A Study on the Application of CO2-Modified Atmosphere Combined with Temperature-Control Technology in Rice Warehouse Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rice Color Measurement
2.3. Fatty Acid Value (FAV)
2.4. Brown Rice Yield (BRY)
2.5. Head Rice Yield (HRY)
2.6. Germination Percentage
2.7. Fungal Diversity
2.8. Determination of Aflatoxin B1 (AFB1) Content
2.9. Metabolomics Analysis
2.9.1. Sample Preparation
2.9.2. Quality Control (QC) Sample
2.9.3. LC-MS/MS Analysis
2.9.4. Metabolomic Data Processing
2.10. Data Processing
3. Results and Discussion
3.1. The Origin Rice Quality Parameters
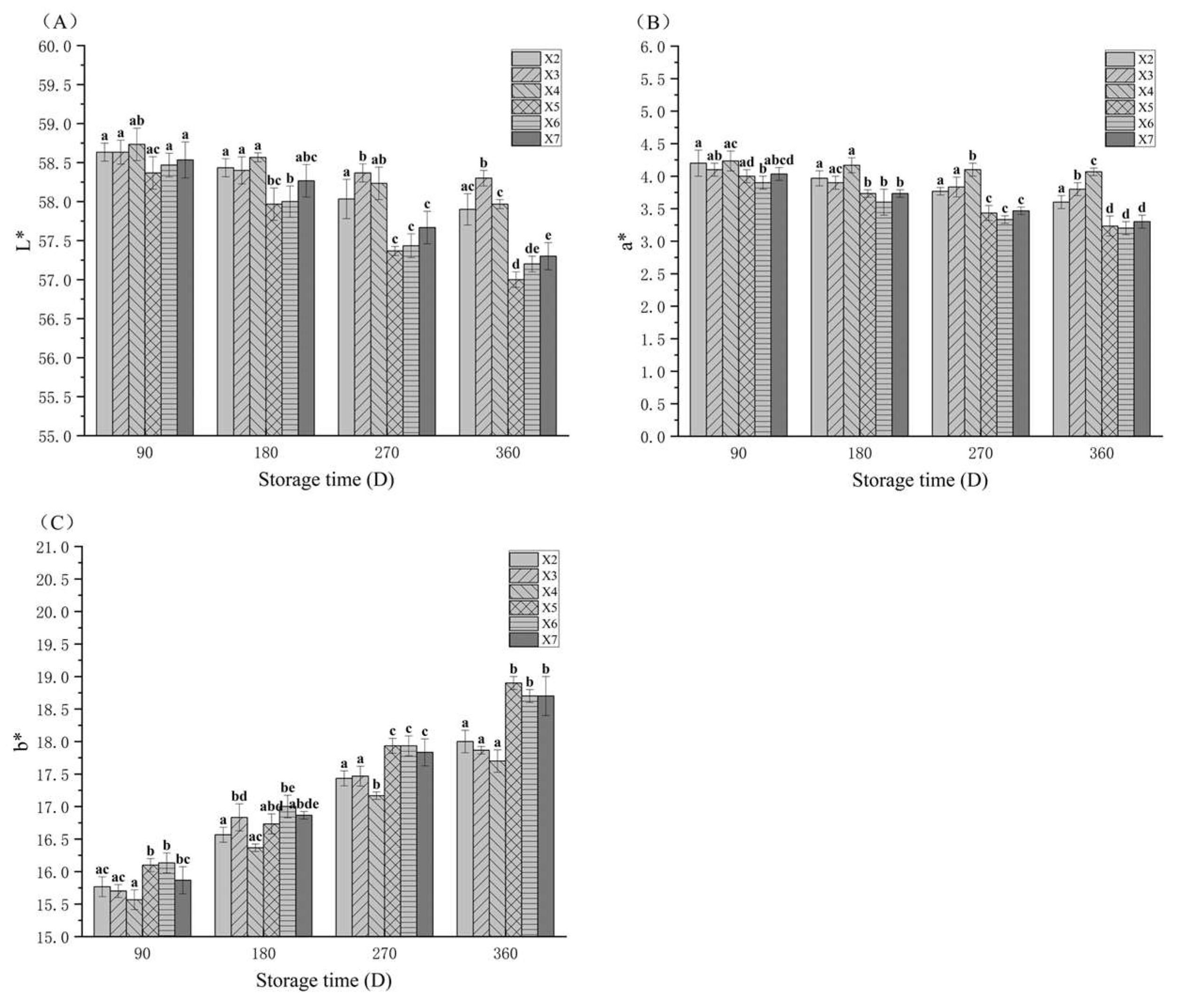
3.2. The Color Changes in Rice During Storage
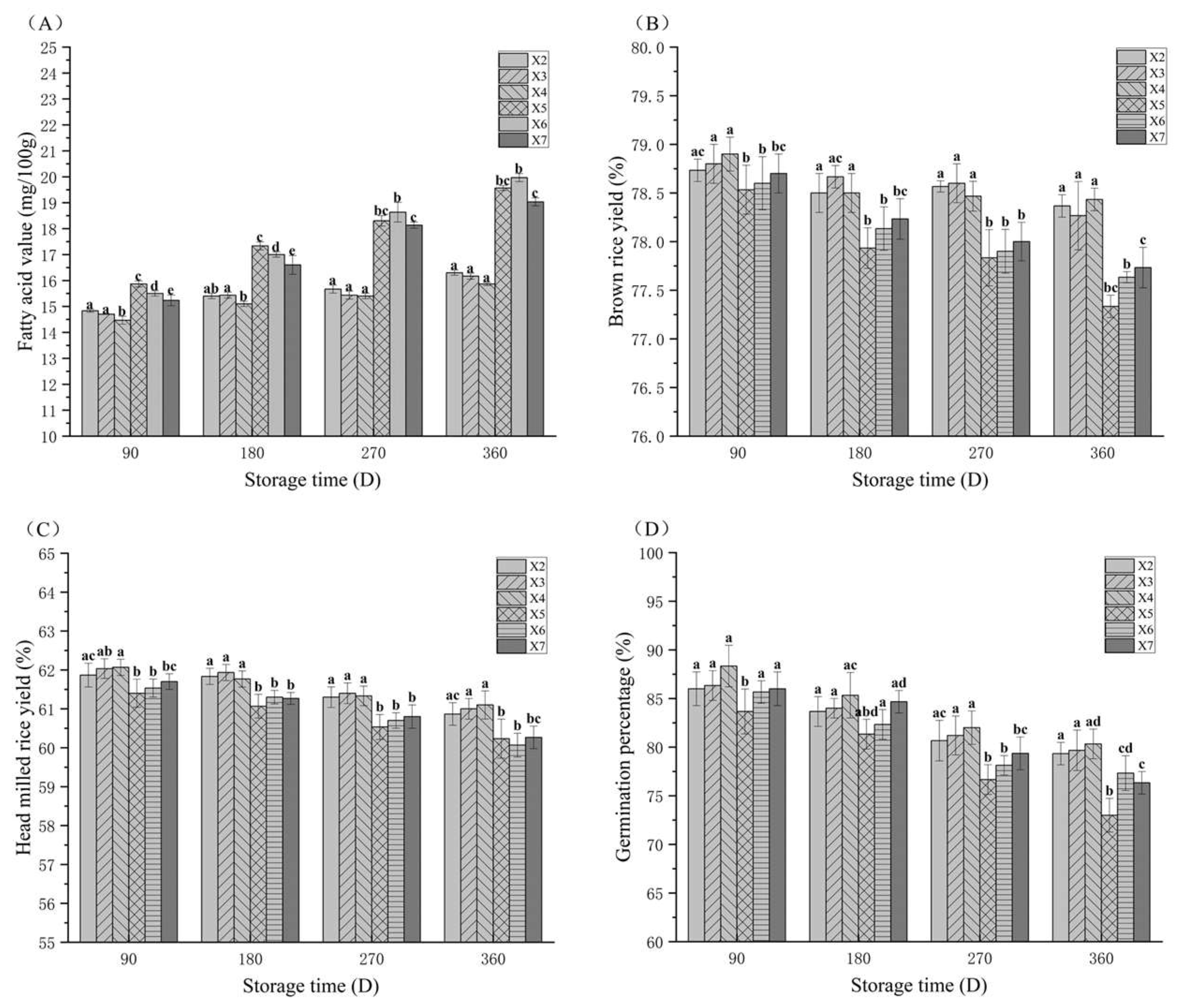
3.3. The Fatty Acid Value (FAV) Changes in Rice During Storage
3.4. The Brown Rice Yield (BRY) Changes in Rice During Storage
3.5. The Head Rice Yield (HRY) Changes in Rice During Storage
3.6. The Germination Percentage Changes in Rice During Storage
3.7. The Diversity of Fungi Changes in Rice During Storage
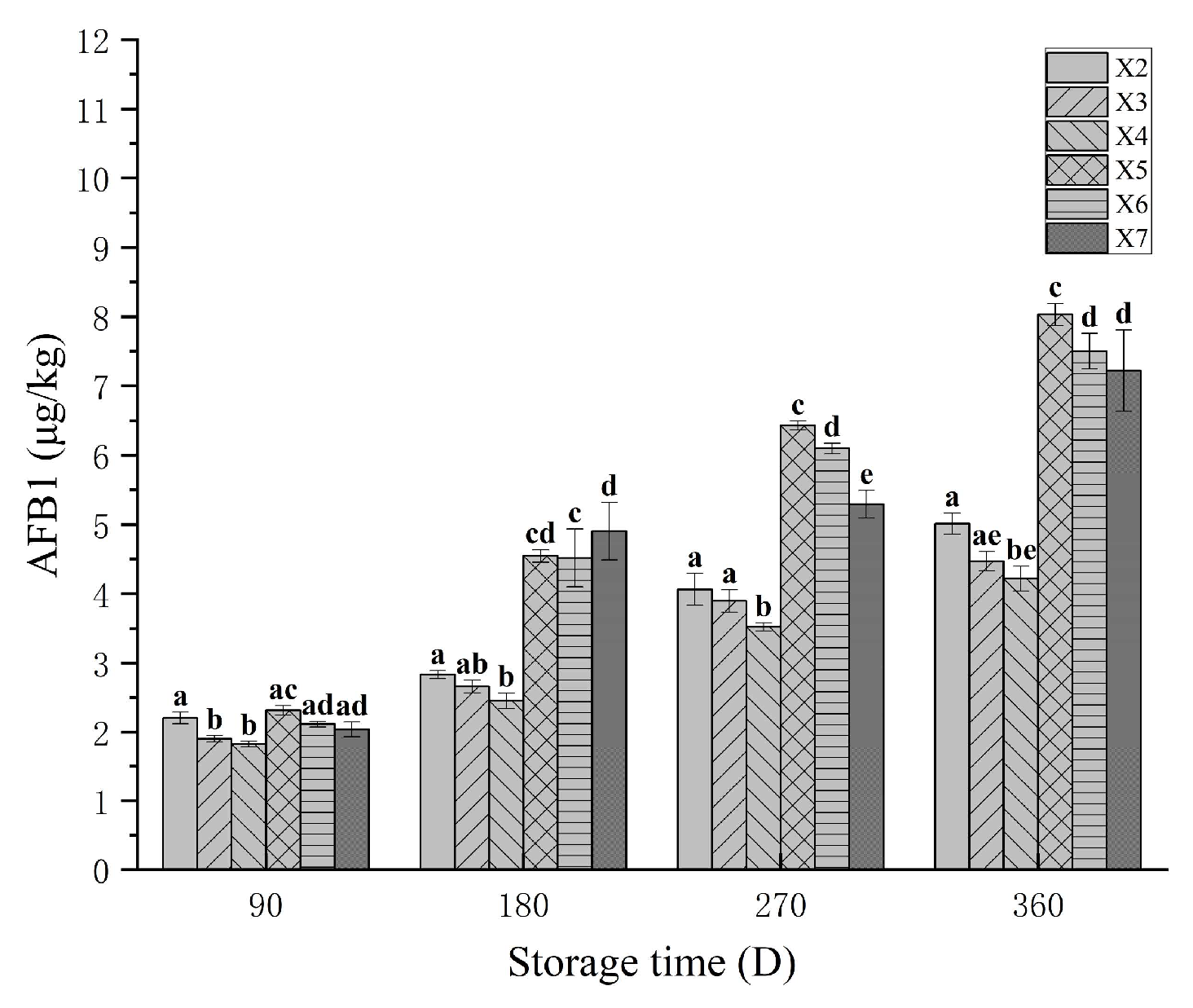
3.8. The AFB1 in Rice During Storage
3.9. Metabolite Analysis of Rice Under Different Storage Conditions
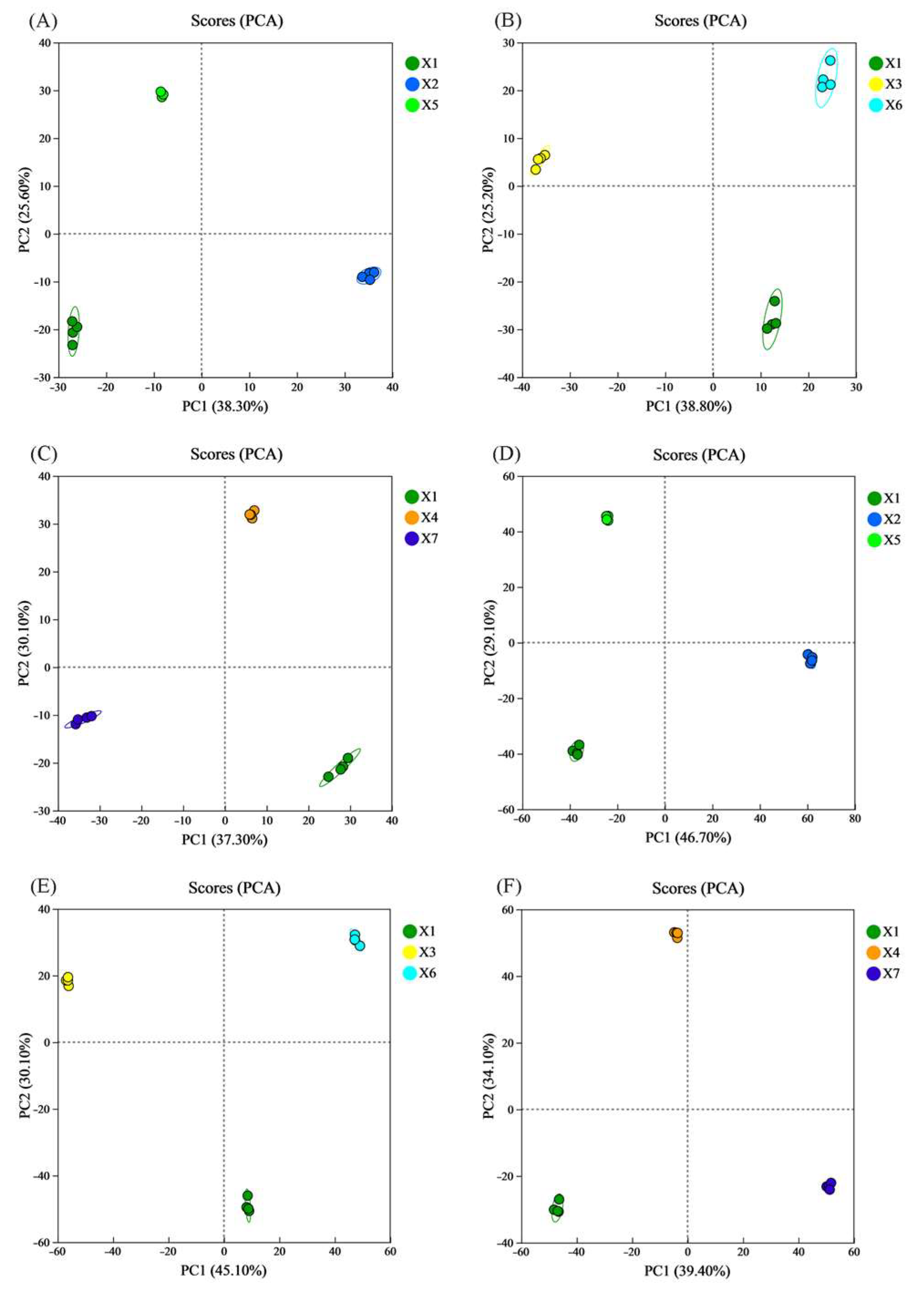
3.9.1. Principal Component Analysis (PCA)
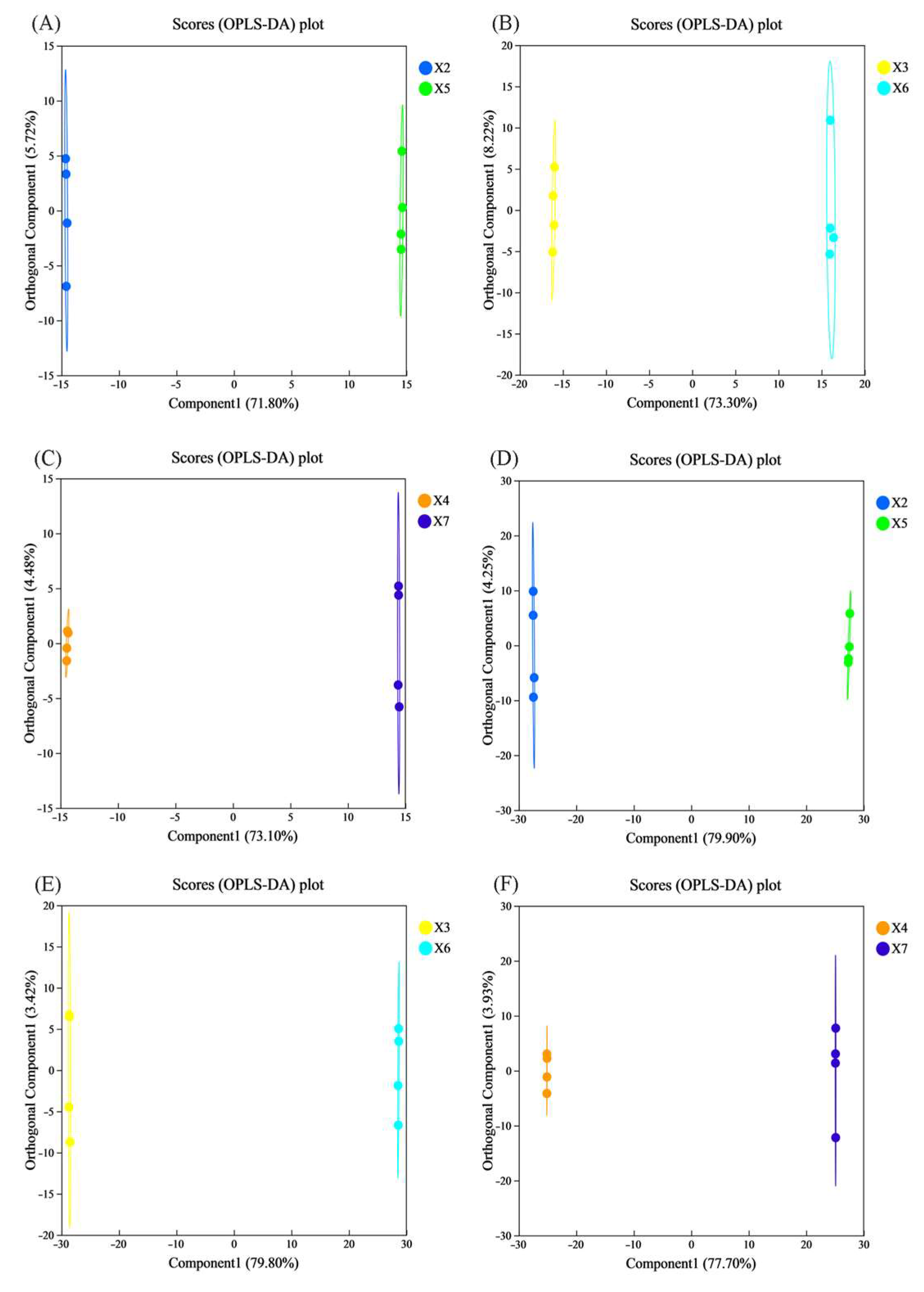
3.9.2. Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA)
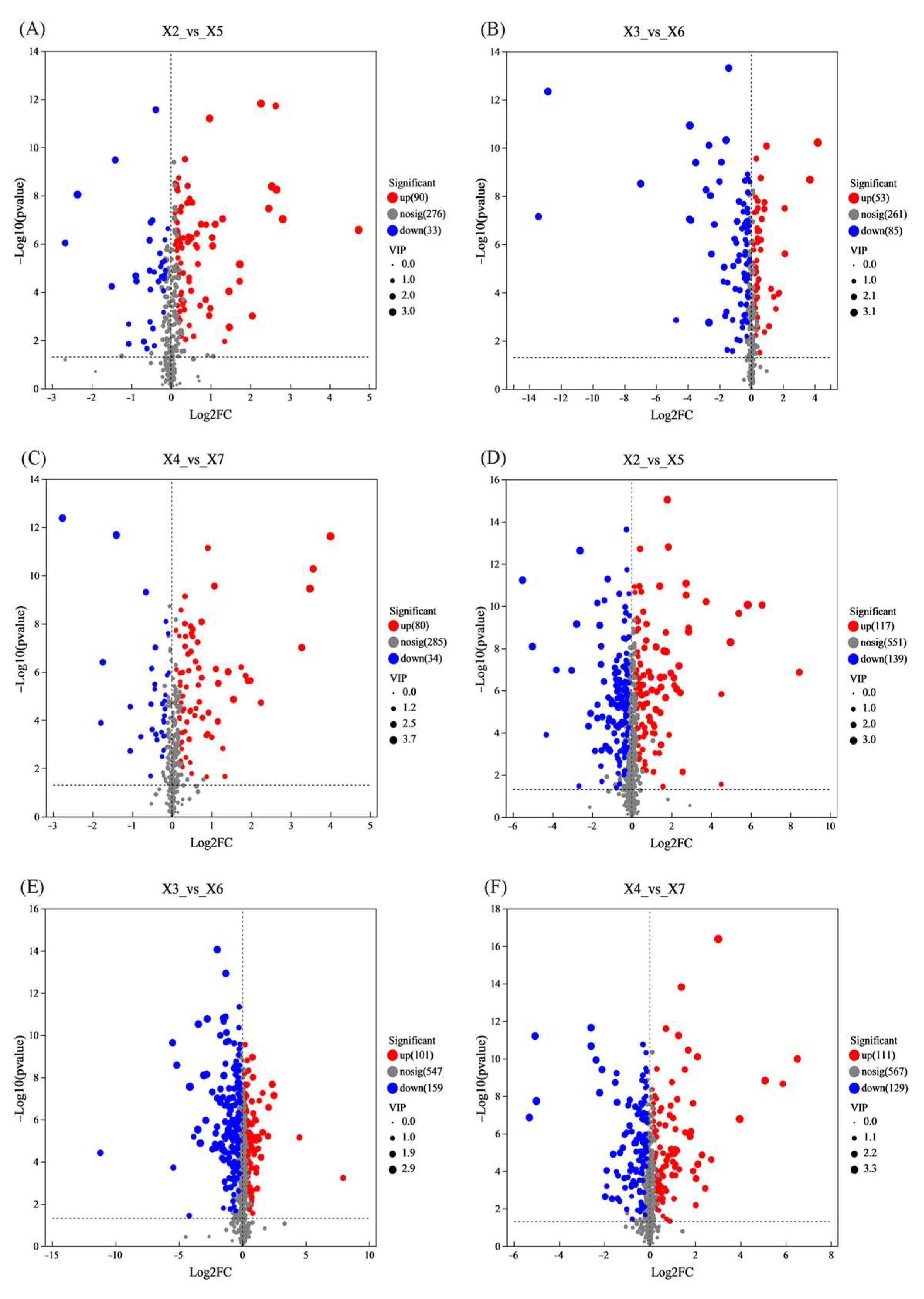
3.9.3. Identification of Key Metabolites in Rice
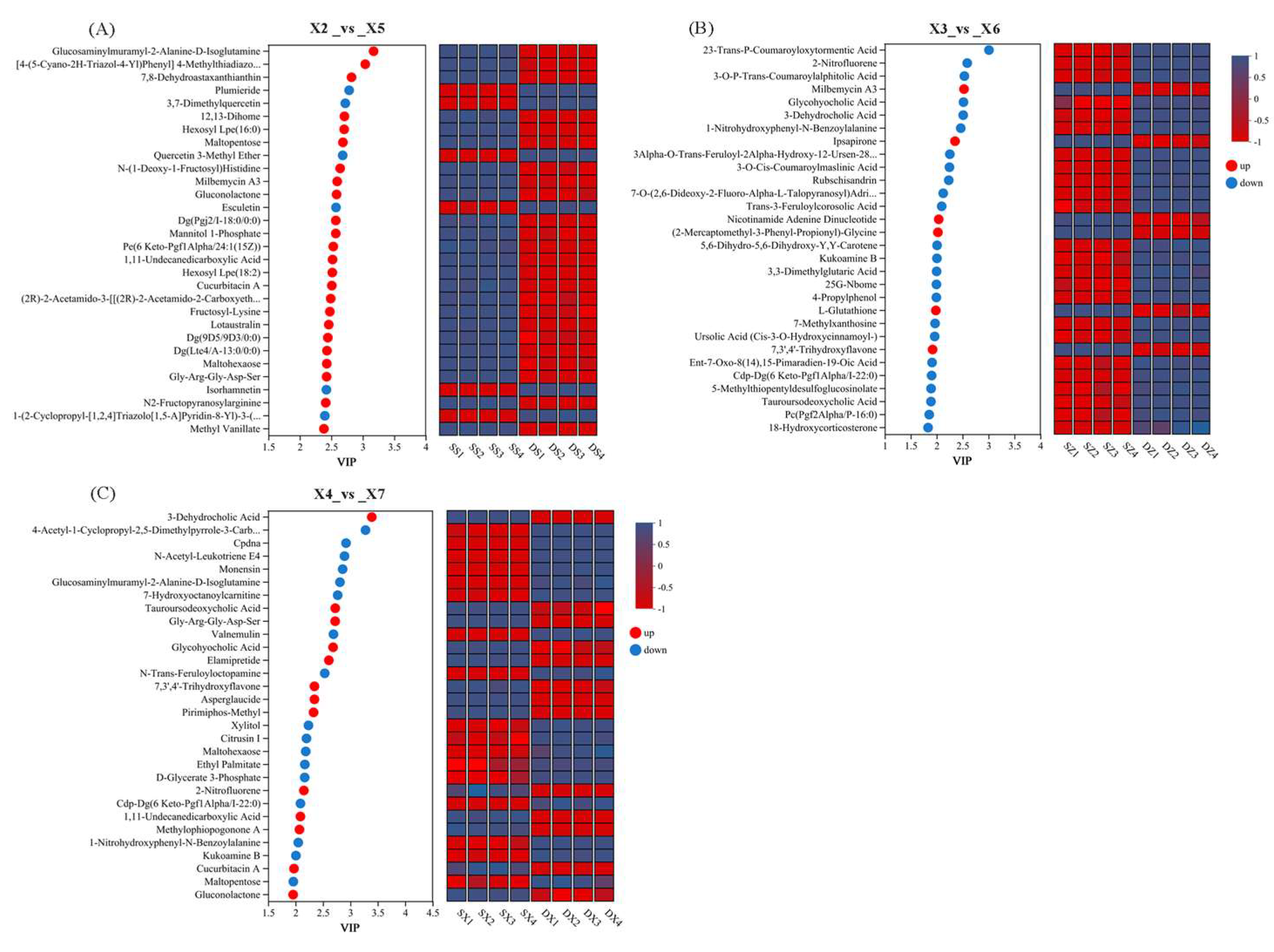
3.9.4. VIP Analysis of Differential Metabolites in Rice
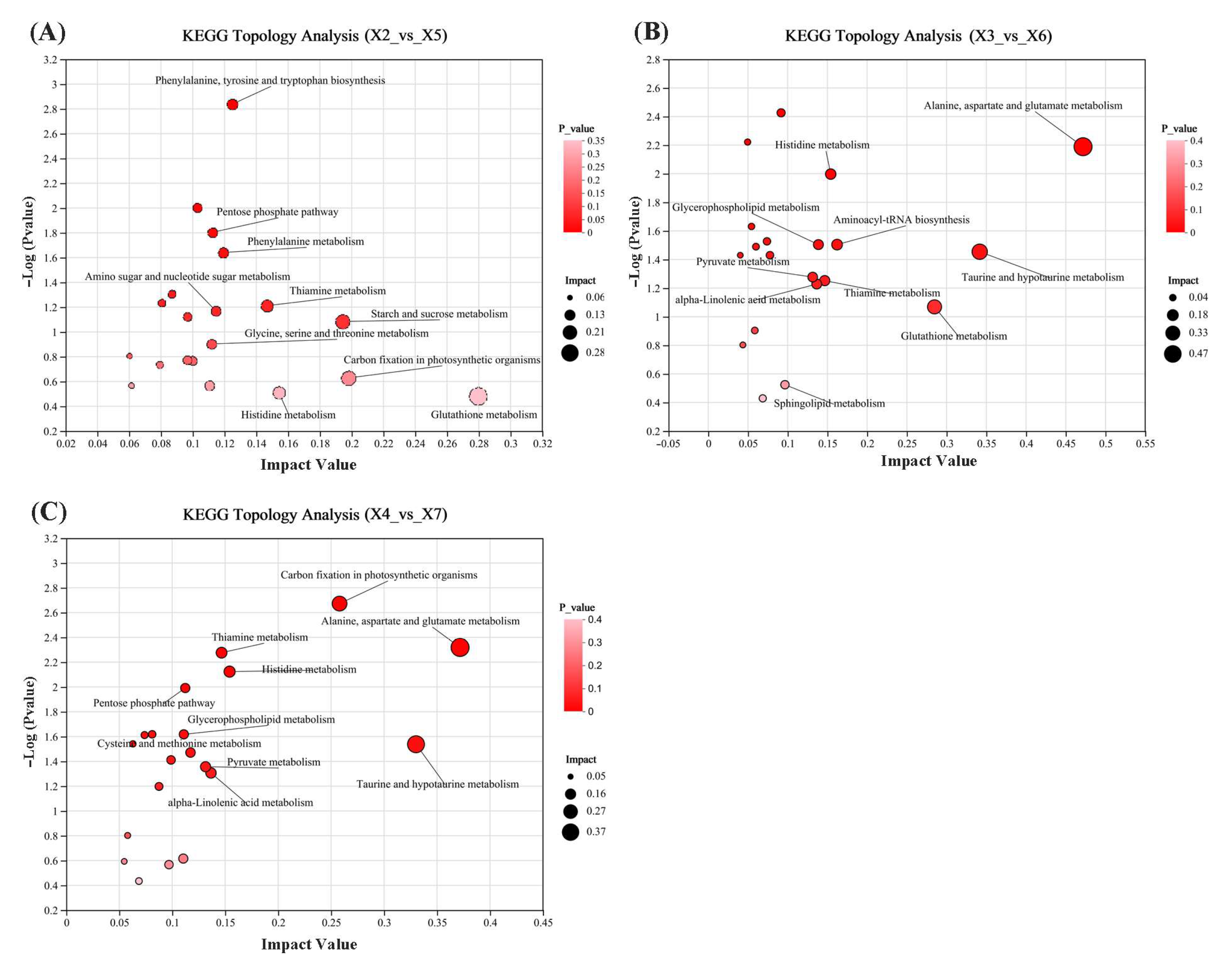
3.9.5. Functional Enrichment of KEGG Metabolic Pathways in Rice
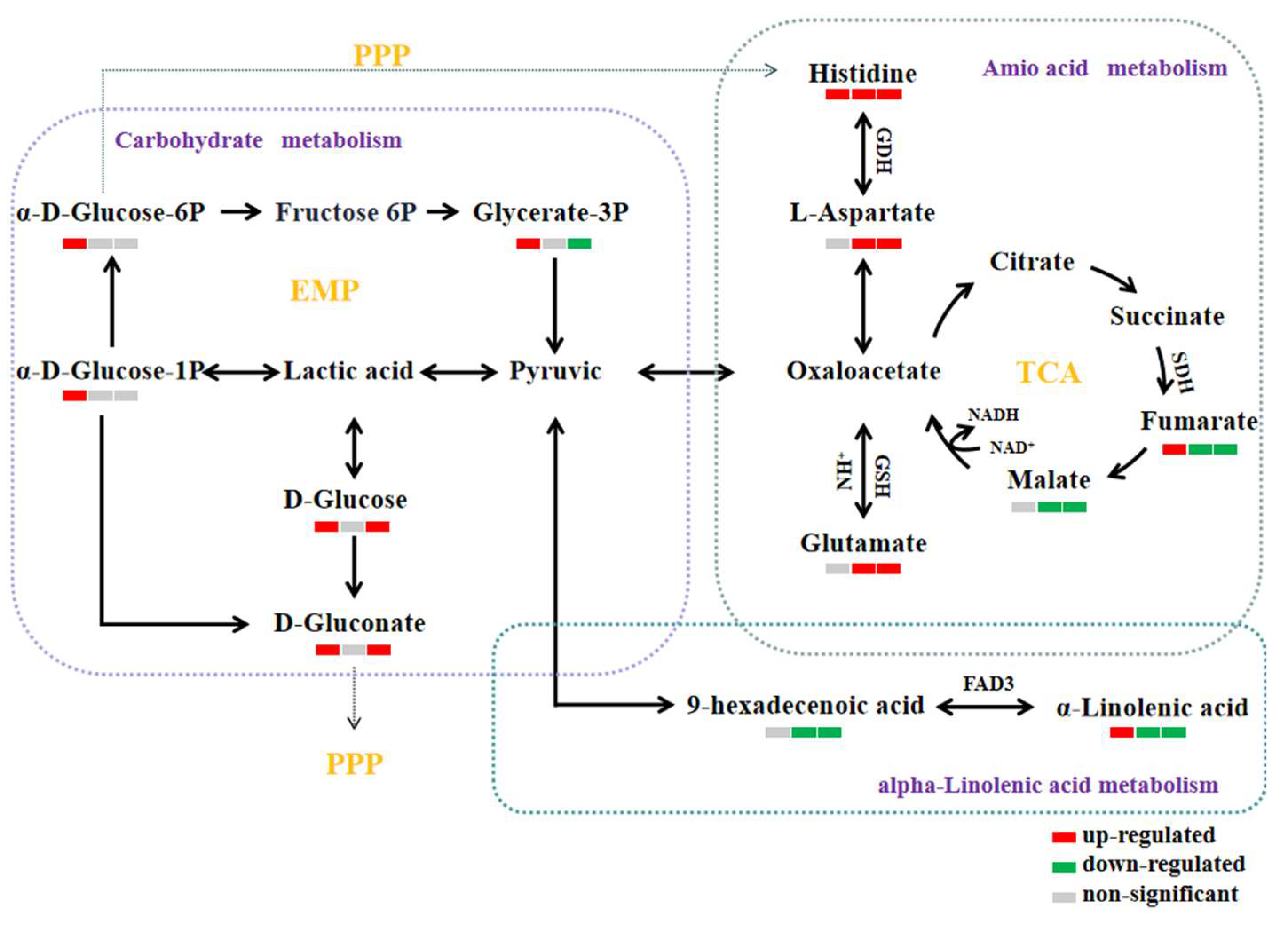
3.10. Analysis of Metabolic Pathways
3.10.1. Amino Acid Metabolism
3.10.2. Carbohydrate Metabolism
3.10.3. Lipid Metabolism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martens, S.; Coradi, P.C.; Maldaner, V.; de Oliveira Carneiro, L.; Teodoro, P.E.; Rodrigues, D.M.; Anschau, K.F.; Teodoro, L.P.R.; Flores, É.M.M. Drying and intermittence processes on the polished and brown rice physicochemical and morphological quality by near-infrared spectroscopy, X-ray diffraction, and scanning electron microscopy. Food Chem. X 2023, 19, 100753. