Optimization of Its Preparation and Evaluation of Fresh and Tender Leaves of Artemisia argyi for Food Usage
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation and Optimization of Edible Raw Materials Derived from the Tender Leaves of A. argyi
2.3. Characteristic Evaluation
2.3.1. Sensory Evaluation
2.3.2. Chlorophyll Detection
2.3.3. Extraction and Determination of Flavonoids and Polyphenols
2.3.4. Volatile Oil Content and Composition Analysis
2.4. Nutritional Composition Analysis
2.5. Acute Oral Toxicity Test
2.5.1. Weight and Toxic Symptoms
2.5.2. Hematology and Biochemical Analysis
2.5.3. Histological Examination
2.6. Secondary Metabolomics Analysis
2.7. 16S rRNA Sequencing
2.8. Statistical Analysis
3. Results
3.1. The Effect of Boiling Treatment on the Quality Evaluation of A. argyi
3.1.1. Sensory Evaluation and Chlorophyll Content
3.1.2. Detection of Active Ingredients and Pharmacological Substances
3.2. The Effect of Drying Treatment on the Quality Evaluation of A. argyi
3.3. The Nutritional Value for Edible Raw Materials of A. argyi
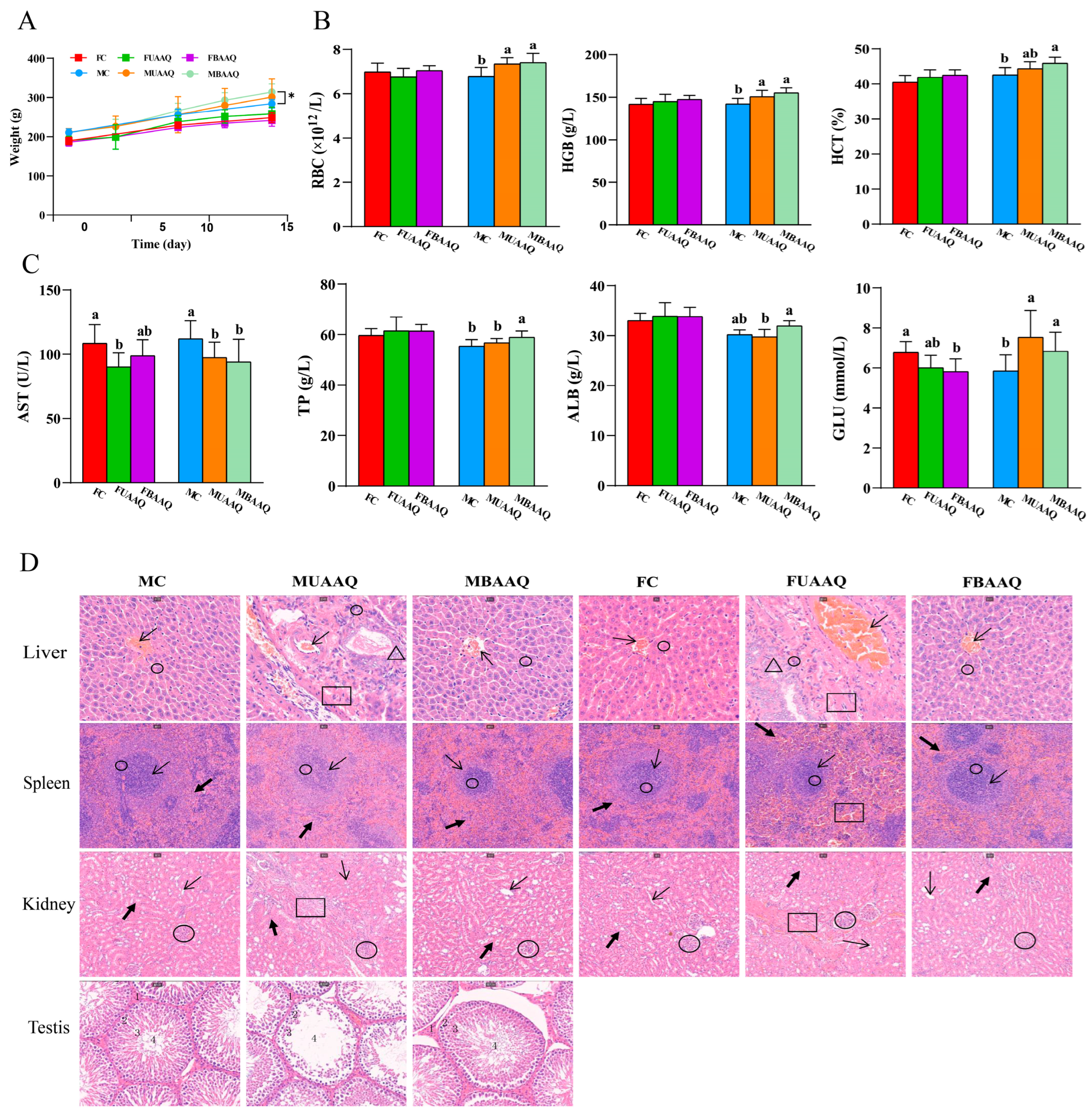
3.4. The Acute Toxicity of Edible Raw Materials of A. argyi in Rats
3.4.1. Effects on Body Weight and Organ Index
3.4.2. Effects on Hematology
3.4.3. Effects on Serum Biochemical Indexes
3.4.4. Histopathological Examination
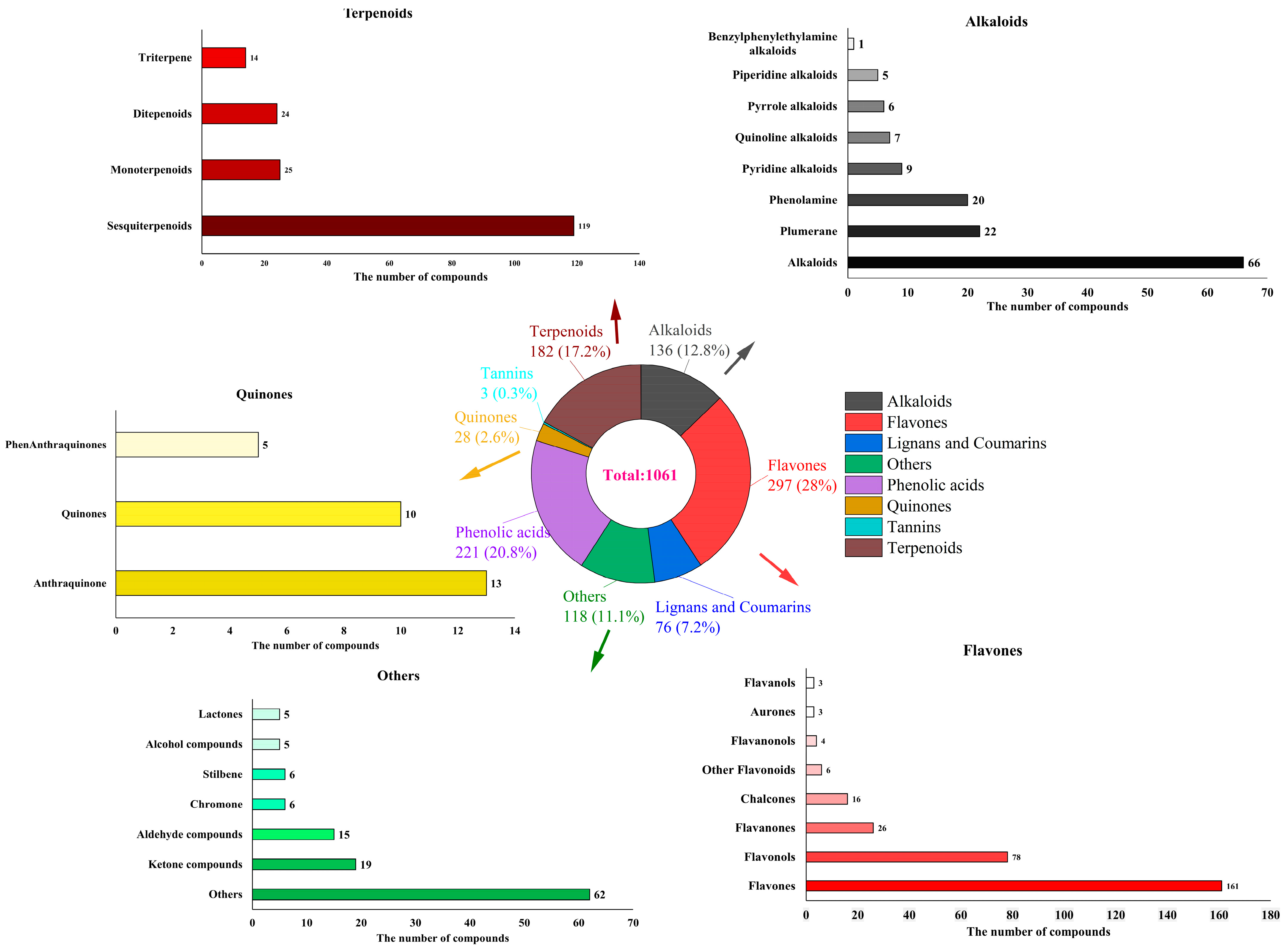
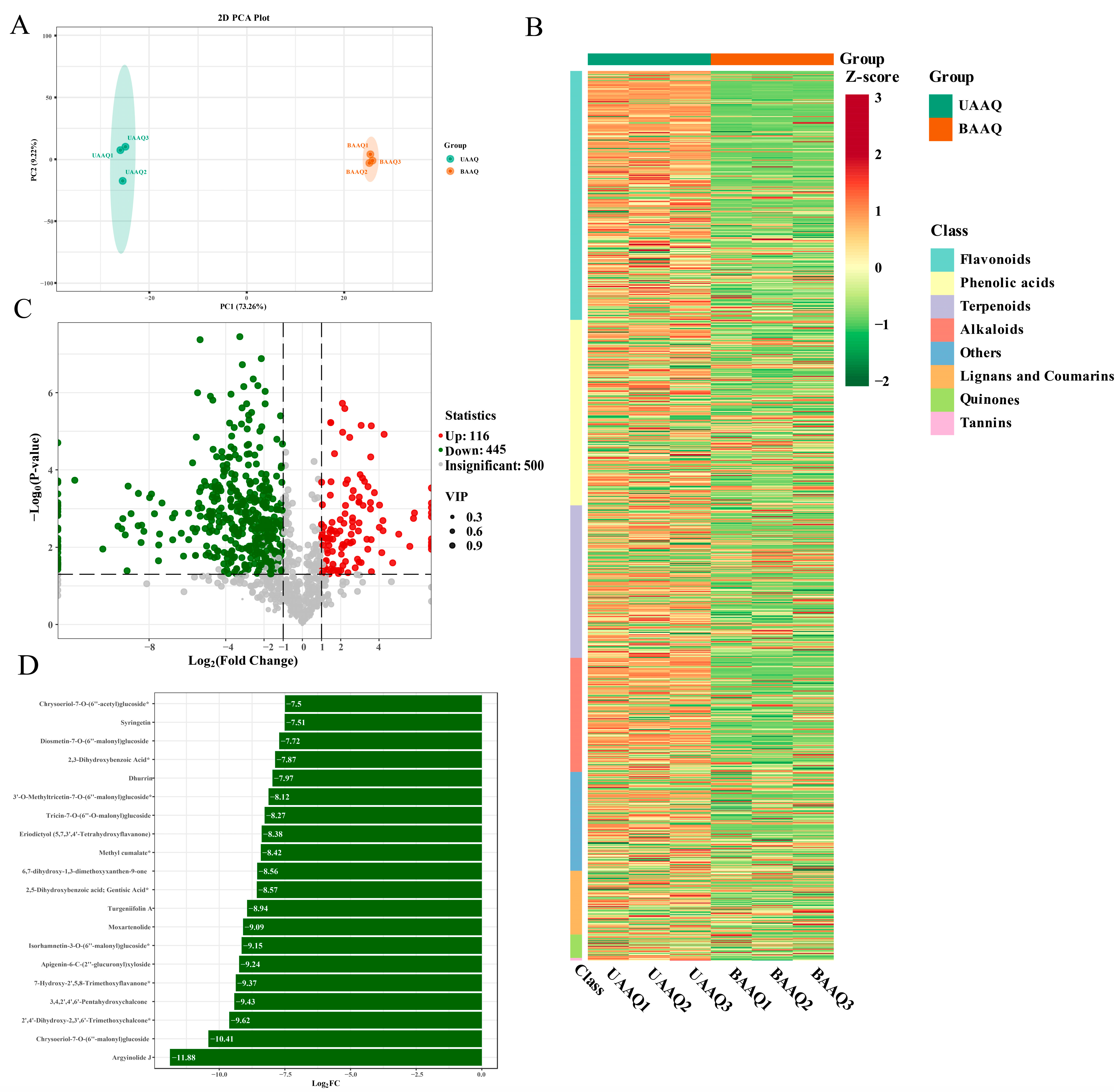
3.5. Secondary Metabolites Profiles in Fresh Tender Leaves of A. argyi
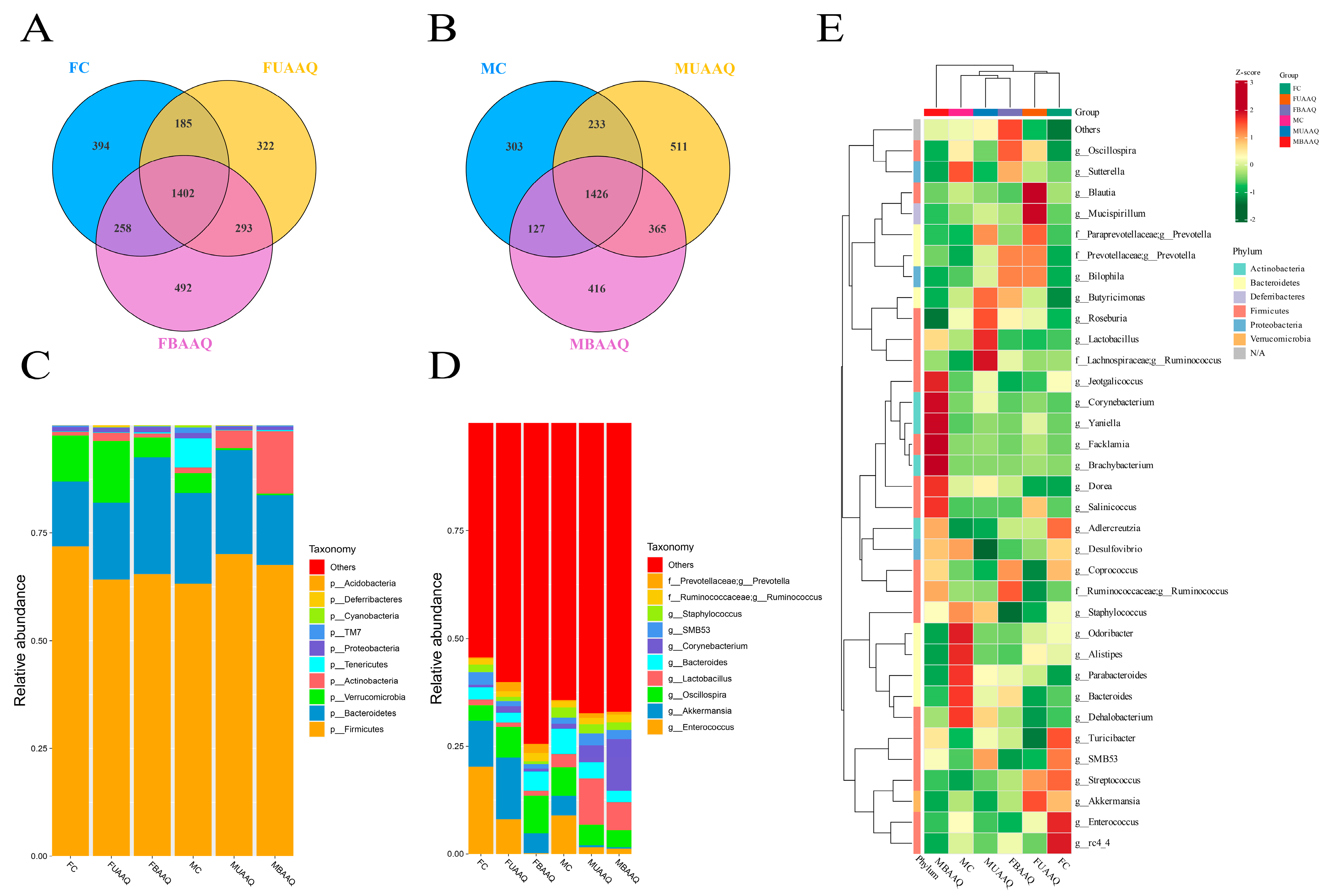
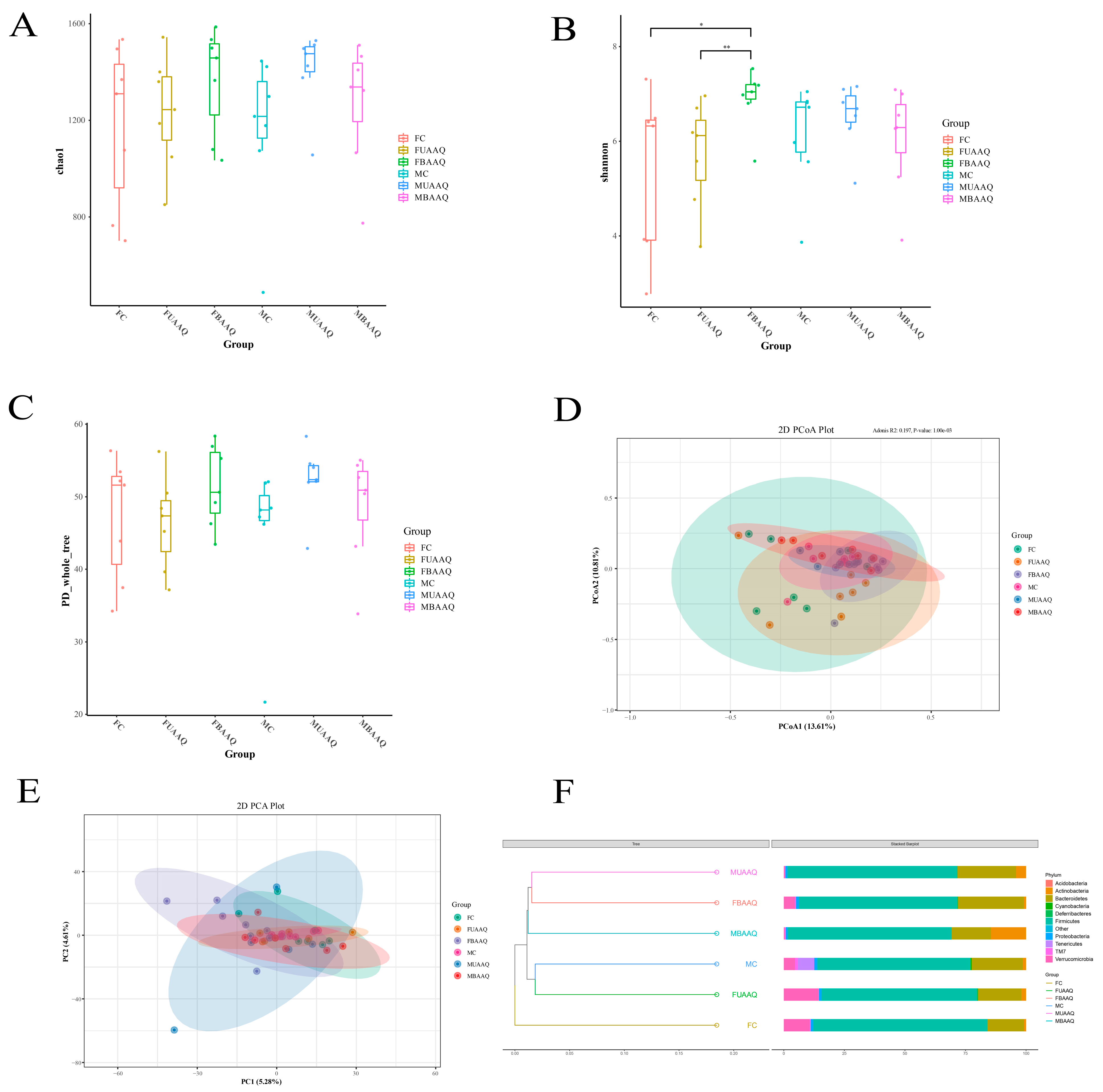
3.6. Edible Raw Materials of A. argyi Modulated Caecal Microbiota Community in Rats
3.6.1. Community Composition of the Caecal Microbiota in Rats
3.6.2. Microbial Diversity of the Caecal Contents in Rats
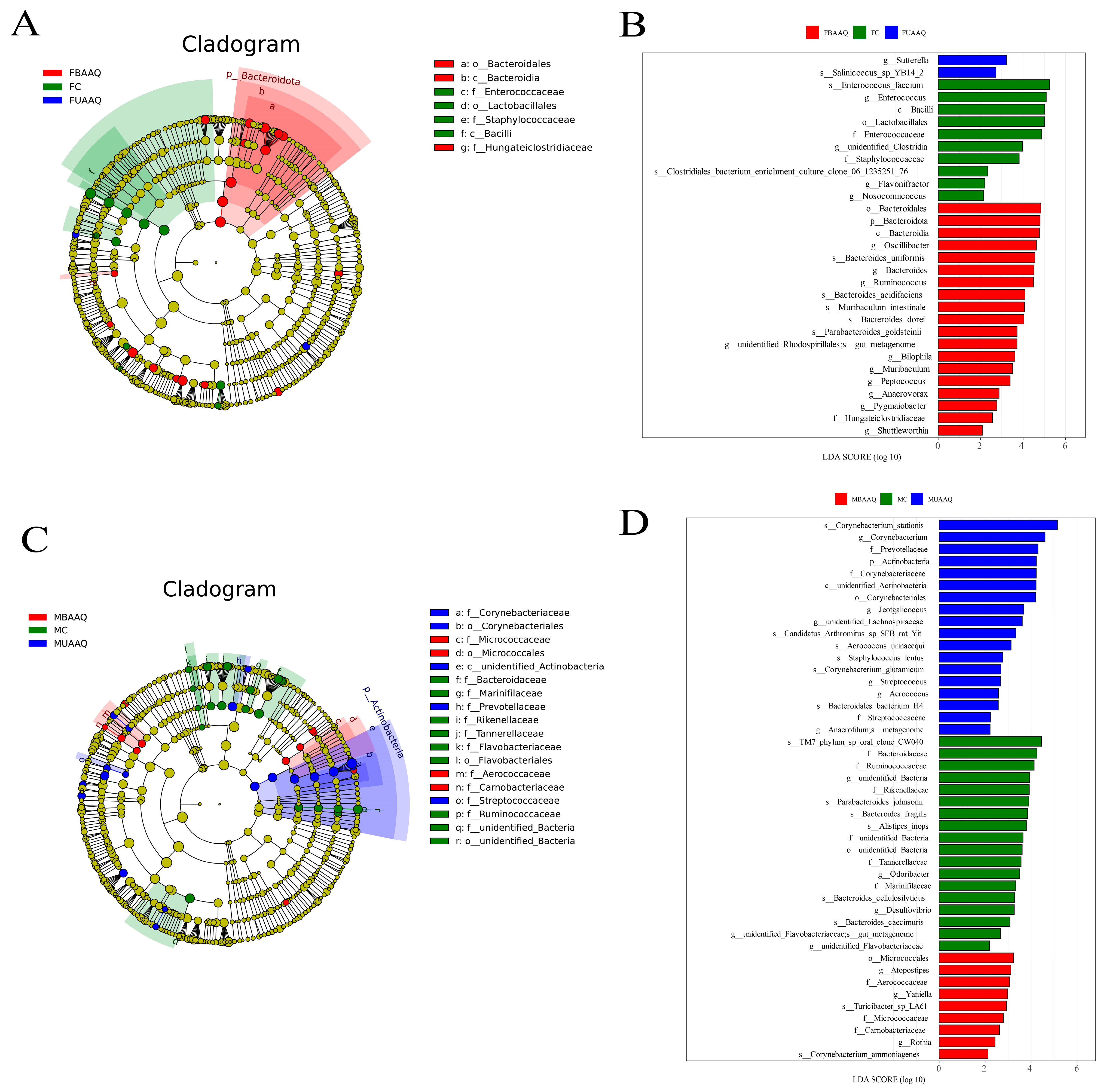
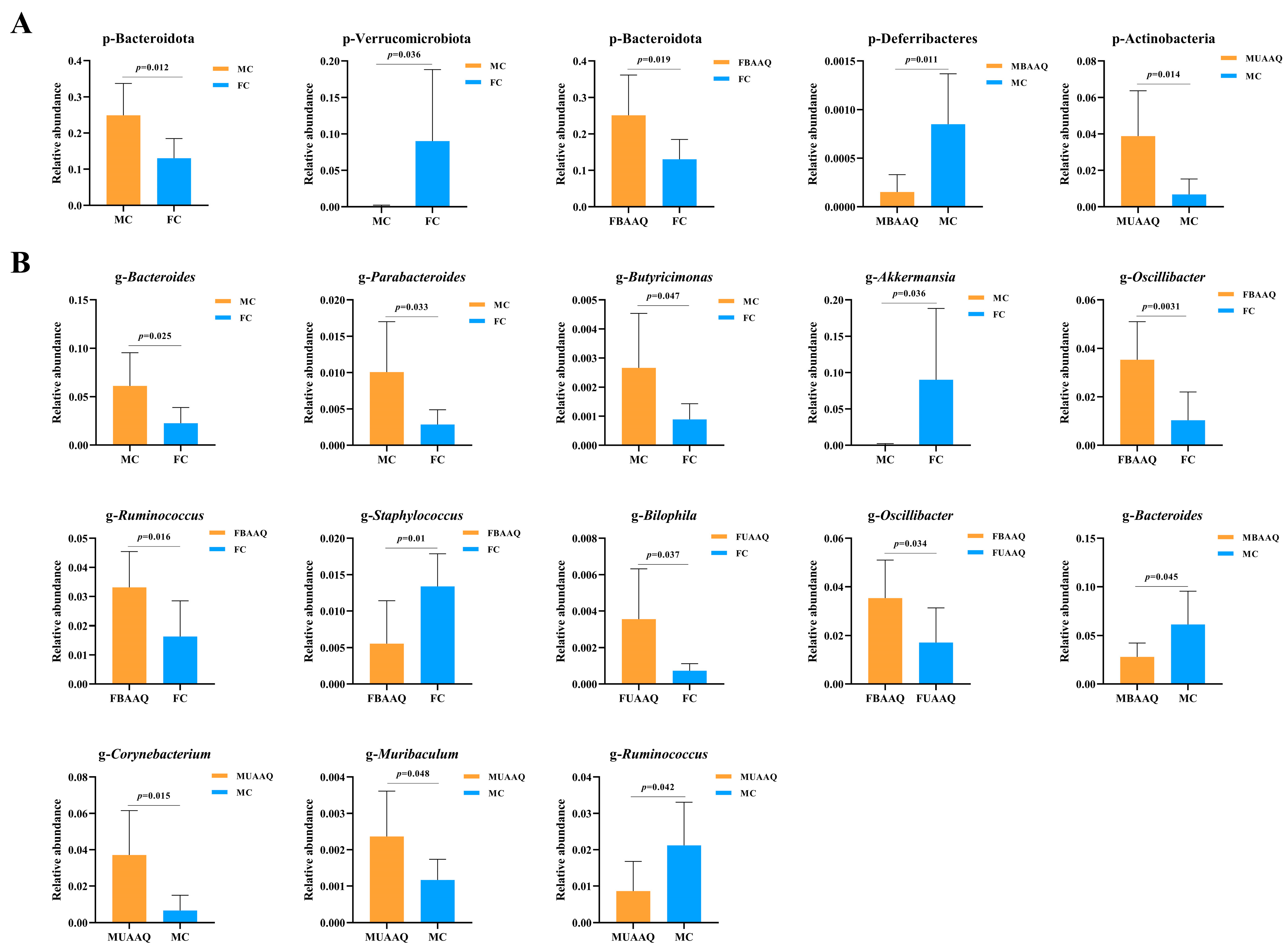
3.6.3. Analysis of Differential Caecal Microbiota in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, D.; Huang, N.N.; Du, X.W. Review of the Chemical Composition and Biological Activities of Essential Oils from Artemisia argyi, Artemisia princeps, and Artemisia montana. Curr. Top. Med. Chem. 2023, 23, 1522–1541. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Jung, Y.; Chun, W.; Yang, B.; Ryu, J.; Lim, C.; Kim, J.H.; Kim, H.; Cho, S.I. Anti-Inflammatory Effects of Artemisia Leaf Extract in Mice with Contact Dermatitis In Vitro and In Vivo. Mediat. Inflamm. 2016, 2016, 8027537. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, S.; Chang, J.; Zang, E.; Liu, Q.; Zhou, B.; Li, C.; Li, M.; Huang, X.; Zhang, Z.; et al. The pharmacophylogenetic relationships of two edible medicinal plants in the genus Artemisia. Front. Plant Sci. 2022, 13, 949743. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.; Huo, T.; Qin, X.; Qin, P.; Chen, G. Edible history tracing and safety investigation of Artemisia argyi Lévl. et Vant. cv. qiai. J. Anhui Agric. Sci. 2024, 52, 144–148. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1991; Volume 76. [Google Scholar]
