Development of Fermented Peach–Apricot Mixed Juice and Study of Its Storage Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inoculum Preparation
2.3. Bacterial Density Counting
2.4. Fermented Juice Preparation
2.5. Selection of Dominant Strains and Determination of Their Proportions
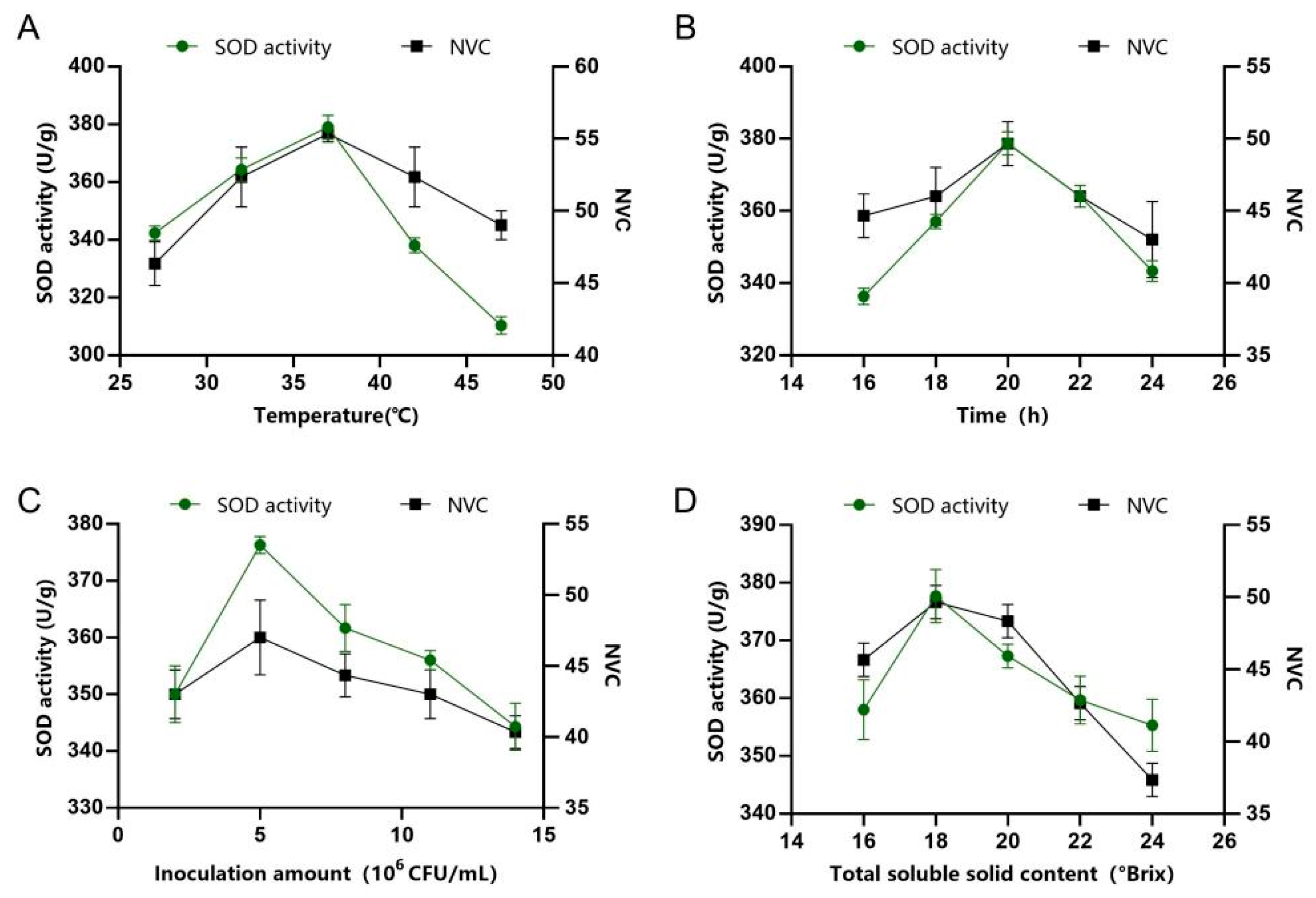
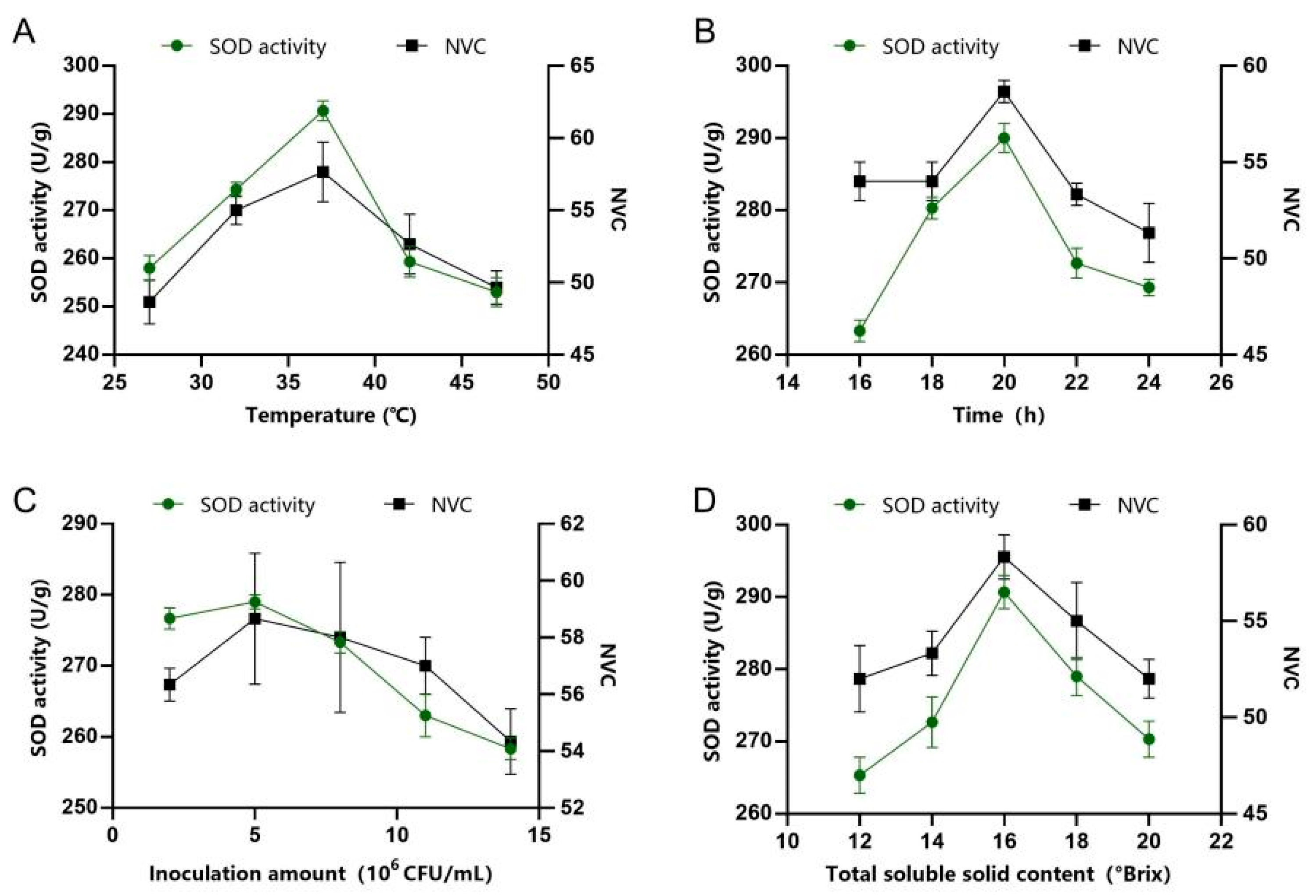
2.6. Single-Factor Experiments
2.7. Response Surface Optimisation
2.8. Determination of Physicochemical Indices
2.9. Sensory Evaluation
2.10. PAMJ Storage Stability Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Determination of Fermentation Strains
3.2. Determination of the Proportions of Fermentation Strains
3.3. Effects of Different Fermentation Conditions on Juice Quality
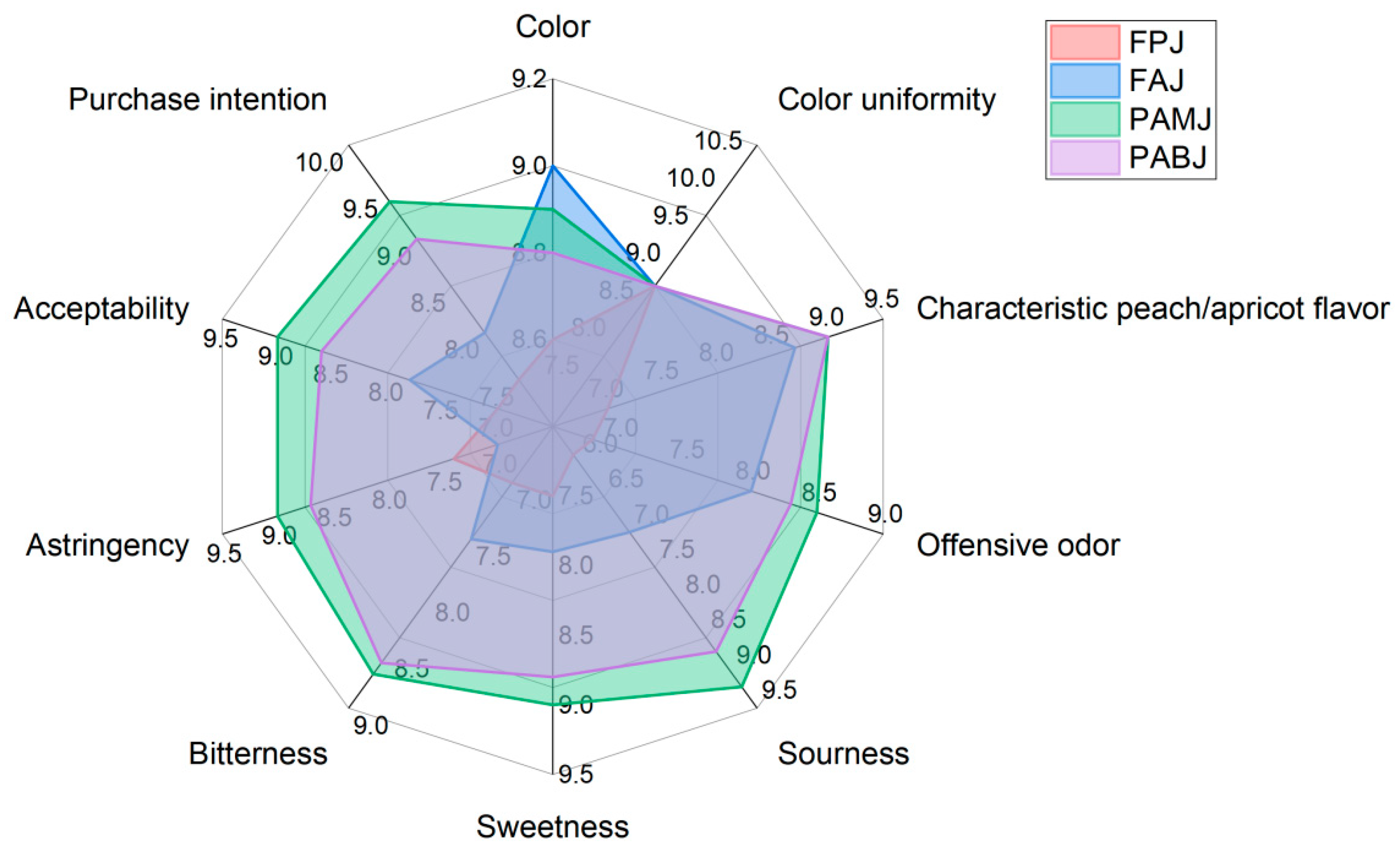
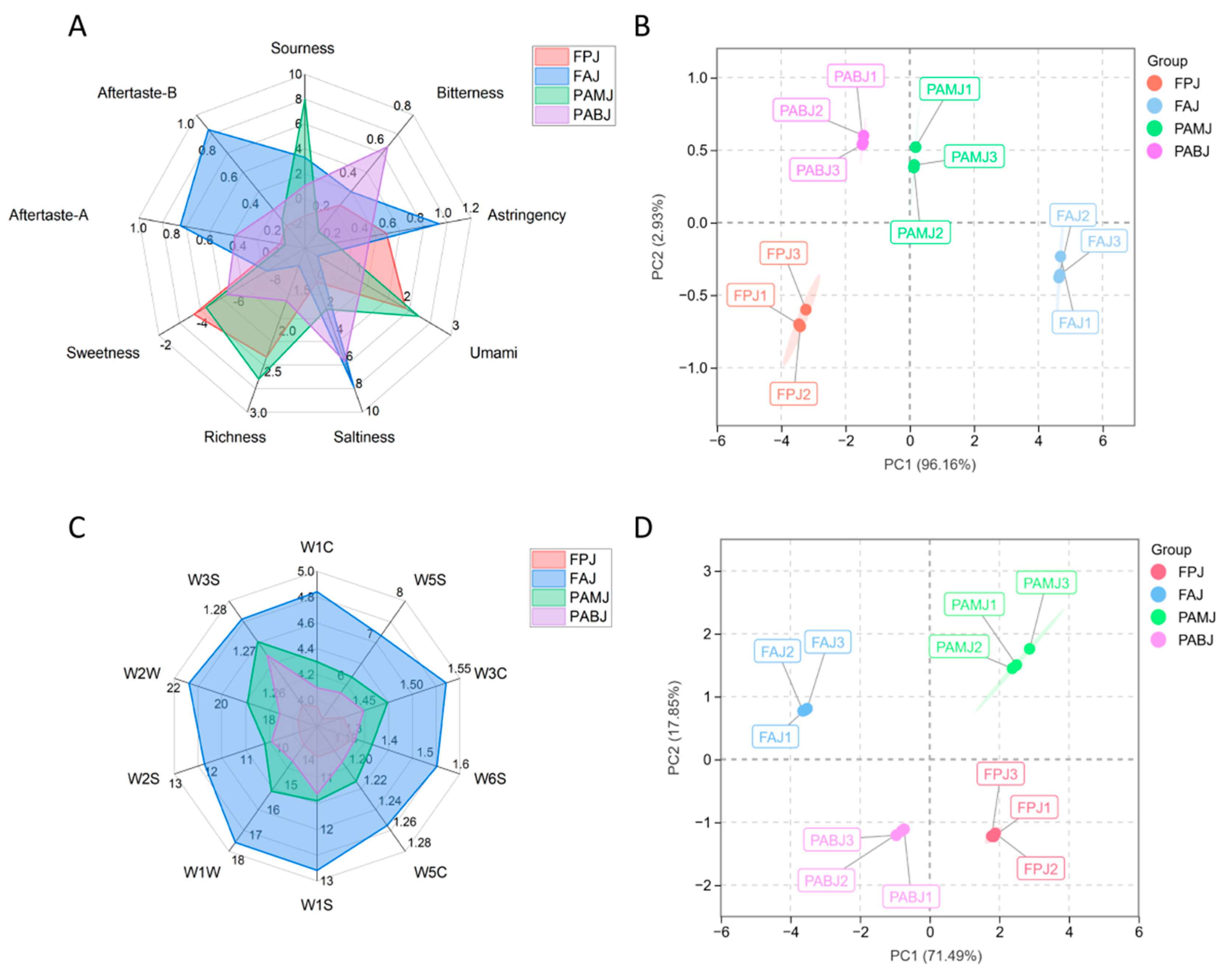
3.4. Comparative Analysis of the Quality of Fermented Juices
3.4.1. Sensory Evaluation Results
3.4.2. ELT Analysis Results
3.4.3. ELN Analysis Results
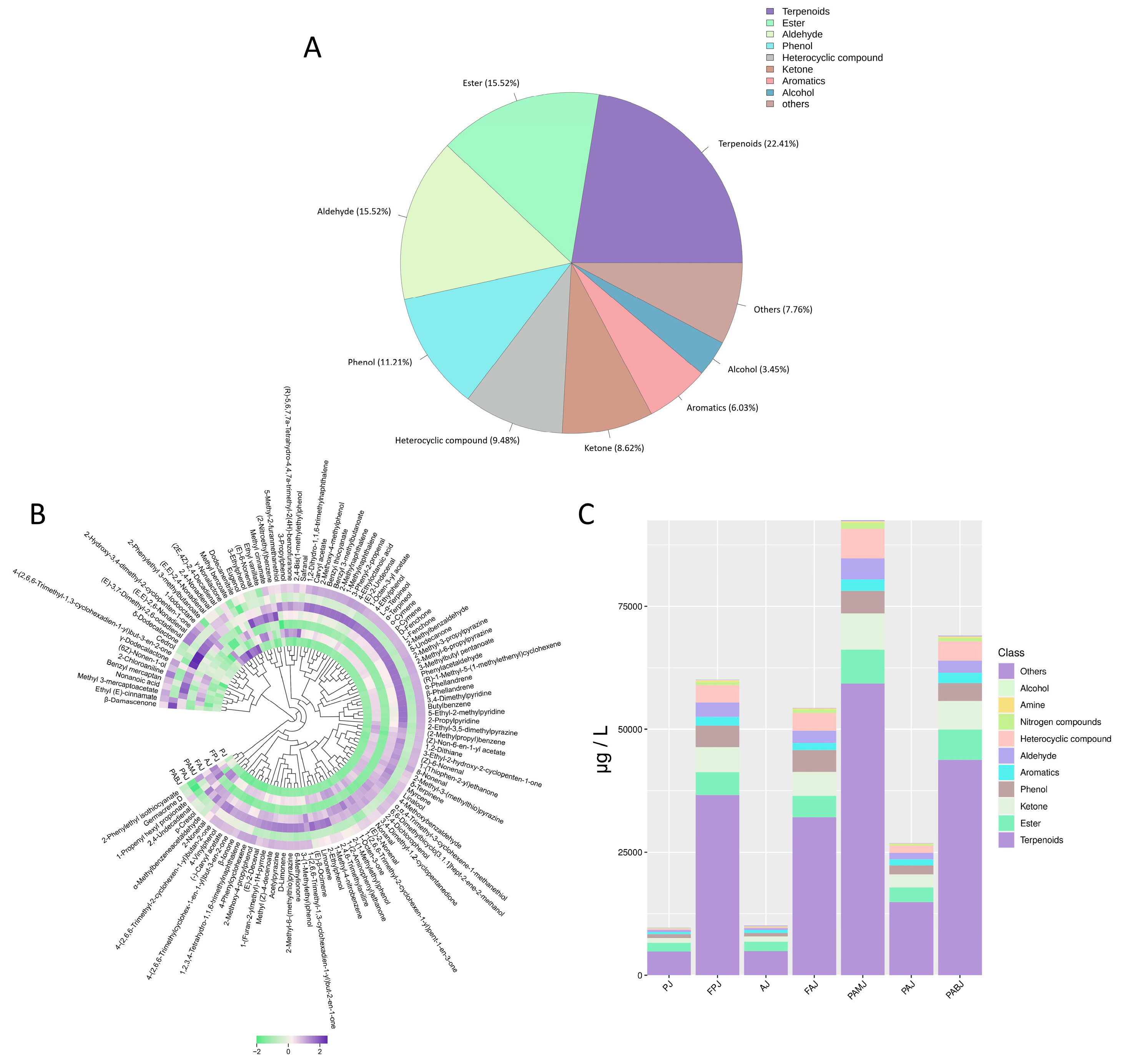
3.4.4. GC–MS Analysis Results
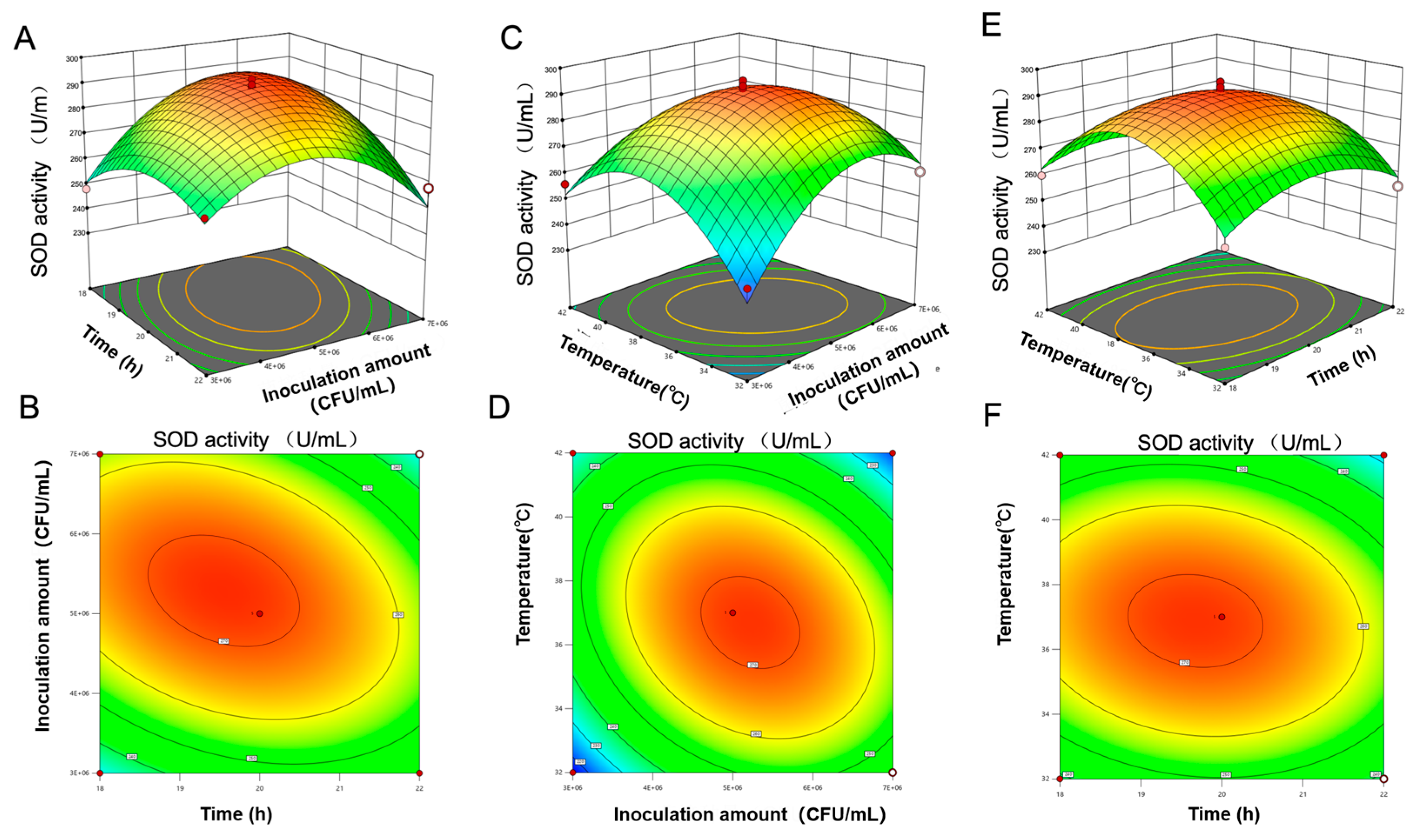
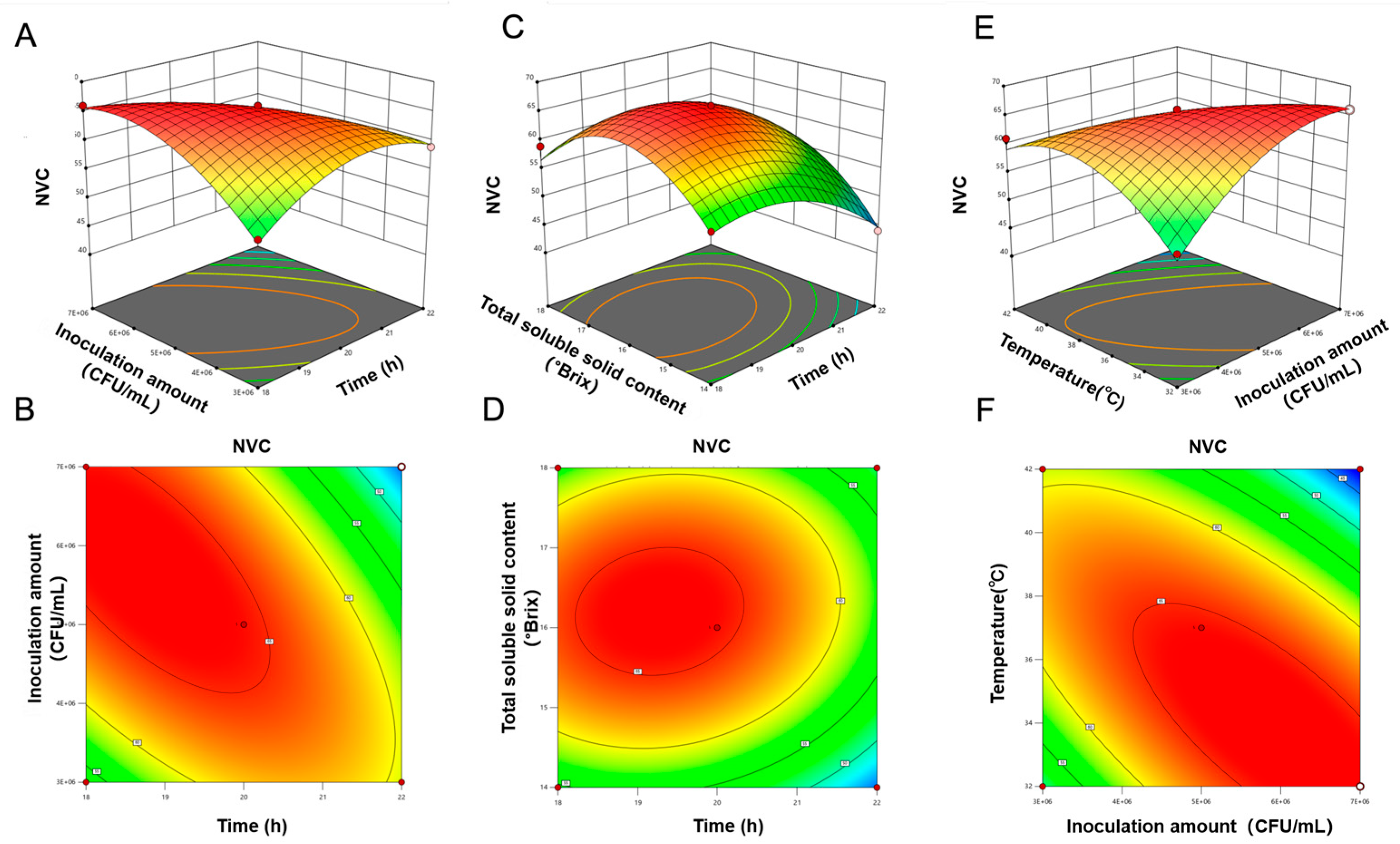
3.4.5. Optimal Fermentation Process of PAMJ Determined Through RSM
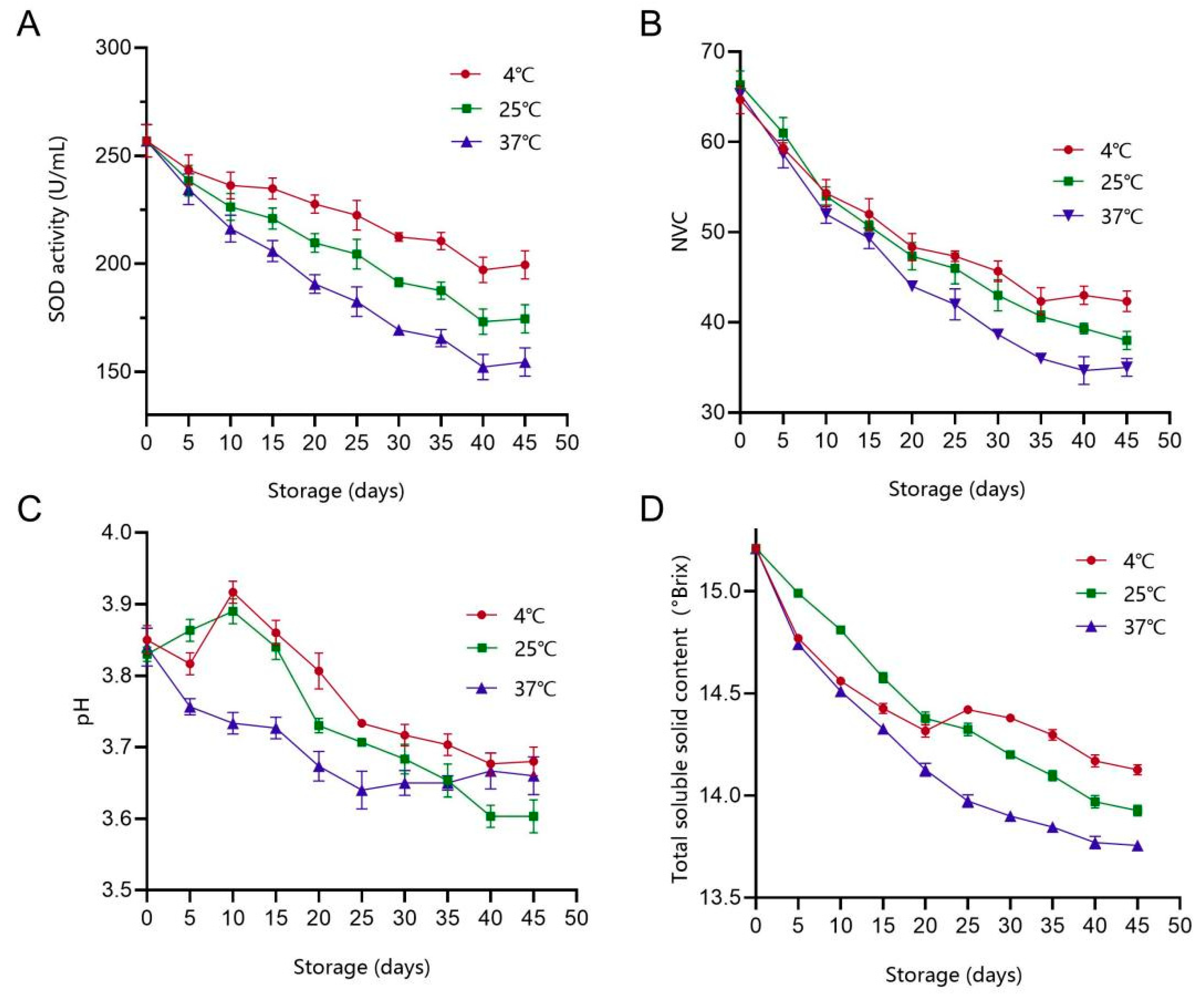
3.5. Storage Stability and Shelf Life of PAMJ
3.5.1. Different Storage Temperatures
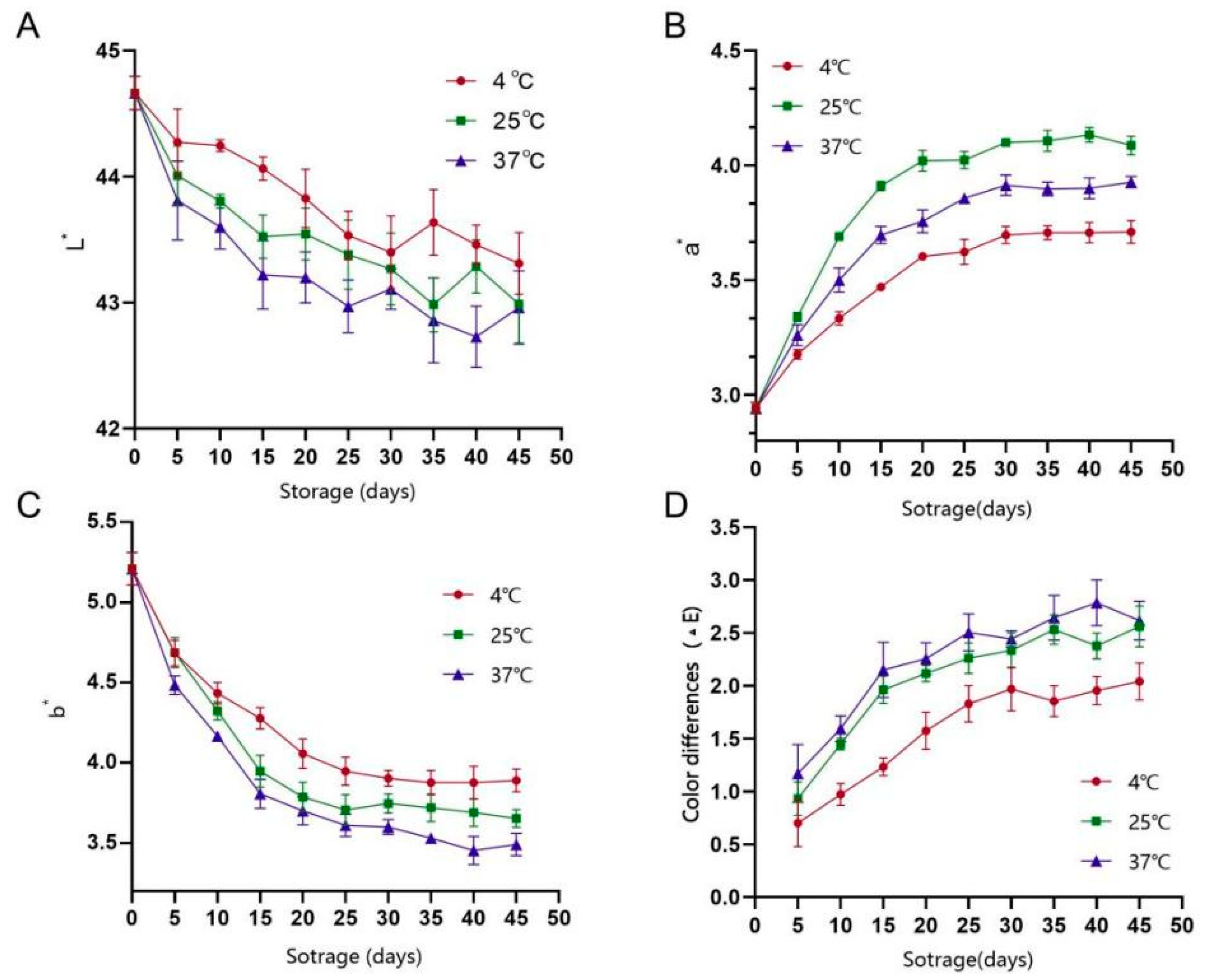
3.5.2. Analysis of Colour Change
3.5.3. Changes in Total Colony Counts During Storage of PAMJ
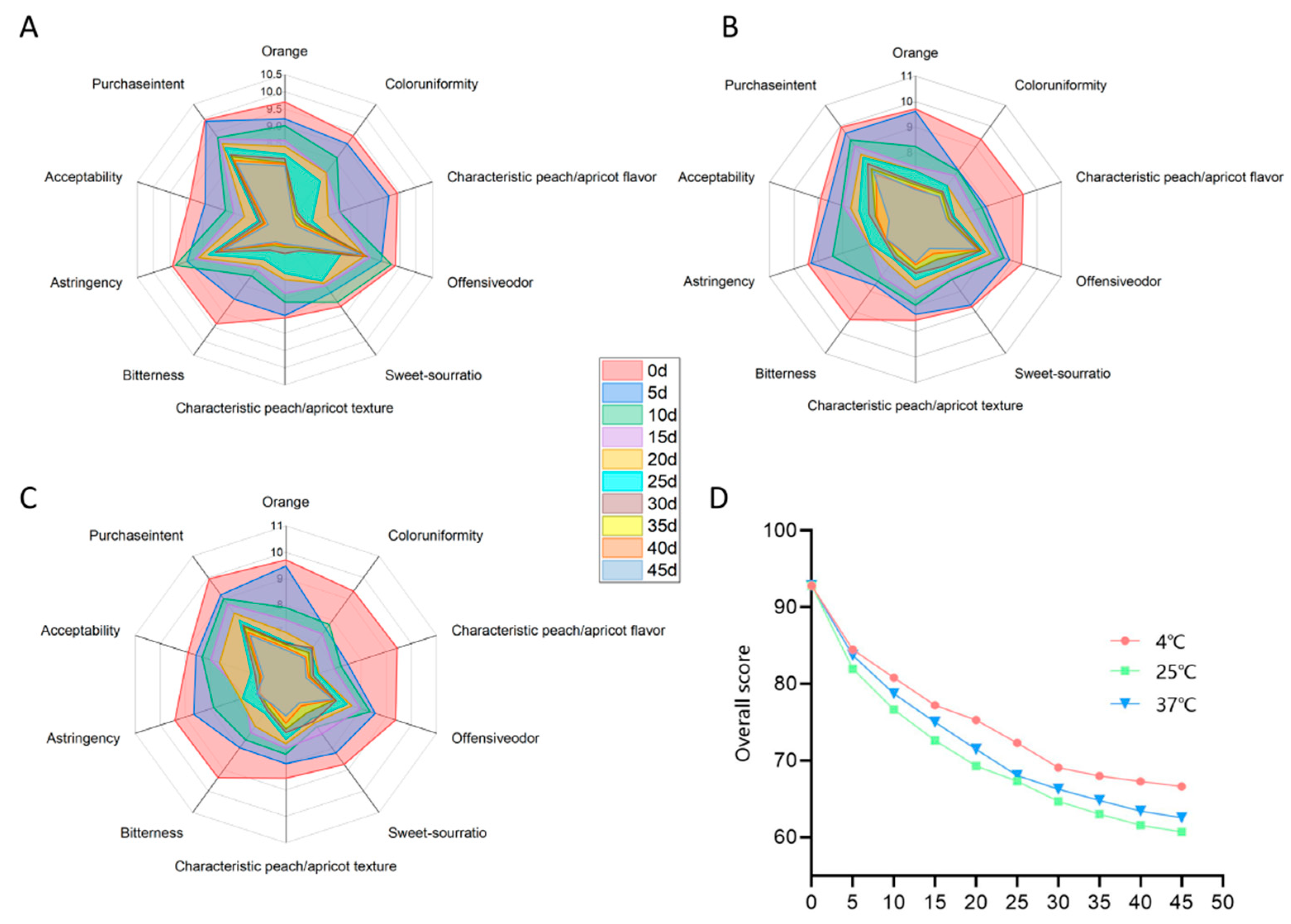
3.5.4. Changes in the Sensory Quality of PAMJ
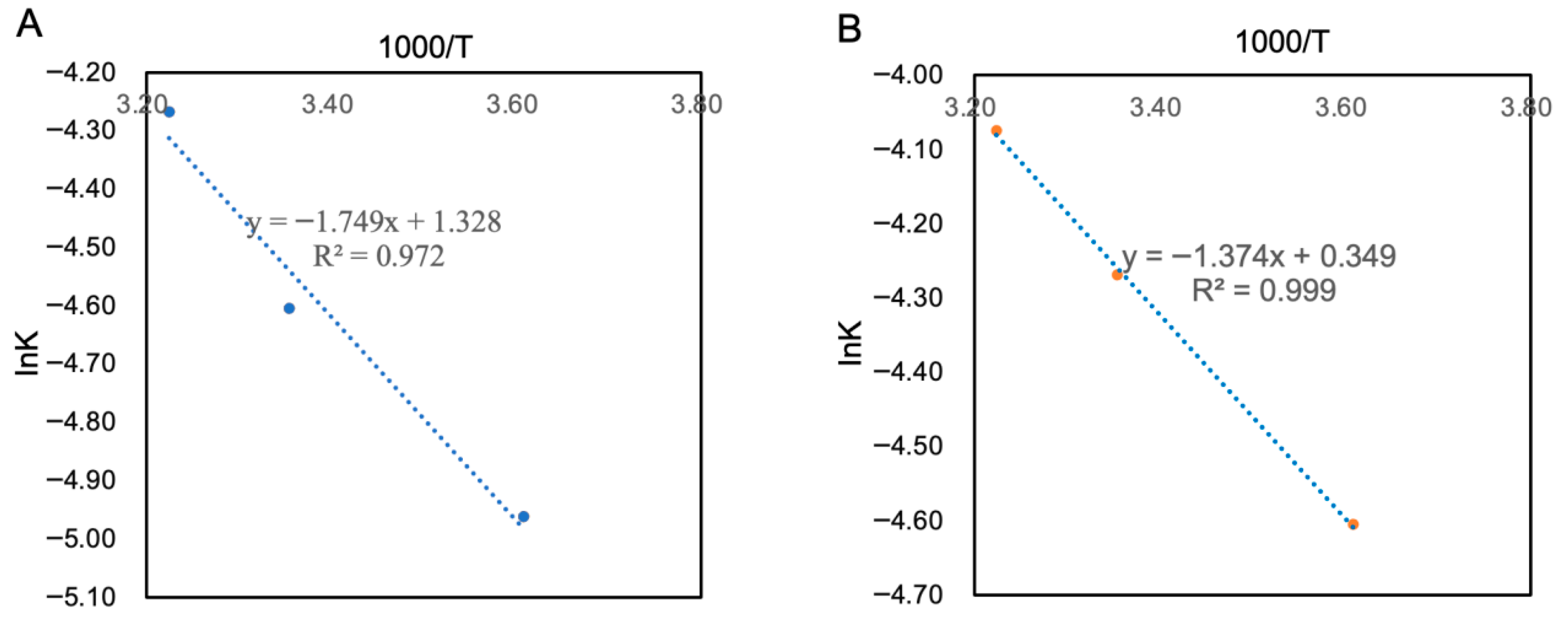
3.5.5. Shelf-Life Prediction for PAMJ
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FPJ | fermented peach juice |
| FAJ | fermented apricot juice |
| PAMJ | fermented peach–apricot mixed juice |
| PABJ | fermented peach–apricot blended juice |
| SOD | superoxide dismutase |
| NVC | number of volatile compounds |
| TSS | total soluble solid |
| VOCs | volatile organic compounds |
| LAB | lactic acid bacteria |
| RSM | response surface methodology |
| ELT | electronic tongue |
| ELN | electronic nose |
| MRS | deMan, Rogosa and Sharpe |
| BBD | Box–Behnken design |
| GC–MS | gas chromatography–mass spectrometry |
| PCA | principal component analysis |
References
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and phytochemical traits of apricots (Prunus armeniaca L.) for application in nutraceutical and health industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Tan, F.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chem. 2022, 366, 130604. [Google Scholar] [CrossRef]
