Dissipation of Triazole Fungicides in Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Protection Products for Field Trial
2.2. Application Rates of Tested Triazoles Active Ingredients
2.3. Field Trials and Sampling
2.4. Cold Storage and Sampling
2.5. Reagents and Materials for Laboratory Analyses
2.6. Sample Preparation
2.7. LC–MS/MS Analysis of Pesticide Residues
2.8. Data Analysis
2.8.1. Fungicide Residue Dissipation Kinetics and Action Pre-Harvest Intervals
2.8.2. Relationship Between Application Rates of Active Ingredient and the Residue Concentration in Apples
2.9. Calculation of Dietary Exposure to Pesticide Residues
3. Results and Discussion
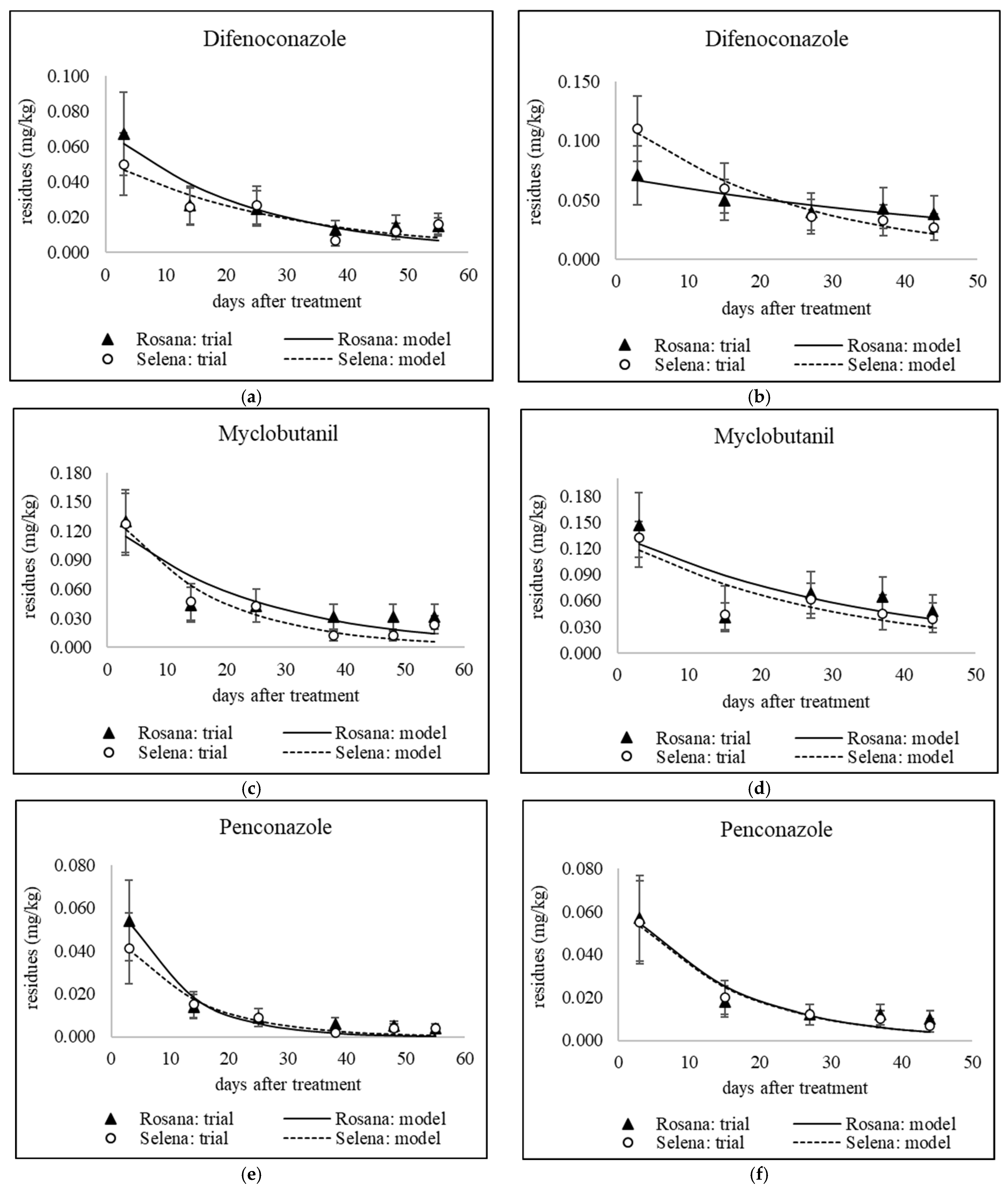
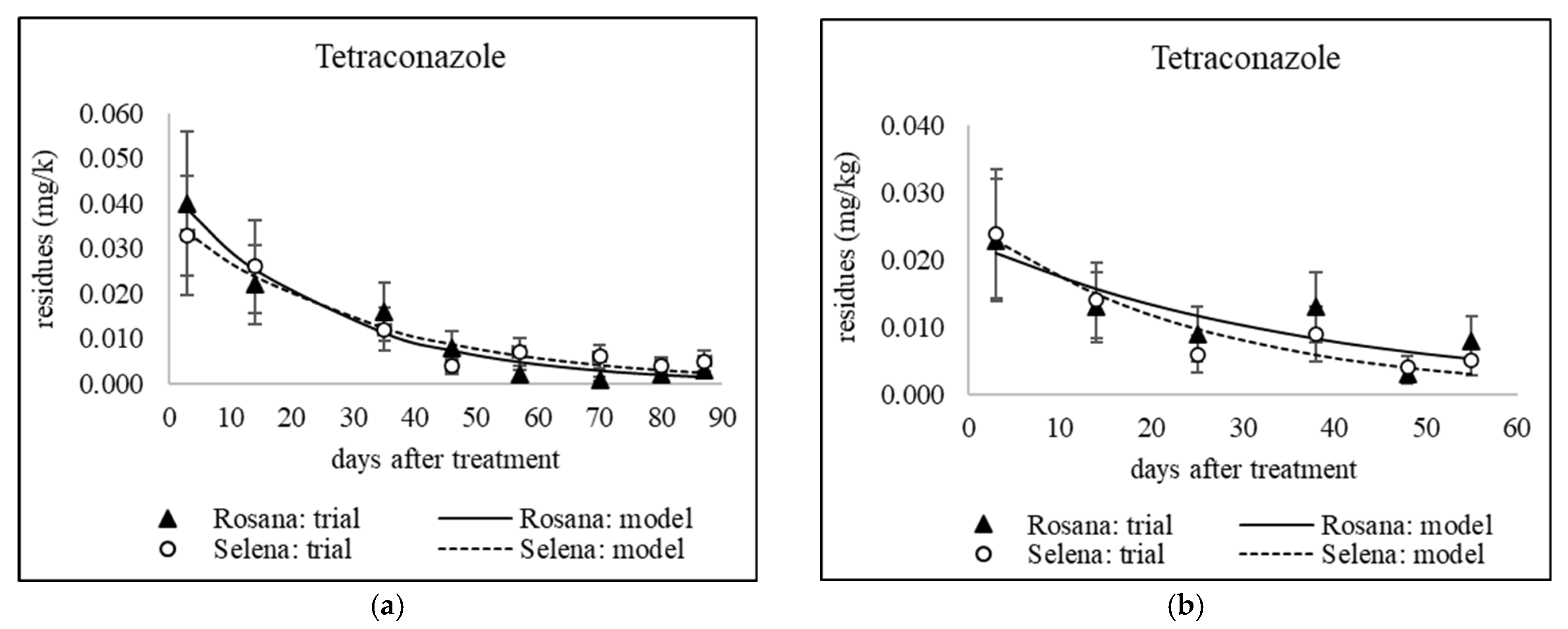
3.1. Dissipation of Triazoles from Different Application Dates
3.1.1. Dissipation Dynamics of Triazoles Applied at a Late Stage
3.1.2. Dissipation Dynamics of Triazoles Applied Early
3.1.3. Low-Residue Production
3.1.4. Non-Residue Production
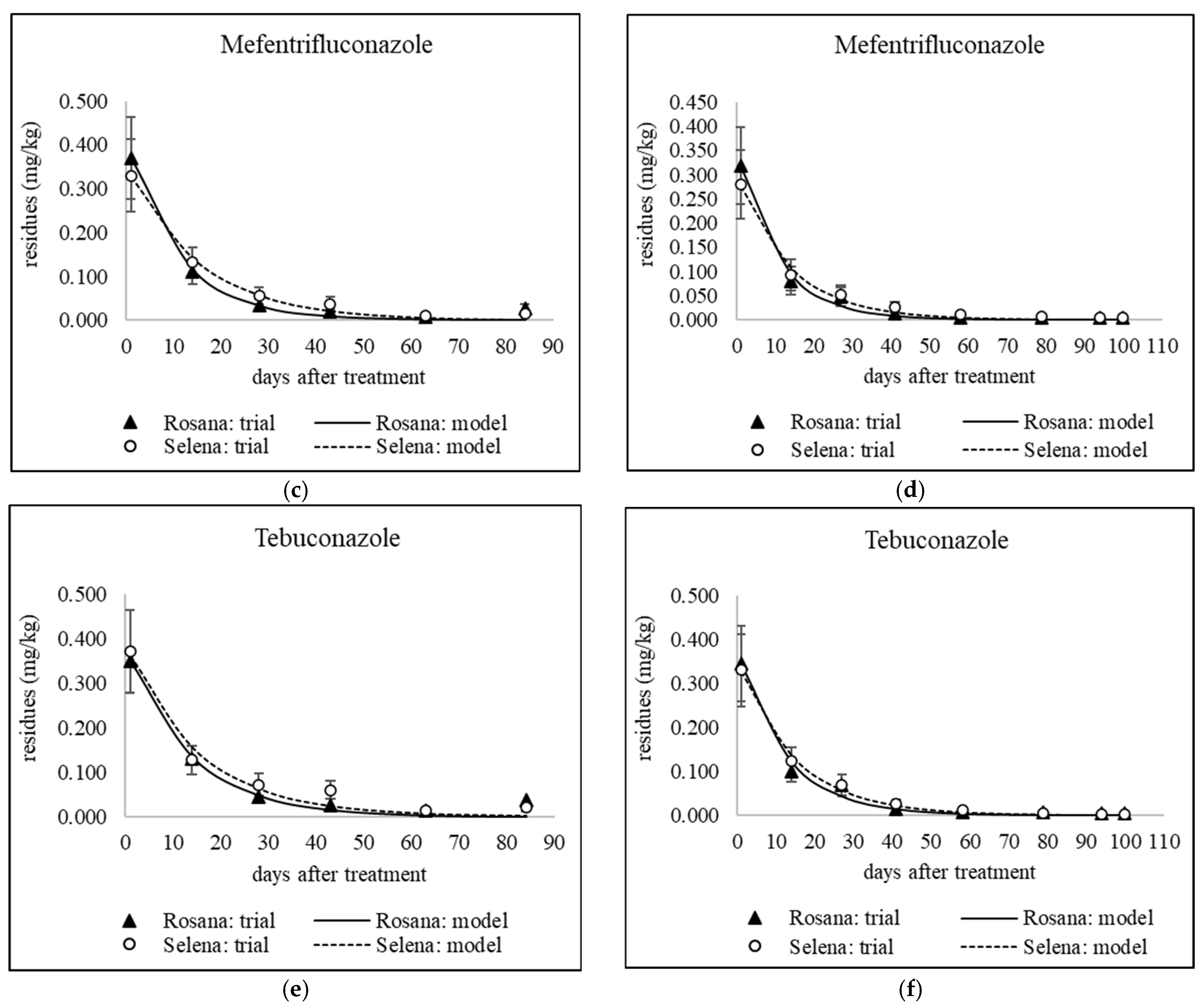
3.2. The Dynamics of Tebuconazole Residues from Pre-Harvest Application to Cold Storage
