Impact of Milk Thistle Cake as the Natural Antioxidant Source on the Mitigation of Oxidative Effects in Goat Milk Induced by Oxidized Linseed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement, Experimental Design, Diets, and Sample Collection
2.1.1. Ethical Statement
2.1.2. Experimental Design and Diets
2.1.3. Sample Collection
2.2. Chemical Analyses
2.2.1. Oxidation State of Linseed Oil
2.2.2. Proximate and Mineral Composition of Milk Thistle Cake and Milk Samples
2.2.3. Hydro-Soluble Compounds of Milk Thistle Cake and Milk Samples
2.2.4. Lipo-Soluble Compounds of Milk Thistle Cake and Milk Samples
2.2.5. Antioxidant Activity of Milk Thistle Cake and Milk Samples
2.2.6. Fat Degradation Parameters of Milk Samples
2.3. Statistical Analysis
3. Results
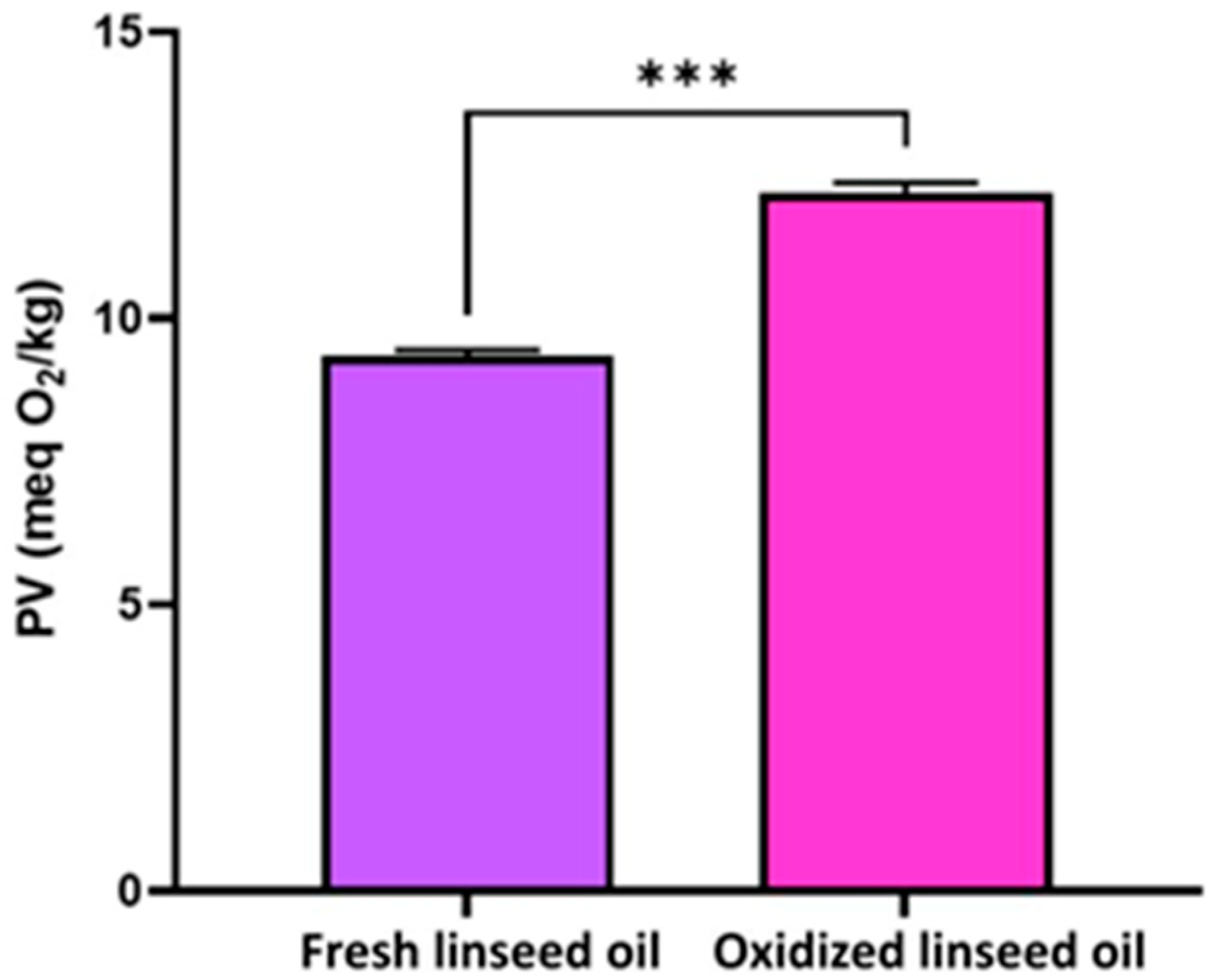
3.1. Oxidation State of the Oils
3.2. Proximate, Mineral, and Lipid Composition of Milk Thistle Cake
3.3. Bioactive Compounds of Milk Thistle Cake
3.4. Influence of Dietary Treatments on Goat Milk Production and Composition
3.4.1. Proximate and Mineral Composition of Goat Milk and Milk Production
3.4.2. Lipid Profile of Goat Milk
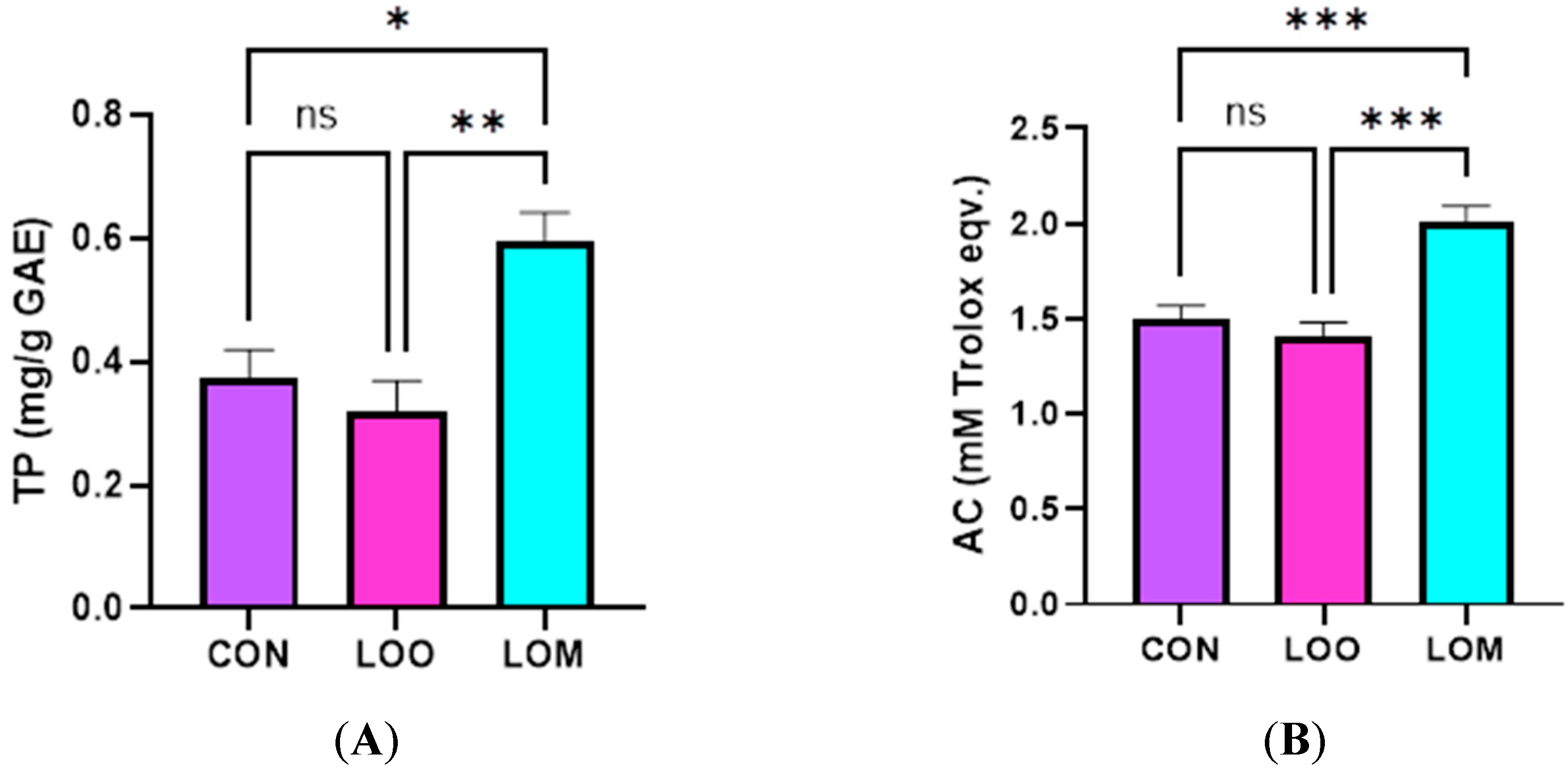
3.5. Antioxidant Potential of Goat Milk
3.6. Lipid Degradation Parameters in Goat Milk
4. Discussion
4.1. Oxidation State of Oils Tested in the Experiment
4.2. Proximate, Mineral, and Lipid Composition and Bioactive Compounds of Milk Thistle Cake
4.3. Effect of Dietary Treatments on Proximate and Mineral Composition of Goat Milk
4.4. Effect of Dietary Treatments on Lipid Composition of Goat Milk
4.5. Effect of Dietary Treatments on Antioxidant Potential of Goat Milk
4.6. Effect of Dietary Treatments on Goat Milk Degradation Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khamisabadi, H. Effects of Silymarin on milk production, liver enzymes, oxidative status and HSP70 gene expression in postparturient Sanjabi ewes. Cell. Mol. Biol. 2020, 66, 76–81. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Priyashantha, H.; Vidanarachchi, J.K.; Kiani, A.; Holman, B.W.B. Effects of nutritional factors on fat content, fatty acid composition, and sensorial properties of meat and milk from domesticated ruminants: An overview. Animals 2024, 14, 840. [Google Scholar] [CrossRef] [PubMed]
- Lambo, M.T.; Liu, R.; Zhang, X.; Zhang, Y.; Li, Y.; Sun, M. Nutritional Evaluation of Milk Thistle Meal as a Protein Feedstuff for Diets of Dairy Cattle. Animals 2024, 14, 1864. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Zhang, H.; Shahid, M.; Ghazal, H.; Shah, A.R.; Niaz, M.; Naz, T.; Ghimire, K.; Goswami, N.; Shi, W.; et al. The Vital Roles of Agricultural Crop Residues and Agro-Industrial By-Products to Support Sustainable Livestock Productivity in Subtropical Regions. Animals 2025, 15, 1184. [Google Scholar] [CrossRef] [PubMed]
- Vlaicu, P.A.; Untea, A.E.; Oancea, A.G. Sustainable poultry feeding strategies for achieving zero hunger and enhancing food quality. Agriculture 2024, 14, 1811. [Google Scholar] [CrossRef]
