Techno-Economic Feasibility of Producing High-Protein Tofu from Chickpeas: Process Design and Nutrient Recovery
Abstract
1. Introduction
2. Background
2.1. Nutritional Composition and Allergenicity
2.2. Functional and Technological Properties of Chickpea Proteins
2.3. Conventional Tofu Production and Processing Constraints
2.4. Process Analogs and Applications of Chickpea-Based Tofu
2.5. Low-Cost Systems and Humanitarian Relevance
2.6. Motivation for Process Adaptation
3. Materials and Methods
3.1. Production of Chickpea Tofu
3.2. Physiochemical Properties
3.3. Calculated Attributes
3.3.1. Conversion of Chickpeas to Okara, Starch, and Tofu
- M0 is the wet mass of raw chickpeas (kg);
- Mi is the wet mass of product i (kg; okara i = 3, starch i = 4, or tofu i = 5) (Figure 1).
3.3.2. Operating Energy Needed
- QB_calculated is the sensible heat required for boiling (kJ);
- M is the mass of chickpea milk (kg);
- Cp is the specific heat capacity (3.8 kJ·kg−1·K−1) [46];
- ΔT is the temperature change (100 K).
- QD_calculated is the thermal energy needed for drying (kJ);
- Mv is the mass of water to be evaporated (kg);
- Hlatent is the latent heat of water (2260 kJ/kg).
- Tofu powder: derived from fresh tofu containing 70% moisture.
- Okara powder: derived from wet okara with 70% moisture, collected during the straining step.
- Starch powder: derived from wet starch sediment containing 55% moisture, following the 1.5 h sedimentation phase.
- Scotta powder: derived from liquid scotta with a soluble solids content of 3 °Bx.
3.4. Fresh Basis Mass Balance of Streams
4. Results
4.1. Raw Chickpea Nutritional Results
4.2. Okara, Starch, and Tofu Nutritional Results
4.3. Mass Balance of the Process
Component Recovery and Partitioning
4.4. Estimated Energy
5. Discussion
5.1. Nutritional Transformation
5.2. Yield Transformation
5.3. Techno-Economic Analysis
5.3.1. Fresh Tofu: Input Cost and Efficiency
5.3.2. Powdered Tofu: Shelf Stability and Cost Trade-Offs
5.3.3. Coproduct Costing and Assumptions
5.3.4. Summary Perspective
5.4. Valorization of Scotta: Drying Feasibility and Alternatives
- Lactic acid fermentation using existing pulse or dairy fermentation cultures, which can yield probiotic beverages or bio-acid solutions as mentioned by Peñas et al. (2006) [54].
- Direct culinary use as a base for soups, porridges, or fortified drinks, especially in institutional feeding settings.
- Microbial conversion for the production of bioactives or micronutrients such as vitamin B12, leveraging known capabilities of Propionibacterium species demonstrated by Yu et al. (2015) [55].
- Composting or animal feed blending, particularly in combination with okara to enhance nutrient recovery in agricultural or livestock systems.
5.5. Environmental Perspective (Conceptual)
5.6. Food Security Relevance (Perspective)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandekar, P.A.; Putman, B.; Thoma, G.; Matlock, M. Cradle-to-grave life cycle assessment of production and consumption of pulses in the United States. J. Environ. Manag. 2022, 302, 114062. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-chemical and functional properties of legume protein, starch, and dietary fiber—A review. Legume Sci. 2022, 4, e117. [Google Scholar] [CrossRef]
- Wiederstein, M.; Baumgartner, S.; Lauter, K. Soybean (Glycine max) allergens—A Review on an Outstanding Plant Food with Allergenic Potential. ACS Food Sci. Technol. 2023, 3, 363–378. [Google Scholar] [CrossRef]
- WITS. World Integrated Trade Solution: Lebanon Imports of Soybeans (HS 120100). Available online: https://today.lorientlejour.com/article/1441371/slight-increase-in-generator-rates-for-the-last-bill-of-the-year.html (accessed on 21 July 2025).
- Cai, T.; Chang, K. Dry tofu characteristics affected by soymilk solid content and coagulation time 1. J. Food Qual. 1997, 20, 391–402. [Google Scholar] [CrossRef]
- Cai, R.; McCurdy, A.; Baik, B.K. Textural property of 6 legume curds in relation to their protein constituents. J. Food Sci. 2002, 67, 1725–1730. [Google Scholar] [CrossRef]
- U.S. Food Drug Administration. Response Letter to GRAS Notice No. GRN 001098: Chickpea Protein Concentrate. 2023. Available online: https://www.fda.gov/media/170786/download (accessed on 2 July 2025).