- Institute of Health, Chinese Academy of Sciences. Food Composition Tables; People’s Health Publishing House: Beijing, China, 1981. [Google Scholar]
- Yang, Y. Chinese Food Composition Standard, 6th ed.; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Kim, J.K.; Shin, E.C.; Lim, H.J.; Choi, S.J.; Kim, C.R.; Suh, S.H.; Kim, C.J.; Park, G.G.; Park, C.S.; Kim, H.K.; et al. Characterization of Nutritional Composition, Antioxidative Capacity, and Sensory Attributes of Seomae Mugwort, a Native Korean Variety of Artemisia argyi H. Lév. & Vaniot. J. Anal. Methods Chem. 2015, 2015, 916346. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (Part I); China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Song, X.; Wen, X.; He, J.; Zhao, H.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Guan, X.; Ge, D.; Li, S.; Huang, K.; Liu, J.; Li, F. Chemical Composition and Antimicrobial Activities of Artemisia argyi Lévl. et Vant Essential Oils Extracted by Simultaneous Distillation-Extraction, Subcritical Extraction and Hydrodistillation. Molecules 2019, 24, 483. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Hu, Y.; Liu, T.; Guo, Y.; Sun, P.; Zheng, J.; Ren, Z.; Wang, Y. Antiviral activities of Artemisia vulgaris L. extract against herpes simplex virus. Chin. Med. 2023, 18, 21. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, H.J.; Chao, J.; Liu, J.F. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J. Ethnopharmacol. 2017, 204, 107–117. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, B.; Li, T.; Xu, Y.; Jin, X.; Guo, X.; Yan, S. Immunomodulatory effect of Artemisia argyi polysaccharide on peripheral blood leucocyte of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 939–946. [Google Scholar] [CrossRef]
- Han, H.M.; Kim, S.J.; Kim, J.S.; Kim, B.H.; Lee, H.W.; Lee, Y.T.; Kang, K.H. Ameliorative effects of Artemisia argyi Folium extract on 2,4-dinitrochlorobenzene-induced atopic dermatitis-like lesions in BALB/c mice. Mol. Med. Rep. 2016, 14, 3206–3214. [Google Scholar] [CrossRef]
- Zhang, P.F.; Shi, B.L.; Su, J.L.; Yue, Y.X.; Cao, Z.X.; Chu, W.B.; Li, K.; Yan, S.M. Relieving effect of Artemisia argyi aqueous extract on immune stress in broilers. J. Anim. Physiol. Anim. Nutr. 2017, 101, 251–258. [Google Scholar] [CrossRef]
