Chemometric Differentiation of Organic Honeys from Southeastern Türkiye Based on Free Amino Acid and Phenolic Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
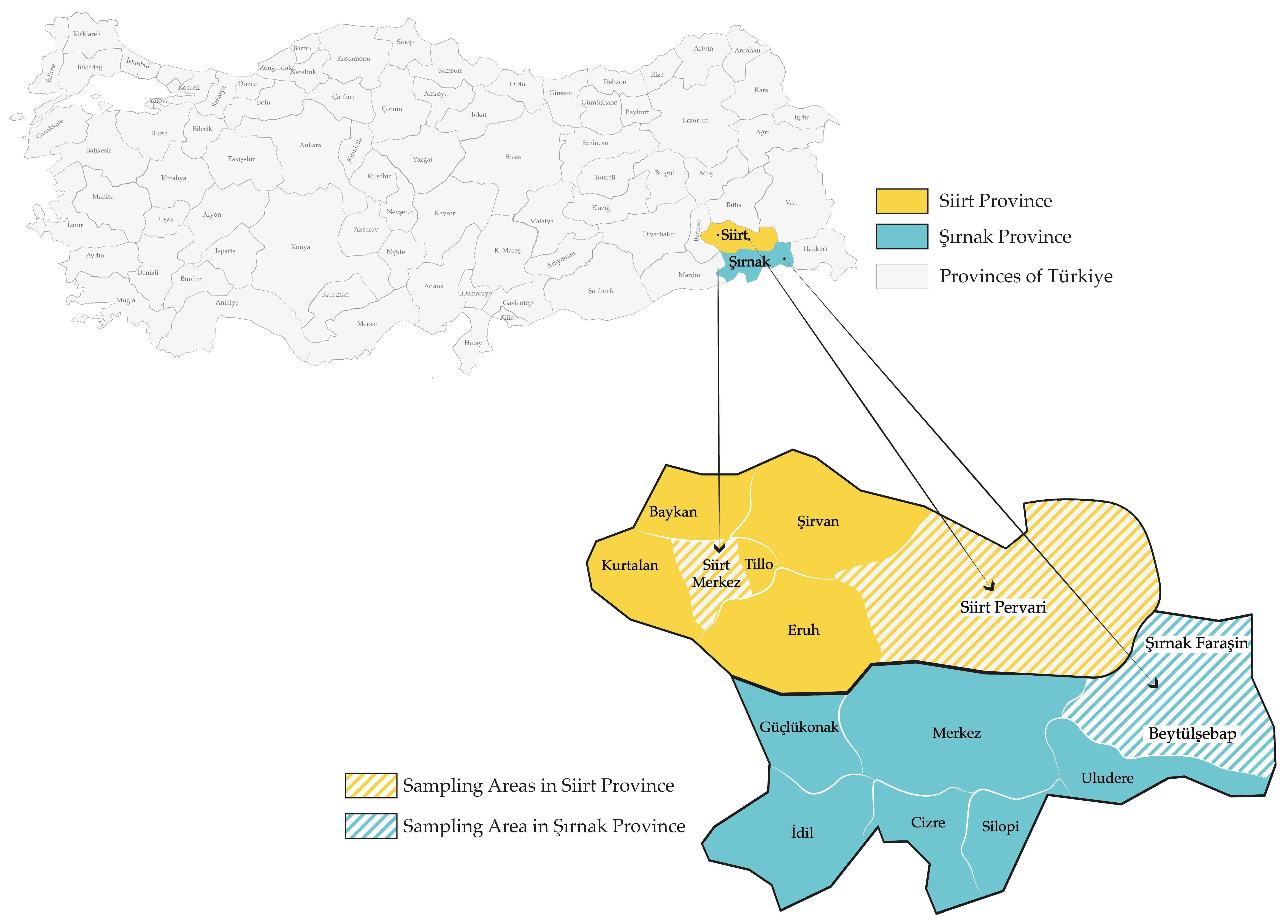
2.2. Honey Samples
2.3. Analysis of Free Amino Acid
2.3.1. Reagents and Chemicals for FAA Analysis
2.3.2. Sample Preparation for FAA Analysis
2.3.3. Free Amino Acid Analysis by UPLC-ESI-MS/MS
2.4. Analysis of Phenolic Compounds
2.4.1. Standards, Reagents, and Calibration
2.4.2. Sample Preparation and Extraction
2.4.3. UPLC-ESI-MS/MS Instrumentation and Conditions
2.5. Data Analysis
3. Results and Discussion
3.1. Determination of Free Amino Acid Content
3.2. Principal Component Analysis and Cluster Analysis for Free Amino Acids
3.3. Identifying Regional Fingerprints for FAA Using Supervised Classification Methods
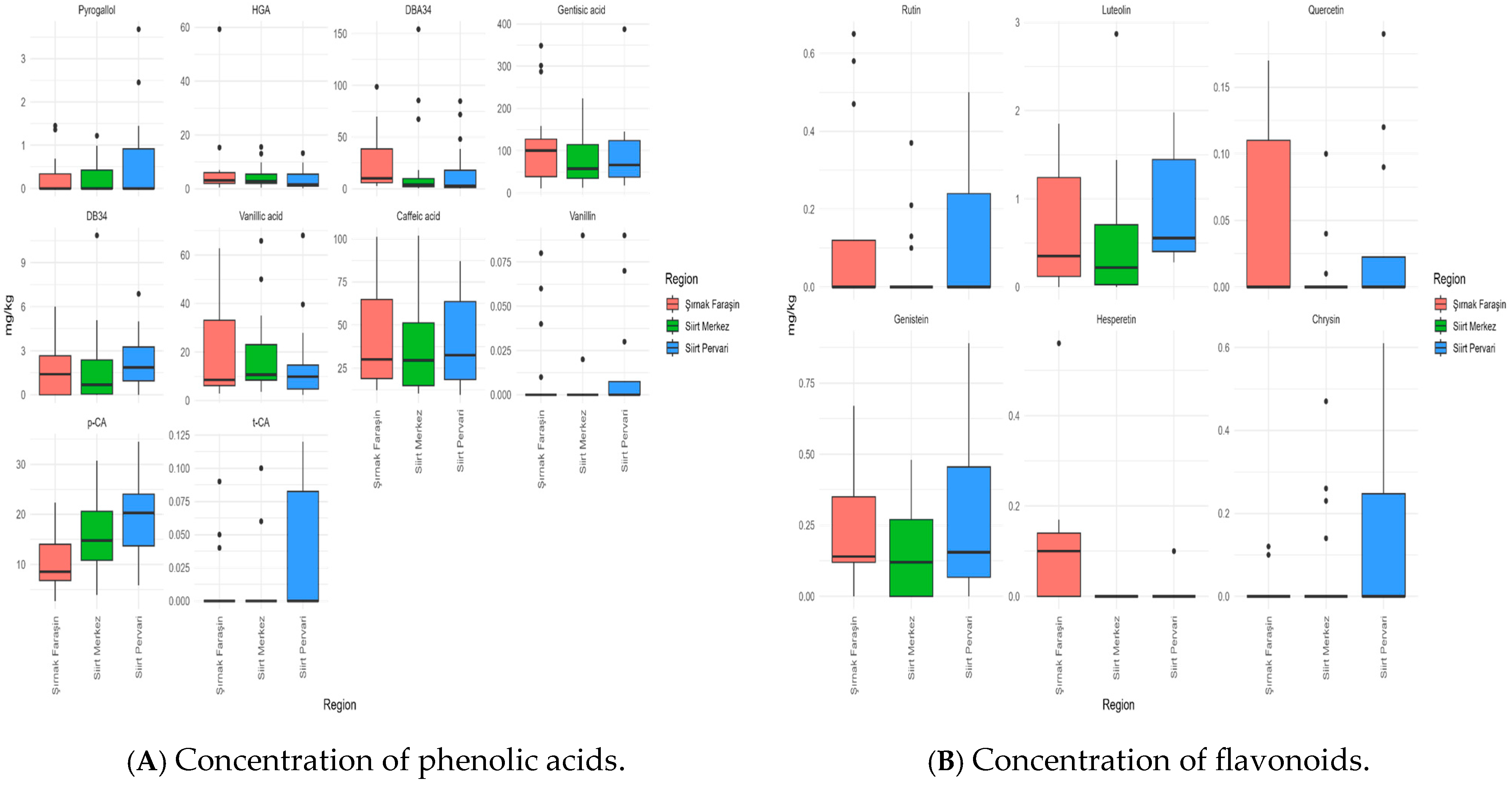
3.4. Determination of Phenolic Compound Profile
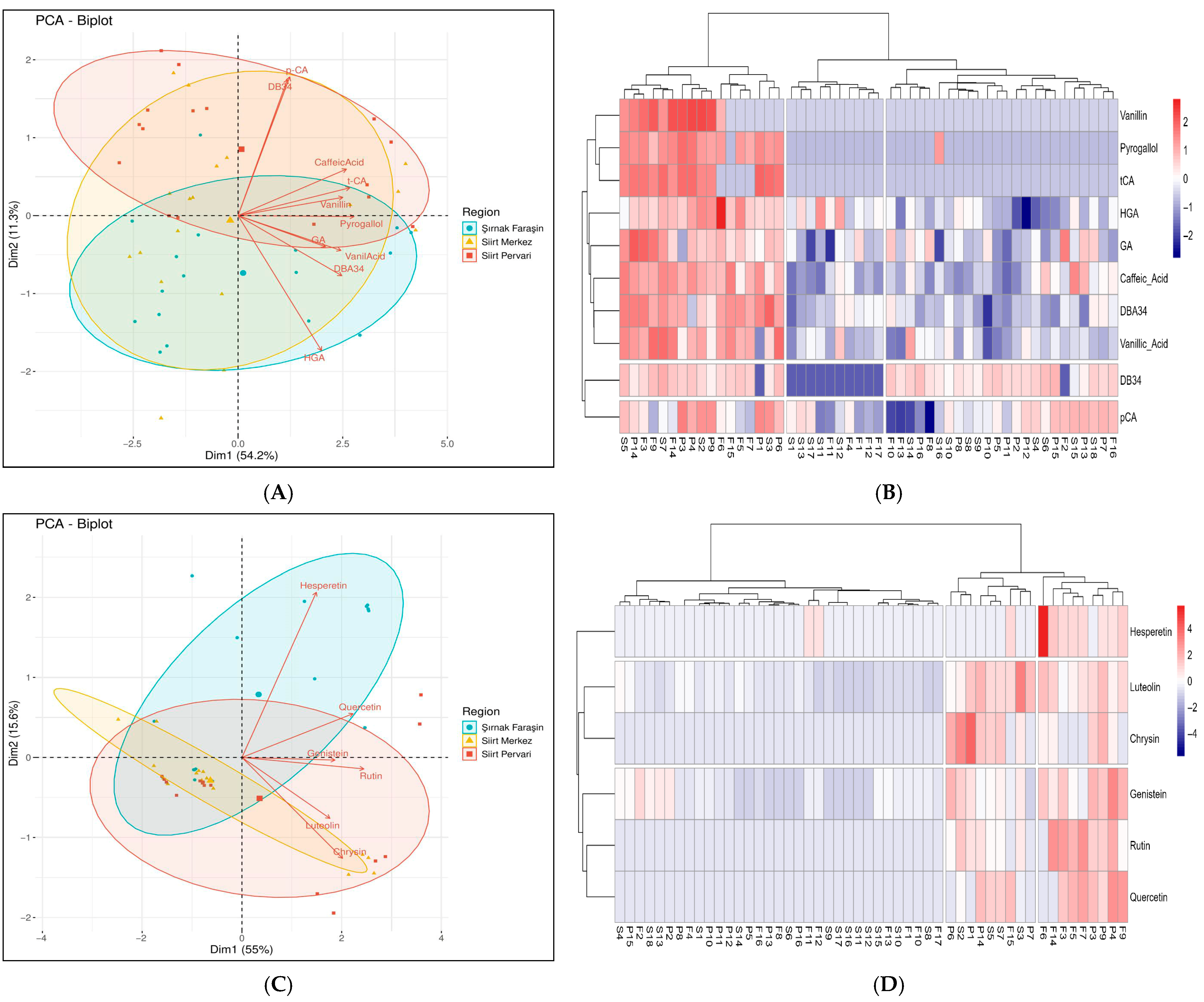