- Acevedo Martinez, K.A.; Yang, M.M.; Gonzalez de Mejia, E. Te chnological properties of chickpea (Cicer arietinum): Production of snacks and health benefits related to type-2 diabetes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3762–3787. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Hong, S.; Zhu, Y.; Garay, A.; Yang, J.; Henderson, D.; Zhang, X.; Xu, Y.; Li, Y. Comprehensive review of chickpea (Cicer arietinum): Nutritional significance, health benefits, techno-functionalities, and food applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70152. [Google Scholar] [CrossRef] [PubMed]
- Pournaki, S.K. Effects of Storage Regimes on Chemistry, Functional Properties, and Vitamin Profile of Different Varieties of Chickpea; South Dakota State University: Brookings, SD, USA, 2024. [Google Scholar]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhu, C.; Singh, R.P.; Chen, W. Chickpea: Its origin, distribution, nutrition, benefits, breeding, and symbiotic relationship with Mesorhizobium species. Plants 2024, 13, 429. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World (SOFI); FAO; IFAD; UNICEF; WFP; WHO: Rome, Italy, 2025; 234p. [Google Scholar]
- Teshome, M.S.; Lema, T.B.; Abessa, T.G.; Mingels, S.; Granitzer, M.; Rameckers, E.; Verbecque, E. Current evidence on the effectiveness of Ready-to-Use Supplementary Foods in children with moderate acute malnutrition: A systematic review and meta-analysis. J. Nutr. Sci. 2023, 12, e130. [Google Scholar] [CrossRef]
- Merrill, R.; de Pee, S.; Ahmed, T.; Kramer, K.; Hossain, N.; Choudhury, N.; Schumacher, B.; Steiger, G.; Minhas, S.; Shamim, A.A. Design, development, and local production of lipid-based nutritional supplements to enhance the complementary feeding diet: A model for collaboration for a feeding trial in Bangladesh. Gates Open Res. 2022, 6, 122. [Google Scholar] [CrossRef]
- Herrera, A.C.; Gonzalez de Mejia, E. Feasibility of commercial breadmaking using chickpea as an ingredient: Functional properties and potential health benefits. J. Food Sci. 2021, 86, 2208–2224. [Google Scholar] [CrossRef]
- Silsin, M.; Khongdan, J.; Paramita, V.D.; Panyoyai, N. Effect of partial replacement of soybean with chickpea to the nutritional and textural properties of tofu. Indones. Food Sci. Technol. J. 2021, 4, 27–31. [Google Scholar] [CrossRef]
- Klupšaitė, D.; Juodeikienė, G. Legume: Composition, protein extraction and functional properties. A review. Chem. Technol. 2015, 66, 5–12. [Google Scholar] [CrossRef]
- Poteraș, C.-B.; Culețu, A.; Manolache, F.-A. Nutritional importance of lentil, lupin, chickpea and soy legumes: A Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2024, 81, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Garcia-Santos, S.; Carloto, D.; Arantes, A.; Lorenzo, J.M.; Silva, J.A.; Santos, V.; Azevedo, J.; Guedes, C.; Ferreira, L. Introducing Mediterranean Lupins in Lamb Diets: Effects on Carcass Composition, Meat Quality, and Intramuscular Fatty Acid Profile. Animals 2022, 12, 1758. [Google Scholar] [CrossRef] [PubMed]
- Snir, B.; Fishman, A.; Glusac, J. Chickpea-Based Milk Analogue Stabilized by Transglutaminase. Foods 2025, 14, 514. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhong, X.; Lu, Y.; Du, X.; Jia, R.; Li, H.; Zhang, M. Changes of soybean protein during tofu processing. Foods 2021, 10, 1594. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Li, B.; Li, L.; Lin, D.; Chen, H.; Liu, Y.; Li, S.; Qin, W.; Liu, J. Research progress in tofu processing: From raw materials to processing conditions. Crit. Rev. Food Sci. Nutr. 2018, 58, 1448–1467. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.C.; Liu, Z. Soymilk and tofu manufacturing. In Handbook of Plant-Based Fermented Food and Beverage Technology; Hui, Y.H., Evranuz, E.O., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 139–162. [Google Scholar]
- Ndatsu, Y.; Olekan, A.A. Effects of different types of coagulants on the nutritional quality tofu produced in the northern part of Nigeria. World J. Dairy Food Sci. 2012, 7, 135–141. [Google Scholar] [CrossRef]
- Kim, C.; Goldsmith, P. The Economics of the Soy Kit as an Appropriate Household Technology for Food Entrepreneurs. Food Nutr. Bull. 2021, 42, 104–115. [Google Scholar] [CrossRef]
- Poysa, V.; Woodrow, L. Stability of soybean seed composition and its effect on soymilk and tofu yield and quality. Food Res. Int. 2002, 35, 337–345. [Google Scholar] [CrossRef]