- Kang, J.Y.; Park, S.K.; Guo, T.J.; Ha, J.S.; Lee, D.S.; Kim, J.M.; Lee, U.; Kim, D.O.; Heo, H.J. Reversal of Trimethyltin-Induced Learning and Memory Deficits by 3,5-Dicaffeoylquinic Acid. Oxid. Med. Cell Longev. 2016, 2016, 6981595. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Lee, D.S.; Park, S.K.; Ha, J.S.; Kim, J.M.; Ha, G.J.; Seo, W.T.; Heo, H.J. Cognitive Function of Artemisia argyi H. Fermented by Monascus purpureus under TMT-Induced Learning and Memory Deficits in ICR Mice. Evid. Based. Complement. Alternat. Med. 2017, 2017, 5809370. [Google Scholar] [CrossRef]
- Chen, C.J.; Luo, D.D.; Miao, Y.H.; Kang, L.P.; Guo, L.P.; Liu, D.H.; Huang, L.Q. Analysis and evaluation of volatile oil content in leaves of different Artemisia argyi germplasm resources. China J. Chin. Mater. Med. 2021, 46, 3814–3823. [Google Scholar] [CrossRef]
- Niu, J.; Yang, Y.; Zhao, L.; Cao, N.; Xiong, X.; Yun, Z.; Yang, F.; Xu, Y.; Tu, H.; Zhong, K.; et al. Sensory Lexicon Construction and Quantitative Descriptive Analysis of Jiang-Flavor Baijiu. Food Sci. Nutr. 2025, 13, e4652. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tsuji, H. Effects of calcium on chlorophyll synthesis and stability in the early phase of greening in cucumber cotyledons. Plant Physiol. 1980, 65, 1211–1215. [Google Scholar] [CrossRef]
- Zulqarnain, A.; Durrani, A.I.; Saleem, H.; Rubab, S. Development of an Ultrasonic-Assisted Extraction Technique for the Extraction of Natural Coloring Substance Chlorophyll from Leaves of Carica papaya. J. Oleo Sci. 2021, 70, 1367–1372. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Liu, Q.; Lin, Y.; Zhang, Z.; Li, S. Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves. Molecules 2022, 27, 2916. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, K.; George, E.; Sathyanarayanan, S.; George, B.P.; Abrahamse, H.; Thamburaj, S.; Thangaraj, P. Phenolics, tannins, flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats, India. Food Sci. Hum. Well 2019, 8, 73–81. [Google Scholar] [CrossRef]
- Dóka, O.; Ficzek, G.; Simon, G.; Zsófi, Z.; Villangó, S.; Végvári, G. Quantification of total polyphenol content in wine lees by conventional optical and photoacoustic spectroscopy. OENO One 2023, 57, 257–264. [Google Scholar] [CrossRef]
- GB 5009.3-2016; National Food Safety Standard—Determination of Moisture in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 12.
- GB 5009.4-2016; National Food Safety Standard—Determination of Ash in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 12.
- GB 5009.5-2016; National Food Safety Standard—Determination of Protein in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 12.
- GB 5009.6-2016; National Food Safety Standard—Determination of Fat in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 16.
- GB/T 5009.10-2003; Determination of Crude Fiber in Plant-based Foods. Ministry of Health of the People’s Republic of China: Beijing, China, 2003; p. 8.