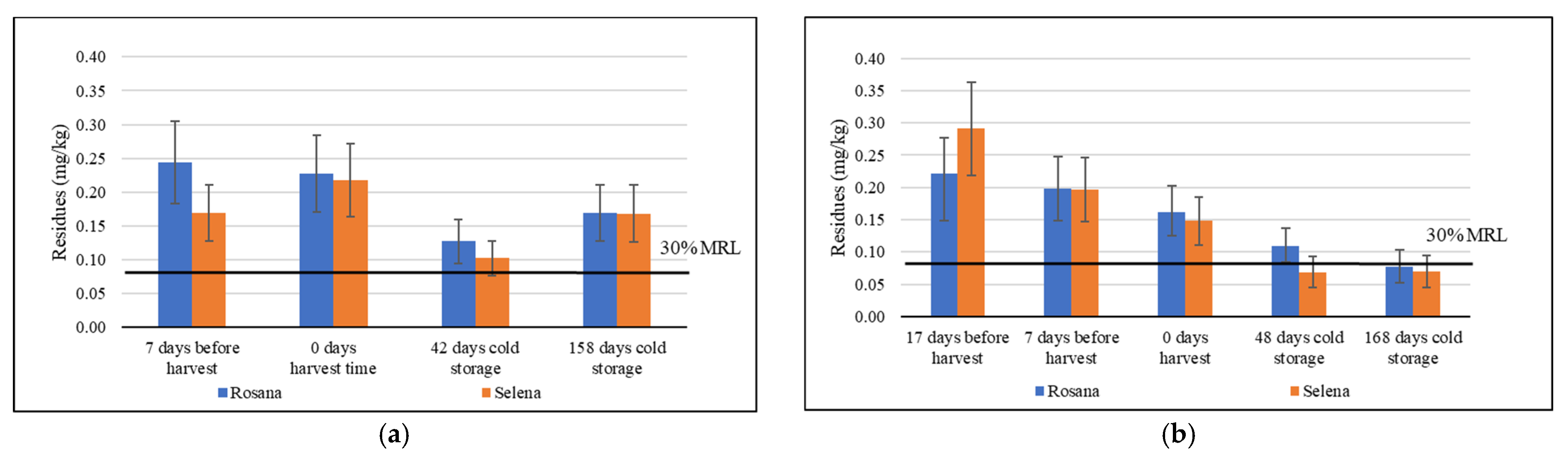
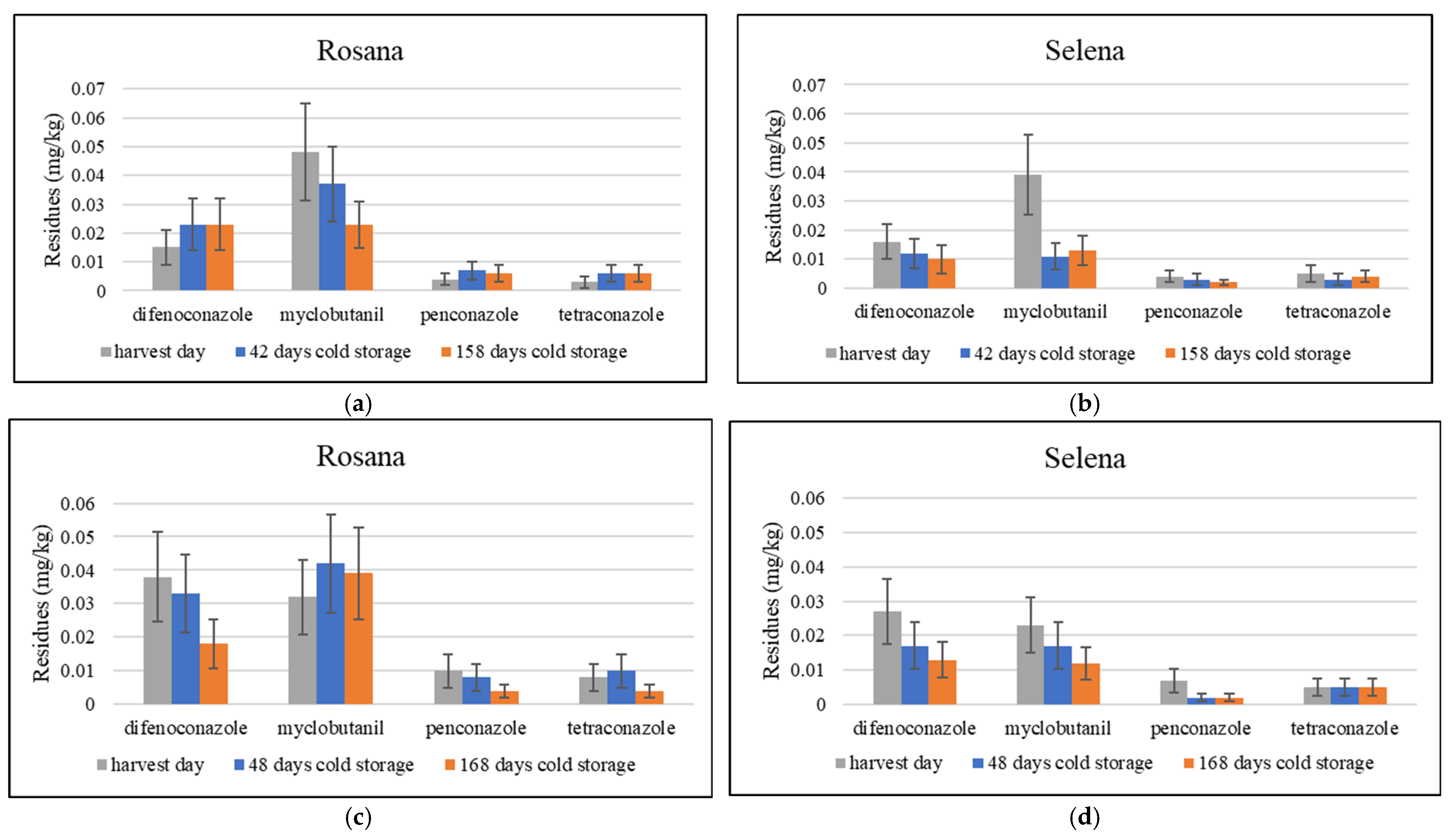
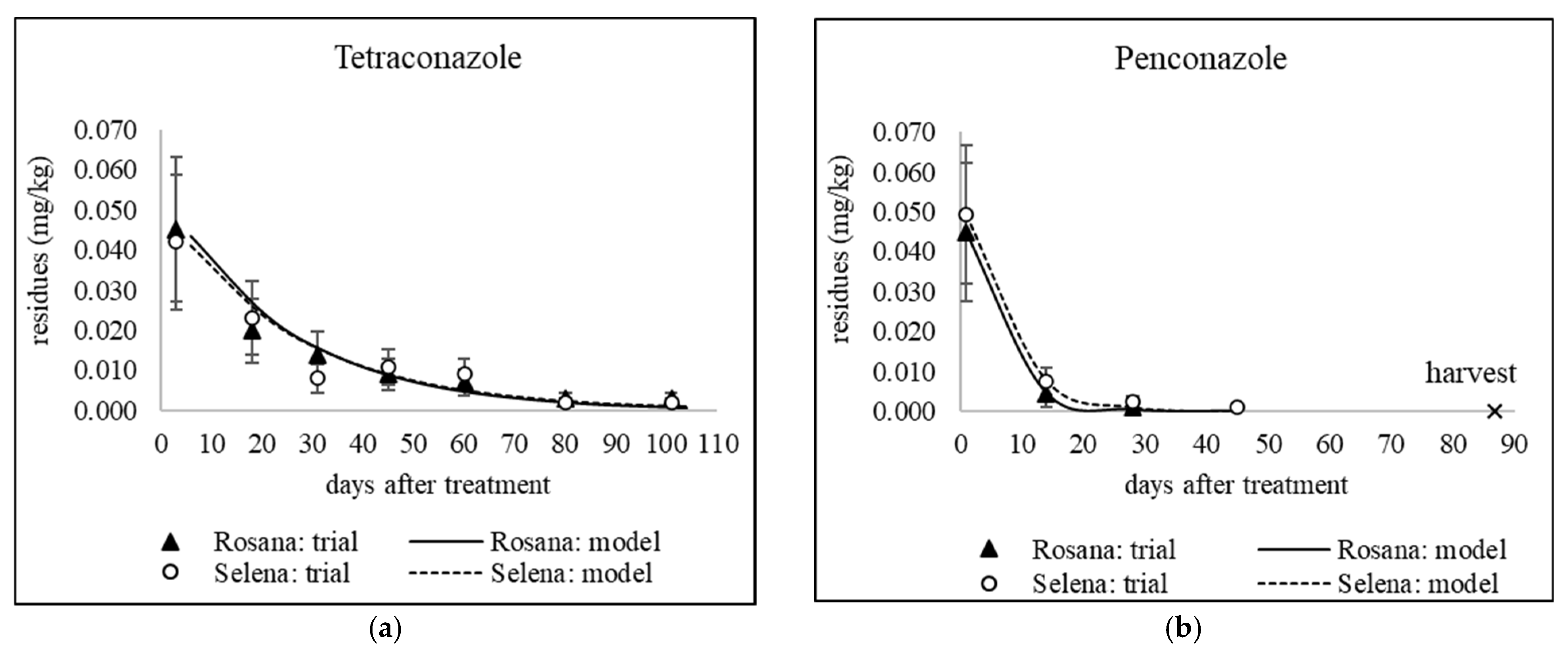
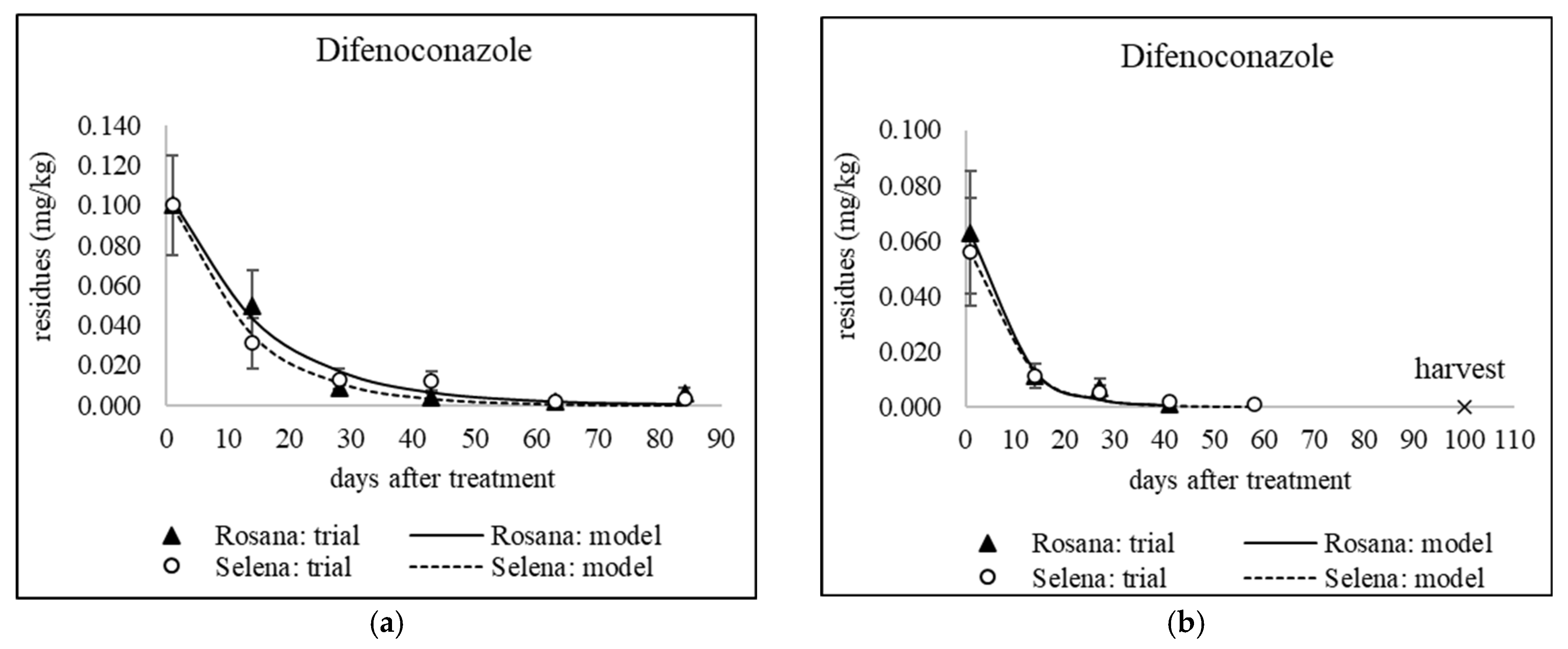
3.3. The Fate of Residues of Difenoconazole, Myclobutanil, Penconazole and Tetraconazole During Cold Storage
3.4. Relationship Between Triazole Application Rates and Residue Concentrations in Apples
3.5. Dietary Risk Assessment
3.6. Risks Associated with Overuse of Azoles
- -
- DMI fungicides should not be used for the entire season; a maximum of four DMI sprays, either alone or in a mixture, are recommended.