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, H.; Hou, D.; Laraib, Y.; Xue, Y.; Shen, Q. Influence of temperature on storage characteristics of different rice varieties. Cereal Chem. 2021, 98, 935–945. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Gong, Z.; Liu, X.; Lv, H.; Zhao, Y. Changes in Quality Characteristics and Metabolite Composition of LowTemperature and Nitrogen-Modified Atmosphere in Indica Rice during Storage. Foods 2024, 13, 2968. [Google Scholar] [PubMed]
- Perišić, V.; Perišić, V.; Hadnađev, M.; Đekić, V.; Dapčević-Hadnađev, T.; Vuković, S.; Vukajlović, F. Impact of diatomaceous earth application on the rheological properties of wheat, triticale and rye flour dough. J. Stored Prod. Res. 2019, 82, 91–97. [Google Scholar] [CrossRef]
- Chen, W.; Xu, H.; Chen, M.; Tang, P.; Wang, K. Spray-Induced Gene Silencing for Postharvest Protection: dsRNA Stability and Insecticidal Efficacy. J. Agric. Food Chem. 2025, 73, 10778–10786. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, T.T.; Baik, O.-D. Disinfestation of stored grains using non-chemical technologies—A review. Trends Food Sci. Technol. 2021, 107, 299–308. [Google Scholar] [CrossRef]
- Lin, J.; Yan, X.; Zhou, Y.; Zhu, G.; Ariyo, O.S.; Wang, S.; Chen, J.; Zhu, Y.; Wang, C.; Li, D.; et al. Modified atmosphere and ozone treatment technologies in stored grain pest control: Mechanism, applications and challenges. Agric. Prod. Process. Storage 2025, 1, 16. [Google Scholar] [CrossRef]
- Navarro, S. The use of modified and controlled atmospheres for the disinfestation of stored products. J. Pest Sci. 2012, 85, 301–322. [Google Scholar] [CrossRef]
- Anuja Gupta, A.G.; Sinha, S.N.; Atwal, S.S. Modified atmosphere technology in seed health management: Laboratory and field assay of carbon dioxide against storage fungi in paddy. Plant Pathol. J. 2014, 13, 193–199. [Google Scholar] [CrossRef]
- Sun, S.; Li, B.; Yang, T.; Luo, F.; Zhao, J.; Cao, J.; Lin, Q. Preservation mechanism of high concentration carbon dioxide controlled atmosphere for paddy rice storage based on quality analyses and molecular modeling tools. J. Cereal Sci. 2019, 85, 279–285. [Google Scholar] [CrossRef]