- GB 5009.83-2016; National Food Safety Standard—Determination of Carotene in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 16.
- GB 5009.86-2016; National Food Safety Standard—Determination of Vitamin C in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 16.
- GB 5009.85-2016; National Food Safety Standard—Determination of Vitamin B2 in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 16.
- Lorenc, W.; Markiewicz, B.; Kruszka, D.; Kachlicki, P.; Barałkiewicz, D. Total Versus Inorganic and Organic Species of As, Cr, and Sb in Flavored and Functional Drinking Waters: Analysis and Risk Assessment. Molecules 2020, 25, 1099. [Google Scholar] [CrossRef] [PubMed]
- GB 15193.3-2014; National Food Safety Standard—Acute Oral Toxicity Test. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2014; p. 28.
- Gu, X.; Xu, G.; Liang, C.; Mektrirat, R.; Wang, L.; Zhang, K.; Meng, B.; Tang, X.; Wang, X.; Egide, H.; et al. Optimization of Fermentation Process of Zanthoxylum bungeanum Seeds and Evaluation of Acute Toxicity of Protein Extract in Mice. Foods 2024, 13, 4004. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, C.; Liu, H.; Hu, Y.; Zhou, Y.; Wu, W.; Qin, S. Polysaccharide extract from Rosa laevigata fruit attenuates inflammatory obesity by targeting redox balance and gut interface in high-fat diet-fed rats. Food Sci. Hum. Well. 2023, 12, 442–453. [Google Scholar] [CrossRef]
- Barbe, J.C.; Garbay, J.; Tempère, S. The Sensory Space of Wines: From Concept to Evaluation and Description. A Review. Foods 2021, 10, 1424. [Google Scholar] [CrossRef]
- Zámboriné Németh, É.; Thi Nguyen, H. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, J.; Wan, C.; Tan, J.; Wu, B.; Zhao, Z. Canonical correlation analysis of factors that influence quality of life among patients with chronic obstructive pulmonary disease based on QLICD-COPD (V2.0). BMJ Open Respir. Res. 2022, 9, e001192. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.E.; Camus, M.S.; Freeman, K.P.; Giori, L.; Hooijberg, E.H.; Jeffery, U.; Korchia, J.; Meindel, M.J.; Moore, A.R.; Sisson, S.C.; et al. ASVCP Guidelines: Principles of Quality Assurance and Standards for Veterinary Clinical Pathology (version 3.0): Developed by the American Society for Veterinary Clinical Pathology’s (ASVCP) Quality Assurance and Laboratory Standards (QALS) Committee. Vet. Clin. Pathol. 2019, 48, 542–618. [Google Scholar] [CrossRef]
- Zhuge, A.; Li, S.; Lou, P.; Wu, W.; Wang, K.; Yuan, Y.; Xia, J.; Li, B.; Li, L. Longitudinal 16S rRNA Sequencing Reveals Relationships among Alterations of Gut Microbiota and Nonalcoholic Fatty Liver Disease Progression in Mice. Microbiol. Spectr. 2022, 10, e0004722. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef]
