R3-O | 8.06 ± 0.03 | 8.04 ± 0.07 | 8.08 ± 0.35 | 7.87 ± 0.18 | 7.83 ± 0.18 | |
| Bilophila | R1-N | 7.05 ± 0.01 b | 7.47 ± 0.03 a | 7.42 ± 0.04 a | 7.16 ± 0.24 b | 7.56 ± 0.03 a |
| R1-O | 5.62 ± 0.01 d | 7.10 ± 0.16 b | 7.56 ± 0.02 a | 7.17 ± 0.10 b | 6.35 ± 0.04 c | |
| R2-N | 7.04 ± 0.02 b | 7.46 ± 0.11 a | 7.36 ± 0.01 a | 7.35 ± 0.03 a | 7.45 ± 0.01 a | |
| R2-O | 7.29 ± 0.05 b | 7.45 ± 0.06 a | 7.49 ± 0.13 a | 7.39 ± 0.08 a | 6.66 ± 0.23 c | |
| R3-N | 7.35 ± 0.05 | 7.45 ± 0.23 | 7.36 ± 0.06 | 7.34 ± 0.10 | 7.43 ± 0.03 | |
| R3-O | 6.99 ± 0.73 c | 7.39 ± 0.01 ab | 7.65 ± 0.04 a | 7.28 ± 0.26 bc | 6.28 ± 0.01 d | |
| C. leptum | R1-N | 7.00 ± 0.06 bc | 7.48 ± 0.09 a | 7.35 ± 0.01 ab | 6.66 ± 0.44 c | 7.26 ± 0.11 ab |
| R1-O | 3.34 ± 0.22 | 3.11 ± 0.30 | 3.55 ± 0.28 | 3.15 ± 0.19 | 3.10 ± 0.18 | |
| R2-N | 7.74 ± 0.08 ab | 8.02 ± 0.20 a | 7.99 ± 0.01 a | 7.79 ± 0.17 ab | 7.60 ± 0.04 b | |
| R2-O | 6.61 ± 0.17 | 6.84 ± 0.47 | 6.82 ± 0.10 | 6.59 ± 0.09 | 6.69 ± 0.02 | |
| R3-N | 7.83 ± 0.01 bc | 8.12 ± 0.12 a | 7.94 ± 0.06 b | 7.77 ± 0.08 c | 7.55 ± 0.05 d | |
| R3-O | 6.71 ± 0.12 | 6.41 ± 0.37 | 6.82 ± 0.30 | 6.60 ± 0.28 | 6.77 ± 0.13 | |
| Enterobacteriaceae | R1-N | 8.44 ± 0.01 a | 8.03 ± 0.12 bc | 8.31 ± 0.11 ab | 7.79 ± 0.33 c | 8.23 ± 0.05 ab |
| R1-O | 7.92 ± 0.02 a | 7.36 ± 0.13 c | 7.41 ± 0.15 c | 7.40 ± 0.09 c | 7.66 ± 0.02 b | |
| R2-N | 8.12 ± 0.03 a | 7.78 ± 0.09 b | 7.97 ± 0.06 ab | 7.87 ± 0.25 b | 7.85 ± 0.04 b | |
| R2-O | 7.59 ± 0.10 a | 7.01 ± 0.23 b | 7.36 ± 0.05 a | 7.36 ± 0.09 a | 7.45 ± 0.06 a | |
| R3-N | 8.06 ± 0.05 a | 7.93 ± 0.07 b | 7.86 ± 0.04 b | 7.87 ± 0.02 b | 7.92 ± 0.01 b | |
| R3-O | 7.32 ± 0.17 a | 6.82 ± 0.09 b | 7.29 ± 0.02 a | 7.22 ± 0.15 a | 7.28 ± 0.15 a | |
| Enterococcus | R1-N | 9.16 ± 0.04 d | 10.00 ± 0.40 b | 9.66 ± 0.04 c | 9.32 ± 0.18 d | 11.63 ± 0.06 a |
| R1-O | 8.73 ± 0.06 a | 6.04 ± 0.03 d | 8.65 ± 0.05 a | 8.45 ± 0.02 b | 7.91 ± 0.15 c | |
| R2-N | 9.17 ± 0.01 cd | 9.87 ± 0.10 b | 9.24 ± 0.26 bc | 8.88 ± 0.51 d | 10.39 ± 0.01 a | |
| R2-O | 8.02 ± 0.02 bc | 6.36 ± 0.10 d | 8.60 ± 0.14 a | 8.20 ± 0.07 b | 7.80 ± 0.30 c | |
| R3-N | 9.06 ± 0.15 bc | 10.03 ± 0.13 a | 9.40 ± 0.01 b | 8.89 ± 0.39 c | 9.87 ± 0.05 a | |