- Musco, N.; Tudisco, R.; Esposito, G.; Iommelli, P.; Totakul, P.; D’Aniello, B.; Lombardi, P.; Amato, R.; Wanapat, M.; Infascelli, F. Effects of Linseed Supplementation on Milk Production, Composition, Odd- and Branched-Chain Fatty Acids, and on Serum Biochemistry in Cilentana Grazing Goats. Animals 2022, 12, 783. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Cívico, M.A.; De La Fuente, M.A.; Núñez Sánchez, N.; Peña Blanco, F.; Martínez Marín, A.L. Effects of dietary concentrate composition and linseed oil supplementation on the milk fatty acid profile of goats. Animal 2018, 12, 2310–2317. [Google Scholar] [CrossRef]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Plata-Pérez, G.; Angeles-Hernandez, J.C.; Morales-Almaráz, E.; Del Razo-Rodríguez, O.E.; López-González, F.; Peláez-Acero, A.; Campos-Montiel, R.G.; Vargas-Bello-Pérez, E.; Vieyra-Alberto, R. Oilseed supplementation improves milk composition and fatty acid profile of cow milk: A meta-analysis and meta-regression. Animals 2022, 12, 1642. [Google Scholar] [CrossRef]
- Thanh, L.P.; Loor, J.J.; Mai, D.T.T.; Hang, T.T.T. Effect of Fish Oil and Linseed Oil on Intake, Milk Yield and Milk Fatty Acid Profile in Goats. Animals 2023, 13, 2174. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.; Abdo, M.M. Crushed flaxseed versus flaxseed oil in the diets of Nubian goats: Effect on feed intake, digestion, ruminal fermentation, blood chemistry, milk production, milk composition and milk fatty acid profile. Anim. Feed Sci. Technol. 2018, 244, 66–75. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Li, Y.; Shi, B.; Guo, X.; Zhao, Y.; Yan, S. Flaxseed Oil and Heated Flaxseed Supplements Have Different Effects on Lipid Deposition and Ileal Microbiota in Albas Cashmere Goats. Animals 2021, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.C.; Ferraz, M.V., Jr.; Susin, I.; Gentil, R.S.; Polizel, D.M.; Ferreira, E.M.; Pires, A.V. Plasma and milk fatty acid profiles in goats fed diets supplemented with oils from soybean, linseed or fish. Small Rumin. Res. 2019, 170, 125–130. [Google Scholar] [CrossRef]
- Vázquez-Añón, M.; Jenkins, T. Effects of feeding oxidized fat with or without dietary antioxidants on nutrient digestibility, microbial nitrogen, and fatty acid metabolism. J. Dairy Sci. 2007, 90, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, M.; Enjalbert, F.; Farizon, Y.; Meynadier, A. Feeding heat-oxidized oil to dairy cows affects milk fat nutritional quality. Animal 2018, 12, 183–188. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Pitino, R.; Simoni, M.; Mavrommatis, A.; De Marchi, M.; Righi, F.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Livestock Mammals’ Performances, Health, and Oxidative Status: A Review of the Literature in the Last 20 Years. Antioxidants 2021, 10, 1461. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Mitsiopoulou, C.; De Marchi, M.; Righi, F. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins in Livestock Animal Products Yield, Quality, and Oxidative Status: A Review. Antioxidants 2021, 10, 780. [Google Scholar] [CrossRef]