- Lian, H.; Luo, K.; Gong, Y.; Zhang, S.; Serventi, L. Okara flours from chickpea and soy are thickeners: Increased dough viscosity and moisture content in gluten-free bread. Int. J. Food Sci. Technol. 2020, 55, 805–812. [Google Scholar] [CrossRef]
- Voss, G.B.; Sousa, V.; Rema, P.; Pintado, M.E.; Valente, L.M. Processed by-products from soy beverage (okara) as sustainable ingredients for Nile tilapia (O. Niloticus) juveniles: Effects on Nutrient Utilization and Muscle Quality. Animals 2021, 11, 590. [Google Scholar] [CrossRef]
- World Food Programme. Update on Food Procurement: Statistical Review; World Food Programme: Rome, Italy, 2020. [Google Scholar]
- FAO. Revitalizing Lebanon’s Chickpea Sector. Available online: https://www.fao.org/one-country-one-priority-product/news-and-events/news/revitalizing-lebanon-chickpea-sector/en (accessed on 17 July 2025).
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Patanè, C.; Iacoponi, E.; Raccuia, S.A. Physico-chemical characteristics, water absorption, soaking and cooking properties of some Sicilian populations of chickpea (Cicer arietinum L.). Int. J. Food Sci. Nutr. 2004, 55, 547–554. [Google Scholar] [CrossRef]
- CXS 171-1989; Codex Standard for Certain Pulses. (Rev. 1–1995). FAO: Rome, Italy; WHO: Geneva, Switzerland, 2025.
- Saio, K.; Koyama, E.; Yamazaki, S.; Watanabe, T. Protein-Calcium-Phytic acid relationships in soybean: Part III. Effect of phytic acid on coagulative reaction in tofu-making. Agric. Biol. Chem. 1969, 33, 36–42. [Google Scholar] [CrossRef][Green Version]
- AOAC. AOAC 930.15. AOAC Official Method 930.15 Loss on Drying (Moisture) for Feeds (at 135 °C for 2 Hours). In Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2023; Volume 22. [Google Scholar]
- Ileleji, K.E.; Garcia, A.A.; Kingsly, A.R.; Clementson, C.L. Comparison of standard moisture loss-on-drying methods for the determination of moisture content of corn distillers dried grains with solubles. J. AOAC Int. 2010, 93, 825–832. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Method Am 5-04. Rapid Determination of Oil/Fat Utilizing High-Temperature Solvent Extraction. 2013. Available online: https://library.aocs.org/Am-5-04/1 (accessed on 2 July 2025).
- AOAC. AOAC 954.01. AOAC Official Method 954.01: Protein (Crude) in Animal Feed and Pet Food: Kjeldahl Method. In Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2023; Volume 22. [Google Scholar]
- Odland, R. Revised Kjeldahl total nitrogen method for feeds and premixes. J. Assoc. Off. Anal. Chem. 1972, 55, 984–985. [Google Scholar] [CrossRef][Green Version]
- Helrich, H. Official methods of analysis. Anim. Feed 1990, 1, 69–90. [Google Scholar][Green Version]
- Fahey, G.C.; Novotny, L.; Layton, B.; Mertens, D.R. Critical factors in determining fiber content of feeds and foods and their ingredients. J. AOAC Int. 2019, 102, 52–62. [Google Scholar] [CrossRef]
- Hagen, C.S.; Peterson, B.; Parr, E.; Estrada, J.; Silva, G.; Greiner, L.L. The impact of floor space allowance and dietary energy level on finishing pigs, from 65 to 120 kg, on growth performance. Transl. Anim. Sci. 2023, 7, txad070. [Google Scholar] [CrossRef]
- Marshall, M.R. Ash Analysis, 4th ed.; Nielsen, S.S., Ed.; Springer: West Lafayette, IN, USA, 2010. [Google Scholar]
- Oguntunde, A.; Akintoye, O. Measurement and comparison of density, specific heat and viscosity of cow’s milk and soymilk. J. Food Eng. 1991, 13, 221–230. [Google Scholar] [CrossRef]
- Singh, R.P.; Heldman, D.R. Introduction to Food Engineering; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Kaur, R.; Prasad, K. Technological, processing and nutritional aspects of chickpea (Cicer arietinum)—A review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas—Composition, nutritional value, health benefits, application to bread and snacks: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, C.; Xu, X.; Kang, F.; Chen, Y.; Zhou, M.; Wang, S.; Li, B. Components analysis and flour preparation of tofu whey. Adv. J. Food Sci. Technol. 2016, 12, 574–578. [Google Scholar] [CrossRef]
- WITS. World Integrated Trade Solution: Lebanon: Imports of Chickpeas (HS 071320). World Integrated Trade Solution. Available online: https://wits.worldbank.org/trade/comtrade/en/country/LBN/year/2023/tradeflow/Imports/partner/ALL/product/071320# (accessed on 2 July 2025).