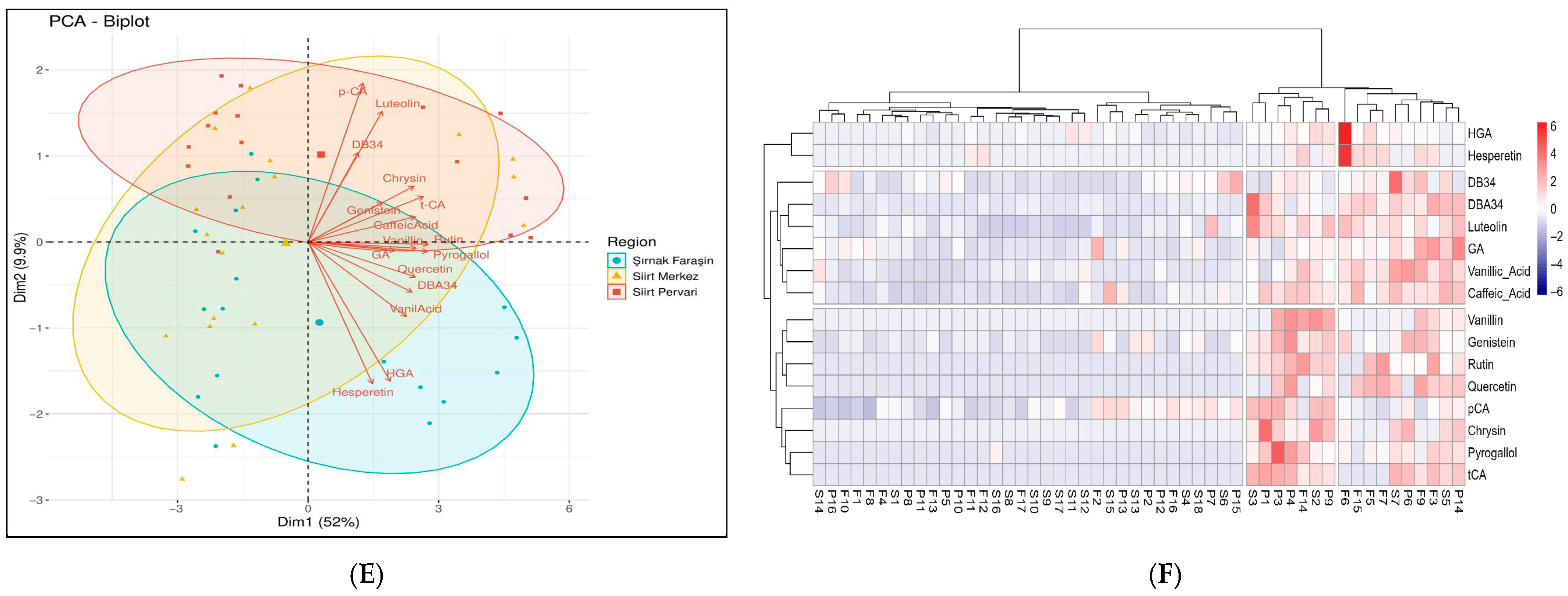
3.5. Principle Component Analysis and Cluster Analysis of Phenolic Profiles
3.5.1. Differentiation Based on Phenolic Acids
3.5.2. Differentiation Based on Flavonoids
3.5.3. The Integrated Analysis of the Complete Phenolic Profile
3.6. Identifying Regional Fingerprints for Phenolic Compounds Using Supervised Classification Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Kuś, P.M. Honey as Source of Nitrogen Compounds: Aromatic Amino Acids, Free Nucleosides and Their Derivatives. Molecules 2020, 25, 847. [Google Scholar] [CrossRef]
- Brugnerotto, P.; Fuente-Ballesteros, A.; Martín-Gómez, B.; Ares, A.M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O.; Bernal, J. Free amino acid profile in Mimosa scabrella honeydew honey from Brazil and chemometric analysis for geographical discrimination. Food Res. Int. 2024, 177, 113856. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. Evaluation of the botanical origin of Estonian uni- and polyfloral honeys by amino acid content. J. Agric. Food Chem. 2008, 56, 10716–10720. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Alvarez-Suarez, A.J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernández, T.Y.; et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Everstine, K.D.; Chin, H.B.; Lopes, F.A.; Moore, J.C. Database of Food Fraud Records: Summary of Data from 1980 to 2022. J. Food Prot. 2024, 87, 100227. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T. Establishing authenticity of honey via comprehensive Romanian honey analysis. Food Chem. 2020, 306, 125595. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I.A. Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Honey Market Presentation. Available online: https://agriculture.ec.europa.eu/document/download/c04a9774-5ba3-41f5-b256-08396b2888ec_en?filename=market-presentation-honey_spring2024_en.pdf (accessed on 21 March 2025).
