Dietary Dityrosine Impairs Glucose Homeostasis by Disrupting Thyroid Hormone Signaling in Pancreatic β-Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Pork Sample Preparation
2.2. Animals and Experimental Design
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Tissue Sample Collection
2.5. Determination of Oxidative Damage and Oxidative Stress Status in Mice
2.6. Analysis of Inflammatory Cytokines and Metabolic Hormones
2.7. Histological Analysis of the Pancreas
2.8. Gene Expression Analysis by RT qPCR
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
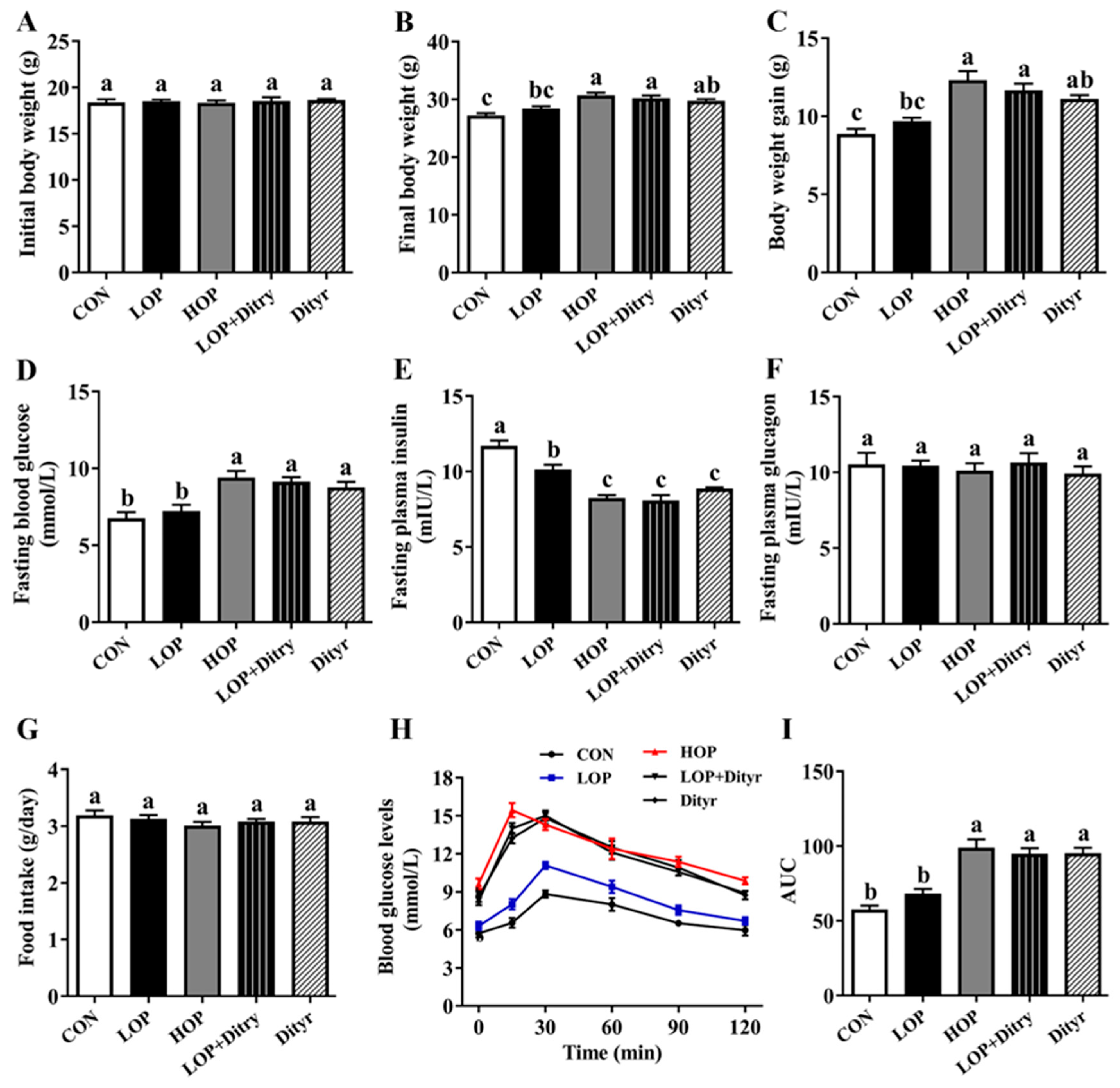
3.1. Dityr Increases Body Weight and Fasting Blood Glucose, Decreased Fasting Plasma Insulin, and Impaired Glucose Tolerance
3.2. Dityr Induces Systemic and Pancreatic Oxidative Damage
3.3. Dityr Disrupts Systemic and Pancreatic Redox Homeostasis
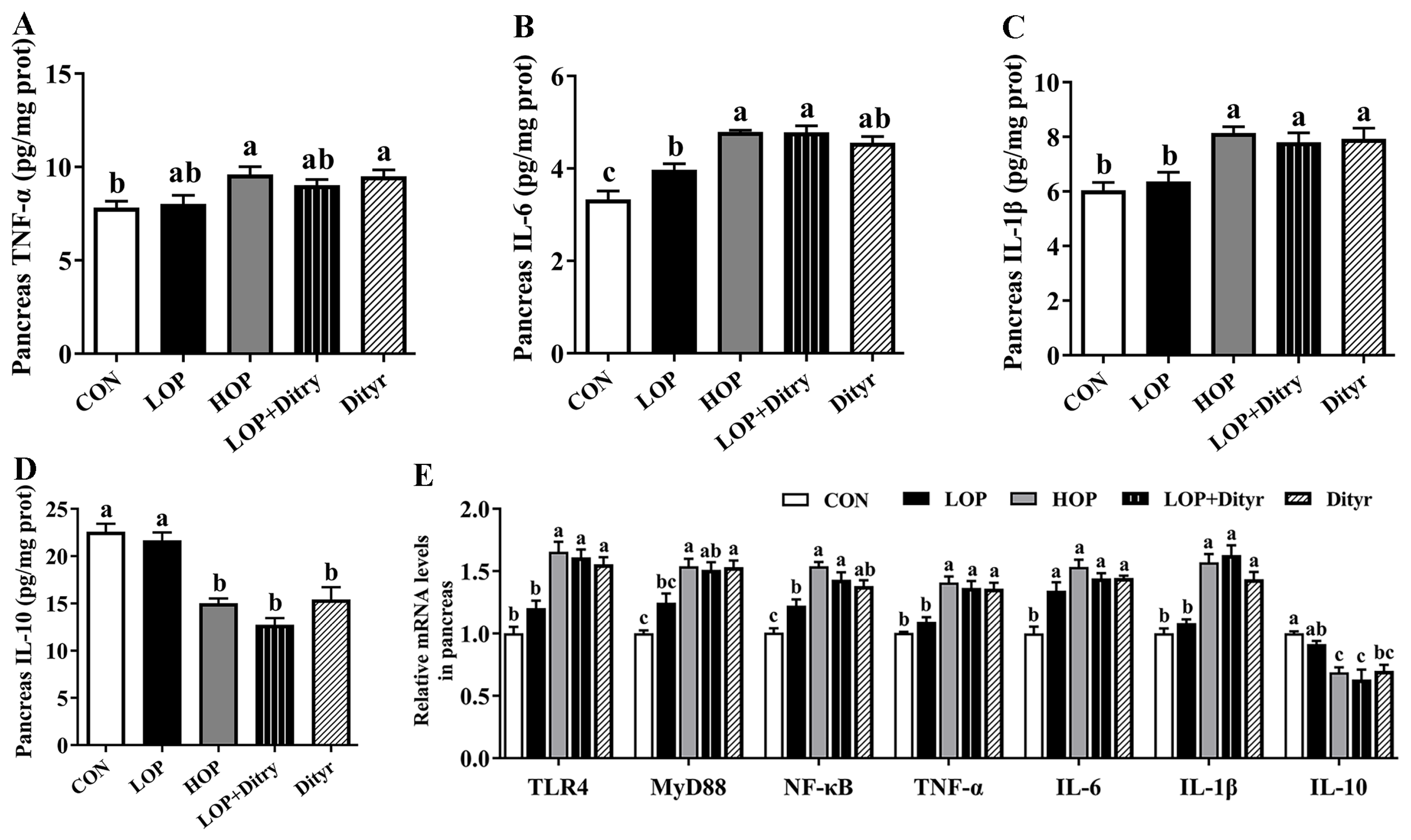
3.4. Dityr Triggers Systemic and Pancreatic Inflammation Response
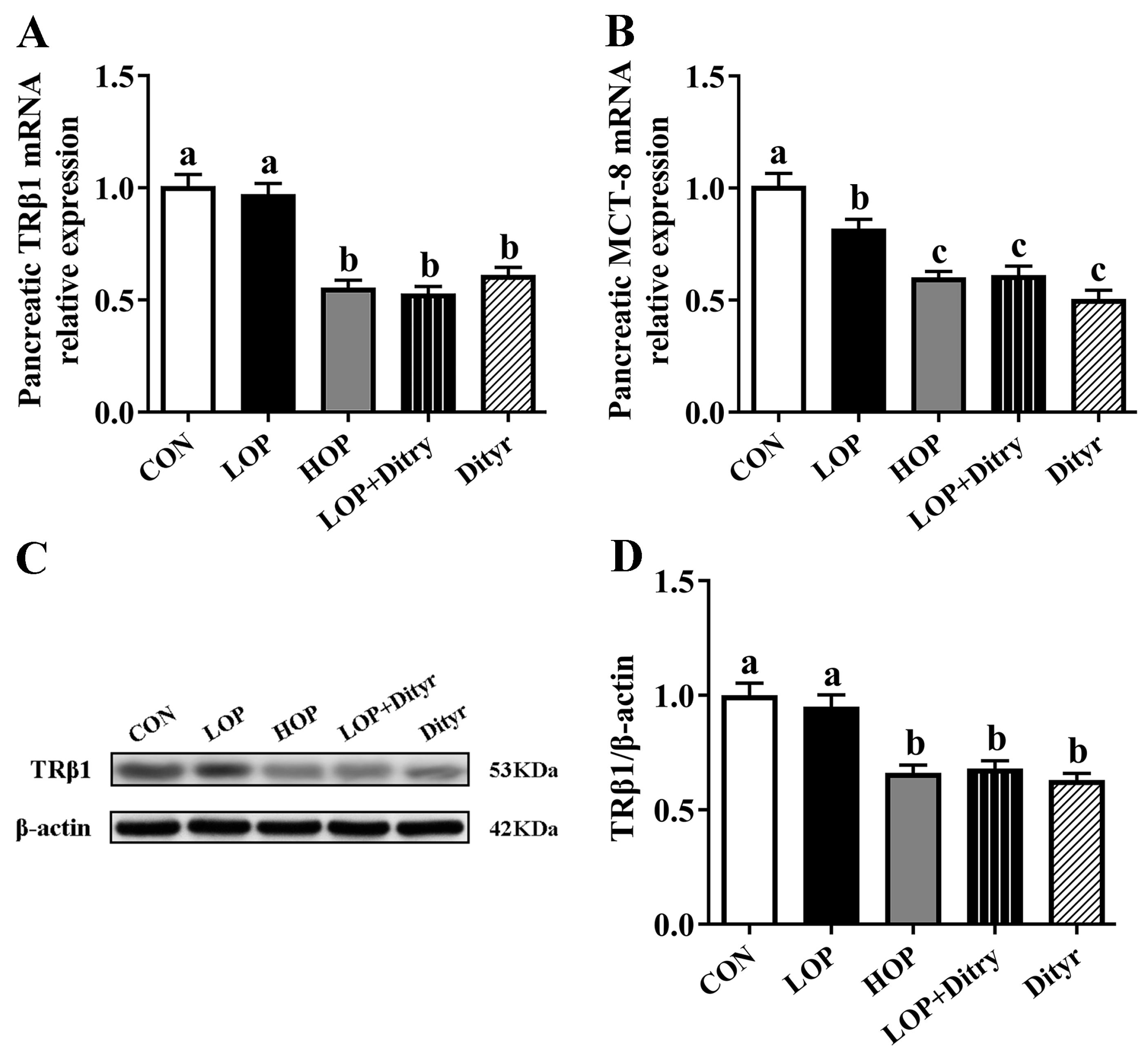
3.5. Dityr Downregulates Pancreatic TRβ1 and MCT-8 to Impair Thyroid Hormone Signaling
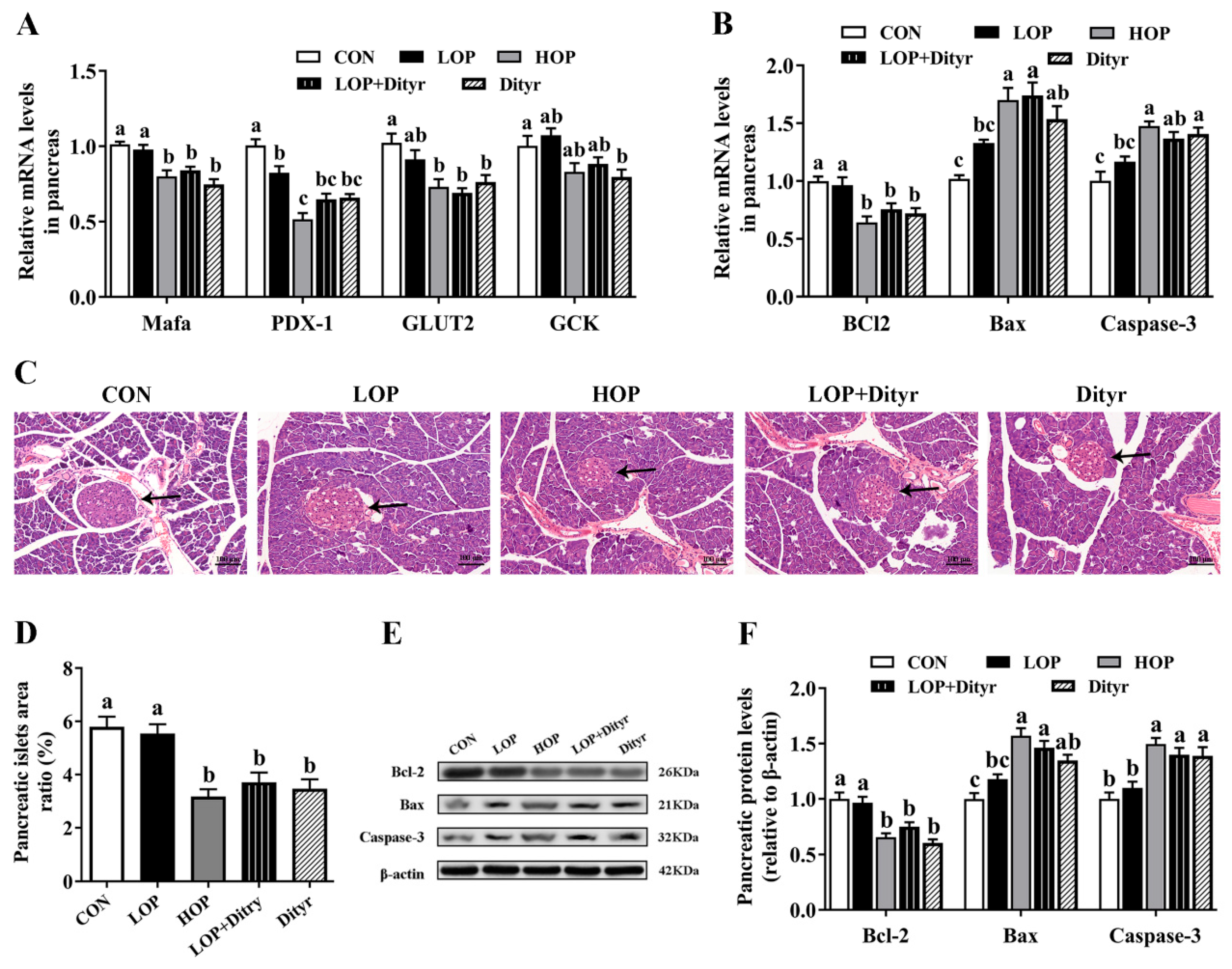
3.6. Dityr Exacerbates Pancreatic β-Cell Dysfunction Through Thyroid Hormone-Mediated Apoptosis and Impaired Insulin Secretion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2D | Type 2 diabetes |
| HTP | High-temperature and high-pressure |
| Dityr | Dityrosine |
| AGEs | Advanced glycation end products |
| AOPPs | Advanced oxidation protein products |
| MDA | Malondialdehyde |
| T-AOC | Total antioxidant capacity |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| ROS | Reactive oxygen species |
