From Desert Greening to Human Health: A Systematic Review of the Extraction, Unique Structure, and Bioactivity of Sea Buckthorn Proanthocyanidins
Abstract
1. Introduction
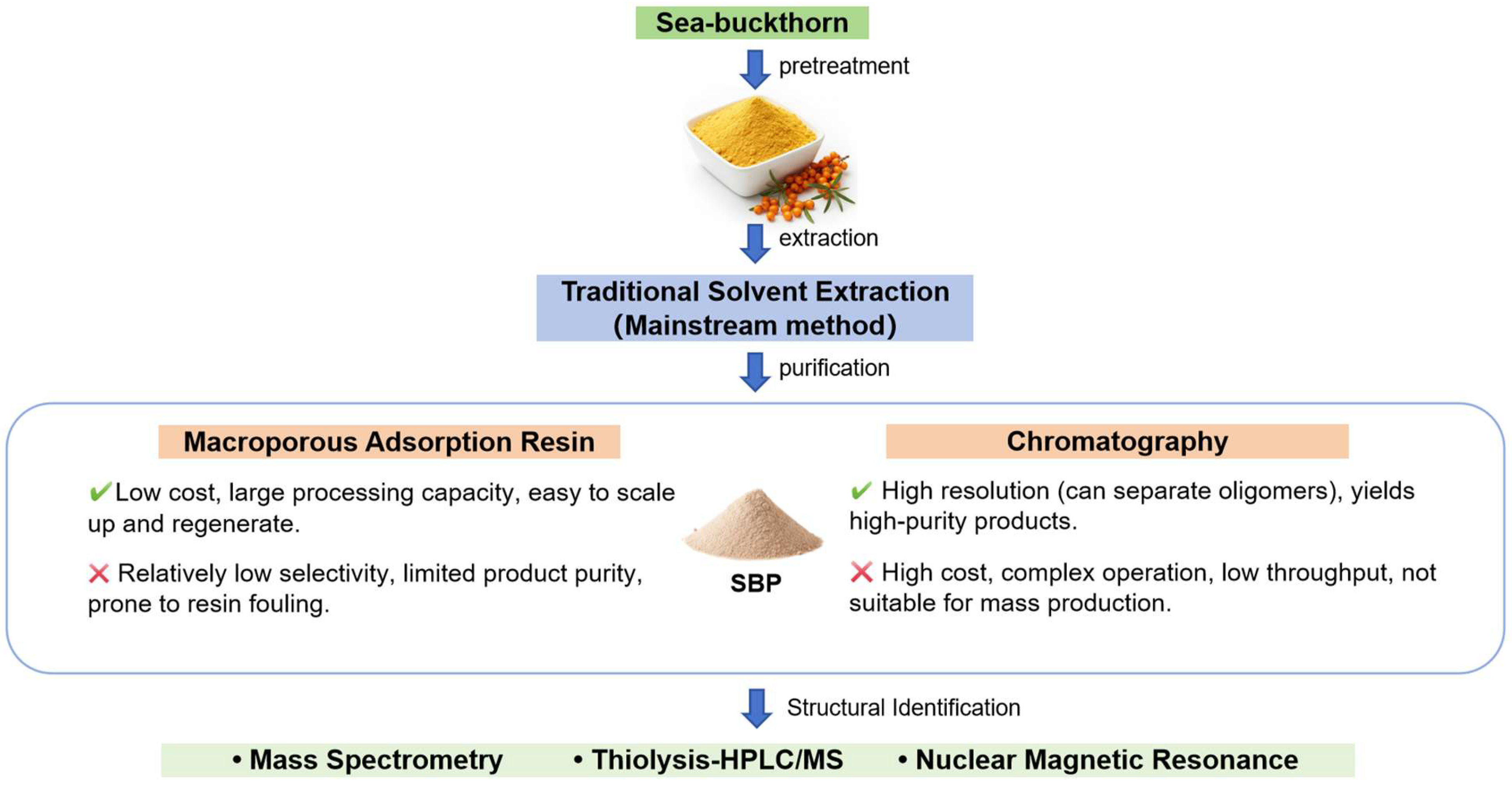
2. Preparation of SBPs
2.1. Extraction of SBPs
2.2. Purification of SBPs
2.2.1. Chromatography
2.2.2. Resin Adsorption
3. Structure and Identification Methods
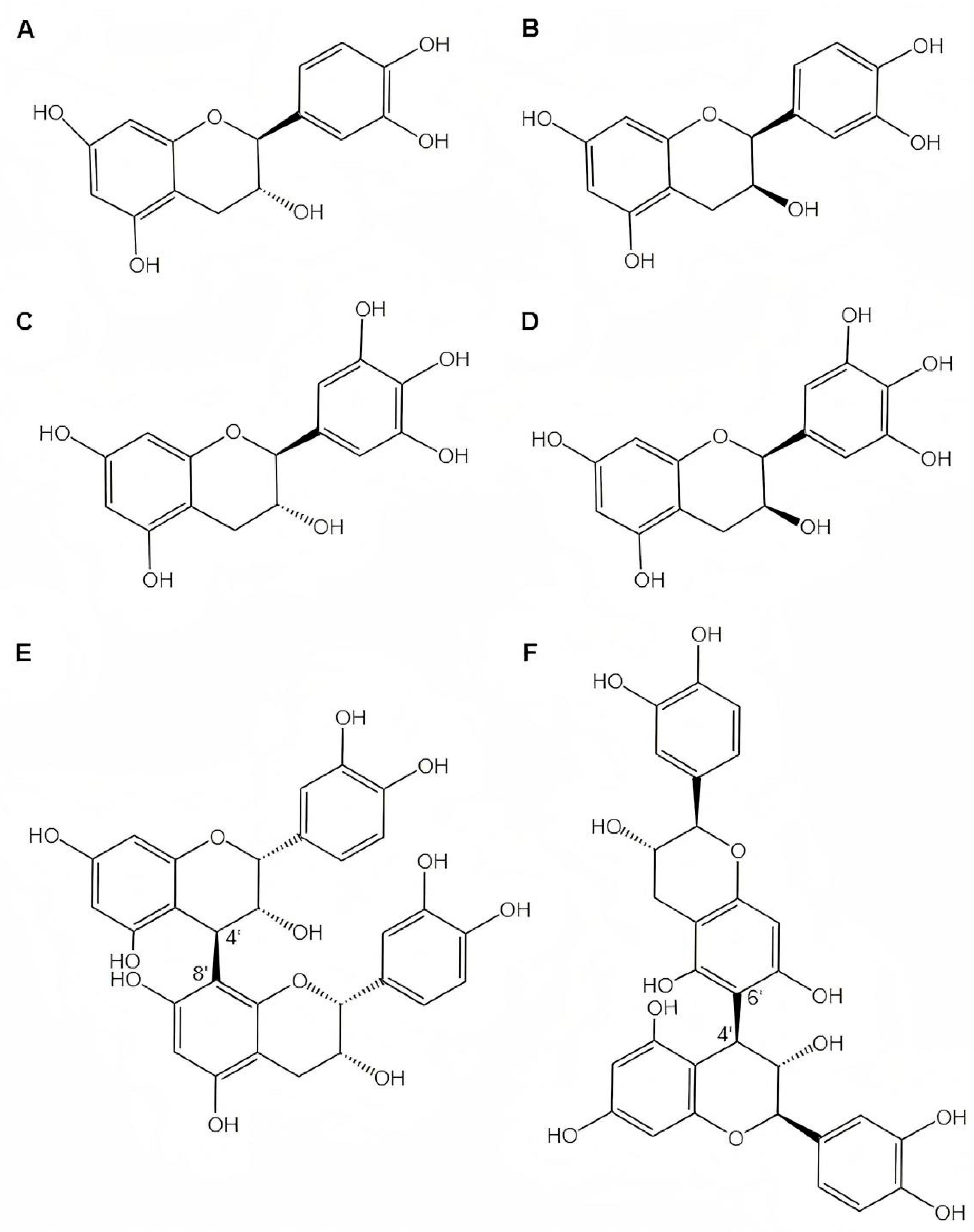
3.1. Monomer Composition
3.2. Linkage Mode and Stereochemistry
3.3. Degree of Polymerization
3.4. The Influence of Geographical and Climatic Factors
3.5. Structure–Activity Relationship
3.6. Structural Identification Methods
3.6.1. Mass Spectrometry Analysis Technology
3.6.2. Nuclear Magnetic Resonance Technology
3.6.3. Thiolysis Coupled Analysis Technology
4. Antioxidant Capacity
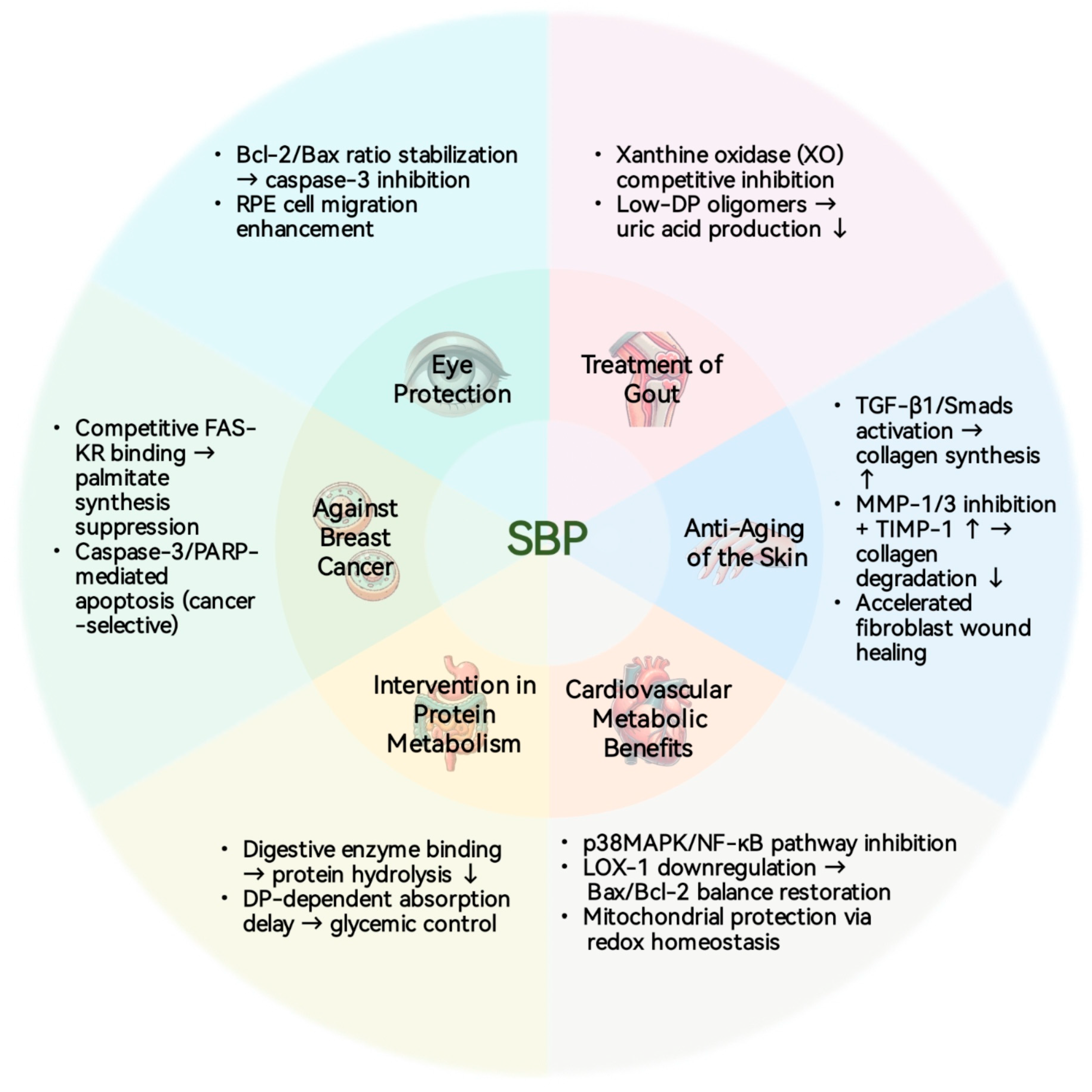
5. Biological Activity
5.1. Cardiovascular and Metabolic Benefits
5.2. Eye Protection
5.3. Anti-Breast Cancer Activity
5.4. Anti-Aging Skin Care
5.5. Treatment of Gout
5.6. Protein Metabolism Intervention
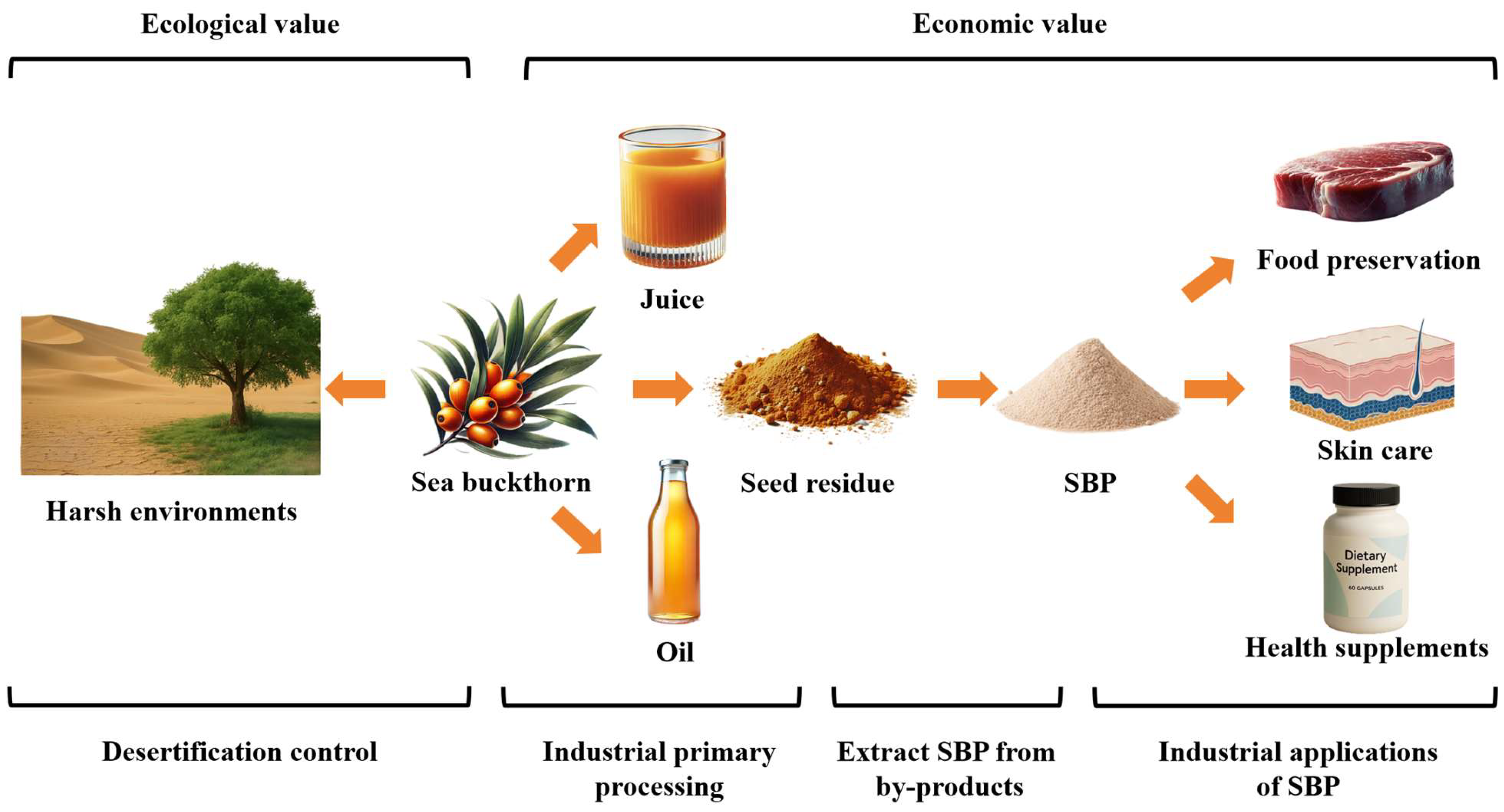
6. Industrial Applications
6.1. Food Preservation
6.2. Skin Care
6.3. Health Supplements
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ADP | Average Degree of Polymerization |
| AMD | Age-Related Macular Degeneration |
| AS | Atherosclerosis |
| BSA | Bovine Serum Albumin |
| C | Catechin |
| Col I | Type I Collagen |
| DES | Deep Eutectic Solvents |
| DP | Degree of Polymerization |
| DW | Dry Weight |
| EC | Epicatechin |
| EGC | Epigallocatechin |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
| FAS | Fatty Acid Synthase |
| FW | Fresh Weight |
| GC | Gallocatechin |
| HILIC-MS | Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry |
| HILIC-UV | Hydrophilic Interaction Liquid Chromatography-Ultraviolet |
| HPLC | High-Performance Liquid Chromatography |
| HSFs | Human Skin Fibroblasts |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| MAE | Microwave-Assisted Extraction |
| MALDI-TOF-MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| NP | Normal-Phase |
| ROS | Reactive Oxygen Species |
| RP | Reversed-Phase |
| RPE | Retinal Pigment Epithelial |
| SBPs | Sea Buckthorn Proanthocyanidins |
| SOD | Superoxide Dismutase |
| UAE | Ultrasound-Assisted Extraction |
| UHPLC-MS/MS | Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry |
| UV | Ultraviolet |
| XO | Xanthine Oxidase |
References
- Mei, D.; Ma, X.; Fu, F.; Cao, F. Research Status and Development Prospects of Sea Buckthorn (Hippophae rhamnoides L.) Resources in China. Forests 2023, 14, 2461. [Google Scholar] [CrossRef]
- Li, G.; Du, S.; Guo, K. Evaluation of Limiting Climatic Factors and Simulation of a Climatically Suitable Habitat for Chinese Sea Buckthorn. PLoS ONE 2015, 10, e0131659. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y. Role of the Different Planting Age of Seabuckthorn Forests to Soil Amelioration in Coal Mining Subsidence Land. Int. J. Coal Sci. Technol. 2014, 1, 192–197. [Google Scholar] [CrossRef]