- Jensen, J.D.; Mørkbak, M.R. The role of gastronomic, externality, and feasibility attributes in consumer demand for organic and local foods: The case of honey and apples. Int. J. Consum. Stud. 2013, 37, 634–641. [Google Scholar] [CrossRef]
- Cosmina, M.; Gallenti, G.; Marangon, F.; Troiano, S. Reprint of “Attitudes towards honey among Italian consumers: A choice experiment approach”. Appetite 2016, 106, 110–116. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273. [Google Scholar] [CrossRef]
- Ballco, P.; Jaafer, F.; de Magistris, T. Investigating the price effects of honey quality attributes in a European country: Evidence from a hedonic price approach. Agribusiness 2022, 38, 885–904. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.X. Chapter 3. Chemical Composition, Characterization, and Differentiation of Honey Botanical and Geographical Origins. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 62, pp. 89–137. [Google Scholar] [CrossRef]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef] [PubMed]
- She, S.; Chen, L.; Song, H.; Lin, G.; Li, Y.; Zhou, J.; Liu, C. Discrimination of geographical origins of Chinese acacia honey using complex 13C/12C, oligosaccharides and polyphenols. Food Chem. 2019, 272, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Nyarko, K.; Boozer, K.; Greenlief, C.M. Profiling of the polyphenol content of honey from different geographical origins in the United States. Molecules 2023, 28, 5011. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Iurlina, M.O.; Saiz, A.I.; Fritz, R.; Manrique, G.D. Major flavonoids of Argentinean honeys: Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chem. 2009, 115, 1141–1149. [Google Scholar] [CrossRef]
- Cometto, P.M.; Faye, P.F.; Di Paola Naranjo, R.D.; Rubio, M.A.; Aldao, M.A. Comparison of free amino acids profile in honey from three Argentinian regions. J. Agric. Food Chem. 2003, 51, 5079–5087. [Google Scholar] [CrossRef]
- Ren, W.; Li, Y.; Yin, Y.; Blachier, F. Overview. In Nutritional and Physiological Functions of Amino Acids in Pigs; Blachier, F., Wu, G., Yin, Y., Eds.; Springer: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Davies, A.M.C. Amino acid analysis of honeys from eleven countries. J. Apic. Res. 1975, 14, 29–39. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; dos Santos, F.A.R.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef]
- Silici, S.; Sagdic, O.; Ekici, L. Evaluation of the phenolic content, antiradical, and antimicrobial activity of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Ulusoy, E.; Kolaylı, S.; Sarıkaya, A.O. Antioxidant and antimicrobial activity of different floral origin honeys from Türkiye. J. Food Biochem. 2010, 34, 321–335. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant-derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2019, 34, 729–741. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, L.; Bao, W.; Ni, J.; Wang, Y.; Huang, Y.; Lyv, J.; Cao, X.; Chen, T.; You, K.; et al. Gentisic acid prevents colorectal cancer metastasis via blocking GPR81-mediated DEPDC5 degradation. Phytomedicine 2024, 129, 155615. [Google Scholar] [CrossRef]
- Picone, G.; Mezzetti, B.; Babini, E.; Capocasa, F.; Placucci, G.; Capozzi, F. Unsupervised principal component analysis of NMR metabolic profiles for the assessment of substantial equivalence of transgenic grapes (Vitis vinifera). J. Agric. Food Chem. 2011, 17, 9271–9279. [Google Scholar] [CrossRef]
- Kivrak, İ. Free Amino Acid Profiles of 17 Turkish Unifloral Honeys. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 855–862. [Google Scholar] [CrossRef]
- Thiele, B.; Stein, N.; Oldiges, M.; Hofmann, D. Direct analysis of underivatized amino acids in plant extracts by LC-MS-MS. In Amino Acid Analysis; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 828, pp. 317–328. [Google Scholar] [CrossRef]
- Kowalski, S.; Kopuncová, M.; Ciesarová, Z.; Kukurová, K. Free amino acids profile of Polish and Slovak honeys based on LC–MS/MS method without the prior derivatisation. J. Food Sci. Technol. 2017, 54, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Kıvrak, Ş.; Kıvrak, İ. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2017, 20, 864–876. [Google Scholar] [CrossRef]
