Safflower Polysaccharides Alleviate TNBS-Induced Colitis by Modulating Gut Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Chemical Composition Analysis and Average Molecular Weight Distribution Determination
2.3. Monosaccharide Composition Analysis of SPS
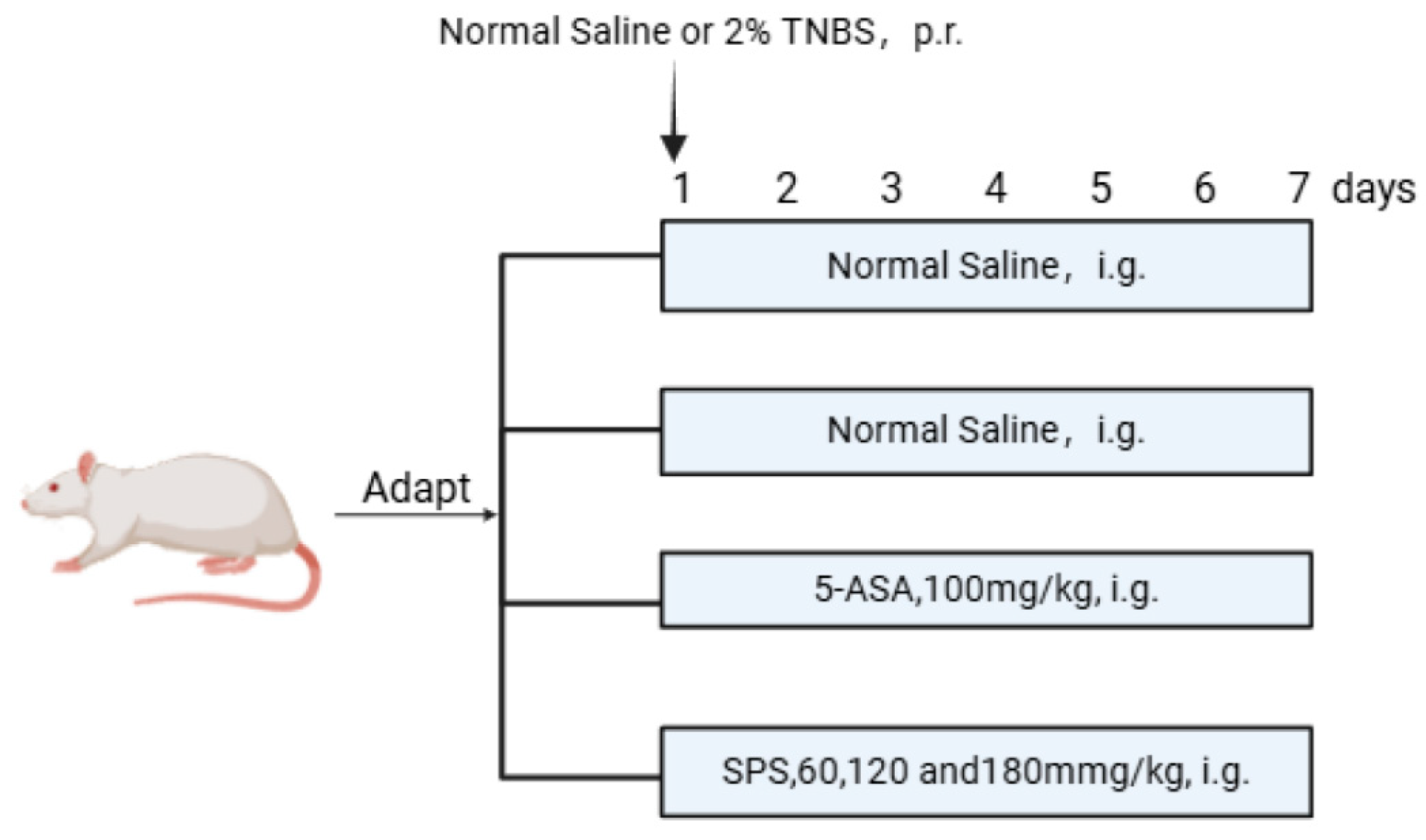
2.4. Establishment of the Rat Colitis Model and Grouping Treatment
2.5. Assessment of Disease Activity Index (DAI)
2.6. Histopathological Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Real-Time Quantitative PCR (qRT-PCR) Analysis
2.9. Western Blot (WB) Analysis
2.10. Flow Cytometry
2.11. Determination of Myeloperoxidase (MPO) Activity
2.12. Cell Culture and Co-Culture
2.13. siRNA Transfection
2.14. Cell Viability Assay
2.15. Statistical Analysis
3. Results
3.1. Physicochemical Properties of SPS
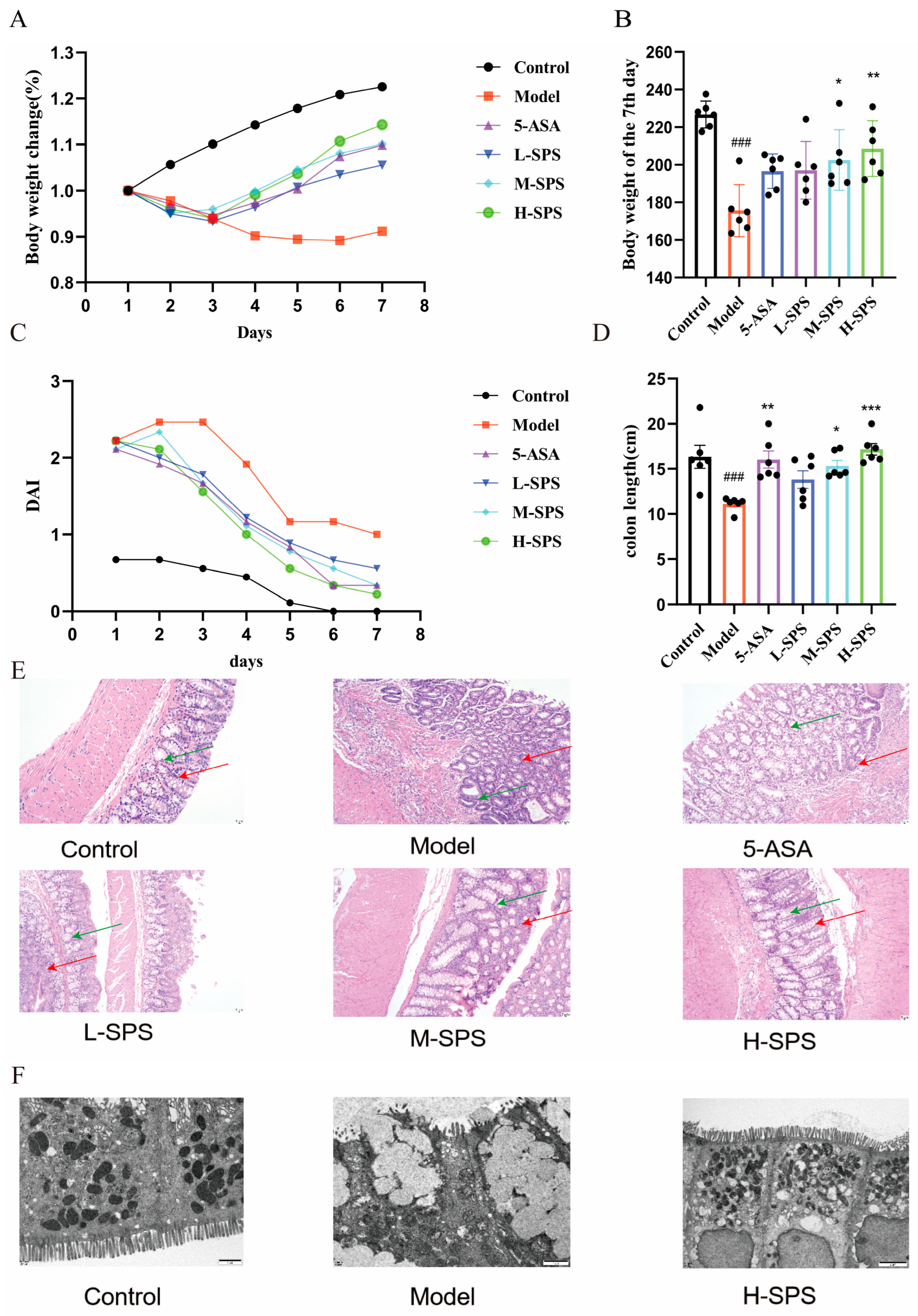
3.2. SPS Ameliorates TNBS-Induced Colitis in Rats
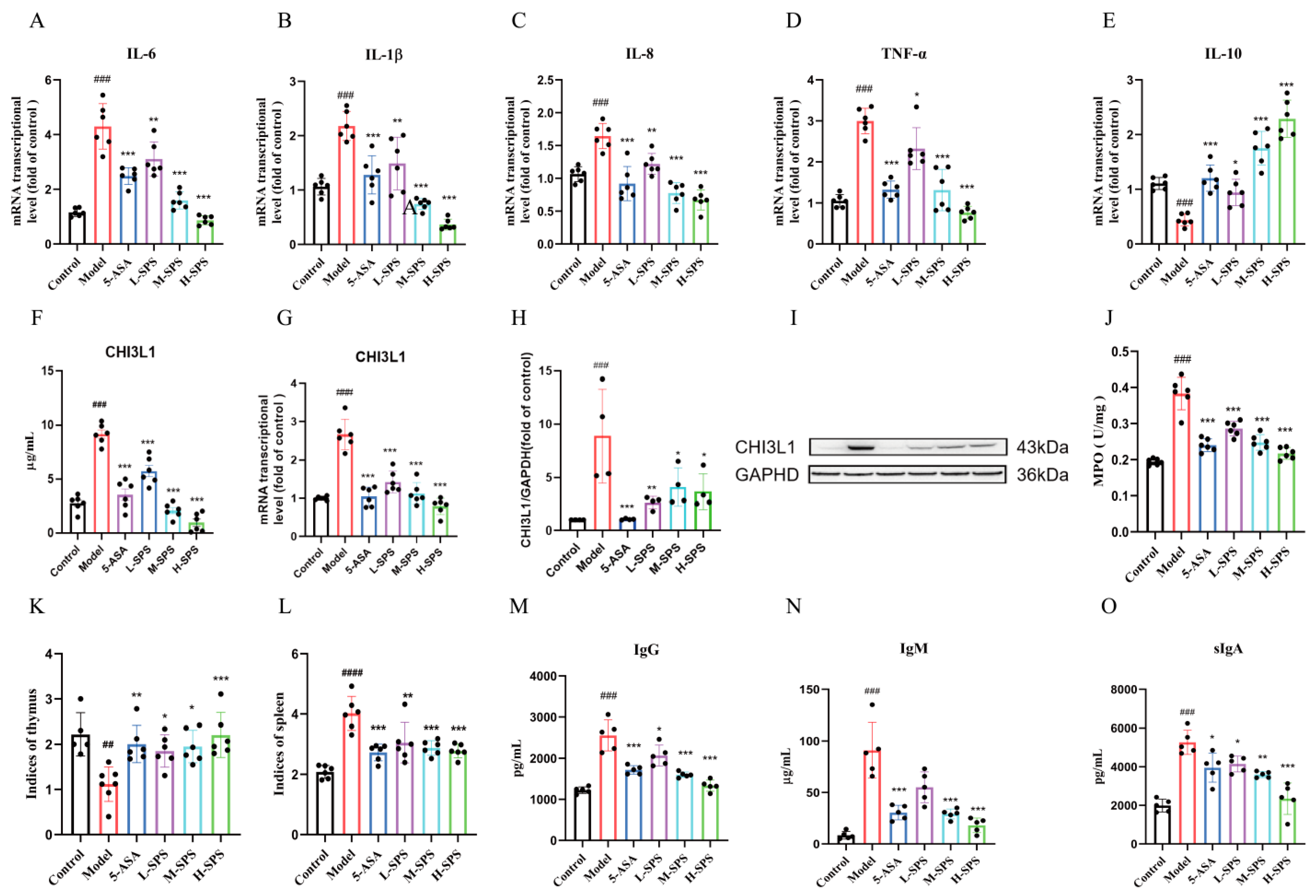
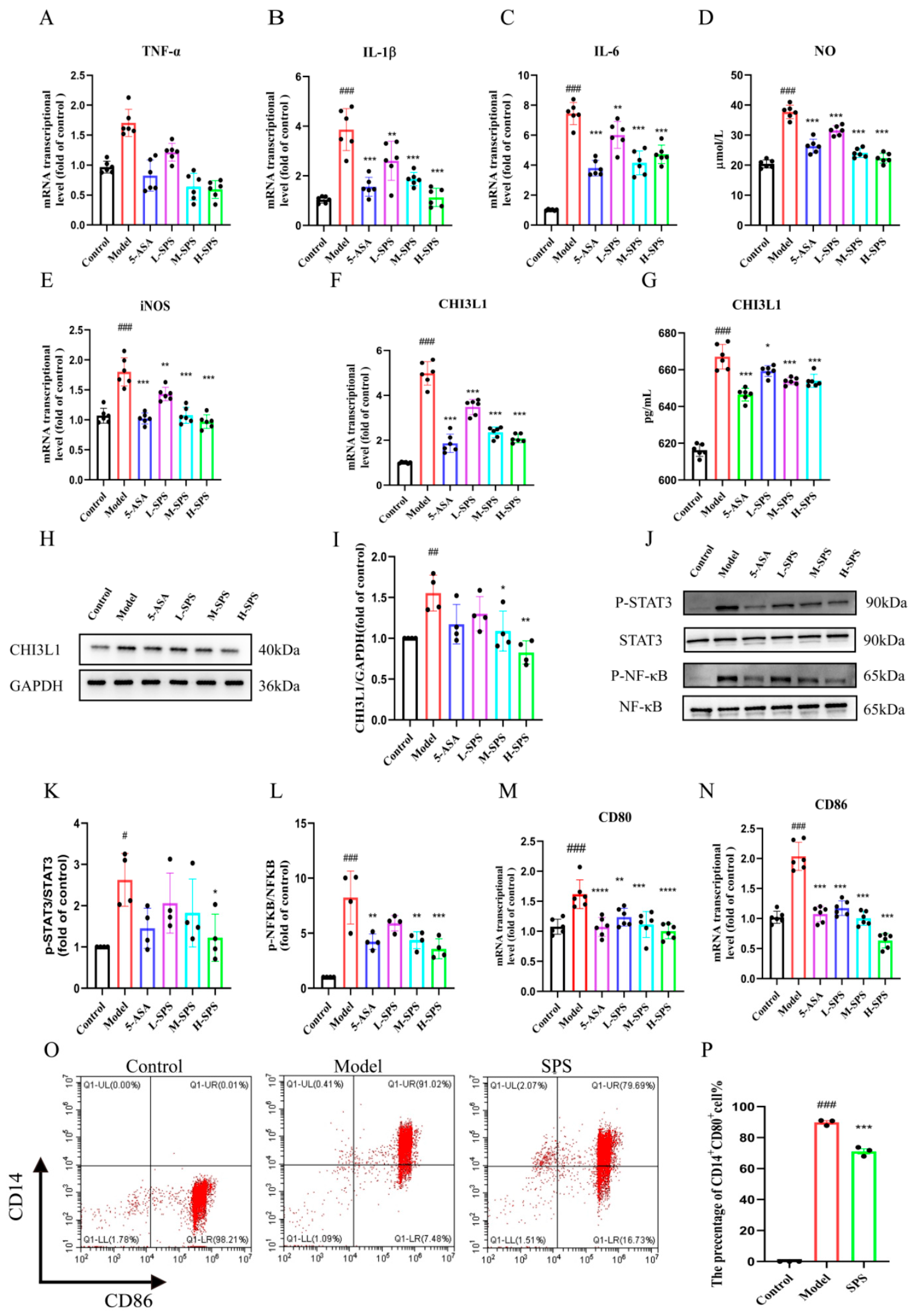
3.3. SPS Alleviates Colonic Inflammation and Modulates Systemic Immunity in Colitis Rats
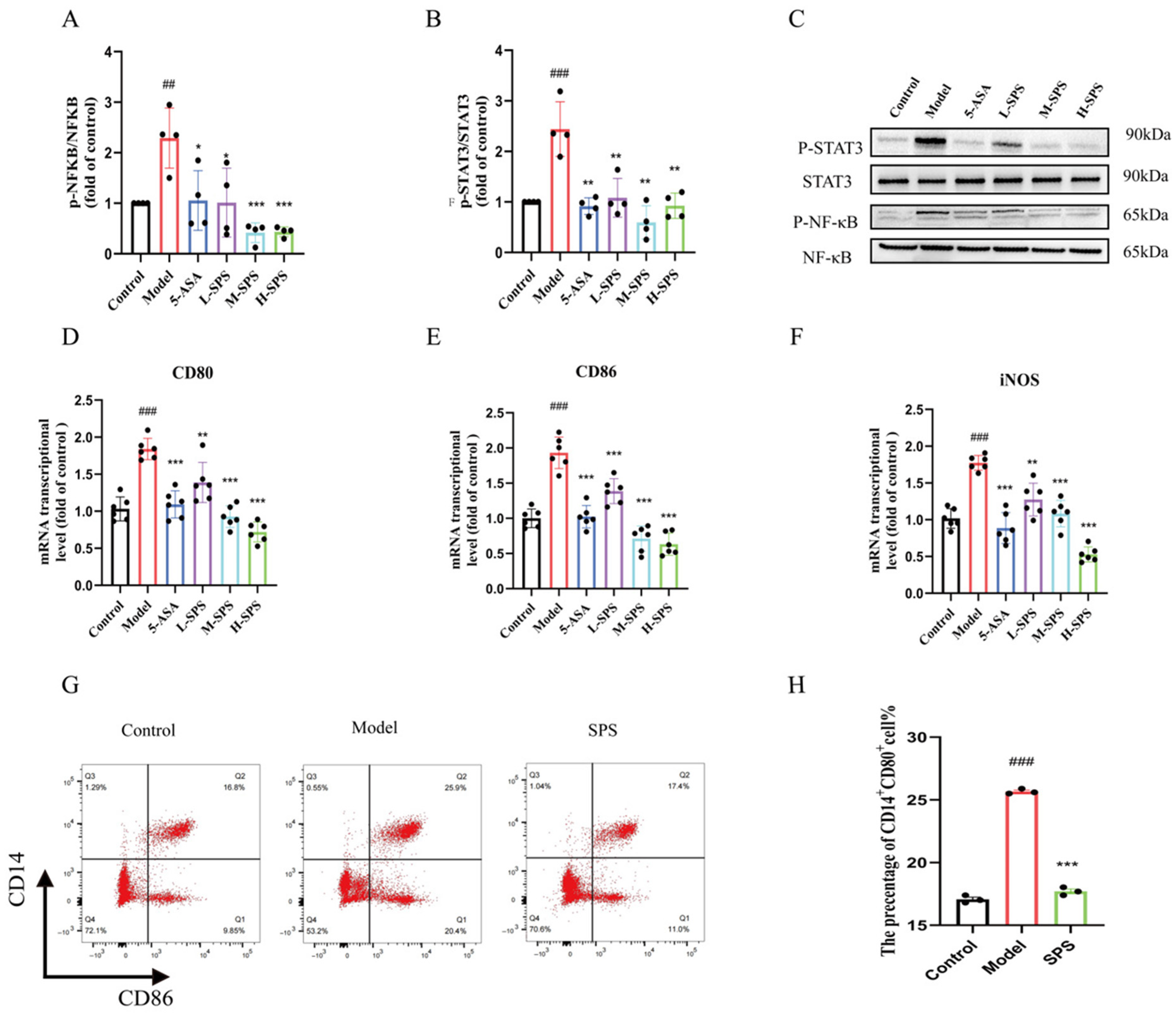
3.4. SPS Regulates Macrophage M1 Polarization by Inhibiting the STAT3/NF-κB Pathway in Colitis Rats
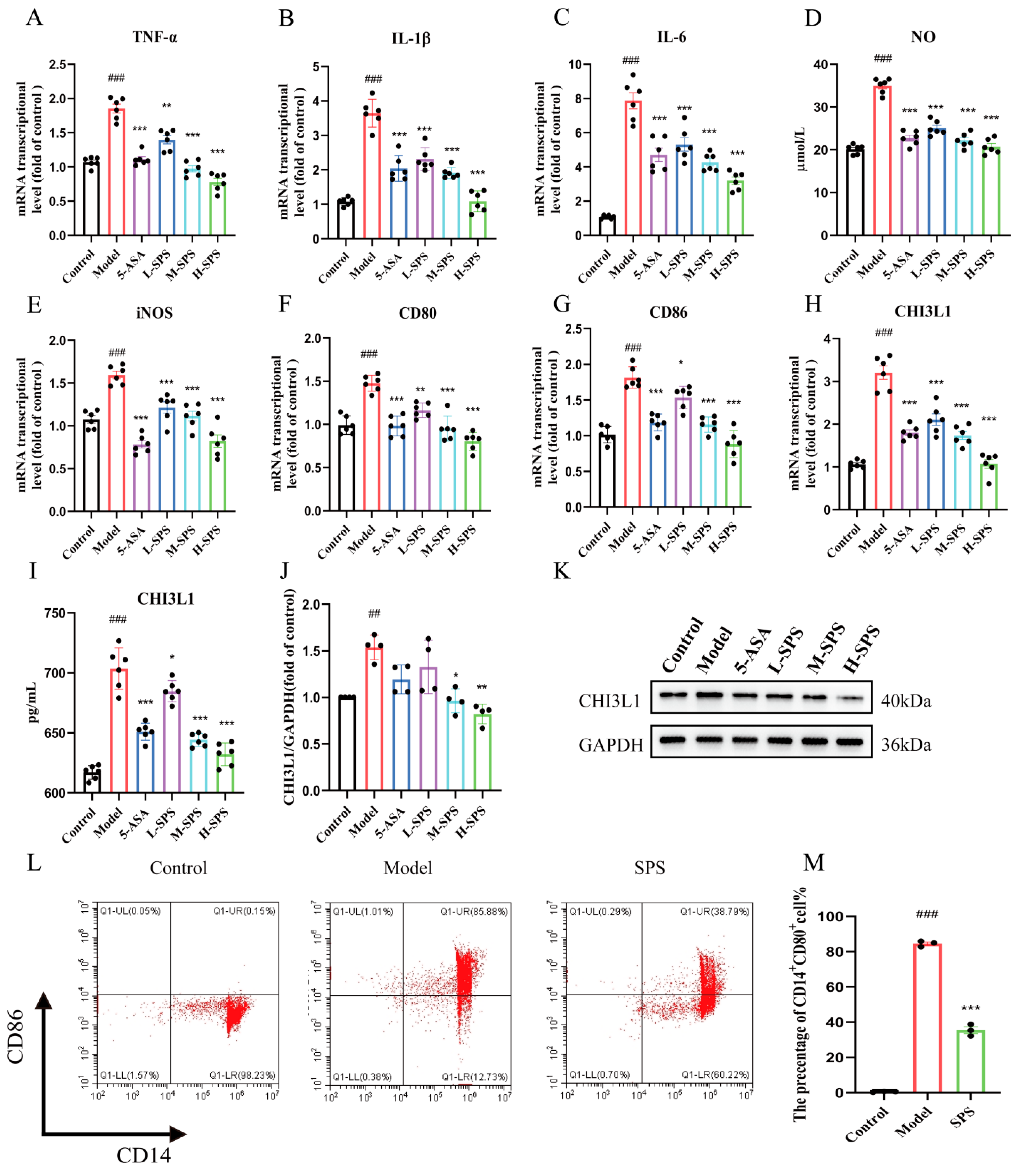
3.5. The Anti-Inflammatory Effects of SPS on Caco-2 Cells
3.6. The Regulation of SPS on LPS-Induced M1 Polarization in THP-1 Cells
3.7. Effect of Inflammatory Damage in Caco-2 Cells on M1 Polarization of Macrophages
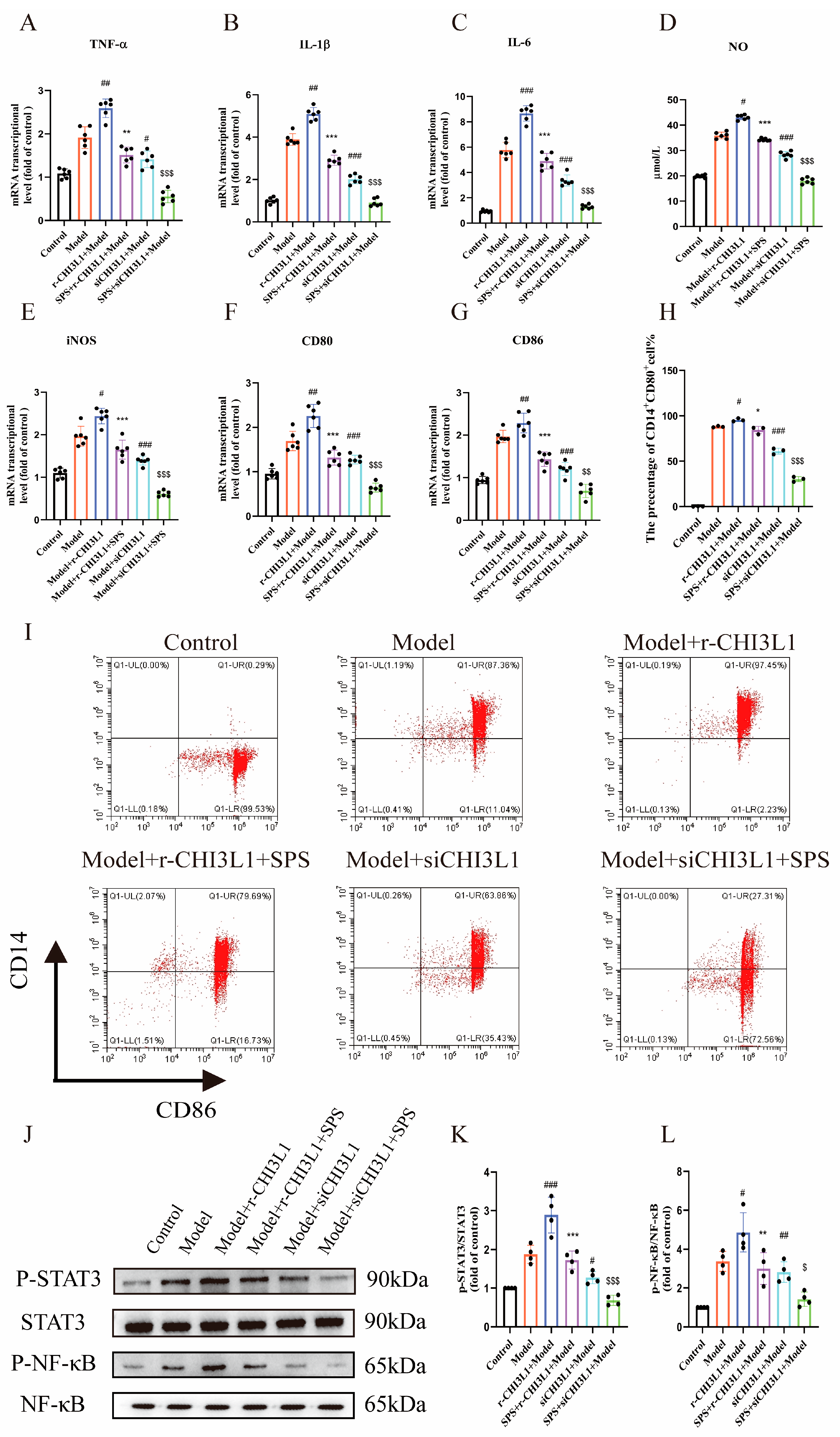
3.8. The Role of CHI3L1 in M1 Macrophage Polarization Induced by Inflammatory-Damaged Caco-2 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q.; Li, S.; Lin, X.; Yang, S.; Zhu, R.; Fu, C.; Zhang, Z. Harnessing nature’s pharmacy: Investigating natural compounds as novel therapeutics for ulcerative colitis. Front. Pharmacol. 2024, 15, 1394124. [Google Scholar] [CrossRef]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef]