- Xue, G.; Wang, T.; Guo, H.; Zhang, N.; Carmalt, C.J.; Hofkens, J.; Lai, F.; Liu, T. Polymer-confined synthesis of gram-scale high-entropy perovskite fluoride nanocubes for improved electrocatalytic reduction of nitrate to ammonia. Nanoscale Horiz. 2025, 10, 135–141. [Google Scholar] [CrossRef]
- Hamzavi, F.; Naseri, B.; Hassanpour, M.; Razmjou, J.; Golizadeh, A. Biology and life table parameters of Callosobruchus maculatus (F.) on Vigna unguiculata (L.) Walp. fertilized with some mineral-and bio-fertilizers. J. Stored Prod. Res. 2022, 97, 101978. [Google Scholar] [CrossRef]
- Han, Q.; Chen, Y.; Liu, X.; Bi, J.; Zhang, W.; Zeng, X.; Wang, P.; Shu, Z. Quality attributes of paddy rice during storage as affected by accumulated temperature. Front. Nutr. 2024, 10, 1337110. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Zhao, Y.; Xu, X.; Li, Y.; Lv, H. Untargeted Lipidomics Reveal Quality Changes in High-Moisture Japonica Brown Rice at Different Storage Temperatures. Foods 2023, 12, 4218. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Whitehead, S.R.; Macarisin, D.; Liu, J.; Burchard, E.; Freilich, S.; Dardick, C.; Droby, S.; Wisniewski, M. Effect of Washing, Waxing and Low-Temperature Storage on the Postharvest Microbiome of Apple. Microorganisms 2020, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Lu, L.; Guo, Z.L.; Zhu, Z.W. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Chen, L.; Lu, W.; Wang, L.; Xing, X.; Chen, Z.; Teng, X.; Zeng, X.; Muscarella, A.D.; Shen, Y.; Cowan, C.A.; et al. Metabolite discovery through global annotation of untargeted metabolomics data. Nat. Methods 2021, 18, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qiao, X.; Guo, J.; Yang, T.; Wang, M.; Ma, Y.; Zhao, S.; Ding, L.; Liu, H.; Wang, J. Related factors based on non-targeted metabolomics methods in minor ischaemic stroke. Front. Endocrinol. 2022, 13, 952918. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Chen, Z.; Wang, F.Z.; Jia, W.; Xu, Z.C. Combined transcriptomic and metabolomic analyses uncover rearranged gene expression and metabolite metabolism in tobacco during cold acclimation. Sci. Rep. 2020, 10, 13. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhang, H.; Hu, M.; Lu, P.; Qu, C. Study on peanut protein oxidation and metabolomics/proteomics analysis of peanut response under hypoxic/re-aeration storage. Food Chem. X 2024, 21, 101173. [Google Scholar] [CrossRef]