- Davies, A.M.C. The application of amino acid analysis to the determination of the geographic origin of honey. J. Food Technol. 1976, 11, 515–523. [Google Scholar]
- Davies, A.M.C.; Harris, R.G. Free Amino Acid Analysis of Honeys from England and Wales: Application to the Determination of the Geographical Origin of Honeys. J. Apic. Res. 1982, 21, 168–173. [Google Scholar] [CrossRef]
- Gilbert, J.; Shephard, M.J.; Wallwork, M.A.; Harris, R.G. Determination of the geographical origin of honeys by multivariate analysis of gas chromatographic data on their free amino acid content. J. Apic. Res. 1981, 20, 125–135. [Google Scholar] [CrossRef]
- Cesare Marincola, F.; Palmas, C.; Lastres Couto, M.A.; Paz, I.; Cremades, J.; Pintado, J.; Bruni, L.; Picone, G. Metabolic Profile of Senegalese Sole (Solea senegalensis) Muscle: Effect of Fish–Macroalgae IMTA-RAS Aquaculture. Molecules 2025, 30, 2518. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Elmore, J.S.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Giroud, B.; Albert, M.; Lheritier, J.; Grenier-Loustalot, M.F. Characterization of honey amino acid profiles using high-pressure liquid chromatography to control authenticity. Anal. Bioanal. Chem. 2004, 378, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, L.; Chang, Q.; Peng, T.; Hu, X.; Fan, C.; Pang, G.; Wang, W. Discrimination of botanical origins for Chinese honey according to free amino acids content by high-performance liquid chromatography with fluorescence detection with chemometric approaches. J. Sci. Food Agric. 2017, 97, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Hermosín, I.; Chicón, R.M.; Cabezudo, M.D. Free amino acid composition and botanical origin of honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Iglesias, M.T.; De Lorenzo, C.; Del Carmen Polo, M.; Martín-Alvarez, P.J.; Pueyo, E. Usefulness of amino acid composition to discriminate between honeydew and floral honeys. Application to honeys froma small geographic area. J. Agric. Food Chem. 2004, 52, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organisation of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 March 2025).
- Republic of Turkey Ministry of Enviroment and Forestry. UN Convention of Biological Diversity Fourth National Report 30/06/2009. Available online: https://www.cbd.int/doc/world/tr/tr-nr-04-en.pdf (accessed on 23 March 2025).
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physicochemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Şenyuva, H.Z.; Gilbert, J.; Silici, S.; Charlton, A.; Dal, C.; Gürel, N.; Cimen, D. Profiling Turkish Honeys to Determine Authenticity Using Physical and Chemical Characteristics. J. Agric. Food Chem. 2009, 57, 3911–3919. [Google Scholar] [CrossRef]
- Silici, S.; Karaman, K. Chemometric approaches for the characterization of turkish rhododendron and honeydew honeys depending on amino acid composition. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 864–877. [Google Scholar] [CrossRef]
- Turkish Agriculture and Forestry Journal. Available online: http://www.turktarim.gov.tr/Haber/761/siirtten-sifa-kaynagi-pervari-bali (accessed on 16 August 2025).
- UNDP Türkiye. Organic Agriculture Cluster. Available online: https://www.undp.org/turkiye/projects/organic-agriculture-cluster (accessed on 16 August 2025).
- Republic of Türkiye Ministry of Industry and Technology. Başarılar: Arıcılık. Available online: https://www.gap.gov.tr/sayfa/basari-hikayeleri/basarilar/aricilik/ (accessed on 16 August 2025).
- Republic of Türkiye Ministry of Agriculture and Forestry Apiculture Research Institute. Beekeeping Statistics by Province in Türkiye. 2024. Available online: https://arastirma.tarimorman.gov.tr/aricilik/Link/2/Aricilik-Istatistikleri (accessed on 16 August 2025).
- Turkish Patent and Trademark Office. Official Statistics. Available online: https://ci.turkpatent.gov.tr/cografi-isaretler/detay/37928 (accessed on 16 August 2025).
- Turkish Patent and Trademark Office. Official Statistics. Available online: https://ci.turkpatent.gov.tr/cografi-isaretler/detay/8563 (accessed on 16 August 2025).