- Karaiskou, C.; Kasapidou, E.; Michailidis, G.; Markantonatos, X.; Basdagianni, Z. Effect of dietary milk thistle (Silybum marianum L.) oil supplementation on animal performance and milk fatty acid composition in dairy ewes. Small Rumin. Res. 2021, 203, 106493. [Google Scholar] [CrossRef]
- Stastnik, O.; Pavlata, L.; Mrkvicova, E. The milk thistle seed cakes and hempseed cakes are potential feed for poultry. Animals 2020, 10, 1384. [Google Scholar] [CrossRef]
- Samee, A.; Amir, R.M.; Ahmad, A.; Watto, F.M.; Ali, M.; Azam, M.T.; Ashraf, H. Effectiveness of milk thistle on human body against diseases: A comprehensive review. Sch. Bull. 2023, 9, 8–18. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Jarošová, M.; Bedrníček, J.; Lorenc, F.; Stupková, A.; Švajner, J.; Smetana, P.; Kyselka, J.; Filip, V.; et al. Milk thistle (Silybum marianum L. Gaertner) oilseed cake flour: Functional, nutritional, and antioxidant characteristics as affected by cultivar and preparation process. Food Biosci. 2025, 63, 105735. [Google Scholar] [CrossRef]
- Tedesco, D.; Domeneghini, C.; Sciannimanico, D.; Tameni, M.; Steidler, S.; Galletti, S. Silymarin, a possible hepatoprotector in dairy cows: Biochemical and histological observations. J. Vet. Med. A 2004, 51, 85–89. [Google Scholar] [CrossRef]
- Onmaz, A.; Ulger, I.; Ayaşan, T. Effects of silymarin (Silybum marianum) supplementation on milk and blood parameters of dairy cattle. S. Afr. J. Anim. Sci. 2017, 47, 758–765. [Google Scholar]
- Ghanem, N.; Mabrok, H.B.; Shedeed, S.M.; El-Wahab, A.; Walaa, M.; Shakweer, W.M.; ElSabaawy, E.H. Physiological, molecular, and immune responses to milk thistle extract administration in goats during peripartum period. Egypt. Pharm. J. 2022, 21, 376–384. [Google Scholar] [CrossRef]
- ElSabaawy, E.H.; Mohamed, M.I.; El-Wahab, A.; Walaa, M.; El-Nomeary, Y.A.; Hassaan, N.A. Effect of milk thistle extract supplementation on growth performance, nutrient digestibility, and blood parameters of growing Shami goats. Egypt. J. Nutr. Feed. 2022, 25, 323–331. [Google Scholar] [CrossRef]
- Boško, R.; Pluháčková, H.; Martiník, J.; Benešová, K.; Svoboda, Z.; Běláková, S.; Pernica, M. Occurrence of mycotoxins in milk thistle: To be included in legislation or not? Mycotoxin Res. 2025, 41, 199–206. [Google Scholar] [CrossRef]
- Vidal, N.P.; Rahimi, J.; Kroetsch, B.; Martínez, M.M. Quality and chemical stability of long-term stored soy, canola, and sunflower cold-pressed cake lipids before and after thermomechanical processing: A 1H NMR study. LWT 2023, 173, 114409. [Google Scholar] [CrossRef]
- European Union. Directive 2010/63 of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L 276, 33–79.