| T3 | Triiodothyronine |
| THs | Thyroid hormones |
| TRβ1 | Thyroid hormone receptor β1 |
| MCT-8 | Monocarboxylate transporter 8 |
| HOP | High-oxidative pork |
| LOP | Low-oxidative pork |
| SPF | Specific pathogen-free |
| CON | Control |
| OGTT | Oral glucose tolerance test |
| AUC | Area under the curve |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| IL-10 | Interleukin-10 |
| LPS | Lipopolysaccharide |
| LBP | LPS-binding protein |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | Bcl-2 associated X protein |
| Caspase-3 | Cysteinyl aspartate specific proteinase-3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NQO1 | NADPH quinone oxidoreductase 1 |
| HO-1 | Heme oxygenase-1 |
| TLR4 | Toll-like receptor 4 |
| MyD88 | Myeloid differentiation factor 88 |
| NF-κB | Nuclear factor kappa beta |
| MafA | V-maf musculoaponeurotic fibrosarcoma oncogene homologue A |
| PDX-1 | Pancreatic duodenal homeobox-1 |
| GLUT2 | Glucose transporter 2 |
| GCK | Glucokinase |
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Papier, K.; Fensom, G.K.; Knuppel, A.; Appleby, P.N.; Tong, T.Y.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat consumption and risk of 25 common conditions: Outcome-wide analyses in 475,000 men and women in the UK Biobank study. BMC Med. 2021, 19, 53. [Google Scholar] [CrossRef]
- Liu, G.; Zong, G.; Hu, F.B.; Willett, W.C.; Eisenberg, D.M.; Sun, Q. Cooking methods for red meats and risk of type 2 diabetes: A prospective study of U.S. women. Diabetes Care 2017, 40, 1041–1049. [Google Scholar] [CrossRef]