- -
- DMIs at the recommended label rates should be used in mixtures or block alternations with a non-cross-resistant fungicide.
- -
- Preventative applications should always be the first choice with DMIs. Curative applications are only recommended when accurate disease warning systems are in place [62].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Němcová, V.; Buchtová, I. Situační a Výhledová Zpráva Ovoce; Ministerstvo Zemědělství: Praha, Czech Republic, 2023; ISBN 978-80-7434-744-3. [Google Scholar]
- FAO. 2024. Agricultural Production Statistics 2010–2023. FAOSTAT Anal. Briefs, No. 96. Rome. Available online: https://openknowledge.fao.org/handle/20.500.14283/cd3755en (accessed on 2 April 2025).
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The Causal Agent of Apple Scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef]
- MacHardy, W.E. Apple Scab: Biology, Epidemiology, and Management; APS Press: St. Paul, MN, USA, 1996; ISBN 978-0-89054-206-4. [Google Scholar]
- Gañán-Betancur, L.; Peever, T.L.; Amiri, A. No Evidence of Resistance to Trifloxystrobin, Triflumizole, and Boscalid in Podosphaera leucotricha Isolates From U.S. Commercial Apple Orchards. Plant Dis. 2021, 105, 2356–2365. [Google Scholar] [CrossRef]
- Taylor, R.A.J. Microorganisms. In Taylor’s Power Law; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–102. ISBN 978-0-12-810987-8. [Google Scholar]
- Büchele, F.; Neuwald, D.A.; Scheer, C.; Wood, R.M.; Vögele, R.T.; Wünsche, J.N. Assessment of a Postharvest Treatment with Pyrimethanil via Thermo-Nebulization in Controlling Storage Rots of Apples. Agronomy 2021, 12, 34. [Google Scholar] [CrossRef]
- Głos, H.; Bryk, H.; Michalecka, M.; Puławska, J. The Recent Occurrence of Biotic Postharvest Diseases of Apples in Poland. Agronomy 2022, 12, 399. [Google Scholar] [CrossRef]
- Florian, V.C.; Puia, C.; Groza, R.; Suciu, L.A.; Florian, T. Study of the Major Pathogens That Lead to Apple Fruit Decay During Storage. Not. Bot. Horti Agrobot. 2018, 46, 538–545. [Google Scholar] [CrossRef]
- Blažek, J.; Kloutvorová, J.; Křelinová, J. Incidence of Storage Diseases on Apples of Selected Cultivars and Advanced Selections Grown with and without Fungicide Treatments. Hortic. Sci. 2006, 33, 87–94. [Google Scholar] [CrossRef]
- Novotný, D.; Lukáš, J.; Brožová, J.; Růžičková, P. Comparison of the Occurrence of Fungi Causing Postharvest Diseases of Apples Grown in Organic and Integrated Production Systems in Orchards in the Czech Republic. Czech Mycol. 2019, 71, 99–121. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Lioliopoulou, F.; Sotiropoulos, T.; Vellios, E. Efficient Control of Apple Scab with Targeted Spray Applications. Agronomy 2020, 10, 217. [Google Scholar] [CrossRef]
- Damos, P.; Colomar, L.-A.; Ioriatti, C. Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are From the Original Concept? Insects 2015, 6, 626–657. [Google Scholar] [CrossRef]
- Didelot, F.; Caffier, V.; Orain, G.; Lemarquand, A.; Parisi, L. Sustainable Management of Scab Control through the Integration of Apple Resistant Cultivars in a Low-Fungicide Input System. Agric. Ecosyst. Environ. 2016, 217, 41–48. [Google Scholar] [CrossRef]
- Trapman, M. Resistance Management in Vf Apple Scab Resistant Organic Apple Orchards. In Proceedings of the 12th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 31 January–2 February 2006; Fördergemeinschaft Ökologischer Obstbau e.V. (FÖKO): Weinsberg, Germany, 2006; pp. 108–112. [Google Scholar]
- Buchleither, S.; Bohr, A.; Späth, S.; Meyer, M.; Haug, P.; König, C. Susceptibility of Scab Resistant Varieties to Apple Scab, Sooty Blotch and Marssonina Coronaria. In Proceedings of the 20th International Conference on Organic Fruit-Growing, Online, 21–23 February 2022; pp. 70–73. [Google Scholar]
- Szpyrka, E.; Walorczyk, S. Dissipation of Difenoconazole in Apples Used for Production of Baby Food. J. Environ. Sci. Health Part B 2017, 52, 131–137. [Google Scholar] [CrossRef] [PubMed]
- FRAC Code List 2024: Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action. Available online: https://www.frac.info/media/kufnaceb/frac-code-list-2024.pdf (accessed on 2 April 2025).
