Effects of Different Citrus Varieties and Harvesting Time on the Quality of Citrus Dark Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
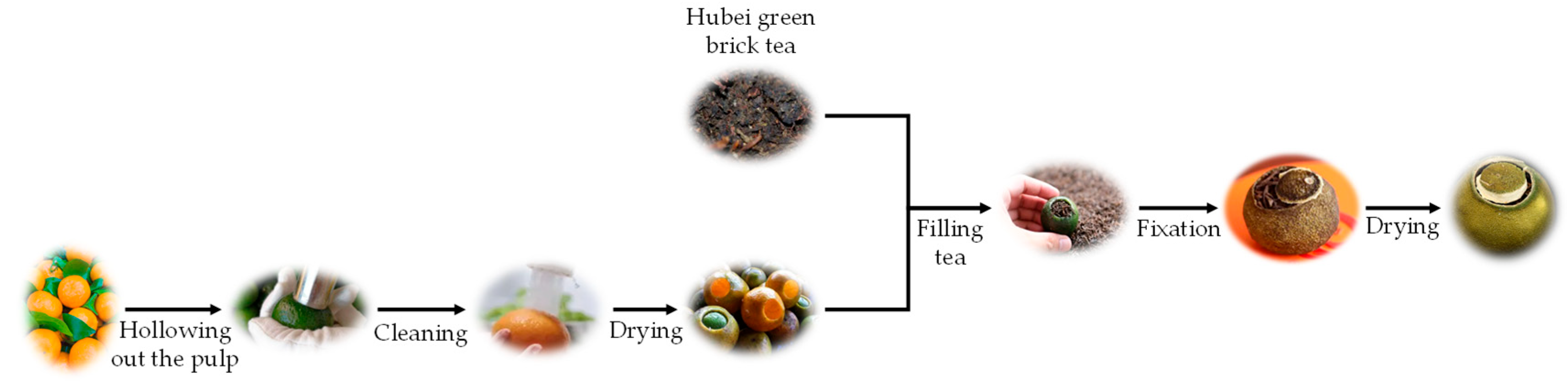
2.2. Processing of Citrus Dark Tea
2.3. Sensory Evaluation of Orange Dark Tea
2.4. Analysis of Major Non-Volatile Compounds in Peach Leaf Orange Dark Tea
2.5. Volatile Content of Peach Leaf Orange Dark Tea
2.6. Antioxidant Capacity of Peach Leaf Orange Dark Tea
2.7. α-Glucosidase and α-Amylase Activities
2.8. Statistical Analysis
3. Results and Discussion
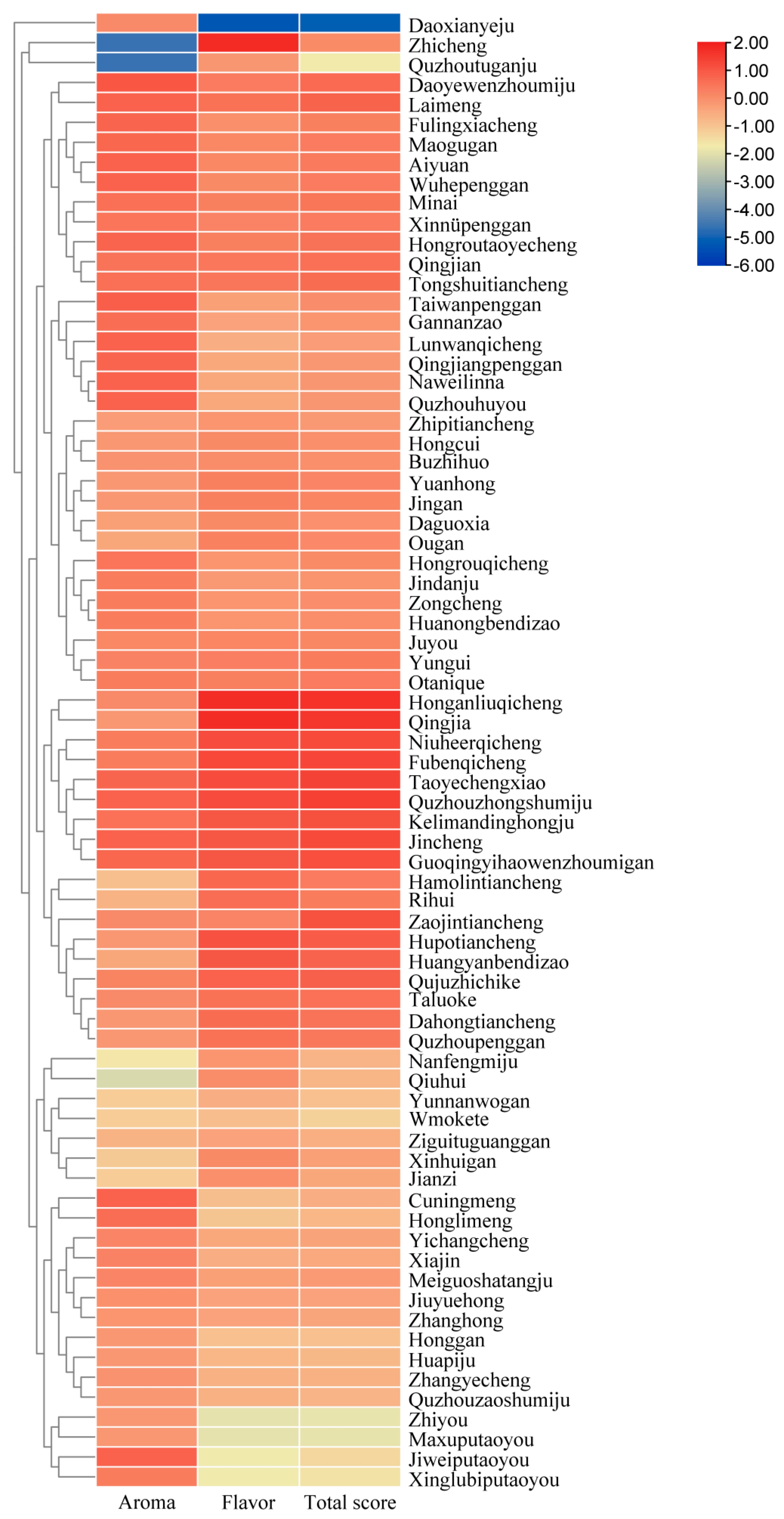
3.1. Effects of Different Citrus Species on Sensory Quality of Citrus Dark Tea
3.2. Effects of Different Citrus Cultivars on Sensory Quality of Citrus Dark Tea
3.3. Effects of Plucking Time on Quality of Peach Leaf Orange Dark Tea
3.3.1. Effects of Harvesting Time on Sensory Quality
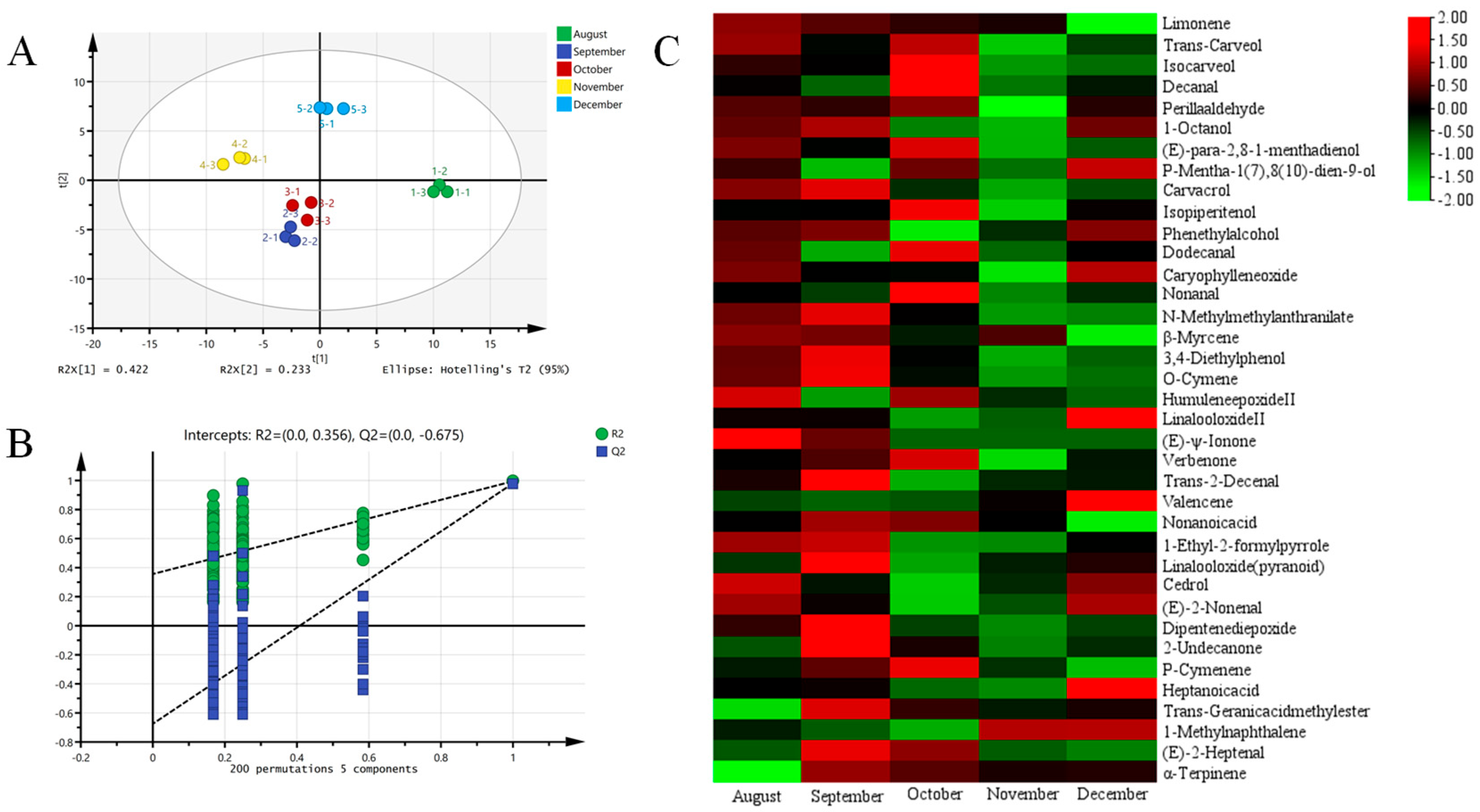
3.3.2. Effects of Harvesting Time on Quality Composition of Flavor
3.3.3. Effects of Harvesting Time on Aroma Compounds
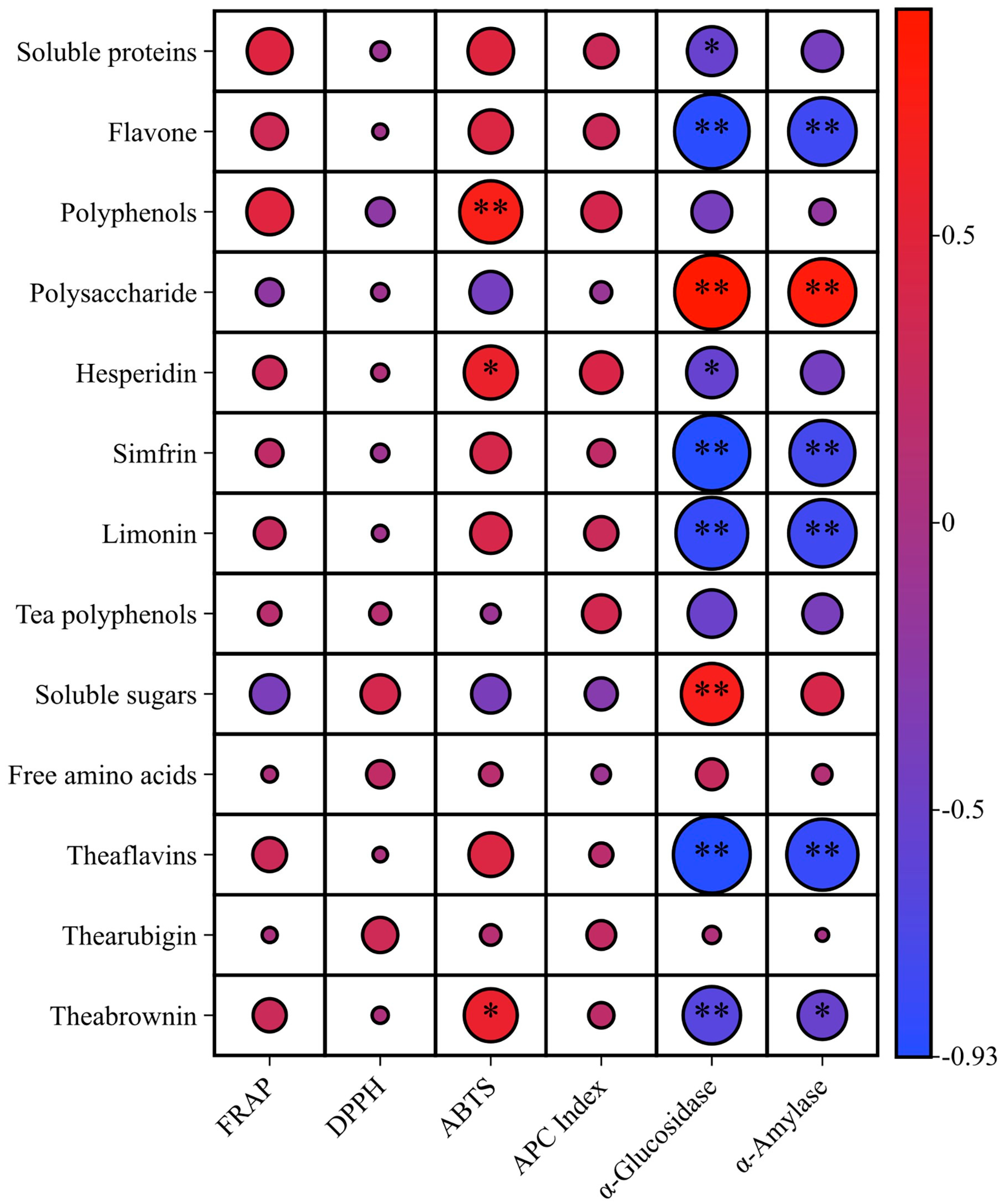
3.3.4. Effects of Harvesting Time on Biological Activities
- (i)
- Antioxidant activity
- (ii)
- Ability to inhibit activity of key enzymes in glycolipid metabolism
- (iii)
- Correlation analysis between bioactivity and functional components of Peach leaf orange dark teas under different orange plucking time conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| ABTS | 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric Reducing Antioxidant Power |
| DPPH | 2, 2-Diphenyl-1-picrylhydrazyl |
| HPLC | High Performance Liquid Chromatography |
| HS-SPME | Headspace Solid-Phase Microextraction |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| DVB | Divinylbenzene |
| CAR | Carboxen |
| PDMS | Polydimethylsiloxane |
| EI | Electron Impact Ionization |
| TE | Trolox equivalents |
| LSD | Least Significant Difference |
| IC50 | Half Maximal Inhibitory Concentration |
| VIP | Variable Importance in Projection |
| APC | Antioxidant Potency Composite |
| ROS | Reactive Oxygen Species |
| TF1 | Theaflavin |
| TF2A | theaflavin-3-gallate |
| TF2B | theaflavin-3′-gallate |
| TF3 | theaflavin-3, 3′-digallate |
References
- Kim, D.; Lee, S.; Park, S.M.; Yun, S.H.; Gab, H.; Kim, S.S.; Kim, H. Comparative Metabolomics Analysis of Citrus Varieties. Foods 2021, 10, 2826. [Google Scholar] [CrossRef]