- Ge, Y.; Li, B.; Yang, Y.; Feng, C.; Tang, X.; Shi, Y.; Le, G.; Sun, J. Oxidized pork induces disorders of glucose metabolism in mice. Mol. Nutr. Food Res. 2021, 65, 2000859. [Google Scholar] [CrossRef] [PubMed]
- Khosravipour, M.; Khosravipour, H. The association between urinary metabolites of polycyclic aromatic hydrocarbons and diabetes: A systematic review and meta-analysis study. Chemosphere 2020, 247, 125680. [Google Scholar] [CrossRef]
- Liu, G.; Zong, G.; Wu, K.; Hu, Y.; Li, Y.; Willett, W.C.; Eisenberg, D.M.; Hu, F.B.; Sun, Q. Meat Cooking Methods and Risk of Type 2 Diabetes: Results from Three Prospective Cohort Studies. Diabetes Care 2018, 41, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Xiong, Y. Intake of Oxidized Proteins and Amino Acids and Causative Oxidative Stress and Disease: Recent Scientific Evidences and Hypotheses. J. Food Sci. 2019, 84, 387–396. [Google Scholar] [CrossRef]
- Akagawa, M. Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches. Free Radic. Res. 2020, 55, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Lin, S.; Li, B.; Yang, Y.; Tang, X.; Shi, Y.; Sun, J.; Le, G. Oxidized pork induces oxidative stress and inflammation by altering gut microbiota in mice. Mol. Nutr. Food Res. 2019, 64, e1901012. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Ding, Y.; Ge, Y.; Xu, Y.; Xie, Y.; Shi, Y.; Le, G. Dityrosine in food: A review of its occurrence, health effects, detection methods, and mitigation strategies. Compr. Rev. Food Sci. Food Saf. 2022, 22, 355–379. [Google Scholar] [CrossRef]