- Wei, F.H.; Xie, W.Y.; Zhao, P.S.; Gao, W.; Gao, F. Echinacea purpurea Polysaccharide Ameliorates Dextran Sulfate Sodium-Induced Colitis by Restoring the Intestinal Microbiota and Inhibiting the TLR4-NF-κB Axis. Nutrients 2024, 16, 1305. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, Q.; Kang, Z.; Huang, J.; Ge, W.; Wan, Q.; Wang, H.; Zhou, W.; Zhao, H.; Liu, D. Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of Tfh/Treg cells. Int. Immunopharmacol. 2022, 111, 109108. [Google Scholar] [CrossRef]
- Ando, I.; Tsukumo, Y.; Wakabayashi, T.; Akashi, S.; Miyake, K.; Kataoka, T.; Nagai, K. Safflower polysaccharides activate the transcription factor NF-kappa B via Toll-like receptor 4 and induce cytokine production by macrophages. Int. Immunopharmacol. 2002, 2, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, J.; Zhang, C.; Zhang, Y.; Wang, R.; Li, X.; Zhang, S. Safflower polysaccharide inhibits the development of tongue squamous cell carcinoma. World J. Surg. Oncol. 2018, 16, 167. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Jia, M.; Lu, D.; Zhang, H.W.; Huang, D.; Liu, S.H.; Lv, C. Safflower Polysaccharide Inhibits AOM/DSS-Induced Mice Colorectal Cancer Through the Regulation of Macrophage Polarization. Front. Pharmacol. 2021, 12, 761641. [Google Scholar] [CrossRef]
- Lin, D.; Xu, C.J.; Liu, Y.; Zhou, Y.; Xiong, S.L.; Wu, H.C.; Deng, J.; Yi, Y.W.; Qiao, M.F.; Xiao, H.; et al. Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L. Polymers 2022, 14, 3510. [Google Scholar] [CrossRef]
