Effects of Growth Stage and Particle Size on the Physical, Chemical, Structural, and Bioactive Properties of Alkaline-Extracted Dietary Fiber from Rice Husk (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Dietary Fiber Extraction
2.4. Physicochemical Properties Analysis
2.4.1. Color, Moisture Content, and Water Activity (aw) Analysis
2.4.2. Water-Holding Capacity (WHC) Analysis
2.4.3. Oil-Holding Capacity (OHC) Analysis
2.4.4. Swelling Capacity (SC) Analysis
2.5. Chemical Composition Analysis
2.6. β-Glucan Content Analysis
2.7. Structural Analysis
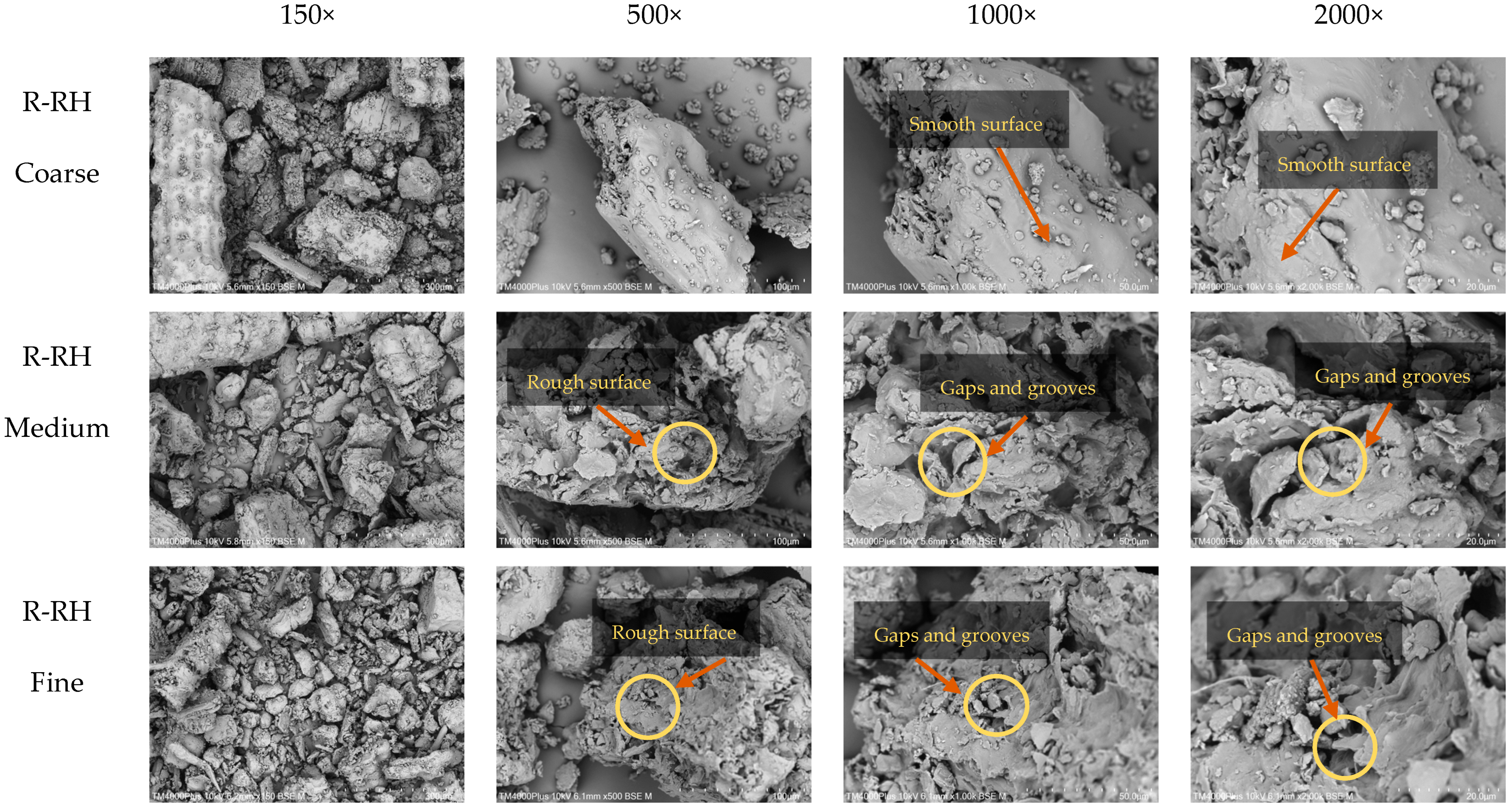
2.7.1. Scanning Electron Microscopy (SEM) Analysis
2.7.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.7.3. X-Ray Diffraction (XRD) Analysis
2.8. Bioactive Compounds and Antioxidant Activities Analysis
2.8.1. Preparation of Sample Extraction
2.8.2. Total Flavonoid Content (TFC) Analysis
2.8.3. Total Phenol Content (TPC) Analysis
2.8.4. DPPH Free Radical Scavenging Assay Analysis
2.8.5. Ferric Reducing Antioxidant Power (FRAP) Assay Analysis
2.8.6. Phytosterol Analysis by GC-MS
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.1.1. Color, Moisture Content, and Water Activity
3.1.2. Water-Holding Capacity (WHC)
3.1.3. Oil-Holding Capacity (OHC)
3.1.4. Swelling Capacity (SC)
3.2. Chemical Compositions
3.3. β-Glucan
3.4. Structural Characterization
3.4.1. Microstructure
3.4.2. FT-IR Spectroscopy
3.4.3. X-Ray Diffraction
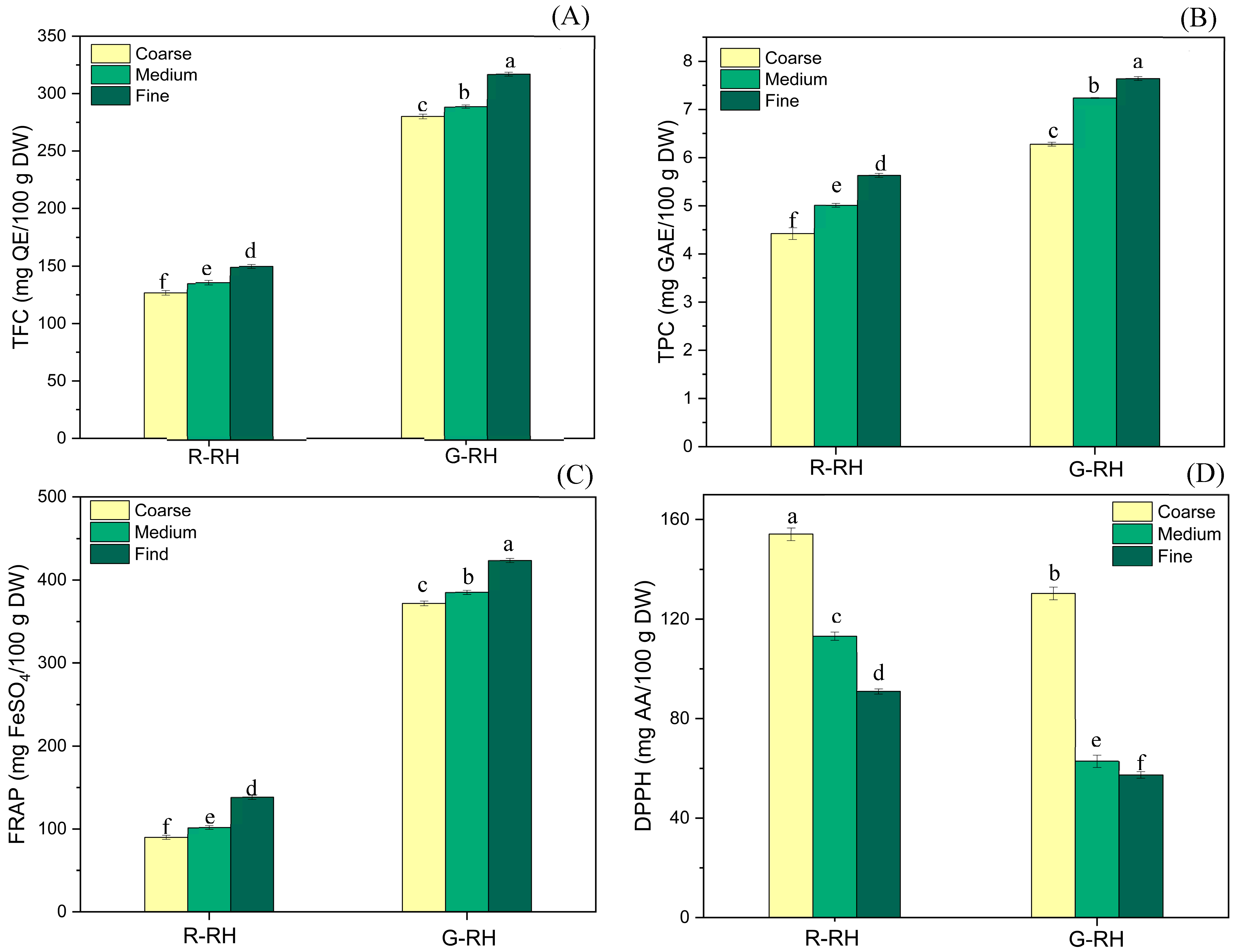
3.5. Bioactive Compounds and Antioxidant Activities
3.6. Phytosterol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, G.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Lipophilic compounds from maize fiber and rice husk residues—An abundant and inexpensive source of valuable phytochemicals. Ind. Crops Prod. 2020, 146, 112203. [Google Scholar] [CrossRef]
- Phuwadolpaisarn, P. Comparison of β-Glucan Content in Milled Rice, Rice Husk and Rice Bran from Rice Cultivars Grown in Different Locations of Thailand and the Relationship between β-Glucan and Amylose Contents. Molecules 2021, 26, 6368. [Google Scholar] [CrossRef] [PubMed]