- Gürbüz, S.; Özenirler, Ç.; Mayda, N.; Gençay Çelemli, Ö.; Özkök, A. Pollen Spectrum of Some Honey Samples Produced in Siirt-Turkey. Hacet. J. Biol. Chem. 2019, 47, 295–303. [Google Scholar] [CrossRef]
- Gürbüz, S.; Gençay Çelemli, Ö.; Özenirler, Ç.; Mayda, N.; Özkök, A.; Sorkun, K. Melissopalnological analysis of honey samples collected from Şirnak City. Uludag Bee J. 2019, 19, 126–135. [Google Scholar] [CrossRef]
- Kıvrak, İ.; Kıvrak, Ş.; Harmandar, M. Free Amino Acid Profiling in the Giant Puffball Mushroom (Calvatia gigantea) Using UPLC-MS/MS. Food Chem. 2014, 158, 88–92. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Seraglio, S.K.T.; Rocha, G.; Balderas, C.B.; Piovezan, M.; Gonzaga, L.V.; Falkenberg, D.D.B.; Fett, R.; de Oliveira, M.A.L.; Costa, A.C.O. Free amino acid determination by GC-MS combined with a chemometric approach for geographical classification of bracatinga honeydew honey (Mimosa scabrella Bentham). Food Control 2017, 78, 383–392. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyńsk, P.; Iwaniuk, P. Analysis of 22 free amino acids in honey from Eastern Europe and Central Asia using LC-MS/MS technique without derivatization step. J. Food Compos. Anal. 2021, 98, 103837. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Cui, Z.; Wang, T.; Liu, T.; Liu, G. Analysis of Free Amino Acid Composition and Honey Plant Species in Seven Honey Species in China. Foods 2024, 13, 1065. [Google Scholar] [CrossRef]
- Conte, L.S.; Miorini, M.; Giomo, A.; Bertacco, G.; Zironi, R. Evaluation of some fixed components for unifloral honey characterization. J. Agric. Food Chem. 1998, 46, 1844–1849. [Google Scholar] [CrossRef]
- Maione, C.; Barbosa, F.; Melgaço Barbosa, R. Predicting the botanical and geographical origin of honey with multivariate data analysis and machine learning techniques: A review. Comput. Electron. Agric. 2019, 157, 436–446. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid and flavonoid composition of Malaysian honeys. J. Food Biochem. 2017, 41, e12282. [Google Scholar] [CrossRef]
- Ganai, A.A.; Farooqi, H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed. Pharmacother. 2015, 76, 30–38. [Google Scholar] [CrossRef] [PubMed]


| Free Amino Acids (FAAs) | Şırnak Faraşin (n = 17) | Siirt Merkez (n = 18) | Siirt Pervari (n = 16) | p-Value * |
|---|---|---|---|---|
| Gly | 5.63 [2.76, 9.33] a | 6.09 [3.28, 8.41] a | 10.16 [6.61, 22.11] b | 0.013 |
| Ala | 3.56 [2.28, 6.33] | 4.18 [2.44, 10.29] | 6.37 [3.85, 9.51] | 0.110 |
| Ser | 2.87 [2.08, 6.27] a | 4.96 [2.83, 8.01] ab | 6.95 [4.35, 11.35] b | 0.036 |
| Pro | 410.3 [310.9, 552,3] | 522.5 [333.5, 759.6] | 495.5 [365.9, 628.7] | 0.223 |
| Val | 15.94 [14.12, 30.45] a | 26.96 [17.43, 46.92] ab | 31.47 [15.34, 43.43] b | 0.059 |
| Thr | 12.33 [9.6, 14.41] | 14.4 [10.6, 20.3] | 14.79 [8.9, 22.8] | 0.320 |
| Cys | 0.99 [0.62, 1.89] | 0.46 [0, 1.56] | 1.01 [0.71, 1.51] | 0.156 |
| Leu | 201.2 [146.7, 235.9] | 300.5 [138.1, 378.8] | 242 [158, 317.2] | 0.242 |
| Ile | 122.4 [78.8, 180.4] a | 182.3 [133.5, 212.5] ab | 199.8 [124.9, 244.9] b | 0.043 |
| Asn | 58.9 [42.5, 73.8] a | 85.1 [61.9, 133.8] b | 87.9 [46.4, 103.6] b | 0.016 |
| Asp | 3.5 [2.61, 4.64] a | 1.54 [1.17, 2.85] b | 3.21 [1.22, 3.77] ab | 0.014 |
| Lys | 199.9 [100.4, 215.1] | 171.8 [119.6, 223.1] | 142.3 [124.8, 195.8] | 0.706 |
| Gln | 12.5 [10.2, 24.9] a | 19.1 [10.1, 31.1] ab | 25.7 [20.1, 36.5] b | 0.024 |
| Glu | 16.5 [13, 21.4] a | 20.8 [10.8, 35.7] ab | 30 [23.3, 42.6] b | 0.010 |
| Met | 8.4 [4.5, 9.9] a | 9.5 [3.7, 12.9] ab | 11.7 [6.1, 19.7] b | 0.047 |