- Chen, J.; Chen, F.; Peng, S.; Ou, Y.; He, B.; Li, Y.; Lin, Q. Effects of Artemisia argyi Powder on Egg Quality, Antioxidant Capacity, and Intestinal Development of Roman Laying Hens. Front. Physiol. 2022, 13, 902568. [Google Scholar] [CrossRef]
- Ruan, Y.; Niu, C.; Zhang, P.; Qian, Y.; Li, X.; Wang, L.; Ma, B. Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities. Gels 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Zhong, W.; Liang, F.; Guo, Y.; Wang, Y.; Wang, Z. Moisturizing and Antioxidant Effects of Artemisia argyi Essence Liquid in HaCaT Keratinocytes. Int. J. Mol. Sci. 2023, 24, 6809. [Google Scholar] [CrossRef]
- Shin, J.M.; Son, Y.J.; Ha, I.J.; Erdenebileg, S.; Jung, D.S.; Song, D.G.; Kim, Y.S.; Kim, S.M.; Nho, C.W. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement. Med. Ther. 2022, 22, 64. [Google Scholar] [CrossRef]
- Ma, Q.; Tan, D.; Gong, X.; Ji, H.; Wang, K.; Lei, Q.; Zhao, G. An Extract of Artemisia argyi Leaves Rich in Organic Acids and Flavonoids Promotes Growth in BALB/c Mice by Regulating Intestinal Flora. Animals 2022, 12, 1519. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Romyasamit, C.; Surachat, K.; Pattaranggoon, N.C.; Suksabay, P.; Permpoon, U.; Nam, T.G.; Sornsenee, P. Phenotypic and Genomic Insights into Schleiferilactobacillus harbinensis WU01, a Candidate Probiotic with Broad-Spectrum Antimicrobial Activity Against ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter) Pathogens. Foods 2025, 14, 1161. [Google Scholar] [CrossRef]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; Yang, Y.; Wei, L.; Wang, C.; Xu, J.; Song, J.; Liu, S.; Bai, J.; Suo, H. Regulatory effects of Poria cocos polysaccharides on gut microbiota and metabolites: Evaluation of prebiotic potential. npj Sci. Food 2025, 9, 53. [Google Scholar] [CrossRef]
- Peng, X.; Wei, Y.; Gong, D.; Zhang, G. Modulatory Role of Hesperetin-Copper(II) on Gut Microbiota in Type 2 Diabetes Mellitus Mice. Foods 2025, 14, 2390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ma, X.; Geng, S.; Jiang, X.; Li, Y.; Hu, L.; Li, J.; Wang, Y.; Han, X. Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury. mSystems 2018, 3, e00137-18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, L.; Li, P.; Yu, J.; Mu, G.; Li, X.; Tuo, Y. Saccharomyces cerevisiae I4 Showed Alleviating Effects on Dextran Sulfate Sodium-Induced Colitis of Balb/c Mice. Foods 2022, 11, 1436. [Google Scholar] [CrossRef] [PubMed]

| Group | UAAQ | BAAQ | ||||
|---|---|---|---|---|---|---|
| Control | Boiling 1 min | Boiling 2 min | Boiling 3 min | Boiling 4 min | Boiling 5 min | |
| After-boiling | / |  |  |  |  |  |