- Hassoun, P.; Bocquier, F. Alimentation des ovins. Aliment. Des Bov. Ovins Et Caprins Éd. Quæ INRA Paris 2007, 6, 126–128. [Google Scholar]
- Untea, A.E.; Varzaru, I.; Saracila, M.; Panaite, T.D.; Oancea, A.G.; Vlaicu, P.A.; Grosu, I.A. Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants 2023, 12, 1084. [Google Scholar] [CrossRef] [PubMed]
- ISO 9622:2013; Milk and Milk Products—Determination of Fat Content—Gerber Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- Untea, A.; Criste, R.C.; Vladescu, L. Development and validation of a microwave digestion–FAAS procedure for Cu, Mn and Zn determination in liver. Rev. Chim. 2012, 63, 341–346. [Google Scholar]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Vlaicu, P.A.; Badea, I.A.; Mironeasa, S. Effects of grape seed oil supplementation to broilers diets on growth performance, meat fatty acids, health lipid indices and lipid oxidation parameters. Agriculture 2021, 11, 404. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Van, I. Determination of bioactive compounds with benefic potential on health in several medicinal plants. Rom. Biotechnol. Lett. 2015, 20, 10773–10783. [Google Scholar]
- Oancea, A.-G.; Untea, A.E.; Dragomir, C.; Radu, G.L. Determination of Optimum TBARS Conditions for Evaluation of Cow and Sheep Milk Oxidative Stability. Appl. Sci. 2022, 12, 6508. [Google Scholar] [CrossRef]
- Codex Alimentarius. FAO/WHO Codex Standard for Named Vegetable Oils. Codex Alimentarius. Amendment 2005. 2011. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/tr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 1 August 2025).
- Janocha, A.; Milczarek, A.; Pietrusiak, D. Impact of Milk Thistle (Silybum marianum [L.] Gaertn.) Seeds in Broiler Chicken Diets on Rearing Results, Carcass Composition, and Meat Quality. Animals 2021, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Haraf, G.; Zając, A.; Krzos, G. Milk Thistle (Silybum marianum (L.) Gaertner) Endosperm as an Alternative Protein Source for a Sustainable Food System (SFS)—Pilot Studies. Sustainability 2023, 15, 14411. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Harrabi, S.; Romdhane, H.; Daassa, M.; Fellah, H. Fatty acid and triacylglycerol compositions of milk thistle seeds growing wild in Tunisia (Silybum marianum L.). Acta Aliment. 2005, 44, 304–310. [Google Scholar] [CrossRef]
- Majidi, M.M.; Shafiei-Koij, F.; Pirnajmedin, F.; Jami, M.; Radan, Z. Fatty acid profile, silymarin content and production properties of milk thistle (Silybum marianum) germplasm under different water environments. Crop Pasture Sci. 2021, 72, 302–310. [Google Scholar] [CrossRef]
- Amanullah, S.M.; Kim, D.H.; Paradhipta, D.H.V.; Lee, H.J.; Joo, Y.H.; Lee, S.S.; Kim, E.T.; Kim, S.C. Effects of essential fatty acid supplementation on in vitro fermentation indices, greenhouse gas, microbes, and fatty acid profiles in the rumen. Front. Microbiol. 2021, 12, 637220. [Google Scholar] [CrossRef]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Colla, G.; Cardarelli, M.; Bonini, P.; Bernardi, J. Phenolic profile and in vitro antioxidant power of different milk thistle (Silybum marianum (L.) Gaertn.) cultivars. Ind. Crops Prod. 2016, 83, 11–16. [Google Scholar] [CrossRef]
- Maaloul, S.; Mejri, F.; Snoussi, A.; Flamini, G.; Boussaid, M.; Hamrouni-Sellami, I. Characterization of Silybum marianum and Silybum eburneum seed oils: Phytochemical profiles and antioxidant properties supporting important nutritional interests. PLoS ONE 2024, 19, e0304021. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Wei, X.; Wei, J.; Liu, Y.; Liu, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Hassanat, F.; Martineau, R.; Gervais, R. Supplementation of increasing amounts of linseed oil to dairy cows fed total mixed rations: Effects on digestion, ruminal fermentation characteristics, protozoal populations, and milk fatty acid composition. J. Dairy Sci. 2012, 95, 4578–4590. [Google Scholar] [CrossRef]