| His | 1.67 [1.03, 2.67] a | 1.06 [0.96, 2.04] a | 2.87 [1.99, 3.97] b | <0.001 |
| Phe | 128.9 [126.4, 225.7] | 159.6 [100.5, 196.9] | 200.3 [142.2, 247.3] | 0.094 |
| Arg | 35.6 [20.3, 50.7] a | 19.7 [12.1, 34.8] a | 56.5 [33.4, 90.2] b | 0.001 |
| Tyr | 20.5 [14.7, 32.1] a | 6.3 [4.5, 8.1] b | 6.6 [3.9, 9.8] b | <0.001 |
| Trp | 9.9 [6.4, 12.5] a | 12.6 [10, 20.6] b | 14.9 [9.9, 20.4] b | 0.013 |
| Total FAAs | 1271.49 | 1569.45 | 1560.03 | |
| Total Essential FAAs | 700.64 | 878.72 | 860.13 |
| Methods | Class (Regions) | Predicted | Total | Accuracy | Precision (PPV) | Recall (TPR) | False Positive Rate (FPR) | False Discovery Rate (FDR) | F1-Score | Matthews Correlation Coefficient (MCC) | Negative Predictive Value (NPV) | True Negative Rate (TNR) | False Negative Rate (FNR) | AUC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Şırnak Faraşin | Siirt Merkez | Siirt Pervari | ||||||||||||||
| PLS-DA | Şırnak Faraşin | 17 | 0 | 0 | 17 | 0.941 | 0.850 | 1.000 | 0.088 | 0.150 | 0.919 | 0.880 | 1.000 | 0.912 | 0.000 | 0.979 |
| Siirt Merkez | 1 | 15 | 2 | 18 | 0.863 | 0.789 | 0.833 | 0.121 | 0.211 | 0.811 | 0.704 | 0.906 | 0.879 | 0.167 | 0.862 | |
| Siirt Pervari | 2 | 4 | 10 | 16 | 0.843 | 0.833 | 0.625 | 0.057 | 0.167 | 0.714 | 0.621 | 0.846 | 0.943 | 0.375 | 0.838 | |
| Total | 20 | 19 | 12 | 51 | 0.824 | 0.824 | 0.819 | 0.089 | 0.176 | 0.815 | 0.735 | 0.917 | 0.911 | 0.181 | 0.893 | |
| RF | Şırnak Faraşin | 15 | 2 | 0 | 17 | 0.902 | 0.833 | 0.882 | 0.088 | 0.167 | 0.857 | 0.783 | 0.939 | 0.912 | 0.118 | 0.969 |
| Siirt Merkez | 1 | 13 | 4 | 18 | 0.804 | 0.722 | 0.722 | 0.152 | 0.278 | 0.722 | 0.571 | 0.848 | 0.848 | 0.278 | 0.838 | |
| Siirt Pervari | 2 | 3 | 11 | 16 | 0.824 | 0.733 | 0.688 | 0.114 | 0.267 | 0.710 | 0.584 | 0.861 | 0.886 | 0.313 | 0.839 | |
| Total | 18 | 18 | 15 | 51 | 0.765 | 0.763 | 0.764 | 0.118 | 0.237 | 0.763 | 0.646 | 0.883 | 0.882 | 0.236 | 0.882 | |
| SVM | Şırnak Faraşin | 13 | 1 | 3 | 17 | 0.902 | 0.929 | 0.765 | 0.029 | 0.071 | 0.839 | 0.777 | 0.892 | 0.971 | 0.235 | 0.931 |
| Siirt Merkez | 1 | 10 | 7 | 18 | 0.765 | 0.714 | 0.556 | 0.121 | 0.286 | 0.625 | 0.465 | 0.784 | 0.879 | 0.444 | 0.759 | |
| Siirt Pervari | 0 | 3 | 13 | 16 | 0.745 | 0.565 | 0.813 | 0.286 | 0.435 | 0.667 | 0.491 | 0.893 | 0.714 | 0.188 | 0.820 | |
| Total | 14 | 14 | 23 | 51 | 0.706 | 0.736 | 0.711 | 0.145 | 0.264 | 0.710 | 0.578 | 0.856 | 0.855 | 0.289 | 0.837 | |
| Phenolic Acids | Şırnak Faraşin (mg/kg) (n = 17) | ND Count | Siirt Merkez (mg/kg) (n = 18) | ND Count | Siirt Pervari (mg/kg) (n = 16) | ND Count | p-Value * |
|---|---|---|---|---|---|---|---|
| Pyrogallol | 0.69 [0.29, 1.38] | 11 | 0.67 [0.58, 1.10] | 13 | 1.23 [0.76, 2.76] | 10 | - |
| HGA | 3.14 [1.71, 6.04] | 2.81 [2.03, 6.37] | 1.6 [1.03, 5.47] | 0.408 | |||
| DBA34 | 10.2 [5.76, 43.3] a | 4.04 [1.93, 12.1] ab | 2.71 [1.11, 31.7] b | 0.049 | |||
| Gentisic acid | 100.6 [38.6, 143.2] | 57.5 [34.3, 120.1] | 66.5 [37.7, 124.5] | 0.301 | |||
| DB34 | 1.4 [0, 3.05] | 6 | 0.68 [0, 2.57] | 5 | 1.86 [0.91, 3.67] | 1 | 0.253 |
| Vanillic acid | 8.52 [5.67, 34] | 10.7 [8.1, 27.1] | 9.83 [4.63, 14.7] | 0.506 | |||
| Caffeic acid | 30 [17.2, 66.9] | 29.5 [14.4, 58.5] | 32.5 [15.1, 63.8] | 0.930 | |||
| Vanillin | 0.05 [0.02, 0.07] | 13 | 0.02 [0.02, 0.09] | 15 | 0.07 [0.04, 0.09] | 12 | - |
| p-CA | 8.54 [6.45, 14] a | 14.78 [10.3, 21.1] b | 20.3 [12.6, 24.9] b | 0.003 | |||
| t-CA | 0.05 [0.04, 0.09] | 14 | 0.08 [0.06, 0.10] | 14 | 0.10 [0.07, 0.11] | 10 | - |