| Soaked water | / |  |  |  |  |  |
| Before drying |  |  |  |  |  |  |
| After drying |  |  |  |  |  |  |
| Powder |  |  |  |  |  |  |
| Chlorophyll extract |  |  |  |  |  |  |
| Chlorophyll a (mg/g) | 1.78 ± 0.15 c | 2.28 ± 0.03 a | 2.26 ± 0.02 ab | 2.21 ± 0.04 ab | 2.14 ± 0.08 b | 2.22 ± 0.03 ab |
| Chlorophyll b (mg/g) | 0.62 ± 0.07 b | 2.03 ± 0.27 a | 2.01 ± 0.23 a | 1.88 ± 0.51 a | 2.46 ± 0.62 a | 2.08 ± 0.51 a |
| Total Chlorophyll (mg/g) | 2.40 ± 0.22 b | 4.31 ± 0.25 a | 4.27 ± 0.20 a | 4.09 ± 0.47 a | 4.61 ± 0.54 a | 4.30 ± 0.48 a |
| Character description (powder) | Color: 2 Aroma intensity: 6 Bitterness intensity: 6 Bitterness persistence: 3 | Color: 6 Aroma intensity: 7 Bitterness intensity: 5 Bitterness persistence: 3 | Color: 6 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 4 | Color: 7 Aroma intensity: 6 Bitterness intensity: 4 Bitterness persistence: 5 | Color: 7 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 6 | Color: 6 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 6 |
| Group | UAAQ | BAAQ | ||||
|---|---|---|---|---|---|---|
| Control | Boiling 1 min | Boiling 2 min | Boiling 3 min | Boiling 4 min | Boiling 5 min | |
| After-boiling | / |  |  |  |  |  |
| Soaked water | / |  |  |  |  |  |
| Before drying |  |  |  |  |  |  |
| After drying |  |  |  |  |  |  |
| Powder |  |  |  |  |  |  |
| Chlorophyll extract |  |  |  |  |  |  |
| Chlorophyll a (mg/g) | 2.15 ± 0.07 | 2.01 ± 0.07 | 2.15 ± 0.12 | 2.01 ± 0.10 | 2.11 ± 0.11 | 2.13 ± 0.13 |
| Chlorophyll b (mg/g) | 1.42 ± 0.21 b | 3.19 ± 0.31 a | 2.55 ± 0.57 a | 3.19 ± 0.34 a | 2.72 ± 0.44 a | 2.67 ± 0.59 a |
| Total Chlorophyll (mg/g) | 3.57 ± 0.28 b | 5.20 ± 0.24 a | 4.70 ± 0.45 a | 5.20 ± 0.25 a | 4.83 ± 0.33 a | 4.80 ± 0.46 a |
| Character description (powder) | Color: 0 Aroma intensity: 8 Bitterness intensity: 7 Bitterness persistence: 7 | Color: 6 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 5 | Color: 5 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 6 | Color: 7 Aroma intensity: 6 Bitterness intensity: 6 Bitterness persistence: 5 | Color: 7 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 6 | Color: 6 Aroma intensity: 6 Bitterness intensity: 6 Bitterness persistence: 6 |
| Powder | Group | Flavonoids (mg/g) | Polyphenols (mg/g) | Volatile Oil (%) | Eucalyptol (‰) | Borneol (‰) | Camphor (‰) | α-Thujone (‰) |
|---|---|---|---|---|---|---|---|---|
| UAAQ | Control | 13.04 ± 0.02 a | 11.59 ± 0.20 a | 0.230 ± 0.010 a | 0.031 ± 0.001 | 0.069 ± 0.001 a | 0.018 ± 0.001 | 0.093 ± 0.002 |
| BAAQ | Boiling 1 min | 11.01 ± 0.02 b | 4.73 ± 0.00 b | 0.173 ± 0.006 b | - | 0.015 ± 0.003 b | 0.012 ± 0.001 | 0.022 ± 0.002 |
| Boiling 2 min | 9.21 ± 0.02 c | 3.49 ± 0.01 c | 0.107 ± 0.006 c | - | - | - | - | |