- Wu, Y.N.; He, S.D.; Pan, T.E.; Miao, X.Y.; Xiang, J.; Ye, Y.K.; Cao, X.D.; Sun, H.J. Enhancement of gamma-aminobutyric acid and relevant metabolites in brown glutinous rice (Oryza sativa L.) through salt stress and low-frequency ultrasound treatments at pre-germination stage. Food Chem. 2023, 410, 135362. [Google Scholar] [CrossRef]
- Zhang, Y.; He, C.; Wu, Y.; Yang, J.; Xuan, H.; Zhu, X. Effect of lipoxygenase activity and red seed coat on rice bran deterioration. J. Sci. Food Agric. 2009, 89, 1904–1908. [Google Scholar] [CrossRef]
- Dou, Z.; Zhou, Y.C.; Zhang, Y.Y.; Guo, W.; Xu, Q.; Gao, H. Influence of nitrogen applications during grain-filling stage on rice (Oryza sativa L.) yield and grain quality under high temperature. Agronomy 2024, 14, 216. [Google Scholar] [CrossRef]
- Shad, Z.M.; Atungulu, G. Physical integrity of long-Grain hybrid, pureline, and medium-grain rice kernels as affected by storage conditions. Appl. Eng. Agric. 2020, 36, 579–588. [Google Scholar] [CrossRef]
- Chao, S.; Mitchell, J.; Fukai, S. Factors determining genotypic variation in the speed of rice germination. Agronomy 2021, 11, 1614. [Google Scholar] [CrossRef]
- Alwan, N.; Bou Ghanem, H.; Dimassi, H.; Karam, L.; Hassan, H.F. Exposure assessment of aflatoxin B1 through consumption of rice in the United Arab Emirates. Int. J. Environ. Res. Public Health 2022, 19, 15000. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Ding, Y.; Cheng, Y.; Xu, S.; Lin, L. Understanding the Changes in Quality of Semi-Dried Rice Noodles during Storage at Room Temperature. Foods 2022, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Kibar, H.; Sönmez, F.; Temel, S. Effect of storage conditions on nutritional quality and color characteristics of quinoa varieties. J. Stored Prod. Res. 2021, 91, 101761. [Google Scholar] [CrossRef]
- Gao, B.; Hu, S.; Jing, L.; Wang, Y.; Zhu, J.; Wang, K.; Li, H.; Sun, X.; Wang, Y.; Yang, L. Impact of elevated CO2 and reducing the source-sink ratio by partial defoliation on rice grain quality-A 3-year free-air CO2 enrichment study. Front. Plant Sci. 2021, 12, 788104. [Google Scholar] [CrossRef]