- Hanigan, M.D.; Souza, V.C.; Martineau, R.; Lapierre, H.; Feng, X.; Daley, V.L. A meta-analysis of the relationship between milk protein production and absorbed amino acids and digested energy in dairy cattle. J. Dairy Sci. 2024, 107, 5587–5615. [Google Scholar] [CrossRef]
- Lamothe, S.; Desroches, V.; Britten, M. Effect of milk proteins and food-grade surfactants on oxidation of linseed oil-in-water emulsions during in vitro digestion. Food Chem. 2019, 294, 130–137. [Google Scholar] [CrossRef]
- Marceddu, R.; Dinolfo, L.; Carrubba, A.; Sarno, M.; Di Miceli, G. Milk Thistle (Silybum Marianum L.) as a Novel Multipurpose Crop for Agriculture in Marginal Environments: A Review. Agronomy 2022, 12, 729. [Google Scholar] [CrossRef]
- Bedrníček, J.; Lorenc, F.; Jarošová, M.; Bártová, V.; Smetana, P.; Kadlec, J.; Jirotková, D.; Kyselka, J.; Petrášková, E.; Bjelková, M.; et al. Milk Thistle Oilseed Cake Flour Fractions: A Source of Silymarin and Macronutrients for Gluten-Free Bread. Antioxidants 2022, 11, 2022. [Google Scholar] [CrossRef]
- Alzarieni, K.Z.; Bani Amer, A.R.; El-Elimat, T.; Kenttämaa, H.I. Characterization of natural cellulosic fiber obtained from the flower heads of milk thistle (Silybum marianum) as a potential polymer reinforcement material. J. Nat. Fibers 2023, 20, 2211289. [Google Scholar] [CrossRef]
- Baye, K.; Guyot, J.P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef]
- Klop, G.; Bannink, A.; van Duinkerken, G.; van Middelaar, C.E.; Dijkstra, J. Variation in phosphorus content of milk from dairy cattle as affected by differences in milk composition. J. Agric. Sci. 2014, 152, 860–869. [Google Scholar] [CrossRef]
- Rai, D.C.; Rathaur, A.; Yadav, A.K. Nutritional and nutraceutical properties of goat milk for human health: A review. Indian J. Dairy Sci. 2022, 75, 1–10. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Guerrini, A. Use of milk thistle in farm and companion animals: A review. Planta Med. 2023, 89, 584–607. [Google Scholar] [CrossRef] [PubMed]
- Shedeed, S.M.; ElSabaawy, E.H.; Mohamed, M.I.; Mabrok, H.B.; Abd El-Wahab, W.M. Impact of feeding ration supplemented with silymarin-rich extract on milk quality of goat and utilization of milk in producing functional soft-cheese. Egypt. J. Chem. 2023, 66, 205–215. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Stanton, C. Milk intelligence: Mining milk for bioactive substances associated with human health. Int. Dairy J. 2011, 21, 394–401. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef]
- Michlova, T.; Uhrinova, I.; Chodova, D.; Hanusova, E.; Bulla, J. Factors influencing the content of vitamins A and E in sheep and goat milk. Czech J. Food Sci. 2015, 33, 58–64. [Google Scholar] [CrossRef]
- Santos, G.T.; Del Valle, T.A.; Pereira, M.N.; Detmann, E.; Valadares Filho, S.C.; Valadares, R.F.D. Citrus pulp as a dietary source of antioxidants for lactating Holstein cows fed highly polyunsaturated fatty acid diets. Asian-Australas. J. Anim. Sci. 2014, 27, 1104–1112. [Google Scholar] [CrossRef]
- Roussis, I.G.; Tzimas, P.C.; Soulti, K. Antioxidant activity of white wine extracts and some phenolic acids toward corn oil oxidation. J. Food Process. Preserv. 2008, 32, 535–545. [Google Scholar] [CrossRef]