| Boiling 3 min | 7.23 ± 0.02 d | 3.04 ± 0.01 d | 0.100 ± 0.010 c | - | - | - | - | |
| Boiling 4 min | 5.72 ± 0.03 e | 2.93 ± 0.00 e | 0.083 ± 0.006 d | - | - | - | - | |
| Boiling 5 min | 4.92 ± 0.02 f | 2.66 ± 0.01 f | 0.063 ± 0.012 e | - | - | - | - |
| Group | 25 °C Drying | 40 °C Drying | 65 °C Drying | Freeze-Drying | Control |
|---|---|---|---|---|---|
| Samples | BAAQ | UAAQ | |||
 |  |  |  |  | |
| Character description (powder) | Color: 8 Aroma intensity: 5 Bitterness intensity: 6 Bitterness persistence: 5 | Color: 7 Aroma intensity: 5 Bitterness intensity: 5 Bitterness persistence: 6 | Color: 6 Aroma intensity: 4 Bitterness intensity: 4 Bitterness persistence: 5 | Color: 3 Aroma intensity: 6 Bitterness intensity: 5 Bitterness persistence: 6 | Color: 1 Aroma intensity: 8 Bitterness intensity: 8 Bitterness persistence: 7 |
| Chlorophyll a (mg/g) | 2.09 ± 0.11 a | 1.91 ± 0.16 ab | 1.75 ± 0.17 b | 1.11 ± 0.15 c | 1.30 ± 0.08 c |
| Chlorophyll b (mg/g) | 1.01 ± 0.14 a | 0.85 ± 0.12 ab | 0.79 ± 0.13 b | 0.51 ± 0.06 b | 0.74 ± 0.06 c |
| Total Chlorophyll (mg/g) | 3.10 ± 0.25 a | 2.76 ± 0.29 ab | 2.55 ± 0.30 b | 1.62 ± 0.21 c | 2.04 ± 0.14 c |
| Flavonoids (mg/g) | 13.10 ± 0.05 c | 15.05 ± 0.02 b | 12.77 ± 0.02 d | 13.04 ± 0.09 c | 19.00 ± 0.02 a |
| Polyphenols (mg/g) | 5.79 ± 0.00 d | 5.97 ± 0.00 c | 5.23 ± 0.25 e | 6.70 ± 0.00 b | 14.24 ± 0.00 a |
| Volatile oil (%) | 0.390 ± 0.026 c | 0.463 ± 0.021 b | 0.370 ± 0.010 c | 0.470 ± 0.010 b | 1.027 ± 0.006 a |
| Eucalyptol (‰) | - | - | - | - | 0.015 ± 0.000 |
| Borneol (‰) | - | - | - | - | 0.075 ± 0.002 |
| Camphor (‰) | - | - | - | - | 0.114 ± 0.002 |
| α-Thujone (‰) | - | - | - | - | 1.303 ± 0.021 |
| Groups | Body Weight (g) | Death/Treated Rats | Clinical Signs | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 14 | ||||
| Female | FC | 189.36 ± 9.34 | 229.03 ± 8.62 ab | 248.54 ± 12.62 ab | 0/10 | None |
| FUAAQ | 190.41 ± 10.65 | 238.04 ± 16.23 a | 258.11 ± 17.48 a | 3/10 | After the oral administration, 3 died within the first week, and 1 experienced difficulty to breath, but recovered. | |
| FBAAQ | 186.18 ± 10.77 | 223.56 ± 8.15 b | 241.34 ± 15.04 b | 0/10 | None | |
| Male | MC | 210.47 ± 9.85 | 255.92 ± 14.41 | 284.10 ± 11.02 b | 0/10 | None |
| MUAAQ | 211.32 ± 9.77 | 256.18 ± 45.96 | 301.29 ± 46.02 ab | 1/10 | After the oral administration, 1 died within the first week, and 4 showed symptoms of difficulty to breath, abdominal distension, and diarrhea. Three of 4 recovered, and the remaining one maintained the diarrhea and lost body weight until the end of the test. | |
| MBAAQ | 211.29 ± 6.43 | 266.07 ± 19.02 | 313.94 ± 20.71 a | 0/10 | None | |