- Maina, M.B.; Al-Hilaly, Y.K.; Serpell, L.C. Dityrosine cross-linking and its potential roles in Alzheimer’s disease. Front. Neurosci. 2023, 17, 1132670. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; Li, Z.Q.; Cheng, X.R.; Ran, Y.M.; Wu, S.J.; Shi, Y.; Le, G. Dityrosine administration induces dysfunction of insulin secretion accompanied by diminished thyroid hormones T3 function in pancreas of mice. Amino Acids 2017, 49, 1401–1414. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Tang, X.; Cheng, X.R.; Wang, F.F.; Li, Z.Q.; Wu, S.J.; Kou, X.R.; Shi, Y.H.; Le, G.W. Effects of dietary oxidized tyrosine products on insulin secretion via the thyroid hormone T3-regulated TRβ1–AKT–mTOR pathway in the pancreas. RSC Adv. 2017, 7, 54610–54625. [Google Scholar] [CrossRef]
- Eom, Y.S.; Wilson, J.R.; Bernet, V.J. Links between thyroid disorders and glucose homeostasis. Diabetes Metab. J. 2022, 46, 239–256. [Google Scholar] [CrossRef]
- Crunkhorn, S.; Patti, M.E. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid® 2008, 18, 227–237. [Google Scholar] [CrossRef]
- Visser, W.E.; van Mullem, A.A.A.; Visser, T.J.; Peeters, R.P. Different causes of reduced sensitivity to thyroid hormone: Diagnosis and clinical management. Clin. Endocrinol. 2013, 79, 595–605. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gil-Guzman, E.; Mahran, A.M.; Rakesh; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J. Androl. 2001, 22, 568–574. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.-C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Kieser, K.J.; Kagan, J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017, 17, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Ashayeri Ahmadabad, R.; Mirzaasgari, Z.; Gorji, A.; Khaleghi Ghadiri, M. Toll-Like Receptor Signaling Pathways: Novel Therapeutic Targets for Cerebrovascular