- Żuchowski, J. Phytochemistry and Pharmacology of Sea Buckthorn (Elaeagnus Rhamnoides; Syn. Hippophae rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2023, 22, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Gu, L.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Mellway, R.D.; Tran, L.T.; Prouse, M.B.; Campbell, M.M.; Constabel, C.P. The Wound-, Pathogen-, and Ultraviolet B-Responsive MYB134 Gene Encodes an R2R3 MYB Transcription Factor That Regulates Proanthocyanidin Synthesis in Poplar. Plant Physiol. 2009, 150, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Liao, H.; Li, C.; Chen, M. Anti-Inflammatory Effect of Lipophilic Grape Seed Proanthocyanidin in RAW 264.7 Cells and a Zebrafish Model. J. Funct. Foods 2020, 75, 104217. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Okshevsky, M.; Déziel, E.; Tufenkji, N. Proanthocyanidin Interferes with Intrinsic Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Adv. Sci. 2019, 6, 1802333. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary Proanthocyanidins: Occurrence, Dietary Intake, Bioavailability, and Protection against Cardiovascular Disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-Targeted Prevention and Therapy of Cancer by Proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef]
- Yuan, C.; Ren, H.; Hu, K.; Chen, L.; Yue, K.; He, K.; Yu, Q.; Wang, N.; Zhang, G. Effect of Proanthocyanidins on Cognitive Improvement in Thyroxin-Induced Aging Mice. Food Funct. 2025, 16, 207–218. [Google Scholar] [CrossRef]
- Grases, F.; Prieto, R.M.; Fernández-Cabot, R.A.; Costa-Bauzá, A.; Sánchez, A.M.; Prodanov, M. Effect of Consuming a Grape Seed Supplement with Abundant Phenolic Compounds on the Oxidative Status of Healthy Human Volunteers. Nutr. J. 2015, 14, 94. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Yuen, M.; Yuen, T.; Yuen, H.; Peng, Q. Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea Buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts. Antioxidants 2022, 11, 1900. [Google Scholar] [CrossRef]
- Yang, W.; Laaksonen, O.; Kallio, H.; Yang, B. Proanthocyanidins in Sea Buckthorn (Hippophaë rhamnoides L.) Berries of Different Origins with Special Reference to the Influence of Genetic Background and Growth Location. J. Agric. Food Chem. 2016, 64, 1274–1282. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Triterpenoids, Phenolic Compounds, Macro- and Microelements in Anatomical Parts of Sea Buckthorn (Hippophaë rhamnoides L.) Berries, Branches and Leaves. J. Food Compos. Anal. 2021, 103, 104107. [Google Scholar] [CrossRef]
- Rösch, D.; Mügge, C.; Fogliano, V.; Kroh, L.W. Antioxidant Oligomeric Proanthocyanidins from Sea Buckthorn (Hippophaë rhamnoides) Pomace. J. Agric. Food Chem. 2004, 52, 6712–6718. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yuen, M.; Yuen, T.; Yuen, H.; Wang, M.; Peng, Q. Regulatory Effect of Sea-Buckthorn Procyanidins on Oxidative Injury HUVECs. Front. Nutr. 2022, 9, 850076. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, F.; Ouyang, J.; Wang, X.; Ma, X. Inhibitory Effects of Sea Buckthorn Procyanidins on Fatty Acid Synthase and MDA-MB-231 Cells. Tumor Biol. 2014, 35, 9563–9569. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Huo, Y.; Zhou, F.; Wu, W.; Lu, F.; Yang, X.; Guo, X.; Chen, P.; Deng, Q.; et al. Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in Vivo. Nutrients 2016, 8, 245. [Google Scholar] [CrossRef]