- Qi, M.; Chu, S.; Wang, W.; Fu, X.; Jiang, C.; Zhang, L.; Ali, M.H.; Lu, Y.; Jia, M.; Ubul, D.; et al. Safflower polysaccharide ameliorates acute ulcerative colitis by regulating STAT3/NF-κB signaling pathways and repairing intestinal barrier function. Biomed. Pharmacother. 2024, 174, 116553. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Goto, R.; Fujimoto, M.; Okada, N.; Hardt, W.D. The Bactericidal Lectin RegIIIβ Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host Microbe 2017, 21, 195–207. [Google Scholar] [CrossRef]
- Kamioka, M.; Goto, Y.; Nakamura, K.; Yokoi, Y.; Sugimoto, R.; Ohira, S.; Kurashima, Y.; Umemoto, S.; Sato, S.; Kunisawa, J.; et al. Intestinal commensal microbiota and cytokines regulate Fut2+ Paneth cells for gut defense. Proc. Natl. Acad. Sci. USA 2022, 119, e2115230119. [Google Scholar] [CrossRef]
- Yoshida, M.; Arzili, R.; Nikolić, M.Z. Immune-epithelial cell interactions in lung development, homeostasis and disease. Int. J. Biochem. Cell Biol. 2025, 178, 106703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, C.; Li, H.B. The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases. Sci. China Life Sci. 2024, 67, 41–50. [Google Scholar] [CrossRef]
- Takahashi, D.; Kimura, S.; Hase, K. Intestinal immunity: To be, or not to be, induced? That is the question. Int. Immunol. 2021, 33, 755–759. [Google Scholar] [CrossRef]
- Adams, R.C.; MacDonald, K.P.A.; Hill, G.R. The contribution of the monocyte-macrophage lineage to immunotherapy outcomes. Blood 2025, 145, 1010–1021. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C.H. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019, 95, 760–773. [Google Scholar] [CrossRef]