- Tesh, S.A.; Tesh, J.M.; Fegert, I.; Buesen, R.; Schneider, S.; Mentzel, T.; Van Ravenzwaay, B.; Stinchcombe, S. Innovative Selection Approach for a New Antifungal Agent Mefentrifluconazole (Revysol®) and the Impact upon Its Toxicity Profile. Regul. Toxicol. Pharmacol. 2019, 106, 152–168. [Google Scholar] [CrossRef]
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole Fungicides Affect Mammalian Steroidogenesis by Inhibiting Sterol 14 Alpha-Demethylase and Aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef]
- Wang, W.; Fang, Y.; Imran, M.; Hu, Z.; Zhang, S.; Huang, Z.; Liu, X. Characterization of the Field Fludioxonil Resistance and Its Molecular Basis in Botrytis Cinerea from Shanghai Province in China. Microorganisms 2021, 9, 266. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Raddatz, C.; Kutz, S. Relative Abundance and Fungicide Resistance Patterns of Botrytis cinerea and B. pseudocinerea on Apple in Northern Germany. J. Plant Dis. Prot. 2018, 125, 501–504. [Google Scholar] [CrossRef]
- Zhao, H.; Kim, Y.K.; Huang, L.; Xiao, C.L. Resistance to Thiabendazole and Baseline Sensitivity to Fludioxonil and Pyrimethanil in Botrytis Cinerea Populations from Apple and Pear in Washington State. Postharvest Biol. Technol. 2010, 56, 12–18. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC); European Chemicals Agency (ECHA); European Environment Agency (EEA); European Medicines Agency (EMA); European Commission’s Joint Research Centre (JRC). EFSA Impact of the Use of Azole Fungicides, Other than as Human Medicines, on the Development of Azole-resistant Aspergillus spp. EFSA J. 2025, 23, e9200. [Google Scholar] [CrossRef]
- Anamika, Y.; Kusum, J.; Yue, W.; Kalpana, P.; Hardeep, K.; Kumar, S.K.; Vandana, T.; Ashutosh, S.; Xu, J. Chowdhary Anuradha Candida Auris on Apples: Diversity and Clinical Significance. mBio 2022, 13, e00518-22. [Google Scholar] [CrossRef]
- Pandey, M.; Haskell, C.L.; Cowell, J.D.; Amiri, A. Sensitivity to the Demethylation Inhibitor Difenoconazole Among Baseline Populations of Various Penicillium Spp. Causing Blue Mold of Apples and Pears. J. Fungi 2025, 11, 61. [Google Scholar] [CrossRef]
- Stevic, M.; Vuksa, P.; Elezovic, I. Resistance of Venturia inaequalis to Demethylation Inhibiting (DMI) Fungicides. Zemdirbyste 2010, 97, 65–72. [Google Scholar]
- Villani, S.M.; Biggs, A.R.; Cooley, D.R.; Raes, J.J.; Cox, K.D. Prevalence of Myclobutanil Resistance and Difenoconazole Insensitivity in Populations of Venturia inaequalis. Plant Dis. 2015, 99, 1526–1536. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Zambounis, A.; Lioliopoulou, F.; Vellios, E. Detection of Venturia inaequalis Isolates with Multiple Resistance in Greece. Microorganisms 2022, 10, 2354. [Google Scholar] [CrossRef]
- Hoffmeister, M.; Zito, R.; Böhm, J.; Stammler, G. Mutations in Cyp51 of Venturia inaequalis and Their Effects on DMI Sensitivity. J. Plant Dis. Prot. 2021, 128, 1467–1478. [Google Scholar] [CrossRef]
- Kracíková, M.; Haňáčková, Z.; Lišková, P. Monitoring Rezistence Venturia inaequalis k Cyprodinylu a Difenokonazolu v ČR v Letech 2020–2022. VPO 2022, 28, 25–34. [Google Scholar] [CrossRef]
- Ishii, H.; Watanabe, H.; Yamaoka, Y.; Schnabel, G. Sensitivity to Fungicides in Isolates of Colletotrichum Gloeosporioides and C. Acutatum Species Complexes and Efficacy against Anthracnose Diseases. Pestic. Biochem. Physiol. 2022, 182, 105049. [Google Scholar] [CrossRef] [PubMed]
- EU Pesticides Database. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 6 March 2025).
- Regulation EC. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Available online: https://eur-lex.europa.eu/eli/reg/2005/396/oj/eng (accessed on 6 March 2025).
- Paušič, A.; Roškarič, M.; Lešnik, M. Preharvest Treatments with Low-Risk Plant Protection Products Can Help Apple Growers Fulfill the Demands of Supermarket Chains Regarding Pesticide Residues and Marketing Apples under 0-Residue Brands. Agronomy 2023, 13, 1151. [Google Scholar] [CrossRef]
- Government Regulation No. 80/2023 Coll. Ordinance Establishing the Conditions for the Implementation of Agro-Environmental and Climate Measures. Available online: https://www.zakonyprolidi.cz/cs/2023-80 (accessed on 10 April 2025).
- UKZUZ Authorised Plant Protection Products. Available online: https://mze.gov.cz/public/app/eagriapp/POR/Vyhledavani.aspx?type=0&vyhledat=A&stamp=1712491727669 (accessed on 3 April 2025). (In Czech)
- EPA Pesticide Product and Label System. Available online: https://ordspub.epa.gov/ords/pesticides/f?p=PPLS:1 (accessed on 23 April 2025).
- Schusterova, D.; Stara, J.; Kocourek, F.; Hrbek, V.; Mraz, P.; Kosek, V.; Vackova, P.; Kocourek, V.; Hajslova, J.; Horska, T. Dissipation Study of Ten Insecticides in Apples under Field Conditions. J. Sci. Food Agric. 2025, 105, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Schusterova, D.; Horska, T.; Skalsky, M.; Stara, J.; Ourednickova, J.; Uttl, L.; Kocourek, V.; Hajslova, J. Three-Year Monitoring Study of Pesticide Dissipation in Pears. J. Food Compos. Anal. 2024, 126, 105863. [Google Scholar] [CrossRef]
- Sadło, S.; Walorczyk, S.; Grodzicki, P.; Piechowicz, B. Usage of the Relationship between the Application Rates of the Active Ingredient of Fungicides and Their Residue Levels in Mature Apples to Creating a Coherent System of MRLs. J. Plant Dis. Prot. 2016, 123, 101–108. [Google Scholar] [CrossRef][Green Version]
- European Food Safety Authority (EFSA); Cabrera, L.C.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Pastor, P.M. The 2022 European Union Report on Pesticide Residues in Food. EFSA J. 2024, 22, e8753. [Google Scholar] [CrossRef]
- An, Q.; Wu, Y.; Li, D.; Hao, X.; Pan, C.; Rein, A. Development and Application of a Numerical Dynamic Model for Pesticide Residues in Apple Orchards. Pest Manag. Sci. 2022, 78, 2679–2692. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Wang, B.; Zhang, J.; Nie, J. Dynamic Degradation of Penconazole and Its Effect on Antioxidant Enzyme Activity and Malondialdehyde Content in Apple Fruit. Sci. Hortic. 2022, 300, 111053. [Google Scholar] [CrossRef]