Disorders. Int. J. Mol. Sci. 2021, 22, 6153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Yan, B.; Zhang, T.; Gao, Y.; Shi, Y.; Le, G. Health effects of dietary oxidized tyrosine and dityrosine administration in mice with nutrimetabolomic strategies. J. Agric. Food Chem. 2017, 65, 6957–6971. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Lan, J.; Fang, Y.; Pan, Y.; Gu, Z.; Xue, J.; Yang, Y.; Jiang, M.; Ge, Y.; Shen, Q. Dityrosine aggravates hepatic insulin resistance in obese mice by altering gut microbiota and the LPS/TLR4/NF-κB inflammatory pathway. Mol. Nutr. Food Res. 2023, 67, e2300373. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Cohrs, C.M.; Panzer, J.K.; Drotar, D.M.; Enos, S.J.; Kipke, N.; Chen, C.; Bozsak, R.; Schöniger, E.; Ehehalt, F.; Distler, M.; et al. Dysfunction of Persisting β Cells Is a Key Feature of Early Type 2 Diabetes Pathogenesis. Cell Rep. 2020, 31, 107469. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Taguchi, Y.; Tasaki, Y.; Terakado, K.; Kobayashi, K.; Machida, T.; Kobayashi, T. Impaired insulin secretion from the pancreatic islets of hypothyroidal growth-retarded mice. J. Endocrinol. 2010, 206, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, W.; Iwasa, H.; Tumurkhuu, M. Role of the Transcription Factor MAFA in the Maintenance of Pancreatic β-Cells. Int. J. Mol. Sci. 2022, 23, 4478. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Koh, A.; El Khattabi, I.; Li, W.C.; Toschi, E.; Jermendy, A.; Juhl, K.; Mao, K.; Weir, G.C.; Sharma, A.; et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia. 2011, 54, 583–593. [Google Scholar] [CrossRef]