| Total phenolic acids | 163.19 | 120.78 | 136.7 | ||||
| Flavonoids | |||||||
| Rutin | 0.58 [0.30, 0.65] | 12 | 0.17 [0.11, 0.33] | 14 | 0.30 [0.20, 0.39] | 10 | - |
| Luteolin | 0.35 [0.12, 1.3] ab | 2 | 0.22 [0, 0.79] a | 5 | 0.55 [0.38, 1.58] b | 0.034 | |
| Quercetin | 0.14 [0.12, 0.16] | 12 | 0.07 [0.02, 0.10] | 14 | 0.12 [0.10, 0.17] | 12 | - |
| Genistein | 0.14 [0.11, 0.4] | 1 | 0.12 [0, 0.29] | 7 | 0.15 [0.02, 0.52] | 4 | 0.435 |
| Hesperetin | 0.14 [0.12, 0.16] | 8 | - | 18 | 0.10 [0.10, 0.10] | 14 | - |
| Chrysin | 0.11 [0.10, 0.12] | 15 | 0.24 [0.16, 0.42] | 14 | 0.30 [0.21, 0.42] | 10 | - |
| Total flavonoids | 1.46 | 0.82 | 1.52 | ||||
| Total phenolic compounds | 164.65 | 121.60 | 138.22 | ||||
| Methods | Class (Region) | Predicted | Total | Accuracy | Precision (PPV) | Recall (TPR) | False Positive Rate (FPR) | False Discovery Rate (FDR) | F1 Score | Matthews Correlation Coefficient (MCC) | Negative Predictive Value (NPV) | True Negative Rate (TNR) | False Negative Rate (FNR) | AUC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Şırnak Faraşin | Siirt Merkez | Siirt Pervari | ||||||||||||||
| PLS-DA | Şırnak Faraşin | 12 | 5 | 0 | 17 | 0.824 | 0.750 | 0.706 | 0.118 | 0.250 | 0.727 | 0.598 | 0.857 | 0.882 | 0.294 | 0.706 |
| Siirt Merkez | 3 | 11 | 4 | 18 | 0.569 | 0.423 | 0.611 | 0.455 | 0.577 | 0.500 | 0.150 | 0.720 | 0.545 | 0.389 | 0.599 | |
| Siirt Pervari | 1 | 10 | 5 | 16 | 0.706 | 0.556 | 0.313 | 0.114 | 0.444 | 0.400 | 0.241 | 0.738 | 0.886 | 0.688 | 0.584 | |
| Total | 16 | 26 | 9 | 51 | 0.549 | 0.576 | 0.543 | 0.229 | 0.424 | 0.542 | 0.330 | 0.772 | 0.771 | 0.457 | 0.630 | |
| Random Forest | Şırnak Faraşin | 13 | 3 | 1 | 17 | 0.882 | 0.765 | 0.765 | 0.118 | 0.235 | 0.765 | 0.647 | 0.882 | 0.882 | 0.235 | 0.851 |
| Siirt Merkez | 2 | 10 | 6 | 18 | 0.647 | 0.500 | 0.556 | 0.303 | 0.500 | 0.526 | 0.247 | 0.742 | 0.697 | 0.444 | 0.657 | |
| Siirt Pervari | 2 | 7 | 7 | 16 | 0.686 | 0.500 | 0.438 | 0.200 | 0.500 | 0.467 | 0.247 | 0.757 | 0.800 | 0.563 | 0.731 | |
| Total | 17 | 20 | 14 | 51 | 0.588 | 0.588 | 0.586 | 0.207 | 0.412 | 0.586 | 0.380 | 0.794 | 0.793 | 0.414 | 0.746 | |
| SVM | Şırnak Faraşin | 12 | 3 | 2 | 17 | 0.804 | 0.706 | 0.706 | 0.147 | 0.294 | 0.706 | 0.559 | 0.853 | 0.853 | 0.294 | 0.787 |
| Siirt Merkez | 2 | 11 | 5 | 18 | 0.647 | 0.500 | 0.611 | 0.333 | 0.500 | 0.550 | 0.268 | 0.759 | 0.667 | 0.389 | 0.522 | |
| Siirt Pervari | 3 | 8 | 5 | 16 | 0.647 | 0.417 | 0.313 | 0.200 | 0.583 | 0.357 | 0.123 | 0.718 | 0.800 | 0.688 | 0.593 | |
| Total | 17 | 22 | 12 | 51 | 0.549 | 0.541 | 0.543 | 0.227 | 0.459 | 0.538 | 0.317 | 0.777 | 0.773 | 0.457 | 0.634 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürbüz, S.; Kıvrak, Ş. Chemometric Differentiation of Organic Honeys from Southeastern Türkiye Based on Free Amino Acid and Phenolic Profiles. Foods 2025, 14, 3105. https://doi.org/10.3390/foods14173105
Gürbüz S, Kıvrak Ş. Chemometric Differentiation of Organic Honeys from Southeastern Türkiye Based on Free Amino Acid and Phenolic Profiles. Foods. 2025; 14(17):3105. https://doi.org/10.3390/foods14173105
Chicago/Turabian StyleGürbüz, Semra, and Şeyda Kıvrak. 2025. "Chemometric Differentiation of Organic Honeys from Southeastern Türkiye Based on Free Amino Acid and Phenolic Profiles" Foods 14, no. 17: 3105. https://doi.org/10.3390/foods14173105
APA StyleGürbüz, S., & Kıvrak, Ş. (2025). Chemometric Differentiation of Organic Honeys from Southeastern Türkiye Based on Free Amino Acid and Phenolic Profiles. Foods, 14(17), 3105. https://doi.org/10.3390/foods14173105