- Shaw, D.; Tripathi, A.D.; Paul, V.; Agarwal, A.; Mishra, P.K.; Kumar, M. Valorization of essential oils from citrus peel powder using hydro-distillation. Sustain. Chem. Pharm. 2023, 32, 11. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 13. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Haokip, S.W.; Sheikh, K.A.; Das, S.; Devi, O.B.; Singh, Y.D.; Wangchu, L.; Heisnam, P. Unraveling physicochemical profiles and bioactivities of citrus peel essential oils: A comprehensive review. Eur. Food Res. Technol. 2023, 249, 2821–2834. [Google Scholar] [CrossRef]
- Long, W.; Zhang, G.; Dong, Y.; Li, D. Dark tea extract mitigates hematopoietic radiation injury with antioxidative activity. J. Radiat. Res. 2018, 59, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Lu, T.; Xu, X.; He, J.; Liu, P. Analysis of quality and flavor differences in processed green tea with different types of citruses. South. Hortic. 2023, 34, 64–66. [Google Scholar]
- Jiang, J.; Xie, P.; Dong, L.; Chen, F.; Ren, F. Analysis of flavor differences between Xinhui Qingkang tea and orange peel pu-erh tea. Mod. Food Sci. Technol. 2021, 37, 266–274. [Google Scholar]
- Xu, Y.; Liang, P.; Chen, X.; Gong, M.; Zhang, L.; Qiu, X.; Zhang, J.; Huang, Z.; Xu, W. The Impact of Citrus-Tea Cofermentation Process on Chemical Composition and Contents of Pu-Erh Tea: An Integrated Metabolomics Study. Front. Nutr. 2021, 8, 737539. [Google Scholar] [CrossRef]
- Wan, L.; Xiao, G.; Xu, Y.; Chen, W.; Chen, Y.; Wu, J.; Jiang, A. Analysis of Flavonoid Compounds and Antioxidant Activity in Different Citrus Peels. Food Ferment. Ind. 2011, 37, 73–77. [Google Scholar]
- Zhang, Y.; Sun, Y.; Xi, W.; Shen, Y.; Qiao, L.; Zhong, L.; Ye, X.; Zhou, Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Shigeyama, K.; Yoshikawa, M.; Matsumoto, T.; Yonezawa, Y.; Shiota, H.; Ueyama, T.; Sakaguchi, I. Effects of Cultivation Month and Genetic Background on Phenolic Content of Citrus tachibana Peel. Biol. Pharm. Bull. 2018, 41, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Su, M. Chromatography-Mass Spectrometry (Chromatography-Mass Spectrometry) to Explore the Changes of Tangerine Composition in Nanfeng at Different Growth Stages and the Process of Orange Peel Tea. Master’s Thesis, Nanchang University, Nanchang, China, 2022. [Google Scholar]
- Vekiari, S.A.; Protopapadakis, E.E.; Papadopoulou, P.; Papanicolaou, D.; Panou, C.; Vamvakias, M. Composition and seasonal variation of the essential oil from leaves and peel of a Cretan lemon variety. J. Agric. Food. Chem. 2002, 50, 147–153. [Google Scholar] [CrossRef]