- Samsalee, N.; Meerasri, J.; Sothornvit, R. Rice husk nanocellulose: Extraction by high-pressure homogenization, chemical treatments and characterization. Carbohydr. Polym. Technol. Appl. 2023, 6, 100353. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Liu, J.-F.; Cheng, Z.; Wu, N.-N.; Tan, B. Structure, thermal stability, physicochemical and functional characteristics of insoluble dietary fiber obtained from rice bran with steam explosion treatment: Effect of different steam pressure and particle size of rice bran. Food Res. Int. 2024, 187, 114310. [Google Scholar] [CrossRef] [PubMed]
- Akik, C.; El Dirani, Z.; Willis, R.; Truppa, C.; Zmeter, C.; Aebischer Perone, S.; Roswall, J.; Hamadeh, R.; Blanchet, K.; Roberts, B.; et al. Providing continuity of care for people living with noncommunicable diseases in humanitarian settings: A qualitative study of health actors’ experiences in Lebanon. J. Migr. Health 2024, 10, 100269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Ampofo, A.G.; Boateng, E.B. Beyond 2020: Modelling obesity and diabetes prevalence. Diabetes Res. Clin. Pract. 2020, 167, 108362. [Google Scholar] [CrossRef]
- Ma, Z.-Q.; Zhang, N.; Zhai, X.-T.; Tan, B. Structural, physicochemical and functional properties of dietary fiber from brown rice products treated by different processing techniques. LWT 2023, 182, 114789. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Z.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Effect of particle size and microstructure on the physical properties of soybean insoluble dietary fiber in aqueous solution. Food Biosci. 2021, 41, 100898. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Lai, H.-M. Bioactive compounds in rice during grain development. Food Chem. 2011, 127, 86–93. [Google Scholar] [CrossRef]
- Jiamyangyuen, S.; Nuengchamnong, N.; Ngamdee, P. Bioactivity and chemical components of Thai rice in five stages of grain development. J. Cereal Sci. 2017, 74, 136–144. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Huang, Q.; Zia-ud-Din; Zhang, J.; Javaid, A.B. Effects of thermal pre-treatment on physicochemical properties of nano-sized okara (soybean residue) insoluble dietary fiber prepared by wet media milling. J. Food Eng. 2018, 237, 18–26. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Sang, J.; Li, L.; Wen, J.; Liu, H.; Wu, J.; Yu, Y.; Xu, Y.; Gu, Q.; Fu, M.; Lin, X. Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods. LWT 2022, 165, 113737. [Google Scholar] [CrossRef]