- Smit, A.T.; Van Zomeren, A.; Dussan, K.; Riddell, L.A.; Huijgen, W.J.J.; Dijkstra, J.W.; Bruijnincx, P.C.A. Biomass Pre-Extraction as a Versatile Strategy to Improve Biorefinery Feedstock Flexibility, Sugar Yields, and Lignin Purity. ACS Sustain. Chem. Eng. 2022, 10, 6012–6022. [Google Scholar] [CrossRef]
- Kallio, H.; Yang, W.; Liu, P.; Yang, B. Proanthocyanidins in Wild Sea Buckthorn (Hippophaë rhamnoides) Berries Analyzed by Reversed-Phase, Normal-Phase, and Hydrophilic Interaction Liquid Chromatography with UV and MS Detection. J. Agric. Food Chem. 2014, 62, 7721–7729. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, B.; Pan, S.; Yang, E.; Wang, K.; Cenkowski, S.; Hydamake, A.W.; Rao, S. A New Technology for Extraction and Purification of Proanthocyanidins Derived from Sea Buckthorn Bark. J. Sci. Food Agric. 2006, 86, 486–492. [Google Scholar] [CrossRef]
- Fan, J.; Ding, X.; Gu, W. Radical-Scavenging Proanthocyanidins from Sea Buckthorn Seed. Food Chem. 2007, 102, 168–177. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. Sea Buckthorn (Hippophae rhamnoides) Proanthocyanidins Inhibit In Vitro Enzymatic Hydrolysis of Protein. J. Food Sci. 2011, 76, T130–T137. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. Effect of Polymerization on Antioxidant and Xanthine Oxidase Inhibitory Potential of Sea Buckthorn (H. rhamnoides) Proanthocyanidins. J. Food Sci. 2012, 77, C1036–C1041. [Google Scholar] [CrossRef]
- Ma, X.; Yang, W.; Laaksonen, O.; Nylander, M.; Kallio, H.; Yang, B. Role of Flavonols and Proanthocyanidins in the Sensory Quality of Sea Buckthorn (Hippophaë rhamnoides L.) Berries. J. Agric. Food Chem. 2017, 65, 9871–9879. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuen, M.; Li, W.; Yuen, H.; Wang, M.; Smith, D.; Peng, Q. Composition Analysis and Antioxidant Activity Evaluation of a High Purity Oligomeric Procyanidin Prepared from Sea Buckthorn by a Green Method. Curr. Res. Food Sci. 2021, 4, 840–851. [Google Scholar] [CrossRef]
- Sapkota, G. Optimizing Proanthocyanidin Extraction from Grape Seeds in Winery Wastes. Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2018. [Google Scholar]
- Liang, L.; Liu, Y.; Wu, L.; Weng, L.; Qiu, H.; Zhong, W.; Meng, F. Advances in Extraction Protocols, Degradation Methods, and Bioactivities of Proanthocyanidins. Molecules 2024, 29, 2179. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical Solvent Extraction of Procyanidins from Dried Red Grape Pomace. J. Agric. Food Chem. 2010, 58, 4014–4021. [Google Scholar] [CrossRef]
- Downey, M.O.; Hanlin, R.L. Comparison of Ethanol and Acetone Mixtures for Extraction of Condensed Tannin from Grape Skin. S. Afr. J. Enol. Vitic. 2016, 31, 154–159. [Google Scholar] [CrossRef]
- Chen, X.; Song, H.; Zhou, S.; Yuan, C.; Li, J. Exploring Separation Patterns and Mechanisms of Proanthocyanidins in Grape Seeds and Pomace with Diverse Molecular Weights, Compositions, and Structures. Food Chem. X 2023, 20, 101008. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dang, M.; Zhu, W.; Li, C. Galloyl Group in B-Type Proanthocyanidin Dimers Was Responsible for Its Differential Inhibitory Activity on 3T3-L1 Preadipocytes Due to the Strong Lipid Raft-Perturbing Potency. J. Agric. Food Chem. 2021, 69, 5216–5225. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Sun, B.S. Extraction Yields and Anti-oxidant Activity of Proanthocyanidins from Different Parts of Grape Pomace: Effect of Mechanical Treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P.; Jang, J.P. Effects of Water Extraction Temperatures on the Yield, Molecular Weight, and Antioxidant Activity of Proanthocyanidins Extracted from Pinus Radiata Bark. For. Prod. J. 2011, 61, 321–325. [Google Scholar] [CrossRef]