| R3-O | 7.93 ± 0.08 b | 6.93 ± 0.19 d | 8.44 ± 0.04 a | 7.83 ± 0.01 b | 7.47 ± 0.05 c | |
| Faecalibacterium | R1-N | 6.46 ± 0.02 b | 6.97 ± 0.12 a | 6.53 ± 0.05 b | 6.22 ± 0.13 c | 6.14 ± 0.02 c |
| R1-O | 1.78 ± 0.02 c | 2.78 ± 0.14 b | 1.87 ± 0.07 c | 3.14 ± 0.11 a | 3.33 ± 0.15 a | |
| R2-N | 7.06 ± 0.01 a | 7.00 ± 0.24 ab | 6.77 ± 0.03 b | 6.55 ± 0.27 c | 6.63 ± 0.01 c | |
| R2-O | 4.86 ± 0.04 c | 5.72 ± 0.14 a | 5.25 ± 0.10 b | 5.60 ± 0.09 a | 4.94 ± 0.08 c | |
| R3-N | 6.79 ± 0.01 b | 6.99 ± 0.10 a | 6.37 ± 0.04 d | 6.66 ± 0.04 c | 6.44 ± 0.01 d | |
| R3-O | 4.87 ± 0.01 b | 5.25 ± 0.06 a | 5.01 ± 0.11 b | 5.21 ± 0.06 a | 4.96 ± 0.11 ab | |
| Lactobacillus | R1-N | 4.75 ± 0.25 c | 4.68 ± 0.12 c | 4.77 ± 0.08 c | 5.09 ± 0.11 b | 5.37 ± 0.05 a |
| R1-O | 6.00 ± 0.30 b | 5.01 ± 0.21 c | 7.31 ± 0.21 a | 7.25 ± 0.20 a | 7.24 ± 0.03 a | |
| R2-N | 5.30 ± 0.03 a | 4.68 ± 0.23 b | 4.95 ± 0.10 b | 5.46 ± 0.08 a | 4.91 ± 0.06 b | |
| R2-O | 5.81 ± 0.47 b | 5.33 ± 0.15 b | 7.11 ± 0.23 a | 7.07 ± 0.29 a | 7.02 ± 0.26 a | |
| R3-N | 5.04 ± 0.03 c | 4.55 ± 0.07 e | 4.72 ± 0.02 d | 5.28 ± 0.17 b | 5.82 ± 0.01 a | |
| R3-O | 5.92 ± 0.35 b | 4.82 ± 0.31 c | 6.94 ± 0.50 a | 7.05 ± 0.29 a | 6.99 ± 0.08 a | |
| Prevotella | R1-N | 6.02 ± 0.06 a | 5.60 ± 0.06 b | 5.52 ± 0.06 b | 5.17 ± 0.18 c | 5.55 ± 0.10 b |
| R1-O | ND b | 4.33 ± 0.49 a | 4.90 ± 0.29 a | 4.14 ± 0.57 a | 4.34 ± 0.50 a | |
| R2-N | 6.30 ± 0.06 a | 5.80 ± 0.21 b | 5.41 ± 0.19 b | 5.32 ± 0.28 b | 5.59 ± 0.13 b | |
| R2-O | ND b | 4.12 ± 0.54 a | 4.54 ± 0.23 a | 4.54 ± 0.15 a | 4.29 ± 0.09 a | |
| R3-N | 5.71 ± 0.07 ab | 5.79 ± 0.01 a | 5.19 ± 0.11 d | 5.37 ± 0.16 cd | 5.53 ± 0.12 bc | |
| R3-O | 3.95 ± 0.43 | 4.65 ± 0.54 | 4.33 ± 0.13 | 4.40 ± 0.26 | 4.09 ± 0.15 | |
| Roseburia | R1-N | 4.86 ± 0.15 | 5.42 ± 0.15 | 5.57 ± 0.01 | 5.19 ± 0.71 | 5.58 ± 0.05 |
| R1-O | 4.56 ± 0.10 a | 3.75 ± 0.29 b | 4.32 ± 0.19 a | 3.78 ± 0.05 b | 3.72 ± 0.18 b | |
| R2-N | 5.71 ± 0.17 | 5.75 ± 0.16 | 5.80 ± 0.01 | 5.77 ± 0.81 | 6.24 ± 0.01 | |
| R2-O | 5.88 ± 0.66 | 5.39 ± 0.32 | 5.76 ± 0.17 | 5.56 ± 0.21 | 5.73 ± 0.26 | |
| R3-N | 5.41 ± 0.06 | 5.73 ± 0.16 | 5.62 ± 0.03 | 5.70 ± 0.49 | 5.97 ± 0.07 | |
| R3-O | 5.83 ± 0.37 | 5.13 ± 0.17 | 5.91 ± 0.27 | 5.32 ± 0.19 | 5.63 ± 0.44 | |