- Boonarsa, P.; Bunyatratchata, A.; Chumroenphat, T.; Thammapat, P.; Chaikwang, T.; Siripan, T.; Li, H.; Siriamornpun, S. Nutritional Quality, Functional Properties, and Biological Characterization of Watermeal (Wolffia globosa). Horticulturae 2024, 10, 1171. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Tangkhawanit, E.; Kaewseejan, N. Reducing retrogradation and lipid oxidation of normal and glutinous rice flours by adding mango peel powder. Food Chem. 2016, 201, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.R.; Chakraborty, S.; Odaneth, A.; Annapure, U.S. A sequential approach of alkali enzymatic extraction of dietary fiber from rice bran: Effects on structural, thermal, crystalline properties, and food application. Food Res. Int. 2024, 193, 114847. [Google Scholar] [CrossRef]
- Bazán-Colque, R.J.; Ramirez Ascheri, J.L.; Ruiz-Barreto, F.I.; Ramirez Ascheri, D.P. Investigation of the effect of particle size of corn fiber, as potential raw material, on techno-functional and physicochemical properties. J. Cereal Sci. 2023, 112, 103708. [Google Scholar] [CrossRef]
- Liu, K. Effects of particle size distribution, compositional and color properties of ground corn on quality of distillers dried grains with solubles (DDGS). Bioresour. Technol. 2009, 100, 4433–4440. [Google Scholar] [CrossRef]
- Karuppuchamy, V.; Heldman, D.R.; Snyder, A.B. A review of food safety in low-moisture foods with current and potential dry-cleaning methods. J. Food Sci. 2024, 89, 793–810. [Google Scholar] [CrossRef]
- Liu, S.X.; Chen, D.; Plumier, B.; Berhow, M.; Xu, J.; Byars, J.A. Impact of particle size fractions on composition, antioxidant activities, and functional properties of soybean hulls. J. Food Meas. Charact. 2021, 15, 1547–1562. [Google Scholar] [CrossRef]
- Yalegama, L.L.W.C.; Nedra Karunaratne, D.; Sivakanesan, R.; Jayasekara, C. Chemical and functional properties of fibre concentrates obtained from by-products of coconut kernel. Food Chem. 2013, 141, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, X.; Zhou, Z.; Chen, G. Effects of microfluidization process on physicochemical properties of wheat bran. Food Res. Int. 2012, 48, 742–747. [Google Scholar] [CrossRef]