- Raji, R.; Jannatizadeh, A.; Fattahi, R.; Esfahlani, M.A. Investigation of variability of apricot (Prunus armeniaca L.) using morphological traits and microsatellite markers. Sci. Hortic. 2014, 176, 225–231. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Shao, S.; Huang, M.; Gou, N.; Zhang, Y.; Chen, C.; Bai, H.; Qu, J.; Huang, Z.; et al. Metabolomic and transcriptomic analyses reveal novel mechanisms underlying the long-storage trait of apricot (Prunus armeniaca L.). Sci. Hortic. 2023, 318, 112068. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, Z.; He, H.; Shi, L.; Zhu, X.; Cui, K. Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022, 387, 132921. [Google Scholar] [CrossRef] [PubMed]
- Muhialdin, B.J.; Kadum, H.; Zarei, M.; Meor Hussin, A.S. Effects of metabolite changes during lacto-fermentation on the biological activity and consumer acceptability for dragon fruit juice. LWT 2020, 121, 108992. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Ricci, A.; Marrella, M.; Hadj Saadoun, J.; Bernini, V.; Godani, F.; Dameno, F.; Neviani, E.; Lazzi, C. Development of lactic acid-fermented tomato products. Microorganisms 2020, 8, 1192. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, F.; Yu, D.; Liu, X.; Huang, W.; Zhan, J. Dynamic changes in phenolic compounds, colour and antioxidant activity of mulberry wine during alcoholic fermentation. J. Funct. Foods 2015, 18, 254–265. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, C.; Pu, X.; Li, T.; Shi, X.; Wang, B.; Cheng, W. Flavor and functional analysis of Lactobacillus plantarum fermented apricot juice. Fermentation 2022, 8, 533. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Zhang, Q.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Changes in nutritional composition, volatile organic compounds and antioxidant activity of peach pulp fermented by Lactobacillus. Food Biosci. 2022, 49, 101894. [Google Scholar] [CrossRef]
- Tang, S.; Luo, N.; Zeng, Q.; Dong, L.; Zhang, R.; He, S.; Nag, A.; Huang, F.; Su, D. Lychee pulp phenolics fermented by mixed lactic acid bacteria strains promote the metabolism of human gut microbiota fermentation in vitro. Food Funct. 2023, 14, 7672–7681. [Google Scholar] [CrossRef]