- Cho, H.; Kwon, H.Y.; Lee, S.H.; Lee, H.G.; Kang, N.Y.; Chang, Y.T. Development of a Fluorescent Probe for M2 Macrophages via Gating-Oriented Live-Cell Distinction. J. Am. Chem. Soc. 2023, 145, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Kwong, C.H.T.; Gao, C.; Wei, J.; Yue, L.; Zhang, J.; Ye, R.D.; Wang, R. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics 2020, 10, 10106–10119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Wang, Y.; Chen, X.; Wang, C.; Chen, X.; Yuan, X.; Liu, L.; Yang, J.; Zhou, X. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Yoo, S.S.; Kim, S.H.; Son, D.J.; Yun, J.; Han, S.B.; Hong, J.T. Induction of ER stress-mediated apoptosis through SOD1 upregulation by deficiency of CHI3L1 inhibits lung metastasis. Theranostics 2023, 13, 2693–2709. [Google Scholar] [CrossRef]
- Yang, P.S.; Yu, M.H.; Hou, Y.C.; Chang, C.P.; Lin, S.C.; Kuo, I.Y.; Su, P.C.; Cheng, H.C.; Su, W.C.; Shan, Y.S.; et al. Targeting protumor factor chitinase-3-like-1 secreted by Rab37 vesicles for cancer immunotherapy. Theranostics 2022, 12, 340–361. [Google Scholar] [CrossRef]
- Mizoguchi, E.; Sadanaga, T.; Nanni, L.; Wang, S.; Mizoguchi, A. Recently Updated Role of Chitinase 3-like 1 on Various Cell Types as a Major Influencer of Chronic Inflammation. Cells 2024, 13, 678. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.; Hu, X.; Wang, J. Structural characterization and anti-inflammatory activity of a polysaccharide from the lignified okra. Carbohydr. Polym. 2021, 265, 118081. [Google Scholar] [CrossRef]