- Jiao, Y.; Song, Y.; Yan, Z.; Wu, Z.; Yu, Z.; Zhang, D.; Ni, D.; Chen, Y. The New Insight into the Effects of Different Fixing Technology on Flavor and Bioactivities of Orange Dark Tea. Molecules 2023, 28, 1079. [Google Scholar] [CrossRef]
- Li, Y.; Ran, W.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chem. X 2022, 14, 100289. [Google Scholar] [CrossRef]
- Liu, S.; Ai, Z.; Qu, F.; Chen, Y.; Ni, D. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against alpha-amylase, alpha-glucosidase and intestinal glucose uptake. Food Chem. 2017, 234, 168–173. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Ma, L.; Liu, A. Polysaccharides from the peels of Citrus aurantifolia induce apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2017, 101, 680–689. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Shan, C.; Yang, X.; Zhang, Q.; Xu, N.; Xu, L.; Song, W. Effects of five extraction methods on total content, composition, and stability of flavonoids in jujube. Food Chem. X 2022, 14, 8. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.; Tang, F.; Yuan, J.; Liu, Q.; Yao, S. Simultaneous determination of flavonoid and alkaloid compounds in Citrus herbs by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. J. Chromatogr. B 2007, 857, 202–209. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, X.; He, C.; Qiu, A.; Li, Y.; Shu, Q.; Chen, Y.; Ni, D. Withering degree affects flavor and biological activity of black tea: A non-targeted metabolomics approach. LWT 2020, 130, 109535. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed Polyphenol-Rich beverages in the United States. J. Agric. Food. Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qi, J.; Jin, Y.; Li, F.; Wang, J.; Xu, H. Influence of fruit maturity and lactic fermentation on physicochemical properties, phenolics, volatiles, and sensory of mulberry juice. Food Biosci. 2022, 48, 11. [Google Scholar] [CrossRef]
- Hijaz, F.; Gmitter, F.G.; Bai, J.; Baldwin, E.; Biotteau, A.; Leclair, C.; McCollum, T.G.; Plotto, A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, Y.; Li, X.; Ren, J.; Jia, X.; Pan, S.; Li, Z.; He, X.; Hu, Z.; Fan, G. Factors affecting the formation of delayed bitterness in sweet orange (Citrus sinensis (L.) Osbeck) juice. J. Food Compos. Anal. 2024, 132, 12. [Google Scholar] [CrossRef]