- Lisjak, K.; Lelova, Z.; Žigon, U.; Bolta, Š.V.; Teissedre, P.; Vanzo, A. Effect of Extraction Time on Content, Composition and Sensory Perception of Proanthocyanidins in Wine-like Medium and during Industrial Fermentation of Cabernet Sauvignon. J. Sci. Food Agric. 2020, 100, 1887–1896. [Google Scholar] [CrossRef]
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-de-Cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of Sonotrode Ultrasonic-Assisted Extraction of Proanthocyanidins from Brewers’ Spent Grains. Antioxidants 2019, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Impact of Eutectic Solvents Utilization in the Microwave Assisted Extraction of Proanthocyanidins from Grape Pomace. Molecules 2021, 27, 246. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Carle, R.; Stanley, R.A.; Saleh, Z.S. Pilot-Scale Resin Adsorption as a Means to Recover and Fractionate Apple Polyphenols. J. Agric. Food Chem. 2010, 58, 6787–6796. [Google Scholar] [CrossRef] [PubMed]
- Heck, K.L.; Si, L.; Jung, D.J.; Calderón, A.I. Application of Eco-Friendly Natural Deep Eutectic Solvents (NADES) in HPLC for Separation of Complex Natural Products: Current Limitations and Future Directions. J. Pharm. Biomed. Anal. 2024, 244, 116102. [Google Scholar] [CrossRef]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; et al. HPLC Method for the Quantification of Procyanidins in Cocoa and Chocolate Samples and Correlation to Total Antioxidant Capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef]
- Luca, S.V.; Bujor, A.; Miron, A.; Aprotosoaie, A.C.; Skalicka-Woźniak, K.; Trifan, A. Preparative Separation and Bioactivity of Oligomeric Proanthocyanidins. Phytochem. Rev. 2020, 19, 1093–1140. [Google Scholar] [CrossRef]
- Dhull, P.; Dunuweera, S.; Bietsch, J.; Bandu, R.; Wannere, C.; Achanta, S.; Krishnamurthy, D.; Qu, B.; Senanayake, C. Recent Advances and Application of Liquid Chromatography in Pharmaceutical Industry. J. Liq. Chromatogr. Relat. Technol. 2025, 48, 168–187. [Google Scholar] [CrossRef]
- Kostanyan, A.A.; Voshkin, A.A.; Belova, V.V. Analytical, Preparative, and Industrial-Scale Separation of Substances by Methods of Countercurrent Liquid-Liquid Chromatography. Molecules 2020, 25, 6020. [Google Scholar] [CrossRef]
- Zhao, W.; Meng, Y.C.; Yin, Z.P.; Liu, W.H.; Niu, C.J. Study on the Isolation and Purification of Proanthocyanidins from Rhodiola Rose by Macroporous Adsorbent Resin. Adv. Mater. Res. 2011, 236–238, 2053–2057. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, X.; Zhang, J.; Gao, S.; Lu, X. Guide for Application of Macroporous Adsorption Resins in Polysaccharides Purification. eFood 2024, 5, e130. [Google Scholar] [CrossRef]