- He, C.; Qi, J.; Liao, J.; Song, Y.; Wu, C. Excellent hydration properties and oil holding capacity of citrus fiber: Effects of component variation and microstructure. Food Hydrocoll. 2023, 144, 108988. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and modification of hemicellulose from lignocellulosic biomass: A review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Zeng, W.; Huang, Y.; Yang, X. Properties of dietary fiber from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem. 2020, 311, 125873. [Google Scholar] [CrossRef]
- Schmitz, E.; Karlsson, E.N.; Adlercreutz, P. Ultrasound Assisted Alkaline Pre-treatment Efficiently Solubilises Hemicellulose from Oat Hulls. Waste Biomass Valorization 2021, 12, 5371–5381. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef]
- Yang, L.-C.; Hsieh, C.-C.; Lin, W.-C. Characterization and immunomodulatory activity of rice hull polysaccharides. Carbohydr. Polym. 2015, 124, 150–156. [Google Scholar] [CrossRef]
- Montipó, S.; Ballesteros, I.; Fontana, R.C.; Liu, S.; Ballesteros, M.; Martins, A.F.; Camassola, M. Bioprocessing of rice husk into monosaccharides and the fermentative production of bioethanol and lactate. Cellulose 2019, 26, 7309–7322. [Google Scholar] [CrossRef]
- Leković, S.; Marković, S.; Minić, Đ.; Todosijević, J.; Torbica, A.; Đukić, N. B-glucan content variability in seed of barley cultivars. Kragujev. J. Sci. 2023, 111–120. [Google Scholar] [CrossRef]
- Palmer, R.; Cornuault, V.; Marcus, S.E.; Knox, J.P.; Shewry, P.R.; Tosi, P. Comparative in situ analyses of cell wall matrix polysaccharide dynamics in developing rice and wheat grain. Planta 2015, 241, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, H.-M.; Huang, Z.-H.; Zhao, R.-Y. Different aggregation states of barley β-glucan molecules affects their solution behavior: A comparative analysis. Food Hydrocoll. 2020, 101, 105543. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Ditzel, F.I.; Prestes, E.; Carvalho, B.M.; Demiate, I.M.; Pinheiro, L.A. Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr. Polym. 2017, 157, 1577–1585. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind. Crops Prod. 2020, 154, 112627. [Google Scholar] [CrossRef]