- L’Orient Today. Slight Increase in Generator Rates for the Last Bill of the Year. Available online: https://today.lorientlejour.com/article/1441371/slight-increase-in-generator-rates-for-the-last-bill-of-the-year.html (accessed on 20 July 2025).
- Peñas, E.; Préstamo, G.; Polo, F.; Gomez, R. Enzymatic proteolysis, under high pressure of soybean whey: Analysis of peptides and the allergen Gly m 1 in the hydrolysates. Food Chem. 2006, 99, 569–573. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Shen, Y.; Yao, H.; Wang, P.; Ye, K.; Wang, X.; Gu, Q. Enhancing the vitamin B12 production and growth of Propionibacterium freudenreichii in tofu wastewater via a light-induced vitamin B12 riboswitch. Appl. Microbiol. Biotechnol. 2015, 99, 10481–10488. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Life cycle assessment: Blazing a trail for bioresources management. Energy Convers. Manag. X 2021, 10, 100063. [Google Scholar] [CrossRef]
- Finnegan, W.; Yan, M.; Holden, N.M.; Goggins, J. A review of environmental life cycle assessment studies examining cheese production. Int. J. Life Cycle Assess. 2018, 23, 1773–1787. [Google Scholar] [CrossRef]
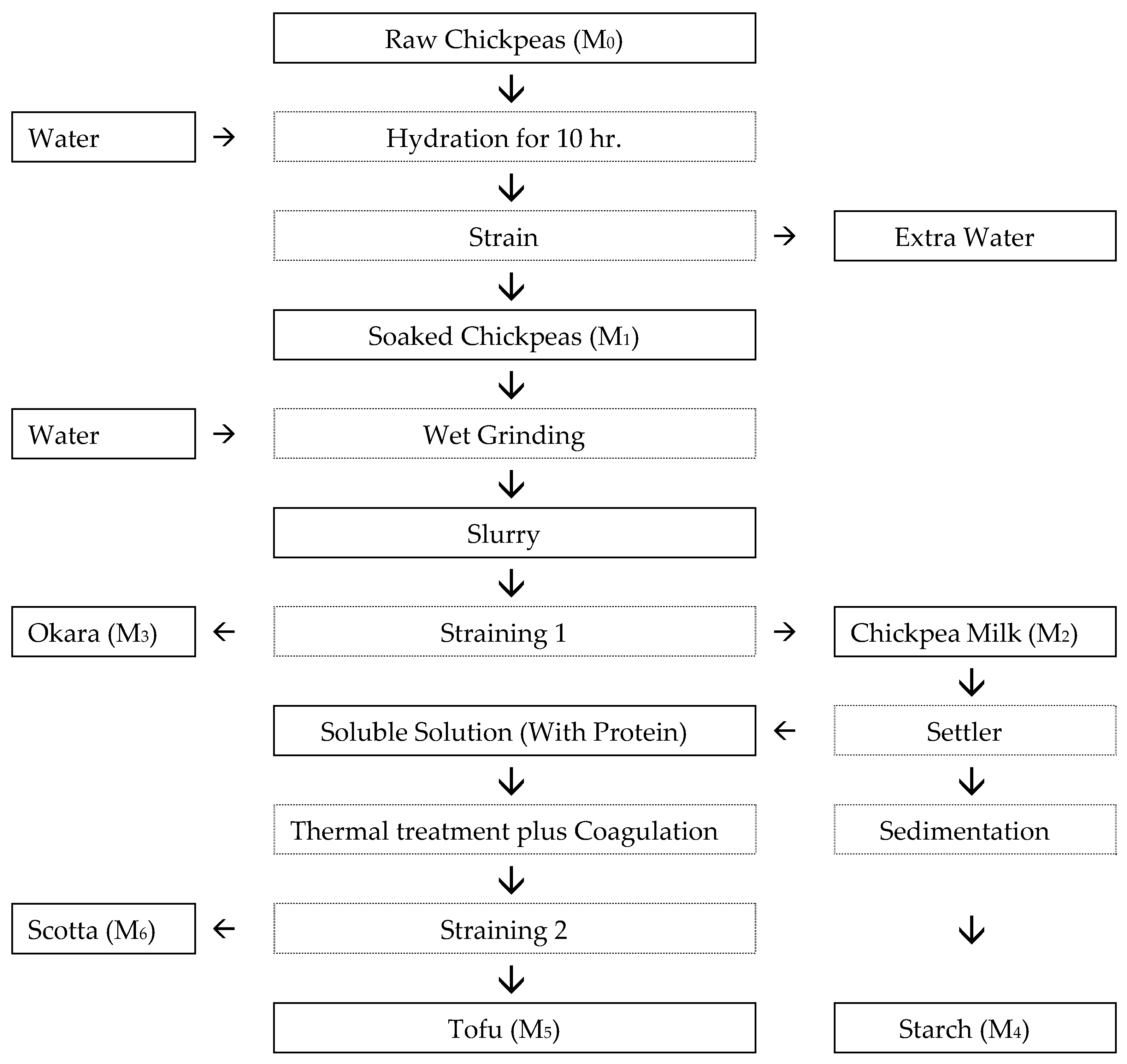
| Step | Name of Operation | Procedural Details * | Reference |
|---|---|---|---|
| 1 | Raw material preparation | Chickpeas (Cicer arietinum L.) were purchased then rinsed with potable water. | |
| 2 | Soaking | Chickpeas were immersed in three parts water (1:3 w:w) at ambient temperature (~22 °C) for 10 h, to attain ~2:1 (soaked/dry) hydration. | [26] |
| 3 | Wet grinding/slurry preparation | A Braun Jug Blender JB3060WH (800 W, max speed 24,000 rpm; De’Longhi Braun Household GmbH, Neu-Isenburg, Germany) was used for homogenization in two phases. First phase: Soaked chickpeas were blended with water at a 1:2 (w/v) ratio until uniform. Second phase: Water was added to reach a final 1:4 (w/v) ratio, and blended until a homogeneous mixture was obtained (~5 min). | [26,36] |
| 4 | Filtration (okara removal) | The slurry was passed through a double-layer muslin cloth; retained fiber (okara) was removed. | [26] |