- Genkawa, T.; Uchino, T.; Inoue, A.; Tanaka, F.; Hamanaka, D. Development of a low-moisture-content storage system for brown rice: Storability at decreased moisture contents. Biosyst. Eng. 2008, 99, 515–522. [Google Scholar] [CrossRef]
- Wang, T.; She, N.; Wang, M.; Zhang, B.; Qin, J.; Dong, J.; Fang, G.; Wang, S. Changes in Physicochemical Properties and Qualities of Red Brown Rice at Different Storage Temperatures. Foods 2021, 10, 2658. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ji, G.; Zhang, J.; Luo, D.; Zhang, F.; Li, L.; Jiang, M.; Zhu, D.; Li, M. Evaluation of Rice Quality Storage Stability: From Variety Screening to Trait Identification. Plants 2025, 14, 356. [Google Scholar] [CrossRef]
- Ziegler, V.; da Silveira, E.B.; Dalla Vecchia, V.; Ferreira, C.D. Chilled paddy rice grains applied directly to industrial processing have a better head rice yield. J. Stored Prod. Res. 2024, 109, 102436. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Wang, C.; Chen, K.; Zhu, Y.; Shen, C.; Ali, J.; Xu, J.; Li, Z. New candidate genes affecting rice grain appearance and milling quality detected by genome-wide and gene-based association analyses. Front. Plant Sci. 2017, 7, 1998. [Google Scholar]
- Liu, S.; Waqas, M.A.; Wang, S.H.; Xiong, X.Y.; Wan, Y.F. Effects of increased levels of atmospheric CO2 and high temperatures on rice growth and quality. PLoS ONE 2017, 12, e0187724. [Google Scholar] [CrossRef]
- Hu, H.; Li, S.; Pan, D.; Wang, K.; Qiu, M.; Qiu, Z.; Liu, X.; Zhang, J. The variation of rice quality and relevant starch structure during long-term storage. Agriculture 2022, 12, 1211. [Google Scholar] [CrossRef]
- Aguiar, R.D.S.; Brito, D.R.; Lopes, M.D.M.; Silva, R.R.; Fidelis, R.R.; Sousa, C.D.; Santos, G.D. Effect of carbon dioxide on quality of rice seeds. Biosci. J. 2015, 31, 1413–1422. [Google Scholar] [CrossRef]
- Hashem, A.S.; Guedes, R.N.C.; Awadalla, H.S. Feeding substrate and temperature interplay determining infestations and losses by the sawtoothed grain beetle (Oryzaephilus surinamensis). J. Stored Prod. Res. 2021, 94, 101887. [Google Scholar] [CrossRef]