- Zhang, C.; Moriguchi, T.; Kajihara, M.; Esaki, R.; Harada, A.; Shimohata, H.; Oishi, H.; Hamada, M.; Morito, N.; Hasegawa, K.; et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell Biol. 2005, 25, 4969–4976. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Zavacki, A.M.; Marinelarena, A.; Hollister-Lock, J.; El Khattabi, I.; Marsili, A.; Weir, G.C.; Sharma, A.; Larsen, P.R.; Bonner-Weir, S. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 2013, 62, 1569–1580. [Google Scholar] [CrossRef]
- Verga Falzacappa, C.; Panacchia, L.; Bucci, B.; Stigliano, A.; Cavallo, M.G.; Brunetti, E.; Toscano, V.; Misiti, S. 3,5,3’-triiodothyronine (T3) is a survival factor for pancreatic beta-cells undergoing apoptosis. J. Cell Physiol. 2006, 206, 309–321. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, F.; Tian, W.; Lai, J.; Qian, L.; Hong, W.; Chen, H.; Li, L.C. Andrographolide promotes pancreatic duct cells differentiation into insulin-producing cells by targeting PDX-1. Biochem. Pharmacol. 2020, 174, 113785. [Google Scholar] [CrossRef] [PubMed]
- Matschinsky, F.M.; Wilson, D.F. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Verga Falzacappa, C.; Mangialardo, C.; Madaro, L.; Ranieri, D.; Lupoi, L.; Stigliano, A.; Torrisi, M.R.; Bouchè, M.; Toscano, V.; Misiti, S. Thyroid hormone T3 counteracts STZ induced diabetes in mouse. PLoS ONE 2011, 6, e19839. [Google Scholar] [CrossRef]
- Verga Falzacappa, C.; Patriarca, V.; Bucci, B.; Mangialardo, C.; Michienzi, S.; Moriggi, G.; Stigliano, A.; Brunetti, E.; Toscano, V.; Misiti, S. The TRβ1 is essential in mediating T3 action on Akt pathway in human pancreatic insulinoma cells. J. Cell Biochem. 2009, 106, 835–848. [Google Scholar] [CrossRef]