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Ye, Q.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269, 118253. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Xu, L.; Du, B.; Song, L. Purification, Characterization and Bioactivities of Polysaccharides Extracted from Safflower (Carthamus tinctorius L.). Molecules 2023, 28, 596. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, X.; Ai, J.; Zhang, C.; Liu, N.; Gao, W. Extraction, Structures, Bioactivities and Structure-Function Analysis of the Polysaccharides From Safflower (Carthamus tinctorius L.). Front. Pharmacol. 2021, 12, 767947. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhu, J.; Li, Y.; Yue, X.; Peng, Y. Almond polysaccharides inhibit DSS-induced inflammatory response in ulcerative colitis mice through NF-κB pathway. Int. J. Biol. Macromol. 2024, 281, 136206. [Google Scholar] [CrossRef]
- Huo, Z.; Li, J.; Li, X.; Xiao, H.; Lin, Y.; Ma, Y.; Li, J.; Yang, H.; Zhang, C. Functional fractions of Astragalus polysaccharides as a potential prebiotic to alleviate ulcerative colitis. Int. J. Biol. Macromol. 2024, 271, 132580. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wang, Y.J.; Bao, Q.W.; Qin, Y.M.; Li, P.P.; Wu, Q.Q.; Xia, C.K.; Wu, D.L.; Xie, S.Z. Polygonatum cyrtonema Hua polysaccharide alleviates ulcerative colitis via gut microbiota-independent modulation of inflammatory immune response. Carbohydr. Polym. 2025, 356, 123387. [Google Scholar] [CrossRef]
- Hu, J.; Mei, Y.; Zhang, H.; Li, J.; Zhang, M.; Li, Y.; Yang, W.; Liu, Y.; Liang, Y. Ameliorative effect of an acidic polysaccharide from Phellinus linteus on ulcerative colitis in a DSS-induced mouse model. Int. J. Biol. Macromol. 2024, 265, 130959. [Google Scholar] [CrossRef]