- Gomdola, D.; McKenzie, E.H.; Bundhun, D.; Jayawardena, R.S. Morpho-molecular characterization of phoma-like fungi from Morus alba in northern Thailand; a novel species (Boeremia albae) and a new host record (B. maritima). Fungal Biol. 2024, 128, 2139–2147. [Google Scholar] [PubMed]
- Femenias, A.; Gatius, F.; Ramos, A.J.; Teixido-Orries, I.; Marín, S. Hyperspectral imaging for the classification of individual cereal kernels according to fungal and mycotoxins contamination: A review. Food Res. Int. 2022, 155, 111102. [Google Scholar] [CrossRef]
- Kushwaha, R.; Singh, V.; Kaur, S.; Kaur, D. Modulating the characteristics of jackfruit seed starches by annealing and autoclaving-cooling modifications. J. Food Process Eng. 2023, 46, e14322. [Google Scholar] [CrossRef]
- Geng, Q.; Hu, J.; Xu, P.; Sun, T.; Qiu, H.; Wang, S.; Song, F.; Shen, L.; Li, Y.; Liu, M.; et al. The Autophagy-Related Protein ATG8 Orchestrates Asexual Development and AFB1 Biosynthesis in Aspergillus flavus. J. Fungi 2024, 10, 349. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Ouma, F.; Luthra, K.; Oduola, A.; Atungulu, G.G. Investigating safe storage conditions to mitigate aflatoxin contamination in rice. Food Control 2024, 163, 110529. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Effect of temperature and relative humidity on growth of Aspergillus and Penicillium spp. and biocontrol activity of Pseudomonas protegens AS15 against aflatoxigenic Aspergillus flavus in stored rice grains. Mycobiology 2018, 46, 287–295. [Google Scholar] [CrossRef]
- Yates, A.G.; Dierksmeier, S.; Couch, Y.; Claridge, T.D.; Probert, F.; Anthony, D.C.; Ruitenberg, M.J. Lesion level and severity acutely influence metabolomic profiles in spinal cord injury. J. Neuropathol. Exp. Neurol. 2025, 84, nlaf082. [Google Scholar] [CrossRef]
- Kadam, S.B.; Barvkar, V.T. COI1 dependent jasmonic acid signalling positively modulates ROS scavenging system in transgenic hairy root culture of tomato. Plant Physiol. Biochem. 2024, 206, 108229. [Google Scholar]