- Frydman, A.; Liberman, R.; Huhman, D.V.; Carmeli-Weissberg, M.; Sapir-Mir, M.; Ophir, R.; Sumner, L.W.; Eyal, Y. The molecular and enzymatic basis of bitter/non-bitter flavor of citrus fruit: Evolution of branch-forming rhamnosyl-transferases under domestication. Plant J. 2013, 73, 166–178. [Google Scholar] [CrossRef]
- Qi, H.; Ding, S.; Pan, Z.; Li, X.; Fu, F. Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 2020, 25, 6027. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; McCollum, G.; Plotto, A.; Manthey, J.A.; Widmer, W.W.; Luzio, G.; Cameron, R. Changes in Volatile and Non-Volatile Flavor Chemicals of “Valencia” Orange Juice over the Harvest Seasons. Foods 2016, 5, 4. [Google Scholar] [CrossRef]
- Hasani, M.; Djalalinia, S.; Khazdooz, M.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Ansari, H.; Qorbani, M.; Heshmat, R. Effect of selenium supplementation on antioxidant markers: A systematic review and meta-analysis of randomized controlled trials. Horm. Int. J. Endocrinol. Metab. 2019, 18, 451–462. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Korompokis, K.; Deleu, L.J.; De Brier, N.; Delcour, J.A. Use of Amylomaltase to Steer the Functional and Nutritional Properties of Wheat Starch. Foods 2021, 10, 303. [Google Scholar] [CrossRef]
- Khan, H.; Jan, F.; Shakoor, A.; Khan, A.; Alasmari, A.F.; Alasmari, F.; Ullah, S.; Al-Harrasi, A.; Khan, M.; Ali, S. Design, synthesis, molecular docking study, and α-glucosidase inhibitory evaluation of novel hydrazide-hydrazone derivatives of 3, 4-dihydroxyphenylacetic acid. Sci. Rep. 2024, 14, 11410. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, B.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Synthesis and Biological Evaluation of 3-Arylcoumarin Derivatives as Potential Anti-Diabetic Agents. J. Enzym. Inhib. Med. Chem. 2018, 34, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Tundis, R.; Sicari, V.; Pellicano, T.M.; Dugay, A.; Deguin, B.; Loizzo, M.R. Impact of Extraction Processes on Phytochemicals Content and Biological Activity of Citrus × Clementina Hort. Ex Tan. Leaves: New Opportunity for Under-Utilized Food By-Products. Food Res. Int. 2020, 127, 108742. [Google Scholar] [CrossRef]