- Amin, Z.; Sheikh, P.A.; Jan, I.; Summuna, B.; Dar, A.A.; Wani, F.F.; Bhat, Z.A. Residues Determination, Risk Assessment, and Dissipation Behavior of Myclobutanil Formulation on Apple. Biomed. Chromatogr. 2024, 38, e5879. [Google Scholar] [CrossRef]
- Li, Y.; Nie, J.; Chang, W.; Xu, G.; Farooq, S.; Liu, M.; Zhang, J. Enantioselective Behavior Analysis of Chiral Fungicide Tetraconazole in Apples with UPLC-MS/MS. Food Control 2020, 116, 107305. [Google Scholar] [CrossRef]
- Guo, C.; Li, J.; Guo, B.; Wang, H. Determination and Safety Evaluation of Difenoconazole Residues in Apples and Soils. Bull. Environ. Contam. Toxicol. 2010, 85, 427–431. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, Y.; Yuan, L.; Ren, X.; Liao, X.; Li, L.; Li, W.; Chen, Z. Enantiomeric Profiling of Mefentrifluconazole in Watermelon across China: Enantiochemistry, Environmental Fate, Storage Stability, and Comparative Dietary Risk Assessment. J. Hazard. Mater. 2021, 417, 125985. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; He, Q.; Lin, H.; Chang, H.; Liu, Y.; Sun, H.; Song, X. Analysis of the Fungicidal Efficacy, Environmental Fate, and Safety of the Application of a Mefentrifluconazole and Pyraclostrobin Mixture to Control Mango Anthracnose. J. Sci. Food Agric. 2023, 103, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, X.; Yang, Y.; Dong, F.; Xu, J.; Wu, X.; Zheng, Y. Dissipation Dynamics and Comparative Dietary Exposure Assessment of Mefentrifluconazole in Rice. Ecotoxicol. Environ. Saf. 2023, 250, 114482. [Google Scholar] [CrossRef] [PubMed]
- Patyal, S.K.; Sharma, I.D.; Chandel, R.S.; Dubey, J.K. Dissipation Kinetics of Trifloxystrobin and Tebuconazole on Apple (Malus domestica) and Soil–A Multi Location Study from North Western Himalayan Region. Chemosphere 2013, 92, 949–954. [Google Scholar] [CrossRef]
- Podbielska, M.; Szpyrka, E.; Piechowicz, B.; Zwolak, A.; Sadło, S. Behavior of Fluopyram and Tebuconazole and Some Selected Pesticides in Ripe Apples and Consumer Exposure Assessment in the Applied Crop Protection Framework. Environ. Monit. Assess. 2017, 189, 350. [Google Scholar] [CrossRef]
- Podbielska, M.; Kus-Liśkiewicz, M.; Jagusztyn, B.; Szpyrka, E. Effect of Microorganisms on Degradation of Fluopyram and Tebuconazole in Laboratory and Field Studies. Environ. Sci. Pollut. Res. 2023, 30, 47727–47741. [Google Scholar] [CrossRef]
- Ticha, J.; Hajslova, J.; Jech, M.; Honzicek, J.; Lacina, O.; Kohoutkova, J.; Kocourek, V.; Lansky, M.; Kloutvorova, J.; Falta, V. Changes of Pesticide Residues in Apples during Cold Storage. Food Control 2008, 19, 247–256. [Google Scholar] [CrossRef]
- Ticha, J.; Hajslova, J.; Kovalczuk, T.; Jech, M.; Honzicek, J.; Kocourek, V.; Lansky, M.; Kloutvorova, J.; Falta, V. Safe Apples for Baby-Food Production: Survey of Pesticide Treatment Regimes Leaving Minimum Residues. Food Addit. Contam. 2007, 24, 605–620. [Google Scholar] [CrossRef]
- Sadło, S.; Walorczyk, S.; Grodzicki, P.; Piechowicz, B. Disappearance of Captan, Boscalid, Pyraclostrobin and Trifloxystrobin Residues in Ripe Apples during Cold Storage under Controlled Atmosphere. Fresenius Environ. Bull. 2016, 25, 195. [Google Scholar]
- Tang, Y.; Hu, K.; Li, X.; Liu, C.; Xu, Y.; Zhang, Z.; Wu, X. Dissipation Dynamics and Dietary Risk Assessment of Four Fungicides as Preservatives in Pear. Agriculture 2022, 12, 630. [Google Scholar] [CrossRef]
- Pogăcean, M.O.; Hlihor, R.M.; Gavrilescu, M. Monitoring Pesticides Degradation in Apple Fruits and Potential Effects of Residues on Human Health. J. Environ. Eng. Landsc. Manag. 2014, 22, 171–182. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyński, P.; Wołejko, E.; Jankowska, M.; Iwaniuk, P.; Hrynko, I.; Rutkowska, E.; Łuniewski, S.; Ilyasova, G.; Jabłońska-Trypuć, A.; et al. Comprehensive Toxicological Multi-Year Study on Pesticides in Apples: Control, Trends and Dietary Risk Assessment. Food Chem. 2025, 464, 141897. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.S.; Sundin, G.W.; Beckerman, J.L. Identification of Resistance to Multiple Fungicides in Field Populations of Venturia inaequalis. Plant Dis. 2011, 95, 921–926. [Google Scholar] [CrossRef] [PubMed]
- FRAC. FRAC Recommendations for SBI Fungicides. Available online: https://www.frac.info/frac-teams/working-groups/sbi-fungicides/recommendations-for-sbi (accessed on 5 April 2025).
- Kildea, S.; Hellin, P.; Heick, T.M.; Byrne, S.; Hutton, F. Mefentrifluconazole Sensitivity amongst European Zymoseptoria tritici Populations and Potential Implications for Its Field Efficacy. Pest Manag. Sci. 2024, 80, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Kadlikova, K.; Vaclavikova, M.; Halesova, T.; Kamler, M.; Markovic, M.; Erban, T. The Investigation of Honey Bee Pesticide Poisoning Incidents in Czechia. Chemosphere 2021, 263, 128056. [Google Scholar] [CrossRef] [PubMed]
- Taenzler, V.; Weyers, A.; Maus, C.; Ebeling, M.; Levine, S.; Cabrera, A.; Schmehl, D.; Gao, Z.; Rodea-Palomares, I. Acute Toxicity of Pesticide Mixtures to Honey Bees Is Generally Additive, and Well Predicted by Concentration Addition. Sci. Total Environ. 2023, 857, 159518. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO. Pesticide Residues in Food 2008: Joint FAO/WHO Meeting on Pesticide Residues; Organisation des Nations Unies pour l’alimentation et l’agriculture, Organisation mondiale de la santé, Eds.; FAO Plant Production and Protection Papers; WHO/FAO: Rome, Italy, 2009; ISBN 978-92-5-106113-8. [Google Scholar]
- Kolberg, D.I.S.; Zechmann, S.; Wildgrube, C.; Sigalov, I.; Scherbaum, E.; Anastassiades, M. Determination of Triazole Derivative Metabo-Lites (TDMs) in Fruit and Vegetables Using the QuPPe Method and Differential Mobility Spec-Trometry (DMS) and Survey of the Residue Sit-Uation in Organic and Conventional Produce. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EurlSrm_meth_TriazoleDerivativeMetabolites.pdf (accessed on 6 September 2025).