- Xie, Q.; Li, H.; Ma, R.; Ren, M.; Li, Y.; Li, J.; Chen, H.; Chen, Z.; Gong, D.; Wang, J. Effect of Coptis chinensis franch and Magnolia officinalis on intestinal flora and intestinal barrier in a TNBS-induced ulcerative colitis rats model. Phytomedicine 2022, 97, 153927. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Bioley, G.; Monnerat, J.; Lötscher, M.; Vonarburg, C.; Zuercher, A.; Corthésy, B. Plasma-Derived Polyreactive Secretory-Like IgA and IgM Opsonizing Salmonella enterica Typhimurium Reduces Invasion and Gut Tissue Inflammation through Agglutination. Front. Immunol. 2017, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chen, H.; Shu, W.; Sun, M.; Fang, L.; Shi, Y.; Pang, Z.; Wu, W.; Liu, Z. Clinical significance of soluble immunoglobulins A and G and their coated bacteria in feces of patients with inflammatory bowel disease. J. Transl. Med. 2018, 16, 359. [Google Scholar] [CrossRef]
- Grönwall, C.; Silverman, G.J. Natural IgM: Beneficial autoantibodies for the control of inflammatory and autoimmune disease. J. Clin. Immunol. 2014, 34 (Suppl. S1), S12–S21. [Google Scholar] [CrossRef]
- Mahida, Y.R.; Rolfe, V.E. Host-bacterial interactions in inflammatory bowel disease. Clin. Sci. 2004, 107, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kong, Y.; Chen, N.; Peng, W.; Zi, R.; Jiang, M.; Zhu, J.; Wang, Y.; Yue, J.; Lv, J.; et al. Identification of Immune-Related Gene Signature and Prediction of CeRNA Network in Active Ulcerative Colitis. Front. Immunol. 2022, 13, 855645. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Y.; Fu, R.; Liu, S.; Li, H.; Shen, T. Atox1 regulates macrophage polarization in intestinal inflammation via ROS-NLRP3 inflammasome pathway. J. Transl. Med. 2024, 22, 497. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, W.; Zou, Y.; Jiao, C.; Zhu, J.; Xu, Q.; Zou, J.; Sun, Y.; Guo, W. Allosterically activating SHP2 by oleanolic acid inhibits STAT3-Th17 axis for ameliorating colitis. Acta Pharm. Sin. B 2024, 14, 2598–2612. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pan, X.; Mao, L.; Pan, H.; Xu, W.; Hu, Y.; Yu, X.; Chen, Z.; Qian, S.; Ye, Y.; et al. Phospho-Tyr705 of STAT3 is a therapeutic target for sepsis through regulating inflammation and coagulation. Cell Commun. Signal. 2020, 18, 104. [Google Scholar] [CrossRef]
- Xu, S.; Deng, K.Q.; Lu, C.; Fu, X.; Zhu, Q.; Wan, S.; Zhang, L.; Huang, Y.; Nie, L.; Cai, H.; et al. Interleukin-6 classic and trans-signaling utilize glucose metabolism reprogramming to achieve anti- or pro-inflammatory effects. Metabolism 2024, 155, 155832. [Google Scholar] [CrossRef]

| Score | Loss of Body Weight (%) | Loose Stools | Bloody Stools |
|---|---|---|---|
| 0 | <1 | olid and granular | negative expression of occult blood |
| 1 | 1–5 | soft and granular | weakly positive expression of occult blood |
| 2 | 5–10 | semi formed loose stool | positive expression of occult blood |
| 3 | 10–15 | unformed loose stool | strongly positive expression of occult blood |
| 4 | ≥15 | had sign of liquid | strongly positive and visible blood |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| IL-6(R) | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| IL-10(R) | CGGGAAGACAATAACTGCACCC | CGGTTAGCAGTATGTTGTCCAGC |
| IL-1β(R) | TTCGACACATGGGATAACGAGG | TTTTTGCTGTGAGTCCCGGAG |
| iNOS(R) | GGAGGACCACCTCTATCAGG | CCTGAACGTAGACCTTGGGT |
| CD80(R) | CCTCAAGTTTCCATGTCCAAGGC | GAGGAGAGTTGTAACGGCAAGG |
| CD86(R) | ACGTATTGGAAGGAGATTACAGCT | TCTGTCAGCGTTACTATCCCGC |
| TNF-α(R) | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| CHI3L1(R) | CCACAGTCCATAGAATCCTCGG | TGCCTGTCCTTCAGGTACTGCA |
| IL-8(R) | CAGGCTTCCTTGTGCAAGTG | TCGAAAGCTGCTATTTCACAG |