- Dai, A.; Zhou, X.; Wu, Z. Design of an Intelligent Controller for a Grain Dryer: A Support Vector Machines for Regression Inverse Model Proportional–Integral–Derivative Controller. Food Sci. Nutr. 2020, 8, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Ain, N.U.; Wu, S.; Li, X.; Li, D.; Zhang, Z. Isolation, Characterization, Pharmacology and Biopolymer Applications of Licorice Polysaccharides: Review. Materials 2022, 15, 3654. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Li, S.; Zhu, Z.; Wang, X.; Cravotto, G. Improving Complexation of Puerarin with Kudzu Starch by Various Ultrasonic Pretreatment: Interaction Mechanism Analysis. Ultrason. Sonochem. 2024, 111, 107095. [Google Scholar] [CrossRef]
- Liu, X.; Feng, B.; Liu, H.; Wang, Y.; Luo, B.; Yang, Y.; Zhang, Q.; Wang, Z.; Xu, Z.; Li, B.; et al. Machine Learning-Assisted FTIR Spectra to Predict Freeze-Drying Curve of Food. LWT 2024, 197, 115894. [Google Scholar] [CrossRef]
- Qu, Y.; Bekard, I.; Hunt, B.; Black, J.; Fabri, L.; Gras, S.L.; Kentish, S.E. The Transition from Resin Chromatography to Membrane Adsorbers for Protein Separations at Industrial Scale. Sep. Purif. Rev. 2024, 53, 351–371. [Google Scholar] [CrossRef]
- Menon, K.R.; Jose, S.; Suraishkumar, G.K. Photon Up-conversion Increases Biomass Yield in Chlorella vulgaris. Biotechnol. J. 2014, 9, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Anjila, P.K.F.; Tharani, G.R.; Sundaramoorthy, A.; Kumar Shanmugam, V.; Subramani, K.; Chinnathambi, S.; Pandian, G.N.; Raghavan, V.; Grace, A.N.; Ganesan, S.; et al. An Ultra-Sensitive Detection of Melamine in Milk Using Rare-Earth Doped Graphene Quantum Dots- Synthesis and Optical Spectroscopic Approach. Microchem. J. 2024, 196, 109670. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Wang, S.; Wei, L.; Cui, Y.-Y.; Chen, Y.-H. Proanthocyanidins: Components, Pharmacokinetics and Biomedical Properties. Am. J. Chin. Med. 2020, 48, 813–869. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antioxidant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Laaksonen, O.; Kallio, H.; Yang, B. Effects of Latitude and Weather Conditions on Proanthocyanidins in Berries of Finnish Wild and Cultivated Sea Buckthorn (Hippophaë rhamnoides L. ssp. Rhamnoides). Food Chem. 2017, 216, 87–96. [Google Scholar] [CrossRef]
- Nie, Y.; Stürzenbaum, S.R. Proanthocyanidins of Natural Origin: Molecular Mechanisms and Implications for Lipid Disorder and Aging-Associated Diseases. Adv. Nutr. 2019, 10, 464–478. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; Van Breemen, R.B. Procyanidins: A Comprehensive Review Encompassing Structure Elucidation via Mass Spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Jaschok-Kentner, B.; Wray, V.; Winterhalter, P. Structure Elucidation of Procyanidin Oligomers by Low-Temperature1 H NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 62–69. [Google Scholar] [CrossRef]
- Nam, J.-W.; Phansalkar, R.S.; Lankin, D.C.; Bisson, J.; McAlpine, J.B.; Leme, A.A.; Vidal, C.M.P.; Ramirez, B.; Niemitz, M.; Bedran-Russo, A.; et al. Subtle Chemical Shifts Explain the NMR Fingerprints of Oligomeric Proanthocyanidins with High Dentin Biomodification Potency. J. Org. Chem. 2015, 80, 7495–7507. [Google Scholar] [CrossRef]
- Brooks, W.C.; Paguigan, N.D.; Raja, H.A.; Moy, F.J.; Cech, N.B.; Pearce, C.J.; Oberlies, N.H. qNMR for Profiling the Production of Fungal Secondary Metabolites. Magn. Reson. Chem. 2017, 55, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Fernández, K.; Kennedy, J.A.; Agosin, E. Characterization of Vitis vinifera L. Cv. Carménère Grape and Wine Proanthocyanidins. J. Agric. Food Chem. 2007, 55, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lv, Y.; Wang, J.; Yang, L.; Shen, S. A Novel Thiolysis-high-performance Liquid Chromatography Method for the Determination of Proanthocyanidins in Grape Seeds. J. Sep. Sci. 2022, 45, 1874–1883. [Google Scholar] [CrossRef]