| Ruminococcus | R1-N | 4.35 ± 0.13 ab | 4.15 ± 0.12 b | 4.58 ± 0.21 a | 3.75 ± 0.02 c | 4.26 ± 0.06 b |
| R1-O | 3.29 ± 0.40 | 3.62 ± 0.25 | 3.73 ± 0.21 | 3.58 ± 0.34 | 3.72 ± 0.03 | |
| R2-N | 7.45 ± 0.06 ab | 7.58 ± 0.20 a | 7.53 ± 0.03 a | 7.00 ± 0.22 c | 7.05 ± 0.19 bc | |
| R2-O | 6.87 ± 0.12 a | 6.92 ± 0.07 a | 6.78 ± 0.18 a | 6.41 ± 0.04 b | 6.40 ± 0.03 b | |
| R3-N | 7.39 ± 0.03 b | 7.72 ± 0.14 a | 7.52 ± 0.03 ab | 7.06 ± 0.10 c | 6.95 ± 0.09 c | |
| R3-O | 6.94 ± 0.02 a | 7.09 ± 0.07 a | 6.92 ± 0.39 a | 6.78 ± 0.20 ab | 6.37 ± 0.06 b | |
| SRB | R1-N | 6.42 ± 0.07 b | 7.95 ± 0.06 a | 8.79 ± 0.19 a | 8.27 ± 0.62 a | 9.17 ± 0.02 a |
| R1-O | 7.43 ± 0.02 e | 8.17 ± 0.02 d | 9.08 ± 0.01 a | 8.61 ± 0.02 c | 8.94 ± 0.10 b | |
| R2-N | 6.83 ± 0.01 c | 7.88 ± 0.78 b | 9.20 ± 0.13 a | 8.88 ± 0.53 a | 9.08 ± 0.04 a | |
| R2-O | 8.93 ± 0.04 ab | 9.09 ± 0.04 a | 9.08 ± 0.05 a | 8.77 ± 0.15 b | 8.86 ± 0.09 b | |
| R3-N | 6.41 ± 0.10 d | 9.04 ± 0.66 bc | 9.17 ± 0.05 b | 8.41 ± 0.51 c | 9.43 ± 0.11 a | |
| R3-O | 9.24 ± 0.60 | 9.00 ± 0.06 | 9.14 ± 0.04 | 8.56 ± 0.15 | 9.52 ± 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhu, M.; Martínez-Cuesta, M.C.; Requena, T. Prebiotic Effect of Polysaccharides and Flavonoids from Passiflora foetida Fruits on the Human Intestinal Microbiota Associated with Obesity. Foods 2025, 14, 3222. https://doi.org/10.3390/foods14183222
Song Y, Zhu M, Martínez-Cuesta MC, Requena T. Prebiotic Effect of Polysaccharides and Flavonoids from Passiflora foetida Fruits on the Human Intestinal Microbiota Associated with Obesity. Foods. 2025; 14(18):3222. https://doi.org/10.3390/foods14183222
Chicago/Turabian StyleSong, Ya, Minqian Zhu, M. Carmen Martínez-Cuesta, and Teresa Requena. 2025. "Prebiotic Effect of Polysaccharides and Flavonoids from Passiflora foetida Fruits on the Human Intestinal Microbiota Associated with Obesity" Foods 14, no. 18: 3222. https://doi.org/10.3390/foods14183222
APA StyleSong, Y., Zhu, M., Martínez-Cuesta, M. C., & Requena, T. (2025). Prebiotic Effect of Polysaccharides and Flavonoids from Passiflora foetida Fruits on the Human Intestinal Microbiota Associated with Obesity. Foods, 14(18), 3222. https://doi.org/10.3390/foods14183222