- Tong, D.; Zhu, K.; Guo, X.; Peng, W.; Zhou, H. The enhanced inhibition of water extract of black tea under baking treatment on alpha-amylase and alpha-glucosidase. Int. J. Biol. Macromol. 2018, 107, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Zhang, B.; Ma, Q.; Hu, H.; Ding, A.; Shang, L.; Zong, Z.; Zhao, W.; Chen, H.; et al. Overexpression of ZlMYB1 and ZlMYB2 increases flavonoid contents and antioxidant capacity and enhances the inhibition of α-glucosidase and tyrosinase activity in rice seeds. Food Chem. 2024, 460, 14. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Raby, K.H.; Tasnim, N.; Islam, M.T.; Chowdhury, M.; Juthi, Z.T.; Mia, M.A.; Jahan, L.; Hossain, A.K.M.Z.; Ahmed, S. Optimization of the Extraction Methods and Evaluation of the Hypoglycemic Effect of Adhatoda Zeylanica Extracts on Artificially Induced Diabetic Mice. Heliyon 2025, 11, e41627. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, Y.; Peng, L.; Wei, K.; Liu, Z.; Wei, X. State-of-the-art review of theabrownins: From preparation, structural characterization to health-promoting benefits. Crit. Rev. Food. Sci. Nutr. 2024, 64, 11321–11340. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Yang, X.; Zhu, F.; Wu, D.; Li, H.; Gan, R. Green extraction, chemical composition, and in vitro antioxidant activity of theabrownins from Kangzhuan dark tea. Curr. Res. Food Sci. 2022, 5, 1944–1954. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 22. [Google Scholar] [CrossRef]
- Ji, Z.; Deng, W.; Chen, D.; Liu, Z.; Shen, Y.; Dai, J.; Zhou, H.; Zhang, M.; Xu, H.; Dai, B. Recent understanding of the mechanisms of the biological activities of hesperidin and hesperetin and their therapeutic effects on diseases. Heliyon 2024, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Insights into the phenolic compounds present in jambolan (Syzygium cumini) along with their health-promoting effects. Int. J. Food Sci. Technol. 2018, 53, 2431–2447. [Google Scholar] [CrossRef]
- Taslimi, P.; Akincioglu, H.; Gulcin, I. Synephrine and phenylephrine act as α-amylase, α-glycosidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase enzymes inhibitors. J. Biochem. Mol. Toxicol. 2017, 31, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 11. [Google Scholar] [CrossRef]
- Peng, J.; Wang, J.; Kong, F.; Liu, Z.; Wang, P.; Gai, C.; Jiang, B.; Mei, W.; Dai, H. New Phragmalin-Type Limonoids from Chukrasia tabularis and their α-Glucosidase Inhibitory Activity. Molecules 2016, 21, 58. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Li, M.; Dong, Y.; Kang, M.; Tao, T.; Li, W.; Zhang, S.; Quan, W.; Liu, Z. Potential anti-hyperglycemic activity of black tea theaflavins through inhibiting α-amylase. Food Chem. X 2024, 22, 12. [Google Scholar] [CrossRef]