- Liu, K.; Li, W.; Yuen, M.; Yuen, T.; Yuen, H.; Wang, M.; Peng, Q. Sea Buckthorn Proanthocyanidins Are the Protective Agent of Mitochondrial Function in Macrophages Under Oxidative Stress. Front. Pharmacol. 2022, 13, 914146. [Google Scholar] [CrossRef]
- Liu, X.; Yuen, M.; Yuen, T.; Yuen, H.; Wang, M.; Peng, Q. Anti-skin Aging Effect of Sea Buckthorn Proanthocyanidins in D-galactose-induced Aging Mice. Food Sci. Nutr. 2024, 12, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 316. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Dorf, N.; Maciejczyk, M. Skin Senescence—From Basic Research to Clinical Practice. Front. Med. 2024, 11, 1484345. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Ong, K.L.; Culbreth, G.T.; Steinmetz, J.D.; Cousin, E.; Lenox, H.; Kopec, J.A.; Haile, L.M.; Brooks, P.M.; Kopansky-Giles, D.R.; et al. Global, Regional, and National Burden of Gout, 1990–2020, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2024, 6, e507–e517. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Muggeo, M. Postprandial Blood Glucose as a Risk Factor for Cardiovascular Disease in Type II Diabetes: The Epidemiological Evidence. Diabetologia 2001, 44, 2107–2114. [Google Scholar] [CrossRef]
- Ma, K.; Yuen, M.; Yuen, T.; Yuen, H.; Peng, Q. Protective Mechanism of Sea Buckthorn Proanthocyanidins Against Hydrogen Peroxide-Introduced Oxidative Damage in Adult Retinal Pigment Epithelial-19. Antioxidants 2024, 13, 1352. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Seol, K.-H.; Seong, P.-N.; Park, B.-Y.; Kim, H.W. Effects of Procyanidin on Meat Quality and Shelf-Life for Preserving Pork Patties during Chilled Storage. Food Sci. Anim. Resour. 2015, 35, 564–571. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Cronobacter Sakazakii Reduction by Blueberry Proanthocyanidins. Food Microbiol. 2014, 39, 127–131. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, Antioxidant and Phytochemical Investigations of Sea Buckthorn (Hippophaë rhamnoides L.) Leaf, Stem, Root and Seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Pal, H.C.; Prasad, R. Dietary Proanthocyanidins Prevent Ultraviolet Radiation-Induced Non-Melanoma Skin Cancer through Enhanced Repair of Damaged DNA-Dependent Activation of Immune Sensitivity. Semin. Cancer Biol. 2017, 46, 138–145. [Google Scholar] [CrossRef]
- Zi, S.; Ma, H.; Li, Y.; Liu, W.; Yang, Q.; Zhao, G.; Lian, S. Oligomeric Proanthocyanidins from Grape Seeds Effectively Inhibit Ultraviolet-Induced Melanogenesis of Human Melanocytes in Vitro. Int. J. Mol. Med. 2009, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
| Buckthorn Part | Extraction Method | Purification Method | Content | Purity | Characterization | Reference |
|---|---|---|---|---|---|---|
| Bark | Aqueous ethanol extraction (Temp: 21 °C, pH: 5.13, ethanol: 65%, Ratio: 1:10 w/v, Time: 90 min) | D3520 macroporous adsorption resin purification (Elution with 30% ethanol) | \ | >95% | [25] | |