- Perciavalle, R.M.; Opferman, J.T. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol. 2013, 23, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as Regulators of Insulin Sensitivity and Metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]

| CON | LOP | HOP | LOP + Dityr | Dityr | |
|---|---|---|---|---|---|
| Plasma | |||||
| Dityr (pg/mL) | 137.71 ± 7.63 c | 151.80 ± 7.24 c | 202.16 ± 7.43 b | 201.23 ± 5.26 b | 249.57 ± 9.59 a |
| AOPPs (pmol/L) | 243.64 ± 4.25 b | 256.58 ± 7.81 b | 298.60 ± 15.40 a | 293.95 ± 6.42 a | 315.21 ± 7.40 a |
| MDA (nmol/mL) | 7.13 ± 0.27 c | 9.96 ± 0.65 b | 13.08 ± 0.68 a | 12.47 ± 0.57 a | 12.47 ± 0.73 a |
| AGEs (ng/L) | 92.92 ± 1.74 b | 93.32 ± 3.66 b | 106.01 ± 2.84 a | 97.04 ± 2.33 ab | 98.21 ± 4.06 ab |
| Pancreas | |||||
| Dityr (pg/mg prot) | 14.09 ± 0.61 b | 17.04 ± 0.64 b | 22.09 ± 1.34 a | 21.58 ± 0.91 a | 23.25 ± 1.11 a |
| AOPPs (pmol/g prot) | 14.73 ± 0.81 c | 17.32 ± 0.73 bc | 25.86 ± 1.16 a | 22.55 ± 1.12 a | 21.25 ± 1.06 ab |
| MDA (nmol/mg prot) | 3.07 ± 0.08 c | 3.94 ± 0.16 bc | 5.30 ± 0.21 a | 4.65 ± 0.30 ab | 4.98 ± 0.17 a |
| AGEs (ng/g prot) | 13.36 ± 0.60 c | 17.92 ± 0.77 b | 22.93 ± 0.46 a | 20.96 ± 0.88 a | 17.99 ± 0.68 b |
| CON | LOP | HOP | LOP + Dityr | Dityr | |
|---|---|---|---|---|---|
| Plasma | |||||
| ROS (RLUs/mL) | 688.45 ± 41.52 b | 938.27 ± 59.13 b | 1318.41 ± 91.63 a | 1293.45 ± 51.89 a | 1199.60 ± 48.01 a |
| T-AOC (U/mL) | 4.50 ± 0.14 a | 4.12 ± 0.11 a | 3.58 ± 0.13 b | 3.35 ± 0.14 b | 3.51 ± 9.13 b |
| SOD (U/mL) | 254.69 ± 10.30 a | 244.55 ± 8.09 a | 196.99 ± 10.02 b | 210.32 ± 11.20 ab | 192.66 ± 10.24 b |