- Rico, D.E.; Scerra, M.; Chilliard, Y.; Salas, E.; Rodríguez-Ramírez, J.; González, L.A. Effect of postruminal supply of linseed oil in dairy cows: 2. Milk fatty acid profile and oxidative stability. J. Dairy Res. 2023, 90, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Onjaiuea, N.; Paengkoum, S.; Taethaisong, N.; Thongpea, S.; Paengkoum, P. Enhancing milk quality and antioxidant status in lactating dairy goats through the dietary incorporation of purple Napier grass silage. Animals 2024, 14, 811. [Google Scholar] [CrossRef]
- Garavaglia, L.; Galletti, S.; Tedesco, D. Silymarin and lycopene administration in periparturient dairy cows: Effects on milk production and oxidative status. N. Z. Vet. J. 2015, 63, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Gholamalian, R.; Mahdavi, A.H.; Riasi, A. Hepatic fatty acids profile, oxidative stability and egg quality traits ameliorated by supplementation of alternative lipid sources and milk thistle meal. J. Anim. Physiol. Anim. Nutr. 2022, 106, 860–871. [Google Scholar] [CrossRef]
| Ingredients in Concentrate (as Feed, %) | CON | LOO | LOM |
|---|---|---|---|
| Maize | 48.4 | 48.4 | 48.4 |
| Triticale | 31.6 | 31.6 | 31.6 |
| Sunflower meal | 10.0 | 10.0 | 0.0 |
| Linseed oil | 7.0 | 0.0 | 0.0 |
| Oxidized linseed oil | 0.0 | 7.0 | 7.0 |
| Milk thistle cake | 0.0 | 0.0 | 10.0 |
| Calcium carbonate | 1.0 | 1.0 | 1.0 |
| Sodium chloride | 1.0 | 1.0 | 1.0 |
| Mineral–vitamin supplement | 1.0 | 1.0 | 1.0 |
| Nutrients in total diet (g/day) | |||
| DM, as fed | 1926.5 | 1926.5 | 1933.1 |
| MFU | 1.6 | 1.6 | 1.7 |
| IDPE | 150.4 | 150.4 | 148.8 |
| IDPN | 159.6 | 159.6 | 151.9 |
| Proximate composition (%) | |||
| Dry matter | 90.3 | 92.3 | 90.2 |
| Crude protein | 13.5 | 13.7 | 12.3 |
| Crude fat | 8.5 | 8.4 | 9.1 |
| Crude fiber | 5.9 | 6.2 | 6.3 |
| Parameter | Milk Thistle Cake | |
|---|---|---|
| Proximate composition (%) | ||
| Dry matter | 92.48 ± 1.21 | |
| Crude protein | 20.58 ± 0.95 | |
| Crude fat | 8.18 ± 0.88 | |
| Crude fiber | 27.84 ± 1.12 | |
| Mineral composition | ||
| Copper (µg/g) | 27.15 ± 1.37 | |
| Iron (µg/g) | 129.3 ± 1.34 | |
| Manganese (µg/g) | 55.15 ± 1.49 | |
| Zinc (µg/g) | 90.96 ± 1.55 | |
| Calcium (%) | 0.91 ± 0.098 | |
| Phosphorus (%) | 0.84 ± 0.13 | |
| Lipid composition (g/100 g) | ||
| Miristic acid | C 14:0 | 0.06 ± 0.001 |
| Palmitic acid | C 16:0 | 9.41 ± 0.198 |
| Palmitoleic acid | C 16:1 | 0.05 ± 0.002 |
| Stearic acid | C 18:0 | 4.85 ± 0.163 |
| Oleic acid | C 18:1 | 33.68 ± 0.148 |
| Linoleic acid | C 18:2n6 | 47.77 ± 0.127 |
| α-linolenic acid | C 18:3n3 | 0.23 ± 0.021 |
| Octadecatetraenoic acid | C18:4n3 | 2.76 ± 0.158 |
| Eicosadienoic acid | C 20:2n6 | 0.51 ± 0.021 |
| Docosadienoic acid | C22:(2n6) | 0.46 ± 0.020 |
| Other fatty acids | 0.30 ± 0.018 | |
| ΣSFA | 14.31 ± 0.362 | |
| ΣMUFA | 33.72 ± 0.150 | |
| ΣPUFA | 51.72 ± 0.306 | |
| Σ Omega 3 | 2.98 ± 0.178 | |
| Σ Omega 6 | 48.73 ± 0.127 | |
| Omega 6/Omega 3 | 16.34 ± 0.936 | |
| Parameters | Milk Thistle Cake |
|---|---|
| Antioxidant potential | |
| α-tocopherol (mg/kg) | 85.75 ± 1.484 |
| γ-tocopherol (mg/kg) | 6.55 ± 0.919 |
| δ-tocopherol (mg/kg) | 4.90 ± 0.565 |
| Total vitamin E (mg/kg) | 97.20 ± 1.838 |
| Lutein (mg/kg) | 2.48 ± 1.435 |
| Total polyphenols (mg/g GAE) | 4.79 ± 0.265 |
| DPPH (mM equiv. Trolox) | 2.45 ± 0.135 |
| Individual polyphenols (mg/kg) | |
| Phenolic acids | |
| Gallic Acid | 72.75 ± 1.343 |
| Vanillic acid | 12.75 ± 1.340 |
| Caffeic acid | 34.75 ± 1.484 |
| Syringic acid | 298.90 ± 13.717 |
| Hydroxybenzoic acid | 49.90 ± 13.838 |
| Protocatechuic acid | 280.45 ± 1.484 |
| Chlorogenic acid | 444.85 ± 14.637 |
| Ferulic acid | 232.75 ± 13.930 |
| Methoxy cinnamic acid | 13.17 ± 1.449 |
| Trans Cinnamic acid | 4.55 ± 1.485 |
| Ellagic acid | 33.65 ± 1.626 |
| Coumaric acid | 10.44 ± 1.491 |
| Flavonoids | |
| Catechin | 54.40 ± 1.697 |
| Epigallocatechin | 452.75 ± 4.454 |