- Wang, M.; Guo, W.; Ke, Z.; Mao, H.; Lv, J.; Qi, L.; Wang, J. Inhibitory mechanisms of galloylated forms of theaflavins on α-glucosidase. Int. J. Biol. Macromol. 2025, 294, 15. [Google Scholar] [CrossRef]
| M | T | O | G | L | P M-T | P M-O | P M-G | P M-L | P T-O | P T-G | P T-L | PO-G | P O-L | P G-L | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soup color | Score | 8.93 | 8.90 | 9.09 | 8.91 | 8.97 | 0.21 | <0.01 | 0.62 | 0.08 | <0.01 | 0.43 | 0.08 | <0.01 | <0.01 | 0.03 |
| Aroma | Score | 22.47 | 20.78 | 22.77 | 22.22 | 23.87 | <0.01 | 0.26 | 0.36 | <0.01 | <0.01 | <0.01 | <0.01 | 0.06 | <0.01 | <0.01 |
| Subdivide | 14.44 | 13.83 | 14.44 | 14.25 | 14.93 | <0.01 | 0.94 | 0.00 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 | |
| Coordination | 8.03 | 7.80 | 8.33 | 7.98 | 8.93 | <0.01 | <0.01 | 0.15 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Taste | Score | 28.98 | 27.41 | 30.19 | 22.56 | 26.07 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Sour | 5.51 | 5.46 | 5.45 | 5.26 | 4.67 | 0.26 | 0.16 | <0.01 | <0.01 | 0.74 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Sweet | 6.08 | 6.19 | 6.06 | 4.86 | 5.67 | - | - | - | - | - | - | - | - | - | - | |
| Bitter | 5.76 | 6.16 | 6.44 | 4.29 | 5.40 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Astringent | 5.96 | 5.78 | 6.24 | 4.25 | 5.67 | 0.03 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.04 | <0.01 | <0.01 | <0.01 | |
| Taste harmony | 5.67 | 5.54 | 6.00 | 3.90 | 4.67 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Score | 60.38 | 57.09 | 62.05 | 53.70 | 58.90 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 |
| Harvest Time | Soup Color | Aroma | Taste | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Aroma | Coordination | Sour | Sweet | Bitter | Astringent | Coordination | |||
| August | 9 ± 0.1 a | 15 ± 0.1 a | 9 ± 0 a | 5.7 ± 0.6 a | 6 ± 0 b | 6 ± 0 b | 5.7 ± 0.2 b | 5.8 ± 0.4 b | 62.1 ± 0.8 c |
| September | 9 ± 0.1 a | 14.9 ± 0.1 a | 8.9 ± 0.1 a | 6 ± 0.3 a | 7 ± 0 a | 7.2 ± 0.4 a | 7.2 ± 0.4 a | 7 ± 0 a | 67.2 ± 0.6 a |
| October | 8.9 ± 0.1 a | 14.9 ± 0.1 a | 8.9 ± 0.1 a | 5.9 ± 0.2 a | 6.1 ± 0.2 b | 6.1 ± 0.9 b | 6.2 ± 0.8 a | 6.1 ± 0.7 b | 63.2 ± 1.8 bc |
| November | 9 ± 0.1 a | 15 ± 0.1 a | 9 ± 0 a | 6.3 ± 0.6 a | 6.1 ± 0.17 b | 6.2 ± 0.7 b | 6.3 ± 0.6 a | 6.3 ± 0.4 b | 64.2 ± 1.2 b |
| December | 9 ± 0.1 a | 14.9 ± 0.2 a | 8.9 ± 0.1 a | 6.1 ± 0.6 a | 6 ± 0 b | 5.8 ± 0.3 b | 6 ± 0 a | 6.0 ± 0.6 b | 62.8 ± 1.2 c |