- Li, Z.; Bhowmik, S.; Sagresti, L.; Brancato, G.; Smith, M.; Benson, D.E.; Li, P.; Merz, K.M., Jr. Simulating metal-imidazole complexes. J. Chem. Theory Comput. 2024, 20, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, Y.; Cheng, C. Production, application and market prospect of L-aspartic acid. Food Ferment. Ind. 2013, 39, 120–124. [Google Scholar]
- Xu, L.; Song, J.Q.; Wang, Y.L.; Liu, X.H.; Li, X.L.; Zhang, B.; Li, A.J.; Ye, X.F.; Wang, J.; Wang, P. Thymol improves salinity tolerance of tobacco by increasing the sodium ion efflux and enhancing the content of nitric oxide and glutathione. BMC Plant Biol. 2022, 22, 31. [Google Scholar] [CrossRef]
- Syeed, S.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, N.A. Control of elevated ion accumulation, oxidative stress, and lipid peroxidation with salicylic acid-induced accumulation of glycine betaine in salinity-exposed Vigna radiata L. Appl. Biochem. Biotechnol. 2021, 193, 3301–3320. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Thalacker-Mercer, A.E.; Gheller, M.E. Benefits and adverse effects of histidine supplementation. J. Nutr. 2020, 150, 2588S–2592S. [Google Scholar] [CrossRef] [PubMed]
- Heidarvand, L.; Millar, A.H.; Taylor, N.L. Responses of the mitochondrial respiratory system to low temperature in plants. Crit. Rev. Plant Sci. 2017, 36, 217–240. [Google Scholar] [CrossRef]
- Xu, Y.; Schmiege, S.C.; Sharkey, T.D. The oxidative pentose phosphate pathway in photosynthesis: A tale of two shunts. New Phytol. 2024, 242, 2453–2463. [Google Scholar] [CrossRef]
- Phégnon, L.; Pérochon, J.; Uttenweiler-Joseph, S.; Cahoreau, E.; Millard, P.; Létisse, F. 6-Phosphogluconolactonase is critical for the efficient functioning of the Pentose phosphate pathway. FEBS J. 2024, 291, 4459–4472. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Q.; Wang, Y.; Yin, J.; Meng, X.; Wang, J.; Zhao, W.; Liu, H.; Zhang, L. Effects of Surfactin Stress on Gene Expression and Pathological Changes in Spodoptera Litura. Sci. Rep. 2024, 14, 30357. [Google Scholar] [CrossRef]
- Ge, C.; Chen, H.; Mei, T.; Tang, X.; Chang, L.; Gu, Z.; Zhang, H.; Chen, W.; Chen, Y.Q. Application of a ω-3 desaturase with an arachidonic acid preference to eicosapentaenoic acid production in Mortierella alpina. Front. Bioeng. Biotechnol. 2018, 5, 89. [Google Scholar] [CrossRef]