- Aihaiti, A.; Zhao, L.; Maimaitiyiming, R.; Wang, L.; Liu, R.; Mu, Y.; Chen, K.; Wang, Y. Changes in volatile flavors during the fermentation of tomato (Solanum lycopersicum L.) juice and its storage stabilization. Food Chem. 2025, 463, 141077. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, R.; Mu, Y.; Lv, M.; Xing, J.; Zheng, L.; Aihaiti, A.; Wang, L. Study on the mechanisms of flavor compound changes during the lactic fermentation process of peach and apricot mixed juice. Foods 2024, 13, 3835. [Google Scholar] [CrossRef]
- Lv, M.; Liu, X.; Liu, R.; Aihaiti, A.; Hong, J.; Zheng, L.; Xing, J.; Cui, Y.; Wang, L. Untargeted metabolomics reveals flavor and metabolic changes in mixed Lactobacillus-fermented black mulberry juice. Food Chem. X 2025, 27, 102367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, J.; Li, J.; Zhang, P.; Tang, F.; Shan, C. Influence of lactic acid bacteria on physicochemical indexes, sensory and flavor characteristics of fermented sea buckthorn juice. Food Biosci. 2022, 46, 101519. [Google Scholar] [CrossRef]
- Lv, M.; Aihaiti, A.; Liu, X.; Tuerhong, N.; Yang, J.; Chen, K.; Wang, L. Development of probiotic-fermented black mulberry (Morus nigra L.) juice and its antioxidant activity in C2C12 cells. Fermentation 2022, 8, 697. [Google Scholar] [CrossRef]
- Pinmanee, P.; Sompinit, K.; Jantimaporn, A.; Khongkow, M.; Haltrich, D.; Nimchua, T.; Sukyai, P. Purification and immobilization of superoxide dismutase obtained from Saccharomyces cerevisiae TBRC657 on bacterial cellulose and its protective effect against oxidative damage in fibroblasts. Biomolecules 2023, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Razola-Díaz, M.d.C.; De Montijo-Prieto, S.; Guerra-Hernández, E.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Gómez-Caravaca, A.M.; Verardo, V. Fermentation of orange peels by lactic acid bacteria: Impact on phenolic composition and antioxidant activity. Foods 2024, 13, 1212. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, K.; Ye, S.; Du, M.; Wang, Z. Effects of different raw materials on bacterial community and flavor compounds in fermented red sour soup. Food Biosci. 2025, 65, 106034. [Google Scholar] [CrossRef]
- Cai, L.; Wang, W.; Tong, J.; Fang, L.; He, X.; Xue, Q.; Li, Y. Changes of bioactive substances in lactic acid bacteria and yeasts fermented kiwifruit extract during the fermentation. LWT 2022, 164, 113629. [Google Scholar] [CrossRef]
- Cao, H.; Bai, M.; Lou, Y.; Yang, X.; Zhao, C.; Lu, K.; Zhang, P. Optimization of the brewing process and analysis of antioxidant activity and flavor of elderberry wine. Fermentation 2023, 9, 276. [Google Scholar] [CrossRef]