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| IL-6(H) | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-10(H) | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| IL-1β(H) | CTCAACTGTGAAATGCCACC | GAGTGATACTGCCTGCCTGA |
| iNOS(H) | CAGAAGTCAAAGTCTCAGACAT | GTCATCTTGTATTGTTGGGCT |
| CD80(H) | CCTCAAGTTTCCATGTCCAAGGC | GAGGAGAGTTGTAACGGCAAGG |
| CD86(H) | ACGTATTGGAAGGAGATTACAGCT | TCTGTCAGCGTTACTATCCCGC |
| TNF-α(H) | CCGAGTCTGGGCAGGTCTA | GTCTGAAGGAGGGGGTAAT |
| CHI3L1(H) | CCACAGTCCATAGAATCCTCGG | TGCCTGTCCTTCAGGTACTGCA |
| TGF-β(H) | CAGGCTTCCTTGTGCAAGTG | TCGAAAGCTGCTATTTCACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Zhu, F.; Aimaier, S.; Zhang, L.; Ali, M.H.; Fan, F.; Lu, Y.; Jia, M.; Wu, D.; Yin, H.; et al. Safflower Polysaccharides Alleviate TNBS-Induced Colitis by Modulating Gut Immunity. Foods 2025, 14, 3199. https://doi.org/10.3390/foods14183199
Jiang C, Zhu F, Aimaier S, Zhang L, Ali MH, Fan F, Lu Y, Jia M, Wu D, Yin H, et al. Safflower Polysaccharides Alleviate TNBS-Induced Colitis by Modulating Gut Immunity. Foods. 2025; 14(18):3199. https://doi.org/10.3390/foods14183199
Chicago/Turabian StyleJiang, Chao, Furong Zhu, Shabaaiti Aimaier, Liang Zhang, Md Hasan Ali, Furong Fan, Yating Lu, Mengwei Jia, Dongsen Wu, Haipeng Yin, and et al. 2025. "Safflower Polysaccharides Alleviate TNBS-Induced Colitis by Modulating Gut Immunity" Foods 14, no. 18: 3199. https://doi.org/10.3390/foods14183199
APA StyleJiang, C., Zhu, F., Aimaier, S., Zhang, L., Ali, M. H., Fan, F., Lu, Y., Jia, M., Wu, D., Yin, H., Wei, J., Chu, S., & Liu, M. (2025). Safflower Polysaccharides Alleviate TNBS-Induced Colitis by Modulating Gut Immunity. Foods, 14(18), 3199. https://doi.org/10.3390/foods14183199