| Epicatechin | 873.75 ± 14.919 |
| Rutin | 371.85 ± 13.228 |
| Quercetin | 7.25 ± 1.343 |
| Stilbenes | |
| Resveratrol | 215.40 ± 1.556 |
| Anthocyanin | |
| Cyanidine-3-glucoside chloride | 21.85 ± 1.626 |
| Total polyphenols | 3486.17 ± 88.996 |
| Parameters | CON | LOO | LOM | SEM | p |
|---|---|---|---|---|---|
| Milk production | |||||
| Milk (mL) | 1017.85 | 1025.43 | 1015.43 | 85.12 | 0.995 |
| Proximate composition (%) | |||||
| Fat | 5.36 | 5.49 | 4.60 | 0.315 | 0.119 |
| Protein | 4.87 | 4.90 | 4.33 | 0.180 | 0.054 |
| Casein | 43.20 a | 43.17 a | 38.26 b | 1.506 | 0.041 |
| Lactose | 4.94 | 4.93 | 5.12 | 0.095 | 0.273 |
| Mineral content | |||||
| Iron (mg/kg) | 13.24 b | 14.06 a,b | 14.93 a | 0.404 | 0.025 |
| Zinc (mg/kg) | 42.66 | 41.32 | 43.23 | 2.731 | 0.880 |
| Calcium (%) | 1.03 | 1.06 | 1.04 | 0.018 | 0.328 |
| Phosphorus (%) | 1.062 b | 1.087 a | 1.086 a | 0.007 | 0.031 |
| Fatty Acids (g/100 g) | CON | LOO | LOM | SEM | p | |
|---|---|---|---|---|---|---|
| Butyric acid | C 4:0 | 0.061 | 0.068 | 0.054 | 0.005 | 0.153 |
| Caproic acid | C 6:0 | 2.750 | 2.728 | 2.755 | 0.113 | 0.647 |
| Nonanoic acid | C 9:0 | 0.222 | 0.216 | 0.272 | 0.027 | 0.286 |
| Capric acid | C 10:0 | 10.174 | 10.426 | 10.456 | 0.443 | 0.882 |
| Undecanoic acid | C 11:0 | 0.485 | 0.392 | 0.486 | 0.046 | 0.280 |
| Lauric acid | C 12:0 | 0.332 | 0.229 | 0.298 | 0.056 | 0.650 |
| Miristic acid | C 14:0 | 9.729 b | 10.921 a | 10.785 a,b | 0.331 | 0.031 |
| Miristoleic acid | C 14:1 | 0.752 | 0.640 | 0.841 | 0.059 | 0.078 |
| Pentadecanoic acid | C 15:0 | 0.234 | 0.242 | 0.258 | 0.013 | 0.400 |
| Pentadecenoic acid | C 15:1 | 1.304 | 1.181 | 1.427 | 0.088 | 0.175 |
| Palmitic acid | C 16:0 | 19.717 b | 23.355 a | 21.314 b | 0.506 | <0.0001 |
| Palmitoleic acid | C 16:1 | 2.120 a | 1.351 b | 2.120 a | 0.103 | <0.0001 |
| Heptadecanoic acid | C 17:0 | 0.661 | 0.640 | 0.703 | 0.039 | 0.534 |
| Heptadecenoic acid | C 17:1 | 0.356 | 0.300 | 0.331 | 0.020 | 0.159 |
| Stearic acid | C 18:0 | 6.111 | 6.689 | 5.684 | 0.459 | 0.328 |
| Cis-oleic acid | C18:1n9c | 25.200 | 22.784 | 23.743 | 0.784 | 0.105 |
| Trans-linoleic acid | C18:2n6t | 3.935 | 3.359 | 3.504 | 0.251 | 0.251 |
| Cis-linoleic acid | C18:2n6c | 4.243 | 3.431 | 3.783 | 0.306 | 0.185 |
| Arachidic acid | C20:0 | 0.269 | 0.162 | 0.231 | 0.023 | 0.102 |
| Gamma-linoleic acid | C18:3n6 | 0.248 | 0.117 | 0.150 | 0.041 | 0.080 |
| Alpha-linolenic acid | C18:3n3 | 1.700 a | 1.340 b | 1.258 b | 0.061 | <0.0001 |
| Conjugated linoleic acid | CLA (c9, t11) | 0.546 a,b | 0.417 b | 0.746 a | 0.067 | 0.008 |
| Octadecatetraenoic acid | C18:4n-3 | 0.195 | 0.140 | 0.142 | 0.017 | 0.050 |
| Eicosadienoic acid | C20:2n6 | 0.119 | 0.074 | 0.154 | 0.024 | 0.086 |
| Eicosatrienoic acid | C20:3n6 | 0.205 | 0.156 | 0.192 | 0.048 | 0.764 |
| Arachidonic acid | C20:4n6 | 0.158 | 0.142 | 0.083 | 0.031 | 0.233 |
| Other fatty acids | 0.736 | 0.656 | 0.669 | 0.050 | 0.473 | |
| ΣSFA | 58.11 b | 63.88 a | 61.06 a,b | 1.123 | 0.005 | |
| ΣMUFA | 29.80 a | 26.28 b | 28.26 a,b | 0.783 | 0.013 | |
| ΣPUFA | 10.80 a | 8.76 b | 9.26 a,b | 0.521 | 0.025 | |
| Omega 3 | 2.02 a | 1.40 b | 1.40 b | 0.062 | <0.0001 | |
| Omega 6 | 9.45 | 7.69 | 8.61 | 0.543 | 0.090 | |
| Omega 6/Omega 3 | 5.03 | 5.28 | 6.22 | 0.383 | 0.089 | |
| Cholesterol | 0.018 | 0.018 | 0.016 | 0.001 | 0.117 | |
| Parameters (mg/kg) | CON | LOO | LOM | SEM | p |
|---|---|---|---|---|---|
| α-tocopherol | 28.18 a | 13.35 b | 27.81 a | 0.220 | <0.0001 |
| γ-tocopherol | 2.08 b | 2.04 b | 2.75 a | 0.108 | <0.0001 |
| δ-tocopherol | 1.75 | 1.70 | 1.72 | 0.020 | 0.241 |
| Total vitamin E | 31.93 a | 17.17 b | 32.28 a | 0.334 | <0.0001 |
| Vitamin A | 4.66 a | 2.02 c | 3.47 b | 0.290 | <0.0001 |