- Oliveira, J.P.D.; Bruni, G.P.; Lima, K.O.; Halal, S.L.M.E.; Rosa, G.S.D.; Dias, A.R.G.; Zavareze, E.D.R. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.-X.; Li, X.-T.; Tang, C.-H. Novel nanoparticles from insoluble soybean polysaccharides of Okara as unique Pickering stabilizers for oil-in-water emulsions. Food Hydrocoll. 2019, 94, 255–267. [Google Scholar] [CrossRef]
- Rezvani, Z.; Goli, S.A.H. Fabrication, physicochemical properties and structural characteristics of nanoparticles from carrot pomace and its insoluble dietary fiber. Food Hydrocoll. 2023, 145, 109131. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinämäki, J.; Krogars, K.; Jörgensen, A.C.; Karjalainen, M.; Colarte, A.I.; Yliruusi, J. Solid-state and mechanical properties of aqueous chitosan-amylose starch films plasticized with polyols. AAPS PharmSciTech 2004, 5, 109–114. [Google Scholar] [CrossRef]
- Nayak, P.P.; Nandi, S.; Datta, A.K. Comparative assessment of chemical treatments on extraction potential of commercial grade silica from rice husk. Eng. Rep. 2019, 1, e12035. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, T.; Wan, H.; Li, M.; Sun, L.; Wang, Z.; Zhang, S. Structural and Physicochemical Characteristics of Rice Bran Dietary Fiber by Cellulase and High-Pressure Homogenization. Appl. Sci. 2019, 9, 1270. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.-C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef]
- Thongbai, B.; Sukboonyasatit, D.; Banlue, K.; Inchuen, S.; Chuenta, W.; Siriamornpun, S.; Suwannarong, S. Cascara Kombucha: The Role of Fermentation and Particle Size in Enhancing Antioxidant and Bioactive Properties. Molecules 2025, 30, 1934. [Google Scholar] [CrossRef] [PubMed]
- Taesuk, N.; Wang, A.; Srikaew, M.; Chumroenphat, T.; Barile, D.; Siriamornpun, S.; Bunyatratchata, A. Phytochemical profiling of Thai plant-based milk alternatives: Insights into bioactive compounds, antioxidant activities, prebiotics, and amino acid abundance. Food Chem. X 2025, 27, 102402. [Google Scholar] [CrossRef]