- Heidler von Heilborn, D.; Reinmüller, J.; Yurkov, A.; Stehle, P.; Moeller, R.; Lipski, A. Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov. J. Fungi 2023, 9, 1031. [Google Scholar] [CrossRef]
- Chorner, Z.; Barbeau, P.A.; Castellani, L.; Wright, D.C.; Chabowski, A.; Holloway, G.P. Dietary α-linolenic acid supplementation alters skeletal muscle plasma membrane lipid composition, sarcolemmal FAT/CD36 abundance, and palmitate transport rates. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
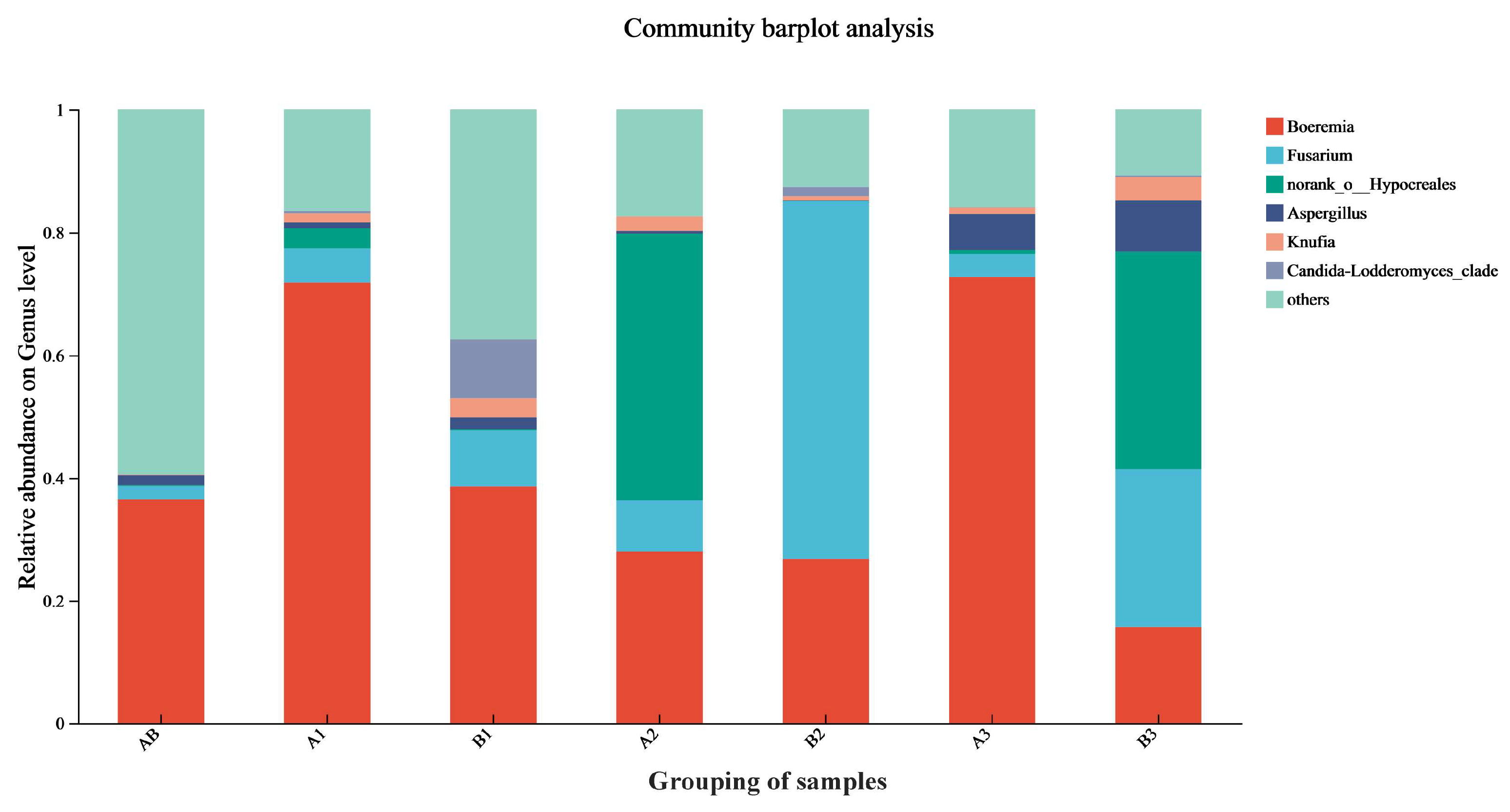
| Sample Information | Sample Name | Sample Grouping |
|---|---|---|
| Origin | AB | AB |
| Experimental warehouse, upper | A1 | A |
| Experimental warehouse, middle | A2 | A |
| Experimental warehouse, lower | A3 | A |
| Control warehouse, upper | B1 | B |
| Control warehouse, middle | B2 | B |
| Control warehouse, lower | B3 | B |
| Quality Indicators | Content | Units |
|---|---|---|
| L* | 58.80 ± 0.17 | - |
| a* | 4.27 ± 0.58 | - |
| b* | 15.53 ± 0.58 | - |
| Fatty acid value | 14.23 ± 0.07 | (mg/100 g) |
| Brown rice yield | 78.93 ± 0.21 | (%) |
| Head milled rice yield | 62.13 ± 0.17 | (%) |
| Germination percentage | 90.33 ± 0.17 | (%) |
| Aflatoxin B1 | 1.66 ± 0.02 | (μg/kg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhao, Y.; Lv, H.; Qi, T.; Song, Y. A Study on the Application of CO2-Modified Atmosphere Combined with Temperature-Control Technology in Rice Warehouse Storage. Foods 2025, 14, 3217. https://doi.org/10.3390/foods14183217
Wang S, Zhao Y, Lv H, Qi T, Song Y. A Study on the Application of CO2-Modified Atmosphere Combined with Temperature-Control Technology in Rice Warehouse Storage. Foods. 2025; 14(18):3217. https://doi.org/10.3390/foods14183217
Chicago/Turabian StyleWang, Shiming, Yan Zhao, Haoxin Lv, Tianjie Qi, and Yongling Song. 2025. "A Study on the Application of CO2-Modified Atmosphere Combined with Temperature-Control Technology in Rice Warehouse Storage" Foods 14, no. 18: 3217. https://doi.org/10.3390/foods14183217
APA StyleWang, S., Zhao, Y., Lv, H., Qi, T., & Song, Y. (2025). A Study on the Application of CO2-Modified Atmosphere Combined with Temperature-Control Technology in Rice Warehouse Storage. Foods, 14(18), 3217. https://doi.org/10.3390/foods14183217