| Seed | Water-acetone (3:7 v/v) extraction (3×, 2 h each), hexane wash | Sephadex LH-20 column chromatography (stepwise elution: H2O → ethanol → H2O/Acetone); further fractionation of polymer fraction | \ | \ | Characterized fractions (e.g., Fraction I: polymeric, ADP 12.2, 81.2% prodelphinidins) | [26] |
| Berry Pulp | Optimized: Acidified Acetone-Water (70:30 v/v, +1% acid), 3× extractions | Sephadex LH-20 column chromatography (Wash: 95% ethanol, Elute: 50% Acetone) | 1.2% DW | 66.2% | ADP 7.4 | [27] |
| Seed Kernel | Same as above | Same as above | 4.6% DW | 70.4% | ADP 5.6 | |
| Seed Coat | Optimized: Acidified Acetone-Water (60:40 v/v, +1% acid), 3× extractions | Same as above | 0.9% DW | 59.5% | ADP 8.2 | |
| Leaves | Same as above | Same as above | 0.6% DW | 60.2% | ADP 10.6 | |
| Seed | Defatted (hexane), then Methanol/Water (7:3 v/v) extraction (5×, 30 min each) | Ethyl acetate wash removes less polar phenolics, then Sephadex LH-20 column chromatography (Stepwise elution: water → 75% ethanol) | 3.4% DW | 68.6% (SPA-2) | ADP 14.7, Prodelphinidins 83.6%. Units: (Epi)Catechin and (Epi)Gallocatechin | [28] |
| Berry (Whole) | Acetone/Water/Acetic Acid (80:19.5:0.5 v/v) extraction (3×, 15 min sonication each) | Sephadex LH-20 column chromatography (Wash: Water; Elute Fractions: I: MeOH/Water 20:80, II: Acetone/Water 70:30, III: Acetone/Water 70:30). Fraction II used for analysis. | \ | \ | DP 2–11 detected (HILIC-MS). Only B-type. Main units: (Epi) Gallocatechin. Dimers 40%, Trimers 40%, Tetramers 20% (molar ratio of DP 2–4). The majority are higher polymers (HILIC-UV). | [24] |
| Berry Puree (seedless) | Same as above | Same as above | 23.0–70.2 mg/100 g FW | \ | Only B-type PAs detected. Main units: (Epi)Catechin and (Epi)Gallocatechin. DP 5–11 not detected significantly. Oligomers (DP 2–4) are 0.3–14.4% of Total PAs. | [29] |
| Berry (Whole, various origins) | Same as above | Sephadex LH-20 column chromatography (Wash: Water; Elute Fractions: I: MeOH/Water 20:80; II: Acetone/Water 70:30; Clean: MeOH) | 0.39–1.94% DW | \ | Only B-type PAs. DP up to 11 detected (HILIC-MS). Oligomers (DP 2–4) are 0.5–5% of total PAs. Significant differences in total PA and oligomer profiles exist between subspecies and locations. | [16] |
| Berry Powder (Qinghai) | Hot water extraction (1:15 m/v, 55 °C, 4 h, 2×) | AB-8 macro-porous resin enrichment (Elution: 30% ethanol), followed by spray drying | \ | 91.5% | LC-MS/MS identified dimers: (−)-epicatechin gallate, procyanidin B, (+)-gallocatechin-(+)-catechin, (+)-gallocatechin dimer. Low degree of polymerization (mostly dimers). UVmax 280 nm. Fourier-Transform Infrared Spectroscopy confirms structure. | [30] |
| Identification Method | Principle and Application | Key Information Provided | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Mass Spectrometry (MS) | Structure identification via molecular and fragment ions. ESI-MS is suitable for oligomers; MALDI-TOF-MS is for high-DP polymers. | Monomer composition, linkage type (A/B-type), degree of polymerization (DP) distribution. | High sensitivity; capable of analyzing complex mixtures and high-DP polymers. | Difficulty in distinguishing stereoisomers; challenging for quantification. | [18,62] |