- Luo, D.; Pang, X.; Xu, X.; Bi, S.; Zhang, W.; Wu, J. Identification of cooked off-flavor components and analysis of their formation mechanisms in melon juice during thermal processing. J. Agric. Food Chem. 2018, 66, 5612–5620. [Google Scholar] [CrossRef]
- Ao, H.; Tang, C.; Lu, Y.; Zhang, Y.; He, L.; Qiu, S.; Yan, Y.; Li, C. Characterization of physicochemical properties, sensory characteristics, and volatile compounds with a special focus on the terpene profile of commercial chinese kiwifruit wines. J. Food Compos. Anal. 2025, 140, 107187. [Google Scholar] [CrossRef]
- Huang, J.; He, W.; Wang, P.; Geng, J.; Zhan, P.; Tian, H. Characterization of aroma profiles in flat peach juice post-thermal sterilization: Insights from comprehensive two-dimensional GC with time-of-flight mass spectrometry (GC×GC-TOF-MS), sensory analysis, and chemometric approaches. J. Food Compos. Anal. 2024, 133, 106398. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Bai, Y.; Fu, C.; Zhou, M.; Gao, B.; Wang, C.; Li, D.; Hu, Y.; Xu, N. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT 2017, 84, 753–763. [Google Scholar] [CrossRef]
- Lu, T.; Song, B.; Yang, J.; Tan, H.; Qiao, H.; Zhi, W.; Chen, R.; Sheng, Z. Lactobacillus HNC7-YLC92 improves the fermentation quality of cassava–acerola cherry beverage. Fermentation 2024, 10, 90. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Z.; Feng, S.; Shi, X.; Qin, L.; Zeng, H. Correlation analysis between microbial communities and flavor compounds during the post-ripening fermentation of traditional chili bean paste. Foods 2024, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sakamoto, M.; Phacharapan, S.; Inoue, N.; Kamitani, Y. Antioxidant characteristic changes, sensory evaluation, processing and storage of functional water modified juice. Food Biosci. 2023, 52, 102468. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.; Niki, E.; Yoshikawa, T. Antioxidant action of fermented grain food supplement: Scavenging of peroxyl radicals and inhibition of plasma lipid oxidation induced by multiple oxidants. Food Chem. 2017, 237, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, M.; Maimaitiyiming, R.; Chen, K.; Tuerhong, N.; Yang, J.; Aihaiti, A.; Wang, L. Development of fermented sea buckthorn (Hippophae rhamnoides L.) juice and investigation of its antioxidant and antimicrobial activity. Front. Nutr. 2023, 10, 1120748. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Aguiló-Aguayo, I.; Bobo, G.; Chung, A.V.; Tiwari, B.K. Effect of storage on total phenolics, antioxidant capacity, and physicochemical properties of blueberry (Vaccinium corymbosum L.) jam. J. Food Process. Preserv. 2018, 42, e13666. [Google Scholar] [CrossRef]