- Albers, C.N.; Johnsen, A.R.; Bollmann, U.E. Urban Areas as Sources of the Groundwater Contaminants N,N-Dimethylsulfamide (N,N-DMS) and 1,2,4-Triazole. Sci. Total Environ. 2023, 881, 163377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-F.; Ying, G.-G. Occurrence, Fate and Ecological Risk of Five Typical Azole Fungicides as Therapeutic and Personal Care Products in the Environment: A Review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef]
- Halešová, T.; Konečná, J.; Václavíková, M.; Karásek, P.; Nováková, E. Výskyt Pesticidních Látek v Řece Punkvě. VTEI 2022, 64, 29. [Google Scholar] [CrossRef]
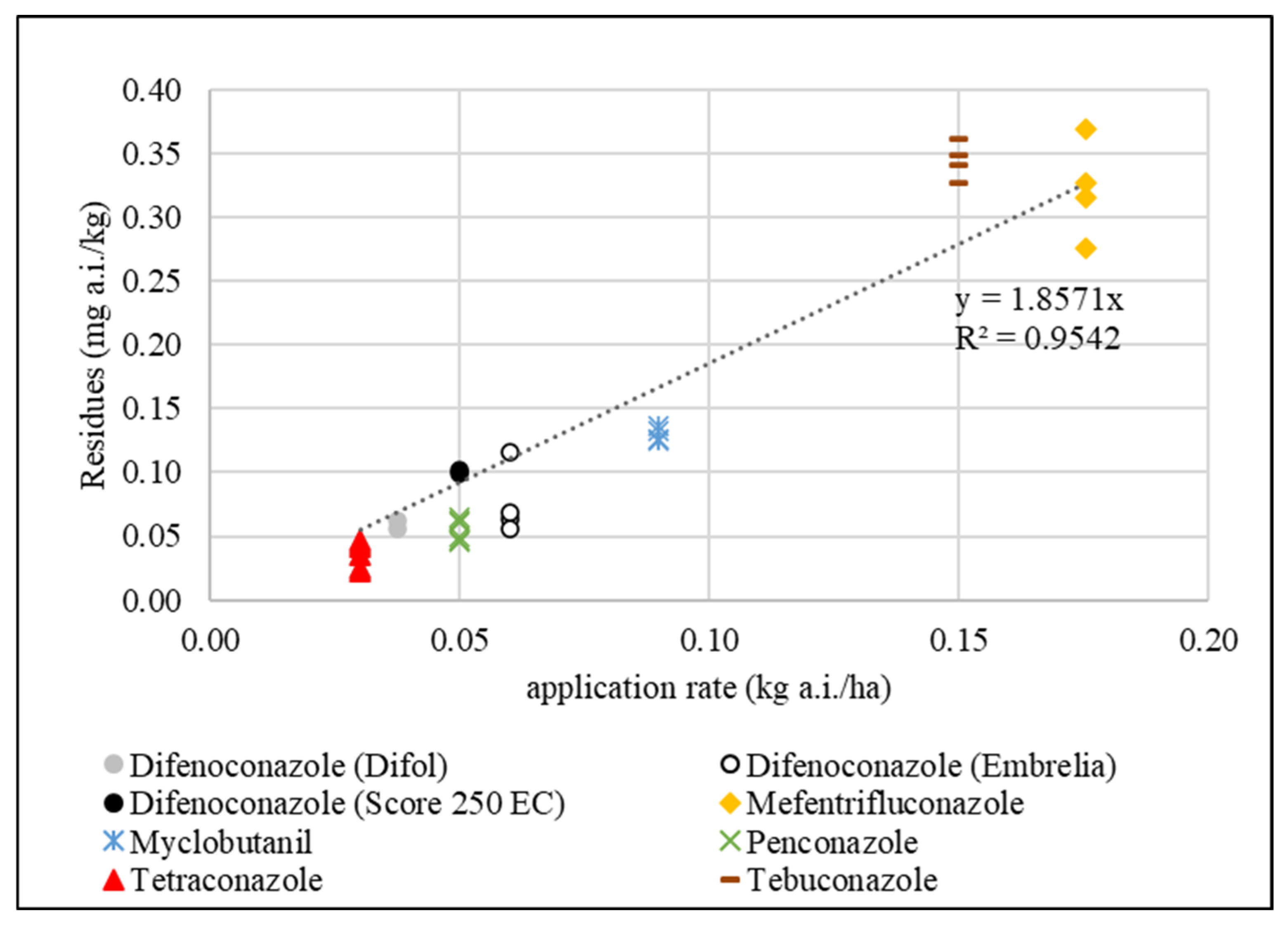
| Trade Name of PPP a | Active Ingredient (a.i.) | Dose of PPP (kg, L/ha) | Content of Triazole a.i. in PPP (g/kg, g/L) | Dose of Triazole a.i. (kg/ha) |
|---|---|---|---|---|
| Embrelia b | difenoconazole + isopyrazam | 1.5 | 40.0 | 0.060 |
| Score 250 EC | difenoconazole | 0.2 | 250 | 0.050 |
| Difol | difenoconazole + folpet | 3.5 | 10.7 | 0.037 |
| Belanty | mefentrifluconazole | 2.34 d | 75.0 | 0.176 |
| Talent c | myclobutanil | 0.45 | 200 | 0.090 |
| Topas 100 EC | penconazole | 0.5 | 100 | 0.050 |
| Domark 10 EC | tetraconazole | 0.3 | 100 | 0.030 |
| Luna Experience | tebuconazole + fluopyram | 0.75 | 200 | 0.150 |
| Year of Trial | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
| PPP | Active Ingredients | Application Date | |||
| Embrelia | difenoconazole | 31 July | 10 August | × | × |
| Score 250 EC | × | × | 30 June | × | |
| Difol | × | × | × | 13 June | |
| Belanty | mefentrifluconazole | × | × | 30 June | 13 June |
| Talent | myclobutanil | 31 July | 10 August | × | × |
| Topas 100 EC | penconazole | 31 July | 10 August | × | 26 June |
| Domark 10 EC | tetraconazole | 29 June | 30 July | 13 June | × |
| Luna Experience | tebuconazole | 14 September | 3 September | 30 June | 13 June |
| Harvest date | 24 September | 23 September | 22 September | 21 September | |
| Analyte | ESI Mode | RT a (min) | MS/MS Transitions (Collision Energy (V)) | LOQ b (mg/kg) | REC c (%) | RSD d (%) | ME e (%) | Linearity (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| difenoconazole | ESI+ | 9.7 + 10.2 | 406.0 > 251.0 (24) 408.0 > 253.0 (24) | 0.001 | 81–87 | 2–9 | 5 | 0.001–0.1 |
| mefentrifluconazole | ESI+ | 9.7 | 398.1 > 182.1 (36) 398.1 > 70.1 (24) | 0.002 | 95–97 | 4–5 | 5 | 0.002–0.1 |
| myclobutanil | ESI+ | 8.1 | 289.1 > 125.0 (40) 289.1 > 70.1 (20) | 0.001 | 92–98 | 2–8 | 3 | 0.001–0.05 |
| penconazole | ESI+ | 9.4 | 284.0 > 159.0 (36) 284.0 > 70.1 (16) | 0.001 | 85–86 | 5–8 | −8 | 0.001–0.05 |
| tebuconazole | ESI+ | 9.5 | 308.1 > 125.0 (44) 308.1 > 70.1 (26) | 0.002 | 86–89 | 1–6 | −9 | 0.002–0.1 |
| tetraconazole | ESI+ | 8.6 | 372.0 > 159.0 (44) 372.0 > 70.1 (20) | 0.002 | 82–87 | 2–4 | 3 | 0.002–0.05 |
| Risk Assessment | Population Class a | Mean BW b (kg) | Unit Weight Consumed (g) |
|---|---|---|---|
| ACUTE | other children | 26.3 | 272.50 |
| adolescents | 45.5 | 450.00 | |
| adults | 74.7 | 409.50 | |
| CHRONIC | other children | 26.0 | 535.00 |
| adolescents | 45.6 | 319.75 | |
| adults | 75.2 | 385.55 |
| Kinetic Model Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Trial PHI) | Cultivar | Active Ingredient | PHI | a MRL | C0 | k | t1/2 | R2 | C(model PHI) | 30% MRL | C(harvest) | APHI(30%) | APHI(0.01) |
| (Day) | (Day) | (mg/kg) | (mg/kg) | (Day−1) | (Day) | (mg/kg) | (Day) | (Day) | |||||
| 2020 (55) | Rosana | Difenoconazole (Embrelia) | 21 | 0.8 | 0.070 | 0.042 | 16.6 | 0.8718 | 0.029 | 0.240 | 0.015 | 21 | 61.9 |
| Selena | 0.052 | 0.033 | 20.8 | 0.8376 | 0.026 | 0.016 | 65.9 | ||||||
| 2021 (44) | Rosana | 0.070 | 0.016 | 43.9 | 0.8571 | 0.050 | 0.038 | 164.3 | |||||
| Selena | 0.120 | 0.040 | 17.5 | 0.9696 | 0.052 | 0.027 | 83.6 | ||||||
| 2022 (84) | Rosana | Difenoconazole (Score 250 EC) | 49 | 0.109 | 0.066 | 10.4 | 0.9803 | 0.004 | 0.006 | 49 | (47.9) 49 | ||
| Selena | 0.107 | 0.080 | 8.7 | 0.9853 | 0.002 | 0.003 | (39.5) 49 | ||||||
| 2023 (100) | Rosana | Difenoconazole (Difol) | 110 | 0.071 | 0.122 | 5.7 | 0.9907 | <0.001 | <0.001 | 110 | (21.4) 110 | ||
| Selena | 0.063 | 0.115 | 6.0 | 0.9942 | <0.001 | <0.001 | (21.2) 110 | ||||||