| Nuclear Magnetic Resonance (NMR) | Precise 3D structure elucidation by analyzing 1H and 13C chemical shifts and coupling constants. | Monomer type, linkage position (C4 → C8/C4 → C6), stereochemistry (2,3-cis/trans). | Provides definitive stereochemical information. | Low sensitivity, severe signal overlap; only suitable for purified, low-DP (<10) samples. | [63,64,65,66] |
| Thiolysis-HPLC/MS | Cleaves interflavan bonds using a nucleophilic reagent (e.g., benzyl mercaptan) to form derivatives for analysis. | Composition and ratio of extension vs. terminal units; calculation of average degree of polymerization (ADP). | Enables compositional analysis of high-DP polymers; relatively accurate for quantification. | Destroys the original structure; cannot provide sequence or stereochemical information. | [26,67] |
| Biological Activity | Study Model | Key Findings and Mechanism | SBP Concentration/Dose | Reference(s) |
|---|---|---|---|---|
| Cardiovascular Benefits | Palmitic acid-damaged Human Umbilical Vein Endothelial Cells (HUVECs) | Restored mitochondrial membrane potential; inhibited the p38MAPK/NF-κB pathway; downregulated LOX-1 expression to reduce apoptosis. | 100 μg/mL | [20] |
| Eye Protection | H2O2-induced AMD model in human RPE cells | Restored cell migration capacity; stabilized the Bcl-2/Bax ratio; inhibited caspase-3 activation to reduce apoptosis. | 50 μg/mL | [22,60,61] |
| Anti-Breast Cancer | Human breast cancer cells (MDA-MB-231) | Competitively inhibited fatty acid synthase (FAS); induced apoptosis via PARP cleavage and caspase-3 activation with selectivity for cancer cells. | IC50 ≈ 50 μg/mL | [21] |
| Anti-Aging Skin Care | H2O2-induced HSFs; D-galactose-induced mouse model | Enhanced SOD and GSH activities; activated TGF-β1/Smads pathway for collagen synthesis; inhibited the MMP system. | In vitro: 50–200 μM; In vivo: 50–200 mg/kg | [15,69] |
| Gout Treatment | Xanthine Oxidase (XO) inhibition assay | Competitively bound to the active site of XO to reduce uric acid production; low-DP fraction was more effective. | Oligomer: Ki ≈ 2.1 μM | [28] |
| Protein Metabolism Intervention | In vitro pepsin digestion model of BSA | Bound to protein and reduced its hydrolysis rate in a DP-dependent manner; high-DP fraction was more potent. | 5–20 mg/mL | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; He, Z.; He, Y.; Peng, Q. From Desert Greening to Human Health: A Systematic Review of the Extraction, Unique Structure, and Bioactivity of Sea Buckthorn Proanthocyanidins. Foods 2025, 14, 3203. https://doi.org/10.3390/foods14183203
Zhou Z, He Z, He Y, Peng Q. From Desert Greening to Human Health: A Systematic Review of the Extraction, Unique Structure, and Bioactivity of Sea Buckthorn Proanthocyanidins. Foods. 2025; 14(18):3203. https://doi.org/10.3390/foods14183203
Chicago/Turabian StyleZhou, Zixin, Zongyi He, Yu He, and Qiang Peng. 2025. "From Desert Greening to Human Health: A Systematic Review of the Extraction, Unique Structure, and Bioactivity of Sea Buckthorn Proanthocyanidins" Foods 14, no. 18: 3203. https://doi.org/10.3390/foods14183203
APA StyleZhou, Z., He, Z., He, Y., & Peng, Q. (2025). From Desert Greening to Human Health: A Systematic Review of the Extraction, Unique Structure, and Bioactivity of Sea Buckthorn Proanthocyanidins. Foods, 14(18), 3203. https://doi.org/10.3390/foods14183203