- Pi, T.-H.; Shiau, C.-Y.; Chang, C.-J.; Sung, W.-C. Studies on the development and quality of fish chiffon cake and its storage stability. J. Aquat. Food Prod. Technol. 2017, 26, 969–978. [Google Scholar] [CrossRef]

| No. | L. paracasei X1 (%) | L. brevis X2 (%) | L. reuteri X3 (%) | L. fermentum X4 (%) | SOD Activity (U/mL) |
|---|---|---|---|---|---|
| 1 | 16 | 15 | 34 | 15 | 162 |
| 2 | 17 | 18 | 31 | 14 | 168 |
| 3 | 18 | 21 | 28 | 13 | 175 |
| 4 | 19 | 14 | 35 | 12 | 143 |
| 5 | 20 | 17 | 32 | 11 | 145 |
| 6 | 21 | 20 | 29 | 10 | 150 |
| 7 | 22 | 13 | 36 | 9 | 126 |
| 8 | 23 | 16 | 33 | 8 | 129 |
| 9 | 24 | 19 | 30 | 7 | 125 |
| No. | L. brevis X1 (%) | L. fermentum X2 (%) | L. plantarum X3 (%) | L. acidophilus X4 (%) | SOD Activity (U/mL) |
|---|---|---|---|---|---|
| 1 | 16 | 15 | 34 | 15 | 315 |
| 2 | 17 | 18 | 31 | 14 | 349 |
| 3 | 18 | 21 | 28 | 13 | 365 |
| 4 | 19 | 14 | 35 | 12 | 328 |
| 5 | 20 | 17 | 32 | 11 | 340 |
| 6 | 21 | 20 | 29 | 10 | 335 |
| 7 | 22 | 13 | 36 | 9 | 324 |
| 8 | 23 | 16 | 33 | 8 | 321 |
| 9 | 24 | 19 | 30 | 7 | 302 |
| No. | L. fermentum X1 (%) | L. acidophilus X2 (%) | L. reuteri X3 (%) | L. paracasei X4 (%) | L. plantarum X5 (%) | L. brevis X6 (%) | SOD Activity (U/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 2 | 8 | 8 | 13 | 34 | 252 |
| 2 | 16 | 4 | 11 | 13 | 9 | 33 | 237 |
| 3 | 17 | 6 | 14 | 7 | 16 | 32 | 212 |
| 4 | 18 | 8 | 6 | 12 | 12 | 31 | 229 |
| 5 | 19 | 10 | 9 | 6 | 8 | 30 | 234 |
| 6 | 20 | 1 | 12 | 11 | 15 | 29 | 224 |
| 7 | 21 | 3 | 15 | 5 | 11 | 28 | 212 |
| 8 | 22 | 5 | 7 | 10 | 7 | 27 | 215 |
| 9 | 23 | 7 | 10 | 4 | 14 | 26 | 223 |
| 10 | 24 | 9 | 13 | 9 | 10 | 25 | 242 |
| FPJ | FAJ | PAMJ | |
|---|---|---|---|
| Fermentation temperature (°C) | 27 | 27 | 27 |
| 32 | 32 | 32 | |
| 37 | 37 | 37 | |
| 42 | 42 | 42 | |
| 47 | 47 | 47 | |
| Fermentation time (h) | 16 | 16 | 16 |
| 18 | 18 | 18 | |
| 20 | 20 | 20 | |
| 22 | 22 | 22 | |
| 24 | 24 | 24 | |
| Inoculum concentration (CFU/mL) | 2 × 106 | 2 × 106 | 2 × 106 |
| 5 × 106 | 5 × 106 | 5 × 106 | |
| 8 × 106 | 8 × 106 | 8 × 106 | |
| 11 × 106 | 11 × 106 | 11 × 106 | |
| 14 × 106 | 14 × 106 | 14 × 106 | |
| Initial TSS content (°Brix) | 10 | 16 | 12 |
| 12 | 18 | 14 | |
| 14 | 20 | 16 | |
| 16 | 22 | 18 | |
| 18 | 24 | 20 |
| No. | Factors | Response Values | ||||
|---|---|---|---|---|---|---|