- Islam, M.A.; Jeong, B.-G.; Kerr, W.L.; Chun, J. Validation of phytosterol analysis by alkaline hydrolysis and trimethylsilyl derivatization coupled with gas chromatography for rice products. J. Cereal Sci. 2021, 101, 103305. [Google Scholar] [CrossRef]
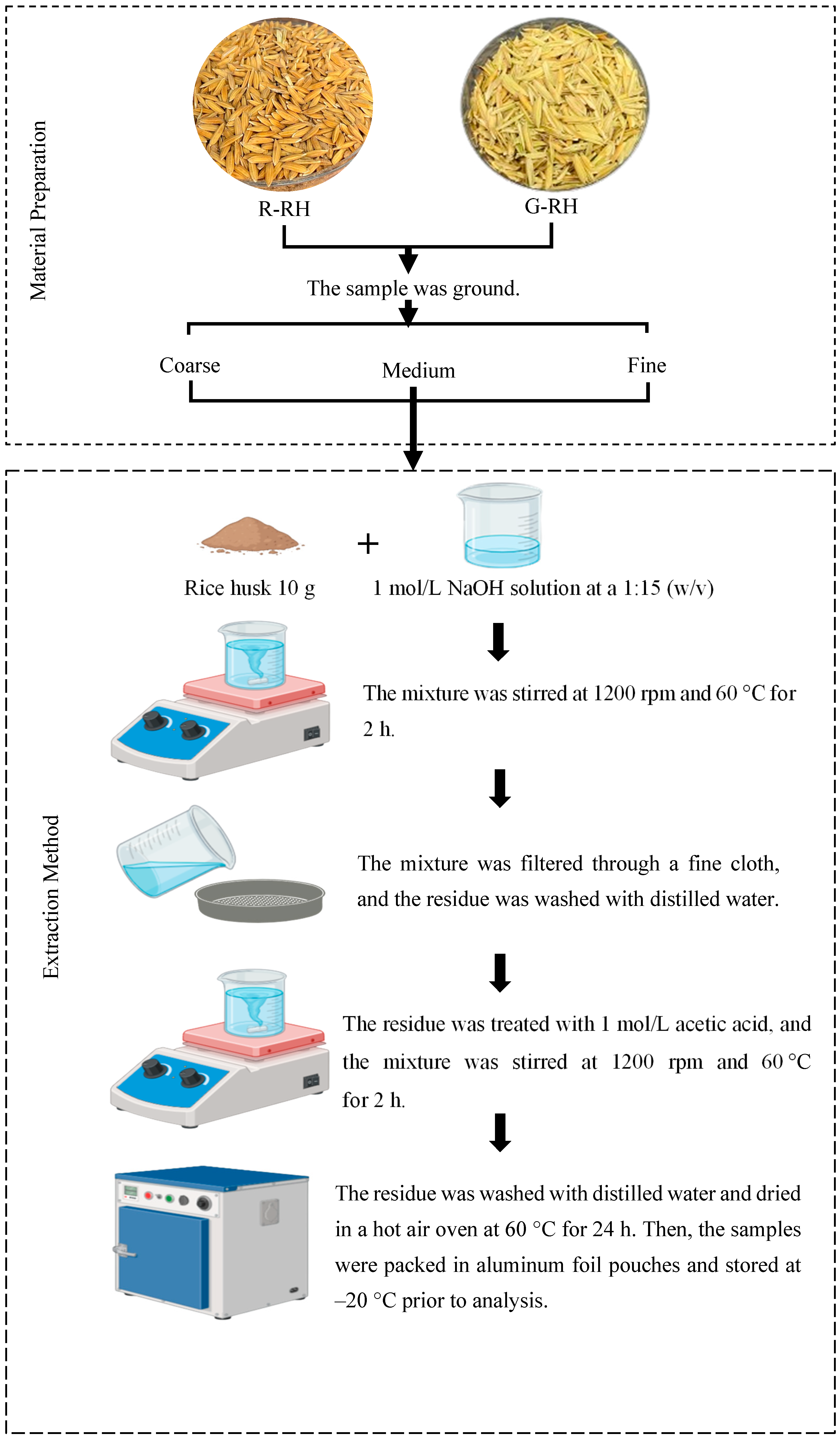


| Growth Stage | Age (Day After Flowering) | Description |
|---|---|---|
| Green rice husk | 22–28 | Rice husk development begins as the grain matures, which is characterized by the hardening and opacification of the endosperm. Physiological maturity is typically indicated when at least one grain on the main panicle exhibits a brown-colored husk. |
| Fully ripe rice husk | 29–35 | Rice husks harvested at the fully ripe stage correspond to grains that have reached their maximum size and hardness. During this stage, the husk color deepens to a darker yellow or brown. |
| Group | Sieves | Color | Moisture Content (g/100 g) | aw ns | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| R-RH | Coarse | 74.42 ± 0.07 b | −1.65 ± 0.04 f | 24.64 ± 0.04 e | 3.21 ± 0.01 a | 0.21 ± 0.01 |
| Medium | 73.08 ± 0.03 c | −0.73 ± 0.03 e | 44.83 ± 0.02 b | 3.15 ± 0.01 c | 0.21 ± 0.01 | |
| Fine | 70.63 ± 0.09 d | 1.99 ± 0.04 b | 46.27 ± 0.03 a | 3.08 ± 0.01 e | 0.21 ± 0.01 | |
| G-RH | Coarse | 78.62 ± 0.06 a | 1.35 ± 0.02 d | 15.84 ± 0.07 f | 3.18 ± 0.01 b | 0.21 ± 0.01 |
| Medium | 69.01 ± 0.03 e | 1.50 ± 0.02 c | 30.45 ± 0.02 d | 3.07 ± 0.03 d | 0.21 ± 0.01 | |
| Fine | 65.19 ± 0.02 f | 2.07 ± 0.03 a | 30.88 ± 0.01 c | 3.04 ± 0.02 f | 0.21 ± 0.01 | |
| Group | Sieves | Water-Holding Capacity (g/g) | Oil-Holding Capacity (g/g) | Swelling Water Capacity (mL/g) |
|---|---|---|---|---|
| R-RH | Coarse | 1.44 ± 0.01 e | 1.51 ± 0.03 g | 0.35 ± 0.03 g |
| Medium | 5.31 ± 0.02 b | 2.35 ± 0.02 d | 0.61 ± 0.02 f | |
| Fine | 5.52 ± 0.03 a | 2.47 ± 0.17 c | 0.74 ± 0.02 d | |
| G-RH | Coarse | 1.24 ± 0.03 f | 1.62 ± 0.01 f | 0.88 ± 0.01 c |
| Medium | 4.55 ± 0.02 d | 3.17 ± 0.03 b | 1.10 ± 0.02 b | |
| Fine | 5.25 ± 0.02 c | 3.44 ± 0.02 a | 1.21 ± 0.01 a |
| Sample | Sieves | Cellulose (g/100 g DW) | Moisture (g/100 g DW) | Ash (g/100 g DW) | Protein (g/100 g DW) | Lipid (g/100 g DW) | β-Glucan (mg/100 g DW) | Campesterol (µg/g DW) | Stigmasterol (µg/g DW) | β-Sitosterol (µg/g DW) |
|---|---|---|---|---|---|---|---|---|---|---|