| 5 | Starch sedimentation and milk decanting | The filtrate was held for 1.5 h; settled starch remained in the vessel while the clarified upper layer was decanted for curding. Starch sedimentation was achieved by manual decanting at a controlled ambient temperature (22–24 °C) without agitation | [7] |
| 6 | Thermal treatment | The decanted milk was heated to 98–100 °C for 3–5 min to denature proteins and inactivate enzymes. | [26] |
| 7 | Coagulation (CaCl2) | Hot milk (≈85 °C) treated with anhydrous CaCl2 at the rate of 2.5 g L−1; gentle stirring for 10 s, then standing 10 min formed curd. | [26] |
| 8 | Molding and passive drainage | Curd was ladled into a cloth-lined perforated mold and allowed to drain by gravity without pressing. | [26] |
| 9 | Refrigerated holding | Blocks were kept at 4 °C for 12 h for whey drainage and cooling prior to further testing. | [6] |
| Stage | Stream | Mean ± SE |
|---|---|---|
| Input | Raw chickpeas | 1 |
| Soak water * | 3 | |
| Intermediate output ** | Soaked chickpeas | 1.98 ± 0.032 |
| Intermediate streams | Okara (fresh) | 1.032 ± 0.055 |
| Decanted milk (pre-coagulation) | 5.126 ± 0.335 | |
| Starch sediment (fresh) | 2.272 ± 0.155 | |
| Final output | Tofu (fresh) | 0.744 ± 0.021 |
| Scotta (whey) | 4.384 ± 0.333 |
| Component | Values (Mean ± SE) |
|---|---|
| Moisture | 13.45 ± 0.17 |
| Protein (d.b.) | 20.52 ± 0.76 |
| Fat (d.b.) | 6.03 ± 0.56 |
| Ash (d.b.) | 5.71 ± 0.98 |
| Fiber (d.b.) | 9.16 ± 0.14 |
| Net Carbohydrates 1 (d.b.) | 58.59 ± 1.24 |
| Total Carbohydrates 2 (d.b.) | 67.74 ± 1.18 |
| Component | Okara (%) | Starch (%) | Tofu (%) |
|---|---|---|---|
| Moisture | 71.42 ± 1.86 | 53.30 ± 2.18 | 68.90 ± 4.94 |
| Protein (d.b.) | 8.54 ± 0.40 | 3.77 ± 0.04 | 56.18 ± 1.38 |
| Fat (d.b.) | 3.15 ± 0.22 | 0.95 ± 0.05 | 12.21 ± 0.59 |
| Ash (d.b.) | 1.50 ± 0.05 | 0.18 ± 0.01 | 6.29 ± 1.43 |
| Fiber (d.b.) | 10.27 ± 0.39 | 0.89 ± 0.03 | 1.55 ± 0.22 |
| Net Carbohydrates 1 (d.b.) | 76.54 ± 0.31 | 94.22 ± 0.06 | 21.27 ± 2.38 |
| Total Carbohydrates 2 (d.b.) | 86.81 ± 0.64 | 95.11 ± 0.06 | 22.82 ± 2.37 |