| Time (h) | Inoculum Concentration (×106 CFU/mL) | TSS (°Brix) | Temperature (°C) | SOD Activity (U/mL) | NVC (Types) | |
| 1 | 20 | 5 | 16 | 42 | 293 | 70 |
| 2 | 20 | 5 | 14 | 32 | 245 | 46 |
| 3 | 18 | 5 | 16 | 37 | 255 | 61 |
| 4 | 20 | 5 | 16 | 37 | 292 | 65 |
| 5 | 20 | 7 | 16 | 37 | 235 | 42 |
| 6 | 20 | 3 | 16 | 37 | 256 | 61 |
| 7 | 22 | 5 | 16 | 32 | 235 | 47 |
| 8 | 20 | 7 | 16 | 37 | 260 | 66 |
| 9 | 20 | 5 | 16 | 37 | 288 | 66 |
| 10 | 20 | 5 | 16 | 37 | 290 | 66 |
| 11 | 18 | 3 | 16 | 37 | 248 | 54 |
| 12 | 20 | 7 | 14 | 37 | 252 | 47 |
| 13 | 22 | 3 | 16 | 37 | 263 | 59 |
| 14 | 20 | 7 | 16 | 32 | 260 | 55 |
| 15 | 42 | 6 | 16 | 37 | 265 | 53 |
| 16 | 20 | 3 | 14 | 37 | 240 | 49 |
| 17 | 22 | 5 | 16 | 32 | 255 | 54 |
| 18 | 20 | 3 | 16 | 32 | 240 | 52 |
| 19 | 20 | 5 | 16 | 37 | 295 | 65 |
| 20 | 20 | 5 | 18 | 32 | 260 | 56 |
| 21 | 20 | 5 | 14 | 32 | 249 | 54 |
| 22 | 18 | 5 | 16 | 42 | 260 | 51 |
| 23 | 22 | 7 | 16 | 37 | 240 | 44 |
| 24 | 20 | 5 | 18 | 42 | 257 | 50 |
| 25 | 18 | 5 | 14 | 37 | 258 | 55 |
| 26 | 18 | 7 | 16 | 37 | 273 | 64 |
| 27 | 22 | 5 | 14 | 37 | 250 | 38 |
| 28 | 20 | 3 | 18 | 37 | 255 | 53 |
| 29 | 18 | 5 | 18 | 37 | 270 | 50 |
| Sample | FPJ/FAJ/PAMJ/PABJ |
|---|---|
| Capillary chromatographic column | DB-5MS (30 m × 0.25 mm × 0.25 µm) |
| Carrier gas | Helium |
| Carrier gas flow rate | 1.2 mL/min |
| Injection mode | Splitless |
| Detector temperature | 250 °C |
| Temperature programme | Initial column temperature of 40 °C held for 3.5 min |
| Heated to 180 °C at 7 °C/min | |
| Heated to 280 °C at 25 °C/min, held for 5 min | |
| Ion source temperature | 150 °C |
| Interface temperature | 280 °C |
| Electron energy | 70 eV |
| Scoring Items | Scoring Criteria | Score |
|---|---|---|
| Appearance | Colour | 0–10 |
| Colour uniformity | 0–10 | |
| Aroma | Characteristic peach/apricot flavour | 0–10 |
| Offensive odour | 0–10 | |
| Taste | Sourness | 0–10 |
| Sweetness | 0–10 | |
| Bitterness | 0–10 | |
| Astringency | 0–10 | |
| Overall acceptability | Acceptability | 0–10 |
| Purchase intention | 0–10 |
| No. | Strain | pH | Initial TSS Content (%) | SOD Activity (U/mL) | NVC (Types) | Sensory Score | Time to 108 CFU/mL (h) |
|---|---|---|---|---|---|---|---|
| 1 | Unfermented | 4.04 ± 0.01 b | 12.00 ± 0.01 b | 113.91 ± 0.10 a | 41 ± 0.75 cd | 80.00 ± 0.63 b | / |
| 2 | L. plantarum | 3.83 ± 0.02 a | 11.85 ± 0.02 c | 137.84 ± 8.64 bc | 46± 1.02 bc | 81.43 ± 1.34 d | 16.32 ± 0.32 b |