| Harvest Time | August | September | October | November | December |
|---|---|---|---|---|---|
| Soluble proteins | 2.96 ± 0.54 a | 2.66 ± 0.21 b | 2.4 ± 0.19 bc | 2.35 ± 0.08 c | 2.5 ± 0.12 bc |
| Flavone | 10.2 ± 1 a | 8.79 ± 0.88 b | 8.28 ± 0.78 b | 5.47 ± 0.89 c | 4.53 ± 1.53 c |
| Polyphenols | 26.5 ± 1.1 ab | 27.3 ± 0.9 a | 25.7 ± 1.9 bc | 24.9 ± 0.4 c | 25.6 ± 1.0 bc |
| Polysaccharide | 30 ± 1.9 b | 31.2 ± 2.8 b | 31.2 ± 2.9 b | 36.9 ± 4.2 a | 38.3 ± 2.1 a |
| Hesperidin | 43.65 ± 3.62 a | 45.83 ± 3.18 a | 44.81 ± 4.35 a | 38.82 ± 4.78 b | 38.05 ± 4.07 b |
| Simfrin | 0.86 ± 0.08 a | 0.83 ± 0.05 a | 0.71 ± 0.04 b | 0.45 ± 0.05 c | 0.38 ± 0.04 d |
| Limonin | 2.02 ± 0.3 a | 1.33 ± 0.26 b | 1.27 ± 0.29 b | 0.97 ± 0.08 c | 0.9 ± 0.04 c |
| Harvest Time | Tea Polyphenols (%) | Free Amino Acids (%) | Soluble Sugars (%) | Theaflavins (mg/g) | Thearubigins (mg/g) | Theabrownins (mg/g) |
|---|---|---|---|---|---|---|
| August | 9.00 ± 0.15 a | 1.71 ± 0.03 ab | 3.45 ± 0.11 c | 0.44 ± 0.01 a | 8.37 ± 0.63 a | 21.49 ± 0.40 ab |
| September | 8.75 ± 0.19 bc | 1.68 ± 0.04 b | 3.38 ± 0.09 c | 0.43 ± 0.02 a | 7.74 ± 0.21 a | 22.75 ± 0.11 a |
| October | 8.76 ± 0.17 bc | 1.74 ± 0.06 a | 3.62 ± 0.08 b | 0.42 ± 0.04 a | 8.39 ± 0.99 a | 22.22 ± 1.07 ab |
| November | 8.61 ± 0.11 c | 1.72 ± 0.01 a | 3.75 ± 0.09 a | 0.38 ± 0.01 b | 8.15 ± 0.84 a | 21.62 ± 1.01 ab |
| December | 8.81 ± 0.25 b | 1.73 ± 0.02 a | 3.66 ± 0.04 b | 0.47 ± 0.04 a | 8.50 ± 1.10 a | 21.26 ± 0.98 b |
| Class | Assay (μg/g) | Proportion (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| August | September | October | November | December | August | September | October | November | December | |
| Alcohols | 584.48 ± 47.69 a | 484.54 ± 19.47 bc | 488.73 ± 29.58 b | 356.02 ± 20.52 d | 401.72 ± 30.53 cd | 39.32 | 43.47 | 38.12 | 24.87 | 38.07 |
| Aldehydes and ketones | 352.28 ± 31.8 a | 333.97 ± 13.98 ab | 355.5 ± 22.46 a | 289.84 ± 16.48 bc | 268.2 ± 20.07 c | 23.70 | 28.98 | 26.28 | 17.85 | 27.69 |
| Olefins | 364.97 ± 18.89 a | 298.16 ± 37.98 b | 309.02 ± 17.84 ab | 287.34 ± 20.17 bc | 228.74 ± 19.81 c | 24.55 | 17.22 | 23.46 | 48.51 | 24.07 |
| Esters | 85.89 ± 4.24 a | 61.04 ± 1.96 b | 48.27 ± 4.14 c | 37.71 ± 3.54 d | 42.07 ± 3.56 cd | 5.78 | 3.87 | 4.80 | 2.51 | 3.76 |
| Others | 99 ± 7.1 a | 93.29 ± 4.89 ab | 82.18 ± 5.38 b | 62.69 ± 5.21 c | 67.4 ± 7.8 c | 6.66 | 6.47 | 7.34 | 6.25 | 6.40 |