| Lutein | 0.340 | 0.360 | 0.341 | 0.023 | 0.783 |
| Astaxanthin | 1.47 | 1.49 | 1.59 | 0.114 | 0.742 |
| Storage Time | Dietary Treatment | CD (mL/g) | CT (mL/g) |
|---|---|---|---|
| t0 | CON | 30.38 | 1.78 |
| LOO | 45.11 | 2.37 | |
| LOM | 36.22 | 1.78 | |
| t1 | CON | 29.52 | 2.06 |
| LOO | 48.17 | 2.74 | |
| LOM | 45.68 | 2.47 | |
| t2 | CON | 26.53 | 2.31 |
| LOO | 46.86 | 3.49 | |
| LOM | 38.91 | 2.54 | |
| Statistical analysis | |||
| Dietary treatment | |||
| CON | 28.81 c | 2.05 b | |
| LOO | 46.72 a | 2.87 a | |
| LOM | 40.27 b | 2.26 a,b | |
| SEM | 1.77 | 0.207 | |
| Storage time | |||
| t0 | 37.24 | 1.98 b | |
| t1 | 41.13 | 2.42 a,b | |
| t2 | 37.44 | 2.78 a | |
| SEM | 1.78 | 0.207 | |
| p values | |||
| Diet | 0.004 | 0.023 | |
| Storage time | 0.264 | 0.035 | |
| Diet x Storage time | 0.521 | 0.848 | |
| Storage Time | Dietary Treatment | 450 nm | 495 nm | 532 nm | MDA, µG/L | p-Anisidine (A.U.) |
|---|---|---|---|---|---|---|
| t0 | CON | 0.301 | 0.145 | 0.116 | 92.36 | 25.01 |
| LOO | 0.343 | 0.179 | 0.148 | 134.97 | 27.14 | |
| LOM | 0.323 | 0.171 | 0.139 | 127.31 | 23.29 | |
| t1 | CON | 0.372 | 0.188 | 0.157 | 148.07 | 76.67 |
| LOO | 0.391 | 0.214 | 0.179 | 176.99 | 94.60 | |
| LOM | 0.397 | 0.209 | 0.180 | 177.72 | 68.01 | |
| t2 | CON | 0.404 | 0.196 | 0.161 | 145.29 | 94.03 |
| LOO | 0.445 | 0.240 | 0.197 | 186.32 | 104.113 | |
| LOM | 0.441 | 0.230 | 0.192 | 182.09 | 73.29 | |
| Statistical analysis | ||||||
| Dietary treatment | ||||||
| CON | 0.359 b | 0.176 b | 0.145 b | 128.57 b | 65.24 a | |
| LOO | 0.393 a | 0.211 a | 0.174 a | 166.09 a | 75.28 a | |
| LOM | 0.387 a | 0.203 a | 0.170 a | 162.37 a | 54.86 b | |
| SEM | 0.006 | 0.004 | 0.003 | 4.514 | 2.873 | |
| Storage time | ||||||
| t0 | 0.322 c | 0.165 c | 0.134 b | 118.21 b | 25.14 c | |
| t1 | 0.387 b | 0.204 b | 0.172 a | 167.59 a | 79.76 b | |
| t2 | 0.430 a | 0.222 c | 0.183 a | 171.24 a | 90.48 a | |
| SEM | 0.004 | 0.004 | 0.003 | 4.521 | 2.875 | |
| p values | ||||||
| Diet | 0.002 | 0.002 | <0.0001 | <0.0001 | <0.0001 | |
| Storage time | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Diet × Storage time | 0.705 | 0.827 | 0.781 | 0.918 | 0.056 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oancea, A.-G.; Dragomir, C.; Vlaicu, P.A.; Varzaru, I.; Saracila, M.; Cismileanu, A.E.; Gras, M.A.; Rotar, M.C.; Untea, A.E. Impact of Milk Thistle Cake as the Natural Antioxidant Source on the Mitigation of Oxidative Effects in Goat Milk Induced by Oxidized Linseed Oil. Foods 2025, 14, 3205. https://doi.org/10.3390/foods14183205
Oancea A-G, Dragomir C, Vlaicu PA, Varzaru I, Saracila M, Cismileanu AE, Gras MA, Rotar MC, Untea AE. Impact of Milk Thistle Cake as the Natural Antioxidant Source on the Mitigation of Oxidative Effects in Goat Milk Induced by Oxidized Linseed Oil. Foods. 2025; 14(18):3205. https://doi.org/10.3390/foods14183205
Chicago/Turabian StyleOancea, Alexandra-Gabriela, Catalin Dragomir, Petru Alexandru Vlaicu, Iulia Varzaru, Mihaela Saracila, Ana Elena Cismileanu, Mihail Alexandru Gras, Mircea Catalin Rotar, and Arabela Elena Untea. 2025. "Impact of Milk Thistle Cake as the Natural Antioxidant Source on the Mitigation of Oxidative Effects in Goat Milk Induced by Oxidized Linseed Oil" Foods 14, no. 18: 3205. https://doi.org/10.3390/foods14183205
APA StyleOancea, A.-G., Dragomir, C., Vlaicu, P. A., Varzaru, I., Saracila, M., Cismileanu, A. E., Gras, M. A., Rotar, M. C., & Untea, A. E. (2025). Impact of Milk Thistle Cake as the Natural Antioxidant Source on the Mitigation of Oxidative Effects in Goat Milk Induced by Oxidized Linseed Oil. Foods, 14(18), 3205. https://doi.org/10.3390/foods14183205