| 3 | L. casei | 3.87 ± 0.01 abc | 11.85 ± 0.16 de | 134.53 ± 8.23 b | 43 ± 0.55 cd | 82.13 ± 0.23 ab | 24.13 ± 0.32 ab |
| 4 | L. acidophilus | 3.87 ± 0.02 de | 11.8 ± 0.22 de | 126.18 ± 10.30 ab | 46 ± 1.17 bc | 84.46 ± 1.46 cd | 20.76 ± 0.24 ab |
| 5 | L. reuteri | 3.83 ± 0.02 g | 11.5 ± 0.11 de | 154.63 ± 7.45 bc | 50 ± 1.03 f | 85.46 ± 0.24 a | 19.96 ± 0.34 cd |
| 6 | P. pentosaceus | 3.89 ± 0.03 fg | 11.6 ± 0.23 de | 132.34 ± 4.62 e | 45 c ± 1.39 ef | 84.26 ± 1.45 a | 19.67 ± 0.43 a |
| 7 | L. brevis | 3.82 ± 0.03 c | 11.5 ± 0.08 bcd | 142.78 ± 2.24 d | 50 c ± 1.67 ab | 85.03 ± 1.34 ab | 17.83 ± 0.23 ab |
| 8 | L. fermentum | 3.82 ± 0.02 cd | 11.5 ± 0.06 a | 146.12 ± 13.31 a | 49 ± 1.42 de | 88.80 ± 0.52 cd | 18.70 ± 0.34 ab |
| 9 | P. acidilactici | 3.84 ± 0.03 d | 11.8 ± 0.07 b | 129.19 ± 5.34 ab | 46 ± 0.08 e | 84.89 ± 1.42 cd | 19.77 ± 0.62 bc |
| 10 | L. paracasei | 3.82 ± 0.02 a | 11.6 ± 0.11 abc | 153.76 ± 4.45 cd | 48 ± 1.41 de | 85.46 ± 0.34 abc | 18.85 ± 0.41 a |
| No | Strain | pH | Initial TSS Content (%) | SOD Activity (U/mL) | NVC (Types) | Sensory Score | Time to 108 CFU/mL (h) |
|---|---|---|---|---|---|---|---|
| 1 | Unfermented | 3.62 ± 0.00 bc | 16.6 ± 0.08 ab | 287.91 ± 0.00 i | 43 ± 0.75 ef | 80.00 ± 0.82 e | / |
| 2 | L. plantarum | 3.46 ± 0.00 fg | 16.1 ± 0.14 de | 327.84 ± 8.98 ef | 49 ± 0.0.75 c | 85.43 ± 1.50 e | 20.32 ± 0.45 ab |
| 3 | L. casei | 3.48 ± 0.01 ab | 16.1 ± 0.26 de | 324.53 ± 8.16 de | 45 ± 1.02 a | 82.13 ± 0.30 a | 19.13 ± 0.19 ef |
| 4 | L. acidophilus | 3.47 ± 0.00 defg | 16.1 ± 0.08 e | 326.18 ± 10.90 gh | 52 ± 0.55 cd | 84.46 ± 1.48 ef | 18.76 ± 0.42 f |
| 5 | L. reuteri | 3.48 ± 0.01 g | 16.5 ± 0.12 de | 314.63 ± 7.59 ef | 49 ± 1.42 ef | 82.46 ± 0.53 fg | 20.96 ± 0.85 ab |
| 6 | P. pentosaceus | 3.48 ± 0.01 fg | 16.3 ± 0.00 cd | 302.34± 4.62 i | 45 ± 1.03e | 85.26 ± 1.03 g | 19.67 ± 0.42 def |
| 7 | B. thermophilum | 3.48 ± 0.01 cde | 16.5± 0.19 bc | 322.78 ± 2.56 fg | 48 ± 1.72 bc | 81.03 ± 1.18 de | 20.83 ± 0.51 def |
| 8 | L. fermentum | 3.47 ± 0.02 cd | 16.2 ± 0.17 de | 333.12 ± 13.31 e | 51 ± 1.39 ab | 88.80 ± 0.64 c | 24.70 ± 0.33 cd |
| 9 | L. brevis | 3.47 ± 0.01 def | 16.1 ± 0.14 ab | 329.19 ± 5.65 h | 50 ± 1.67 ab | 84.89 ± 1.42 fg | 19.77 ± 0.16 a |
| 10 | L. delbrueckii | 3.55 ± 0.02 def | 16.3 ± 0.08 a | 313.76 ± 4.56 cd | 46 ± 1.51 cd | 82.46 ± 0.87 fg | 20.85 ± 0.43 de |
| Source of Variation | SOD Activity (U/mL) | NVC (Compounds) | ||
|---|---|---|---|---|
| F Value | p Value | F Value | p Value | |
| Regression model | 83.15 | <0.0001 | 48.55 | <0.0001 |