| Product | Conversion Value (kg Raw Chickpeas/kg Product) |
|---|---|
| Soaked chickpeas | 0.523 ± 0.009 |
| Okara powder | 3.26 ± 0.154 |
| Wet okara (70% moisture equivalent) | 1.03 ± 0.515 |
| Starch powder | 7.575 ± 0.518 |
| Wet starch (50% moisture equivalent) | 2.273 ± 0.155 |
| Tofu powder | 4.957 ± 0.152 |
| Wet tofu (70% moisture equivalent) | 1.487 ± 0.507 |
| Scotta (3 °Bx) | 0.289 ± 0.038 |
| Scotta powder | 9.649 ± 1.277 |
| Nutrient | Okara (%) | Starch (%) | Tofu (%) | Sum (%) |
|---|---|---|---|---|
| Protein | 13.45 ± 1.44 | 2.56 ± 0.20 | 59.09 ± 2.90 | 75.09 ± 3.13 |
| Fat | 16.67 ± 1.92 | 2.20 ± 0.27 | 43.22 ± 3.22 | 90.37 ± 5.16 |
| Fiber | 34.48 ± 0.75 | 1.30 ± 0.07 | 3.51 ± 0.49 | 39.29 ± 0.74 |
| Net Carbohydrate 1 | 40.28 ± 2.58 | 21.59 ± 1.83 | 8.41 ± 0.54 | 77.53 ± 4.48 |
| Product | Energy Required (kWh/kg Product) |
|---|---|
| Tofu (70% moisture) | 0.798 |
| Tofu powder | 4.109 |
| Okara powder | 1.465 |
| Starch powder | 0.767 |
| Scotta powder | 20.298 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimassi, O.; Jaber, L.; Toufeili, I.; Ouaijan, K.; Hamadeh, S. Techno-Economic Feasibility of Producing High-Protein Tofu from Chickpeas: Process Design and Nutrient Recovery. Foods 2025, 14, 3206. https://doi.org/10.3390/foods14183206
Dimassi O, Jaber L, Toufeili I, Ouaijan K, Hamadeh S. Techno-Economic Feasibility of Producing High-Protein Tofu from Chickpeas: Process Design and Nutrient Recovery. Foods. 2025; 14(18):3206. https://doi.org/10.3390/foods14183206
Chicago/Turabian StyleDimassi, Ossama, Lina Jaber, Imad Toufeili, Krystel Ouaijan, and Shady Hamadeh. 2025. "Techno-Economic Feasibility of Producing High-Protein Tofu from Chickpeas: Process Design and Nutrient Recovery" Foods 14, no. 18: 3206. https://doi.org/10.3390/foods14183206
APA StyleDimassi, O., Jaber, L., Toufeili, I., Ouaijan, K., & Hamadeh, S. (2025). Techno-Economic Feasibility of Producing High-Protein Tofu from Chickpeas: Process Design and Nutrient Recovery. Foods, 14(18), 3206. https://doi.org/10.3390/foods14183206