| Systematic Name | Formula | Structure | Classification | BAAQ vs. UAAQ Type | Safety Risk |
|---|---|---|---|---|---|
| Phthalic acid | C8H6O4 | 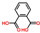 | Phenolic acids | down (0/19167) | Xi |
| 3-Hydroxyanthranilic acid | C7H7NO3 | 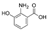 | Alkaloid | down (0/461038) | Xn |
| 3,4-Dihydroxybenzoic acid | C7H6O4 | 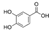 | Phenolic acids | down (0/17677041) | Xi |
| 3-O-Methylgallic acid | C8H8O5 | 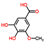 | Phenolic acids | up | Xi |
| 2,5-Dihydroxybenzoic acid | C7H6O4 | 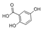 | Phenolic acids | down (0.003 times) | Xi |
| 5,7,3′,4′-Tetrahydroxyflavanone | C15H12O6 | 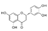 | Flavonoids | down (0.003 times) | Xi |
| Dhurrin | C14H17NO7 | 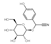 | Alkaloid | down (0.004 times) | Xn |
| 3-amino-2-naphthoic acid | C11H9NO2 | 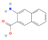 | Alkaloid | down | Xn |
| 2-Nitrophenol | C6H5NO3 | 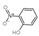 | Phenolic acids | down | Xn |
| Histamine | C5H9N3 | 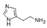 | Alkaloid | down | Xn |
| Benzamide | C7H7NO |  | Alkaloid | down | Xn |
| Isoalantolactone | C15H20O2 | 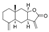 | Terpenoids | down | Xn |
| N-benzylformamide | C8H9NO | 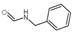 | Alkaloid | down | Xn |
| 3,4-Dihydrocoumarin | C9H8O2 |  | Lignans and coumarins | down | Xn |
| Genipin | C11H14O5 | 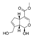 | Terpenoids | down | Xn |
| Turgeniifolin A | C19H18O6 | 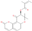 | Lignans and coumarins | down | Xn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Liu, X.; Qin, P.; Wang, L.; Chen, G.; Yang, F. Optimization of Its Preparation and Evaluation of Fresh and Tender Leaves of Artemisia argyi for Food Usage. Foods 2025, 14, 3185. https://doi.org/10.3390/foods14183185
Qin X, Liu X, Qin P, Wang L, Chen G, Yang F. Optimization of Its Preparation and Evaluation of Fresh and Tender Leaves of Artemisia argyi for Food Usage. Foods. 2025; 14(18):3185. https://doi.org/10.3390/foods14183185
Chicago/Turabian StyleQin, Xiaoyan, Xujia Liu, Ping Qin, Lijuan Wang, Guoxun Chen, and Fang Yang. 2025. "Optimization of Its Preparation and Evaluation of Fresh and Tender Leaves of Artemisia argyi for Food Usage" Foods 14, no. 18: 3185. https://doi.org/10.3390/foods14183185
APA StyleQin, X., Liu, X., Qin, P., Wang, L., Chen, G., & Yang, F. (2025). Optimization of Its Preparation and Evaluation of Fresh and Tender Leaves of Artemisia argyi for Food Usage. Foods, 14(18), 3185. https://doi.org/10.3390/foods14183185