| (A) Time | 25.55 | 0.0002 | 49.51 | <0.0001 |
| (B) Inoculum concentration | 3.91 | 0.068 | 3.09 | 0.1004 |
| (C) TSS | 64.35 | <0.0001 | 24.26 | 0.0002 |
| (D) Temperature | 5.33 | 0.0368 | 65.48 | <0.0001 |
| AB | 83.46 | <0.0001 | 58.02 | <0.0001 |
| AC | 0.326 | 0.5771 | 37.14 | <0.0001 |
| AD | 8.15 | 0.0127 | 0.8355 | 0.3761 |
| BC | 1.78 | 0.204 | 1.49 | 0.2431 |
| BD | 60.9 | <0.0001 | 101.1 | <0.0001 |
| CD | 0.0362 | 0.8518 | 0.3714 | 0.552 |
| A2 | 193.32 | <0.0001 | 117.75 | <0.0001 |
| B2 | 448.39 | <0.0001 | 60.02 | <0.0001 |
| C2 | 270.57 | <0.0001 | 240.48 | <0.0001 |
| D2 | 474.42 | <0.0001 | 79.41 | <0.0001 |
| Lack of fit | 0.9235 | 0.5846 | 0.4767 | 0.844 |
| R2 | 0.9631 | - | 0.9738 | - |
| R2Adj | 0.9263 | - | 0.9596 | - |
| Detection Index | Storage Temperature/K | Regression Equation | Reaction Rate Constant (k) | Regression Coefficient (R2) |
|---|---|---|---|---|
| SOD activity | 277.15 (4 °C) | y = 265.01e−0.007 | −0.007 | 0.960 |
| 298.15 (25 °C) | y = 265.01e−0.010 | −0.010 | 0.963 | |
| 310.15 (37 °C) | y = 265.01e−0.014 | −0.014 | 0.951 | |
| NVC | 277.15 (4 °C) | y = 65e−0.010 | −0.010 | 0.958 |
| 298.15 (25 °C) | y = 65e−0.014 | −0.014 | 0.947 | |
| 310.15 (37 °C) | y = 65e−0.017 | −0.017 | 0.951 |
| Storage Temperature (°C) | SOD Activity (U/mL) | NVC (Types) | ||||
|---|---|---|---|---|---|---|
| Predicted Value | Actual Value | Relative Error | Predicted Value | Actual Value | Relative Error | |
| 8 | 90 | 96 | 6.25% | 64 | 69 | 7.25% |
| 28 | 60 | 65 | 7.69% | 46 | 50 | 8.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Zhao, Y.; Yang, Z.; Liu, X.; Liu, R.; Lv, M.; Wang, L. Development of Fermented Peach–Apricot Mixed Juice and Study of Its Storage Stability. Foods 2025, 14, 3128. https://doi.org/10.3390/foods14173128
Lv S, Zhao Y, Yang Z, Liu X, Liu R, Lv M, Wang L. Development of Fermented Peach–Apricot Mixed Juice and Study of Its Storage Stability. Foods. 2025; 14(17):3128. https://doi.org/10.3390/foods14173128
Chicago/Turabian StyleLv, Shun, Yao Zhao, Zeping Yang, Xiaolu Liu, Ruoqing Liu, Mingshan Lv, and Liang Wang. 2025. "Development of Fermented Peach–Apricot Mixed Juice and Study of Its Storage Stability" Foods 14, no. 17: 3128. https://doi.org/10.3390/foods14173128
APA StyleLv, S., Zhao, Y., Yang, Z., Liu, X., Liu, R., Lv, M., & Wang, L. (2025). Development of Fermented Peach–Apricot Mixed Juice and Study of Its Storage Stability. Foods, 14(17), 3128. https://doi.org/10.3390/foods14173128