| R-RH | Coarse | 41.27 ± 0.13 e | 3.27 ± 0.02 b | 3.54 ± 0.02 f | 4.33 ± 0.02 b | 8.76 ± 0.02 a | 66.37 ± 0.06 e | BDL | BDL | 28.98 ± 0.07 e |
| Medium | 76.30 ± 0.35 b | 1.86 ± 0.01 e | 5.21 ± 0.02 e | 2.07 ± 0.02 d | 6.35 ± 0.03 c | 72.78 ± 0.23 d | BDL | BDL | 72.33 ± 0.05 d | |
| Fine | 82.04 ± 0.13 a | 1.55 ± 0.02 f | 6.25 ± 0.03 c | 1.79 ± 0.01 e | 4.96 ± 0.01 e | 85.14 ± 0.97 b | BDL | BDL | 94.68 ± 3.74 c | |
| G-RH | Coarse | 37.49 ± 0.24 f | 4.97 ± 0.01 a | 5.96 ± 0.02 d | 5.01 ± 0.01 a | 7.94 ± 0.03 b | 67.51 ± 1.72 e | BDL | BDL | BDL |
| Medium | 68.02 ± 0.04 d | 3.11 ± 0.02 c | 7.90 ± 0.03 b | 2.73 ± 0.24 c | 5.31 ± 0.02 d | 80.02 ± 1.83 c | BDL | 326.09 ± 1.41 b | 719.46 ± 1.57 b | |
| Fine | 73.16 ± 0.15 c | 3.02 ± 0.01 d | 9.15 ± 0.02 a | 2.58 ± 0.23 c | 4.95 ± 0.03 e | 92.85 ± 2.36 a | 293.25 ± 4.82 a | 375.13 ± 3.98 a | 734.45 ± 4.95 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaikwang, T.; Bunyatratchata, A.; Thammapat, P.; Ratseewo, J.; Siriamornpun, S. Effects of Growth Stage and Particle Size on the Physical, Chemical, Structural, and Bioactive Properties of Alkaline-Extracted Dietary Fiber from Rice Husk (Oryza sativa L.). Foods 2025, 14, 3094. https://doi.org/10.3390/foods14173094
Chaikwang T, Bunyatratchata A, Thammapat P, Ratseewo J, Siriamornpun S. Effects of Growth Stage and Particle Size on the Physical, Chemical, Structural, and Bioactive Properties of Alkaline-Extracted Dietary Fiber from Rice Husk (Oryza sativa L.). Foods. 2025; 14(17):3094. https://doi.org/10.3390/foods14173094
Chicago/Turabian StyleChaikwang, Tipaukson, Apichaya Bunyatratchata, Pornpisanu Thammapat, Jiranan Ratseewo, and Sirithon Siriamornpun. 2025. "Effects of Growth Stage and Particle Size on the Physical, Chemical, Structural, and Bioactive Properties of Alkaline-Extracted Dietary Fiber from Rice Husk (Oryza sativa L.)" Foods 14, no. 17: 3094. https://doi.org/10.3390/foods14173094
APA StyleChaikwang, T., Bunyatratchata, A., Thammapat, P., Ratseewo, J., & Siriamornpun, S. (2025). Effects of Growth Stage and Particle Size on the Physical, Chemical, Structural, and Bioactive Properties of Alkaline-Extracted Dietary Fiber from Rice Husk (Oryza sativa L.). Foods, 14(17), 3094. https://doi.org/10.3390/foods14173094