| GSH-Px (U/mL) | 134.69 ± 5.21 a | 116.55 ± 5.23 a | 84.99 ± 3.61 b | 80.66 ± 2.91 b | 91.66 ± 3.12 b |
| Pancreas | |||||
| ROS (RLUs/mg prot) | 943.45 ± 63.07 b | 1213.27 ± 71.28 b | 1593.41 ± 113.76 a | 1568.45 ± 64.69 a | 1649.60 ± 78.23 a |
| T-AOC (U/mg prot) | 0.65 ± 0.05 a | 0.57 ± 0.02 a | 0.34 ± 0.03 b | 0.39 ± 0.03 b | 0.37 ± 0.03 b |
| SOD (U/mg prot) | 219.01 ± 11.25 a | 223.34 ± 8.73 a | 169.34 ± 9.37 b | 166.67 ± 3.30 b | 176.01 ± 8.81 b |
| GSH-Px (U/mg prot) | 154.67 ± 20.87 a | 143.67 ± 6.80 ab | 100.67 ± 5.11 c | 108.67 ± 7.13 c | 119.34 ± 5.18 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Kou, B.; Zhang, C.; Gu, C.; Cheng, L.; Shi, Y.; Le, G.; Xu, W. Dietary Dityrosine Impairs Glucose Homeostasis by Disrupting Thyroid Hormone Signaling in Pancreatic β-Cells. Foods 2025, 14, 3220. https://doi.org/10.3390/foods14183220
Ge Y, Kou B, Zhang C, Gu C, Cheng L, Shi Y, Le G, Xu W. Dietary Dityrosine Impairs Glucose Homeostasis by Disrupting Thyroid Hormone Signaling in Pancreatic β-Cells. Foods. 2025; 14(18):3220. https://doi.org/10.3390/foods14183220
Chicago/Turabian StyleGe, Yueting, Boyang Kou, Chunyu Zhang, Chengjia Gu, Lin Cheng, Yonghui Shi, Guowei Le, and Wei Xu. 2025. "Dietary Dityrosine Impairs Glucose Homeostasis by Disrupting Thyroid Hormone Signaling in Pancreatic β-Cells" Foods 14, no. 18: 3220. https://doi.org/10.3390/foods14183220
APA StyleGe, Y., Kou, B., Zhang, C., Gu, C., Cheng, L., Shi, Y., Le, G., & Xu, W. (2025). Dietary Dityrosine Impairs Glucose Homeostasis by Disrupting Thyroid Hormone Signaling in Pancreatic β-Cells. Foods, 14(18), 3220. https://doi.org/10.3390/foods14183220