| 2022 (84) | Rosana | Mefentrifluconazole | 28 | 0.4 | 0.404 | 0.090 | 7.7 | 0.9905 | 0.033 | 0.120 | 0.027 | 28 | 54.9 |
| Selena | 0.349 | 0.064 | 10.8 | 0.9927 | 0.058 | 0.015 | 73.6 | ||||||
| 2023 (100) | Rosana | 0.346 | 0.092 | 7.5 | 0.9913 | 0.026 | 0.006 | 51.1 | |||||
| Selena | 0.297 | 0.072 | 9.6 | 0.9903 | 0.040 | 0.006 | 62.8 | ||||||
| 2020 (55) | Rosana | Myclobutanil | 14 | 0.6 | 0.129 | 0.040 | 17.2 | 0.7788 | 0.074 | 0.180 | 0.032 | 14 b | 84.6 |
| Selena | 0.145 | 0.059 | 11.8 | 0.9230 | 0.064 | 0.023 | 60.6 | ||||||
| 2021 (44) | Rosana | 0.136 | 0.028 | 24.4 | 0.5562 | 0.092 | 0.048 | 122.5 | |||||
| Selena | 0.130 | 0.034 | 20.6 | 0.7279 | 0.081 | 0.039 | 101.8 | ||||||
| 2020 (55) | Rosana | Penconazole | 14 | 0.15 | 0.071 | 0.097 | 7.1 | 0.9590 | 0.018 | 0.045 | 0.004 | 14 | 26.8 |
| Selena | 0.051 | 0.076 | 9.1 | 0.9752 | 0.017 | 0.004 | 28.3 | ||||||
| 2021 (44) | Rosana | 0.066 | 0.064 | 10.9 | 0.9181 | 0.027 | 0.010 | 39.5 | |||||
| Selena | 0.065 | 0.064 | 10.8 | 0.9673 | 0.026 | 0.007 | 38.9 | ||||||
| 2023 (87) | Rosana | 0.054 | 0.175 | 4.0 | 0.9997 | 0.005 | <0.001 | 14.0 | |||||
| Selena | 0.057 | 0.141 | 4.9 | 0.9982 | 0.008 | <0.001 | 16.5 | ||||||
| 2020 (87) | Rosana | Tetraconazole | 14 | 0.3 | 0.044 | 0.039 | 18.0 | 0.9625 | 0.025 | 0.090 | 0.003 | 14 | 50.9 |
| Selena | 0.037 | 0.031 | 22.3 | 0.9549 | 0.024 | 0.005 | 56.1 | ||||||
| 2021 (55) | Rosana | 0.023 | 0.027 | 26.0 | 0.7390 | 0.016 | 0.008 | 41.2 | |||||
| Selena | 0.026 | 0.039 | 17.9 | 0.8974 | 0.015 | 0.005 | 32.4 | ||||||
| 2022 (101) | Rosana | 0.044 | 0.040 | 17.2 | 0.9771 | 0.028 | 0.003 | 52.5 | |||||
| Selena | 0.037 | 0.039 | 17.8 | 0.9466 | 0.027 | 0.002 | 52.5 | ||||||
| 2022 (84) | Rosana | Tebuconazole | 14 | 0.3 | 0.375 | 0.073 | 9.5 | 0.9811 | 0.135 | 0.090 | 0.037 | 26.0 | 66.0 |
| Selena | 0.386 | 0.065 | 10.7 | 0.9694 | 0.155 | 0.021 | 29.9 | 75.0 | |||||
| 2023 (100) | Rosana | 0.370 | 0.080 | 8.7 | 0.9877 | 0.121 | 0.006 | 23.6 | 60.4 | ||||
| Selena | 0.349 | 0.067 | 10.4 | 0.9953 | 0.137 | 0.003 | 27.0 | 70.8 | |||||
| (A) | ARfD | Residue at Harvest (Range) | Other Children | Adolescents | Adults |
| mg/kgbw | mg/kg | %ARfD | %ARfD | %ARfD | |
| difenoconazole | 0.16 | 0.003–0.038 | 0.11–1.37 | 0.07–0.92 | 0.04–0.49 |
| mefentrifluconazole | 0.15 | 0.006–0.027 | 0.21–1.04 | 0.14–0.69 | 0.08–0.37 |
| myclobutanil | 0.31 | 0.023–0.048 | 0.42–0.89 | 0.29–0.60 | 0.15–0.32 |
| penconazole | 0.5 | 0.004–0.010 | 0.05–0.12 | 0.03–0.08 | 0.02–0.04 |
| tebuconazole * | 0.03 | 0.003–0.162 | 0.48–31.09 | 0.32–20.84 | 0.17–11.12 |
| tetraconazole | 0.05 | 0.002–0.008 | 0.23–0.92 | 0.16–0.62 | 0.08–0.33 |
| (B) | ADI | Residue at Harvest (Range) | Other Children | Adolescents | Adults |
| mg/kgbw/day | mg/kg | %ADI | %ADI | %ADI | |
| difenoconazole | 0.01 | 0.003–0.038 | 0.72–9.10 | 0.32–4.11 | 0.17–2.11 |
| mefentrifluconazole | 0.035 | 0.006–0.027 | 0.38–1.85 | 0.17–0.83 | 0.09–0.43 |
| myclobutanil | 0.025 | 0.023–0.048 | 2.21–4.61 | 0.99–2.07 | 0.51–1.07 |
| penconazole | 0.03 | 0.004–0.010 | 0.32–0.80 | 0.14–0.36 | 0.08–0.19 |
| tebuconazole * | 0.03 | 0.003–0.162 | 0.20–12.92 | 0.09–5.82 | 0.05–3.02 |
| tetraconazole | 0.004 | 0.002–0.008 | 0.81–3.25 | 0.39–1.54 | 0.22–0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horska, T.; Stara, J.; Kocourek, F.; Uttl, L.; Han, J.; Kocourek, V.; Hajslova, J.; Hanackova, Z.; Schusterova, D. Dissipation of Triazole Fungicides in Apples. Foods 2025, 14, 3210. https://doi.org/10.3390/foods14183210
Horska T, Stara J, Kocourek F, Uttl L, Han J, Kocourek V, Hajslova J, Hanackova Z, Schusterova D. Dissipation of Triazole Fungicides in Apples. Foods. 2025; 14(18):3210. https://doi.org/10.3390/foods14183210
Chicago/Turabian StyleHorska, Tereza, Jitka Stara, Frantisek Kocourek, Leos Uttl, Jingwen Han, Vladimir Kocourek, Jana Hajslova, Zuzana Hanackova, and Dana Schusterova. 2025. "Dissipation of Triazole Fungicides in Apples" Foods 14, no. 18: 3210. https://doi.org/10.3390/foods14183210
APA StyleHorska, T., Stara, J., Kocourek, F., Uttl, L., Han, J., Kocourek, V., Hajslova, J., Hanackova, Z., & Schusterova, D. (2025). Dissipation of Triazole Fungicides in Apples. Foods, 14(18), 3210. https://doi.org/10.3390/foods14183210