| Total | 1486.62 ± 109.71 a | 1271 ± 78.29 b | 1283.69 ± 79.39 b | 1033.6 ± 65.92 c | 1008.13 ± 81.76 c | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Harvest Time | Orange Peel | Orange Tea | ||||
|---|---|---|---|---|---|---|
| FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | |
| August | 34.93 ± 0.61 a | 29.7 ± 1.3 a | 118.91 ± 1.57 a | 267.99 ± 3.82 a | 370.31 ± 4.15 b | 325.15 ± 0.68 a |
| September | 33.26 ± 1.71 ab | 23.91 ± 3.14 b | 118.16 ± 2.04 ab | 264.63 ± 3.85 a | 365.86 ± 4.86 b | 325.29 ± 0.7 a |
| October | 32.08 ± 0.05 b | 23.59 ± 1.99 b | 116.2 ± 0.52 bc | 264.83 ± 2.67 a | 380.41 ± 1.6 a | 325.06 ± 1.95 a |
| November | 31.66 ± 0.44 b | 22.67 ± 0.49 b | 115.52 ± 1.42 c | 261.46 ± 3.74 a | 372.57 ± 4.97 b | 324.28 ± 1.45 a |
| December | 32.02 ± 1 b | 22.55 ± 0.74 b | 115.42 ± 0.96 c | 267.86 ± 5.61 a | 371.15 ± 4.38 b | 324.66 ± 1.12 a |
| Harvest Time | FRAP APC | DPPH APC | ABTS APC | Synthesis APC |
|---|---|---|---|---|
| August | 100.00 | 97.35 | 99.96 | 99.10 |
| September | 98.75 | 96.18 | 100.00 | 98.31 |
| October | 98.82 | 100.00 | 99.93 | 99.58 |
| November | 97.56 | 97.94 | 99.69 | 98.40 |
| December | 99.95 | 97.57 | 99.81 | 99.11 |
| Harvest Time | α-Glucosidase | α-Amylase |
|---|---|---|
| August | 304.7 ± 5.24 d | 471.95 ± 9.09 e |
| September | 339.4 ± 9.49 c | 496.23 ± 9.99 d |
| October | 346.36 ± 10.19 c | 692.13 ± 7.64 c |
| November | 374.79 ± 1.76 b | 764.4 ± 4.88 b |
| December | 388.35 ± 5.51 a | 790.66 ± 2.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Jiao, Y.; Zhang, D.; Yu, Z.; Ni, D.; Huang, H.; Chen, Y. Effects of Different Citrus Varieties and Harvesting Time on the Quality of Citrus Dark Tea. Foods 2025, 14, 3181. https://doi.org/10.3390/foods14183181
Guo F, Jiao Y, Zhang D, Yu Z, Ni D, Huang H, Chen Y. Effects of Different Citrus Varieties and Harvesting Time on the Quality of Citrus Dark Tea. Foods. 2025; 14(18):3181. https://doi.org/10.3390/foods14183181
Chicago/Turabian StyleGuo, Fuwei, Yuanfang Jiao, De Zhang, Zhi Yu, Dejiang Ni, Han Huang, and Yuqiong Chen. 2025. "Effects of Different Citrus Varieties and Harvesting Time on the Quality of Citrus Dark Tea" Foods 14, no. 18: 3181. https://doi.org/10.3390/foods14183181
APA StyleGuo, F., Jiao, Y., Zhang, D., Yu, Z., Ni, D., Huang, H., & Chen, Y. (2025). Effects of Different Citrus Varieties and Harvesting Time on the Quality of Citrus Dark Tea. Foods, 14(18), 3181. https://doi.org/10.3390/foods14183181






