Integrating Sensory Evaluation, Electronic Nose, and Metabolomics to Characterize Aroma in Peach and Nectarine Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sensory Analysis
2.3. E-Nose Measurement
2.4. GC-MS Measurement
2.5. Statistical Analysis
3. Results
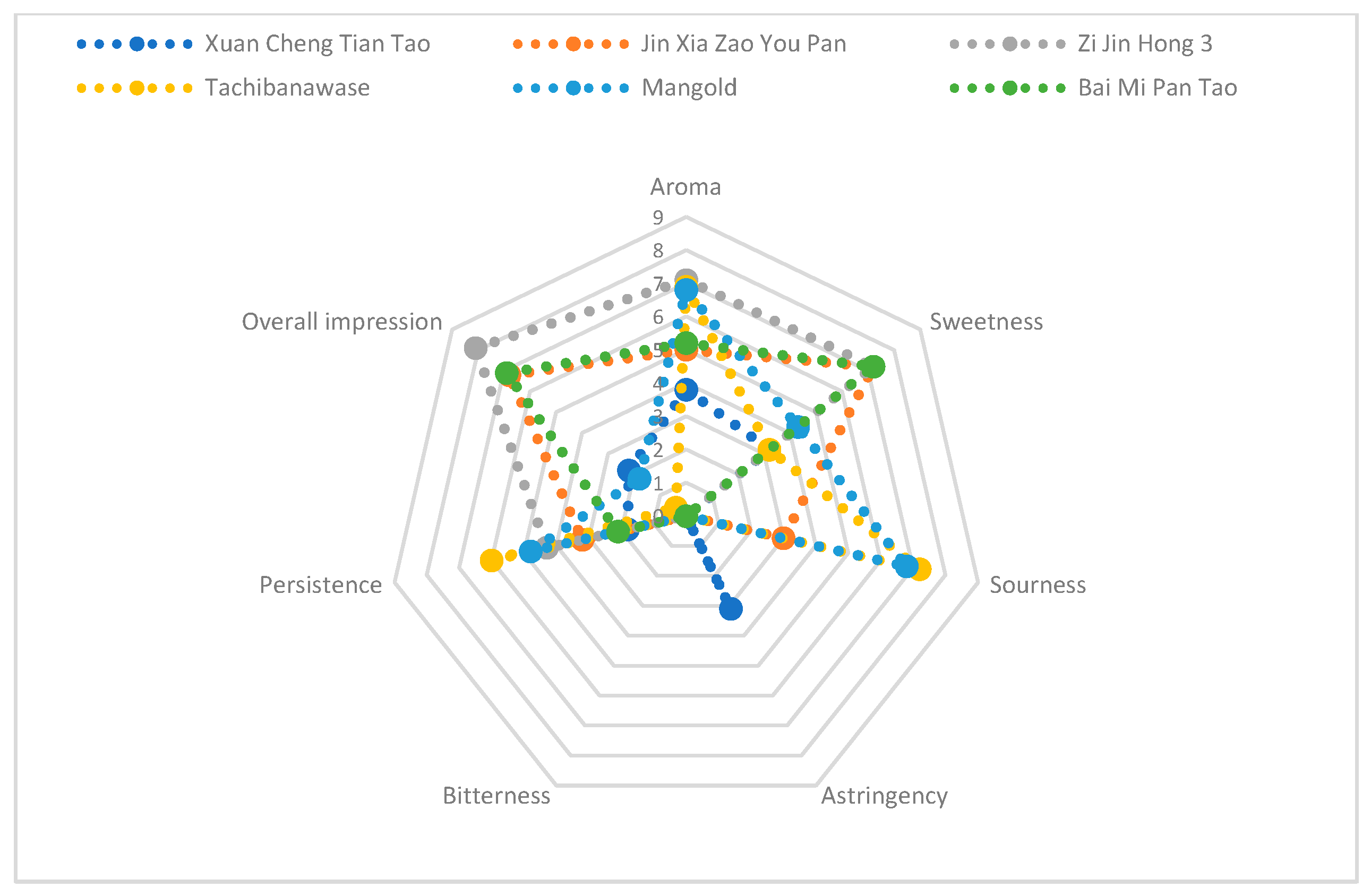
3.1. Sensory Attribute
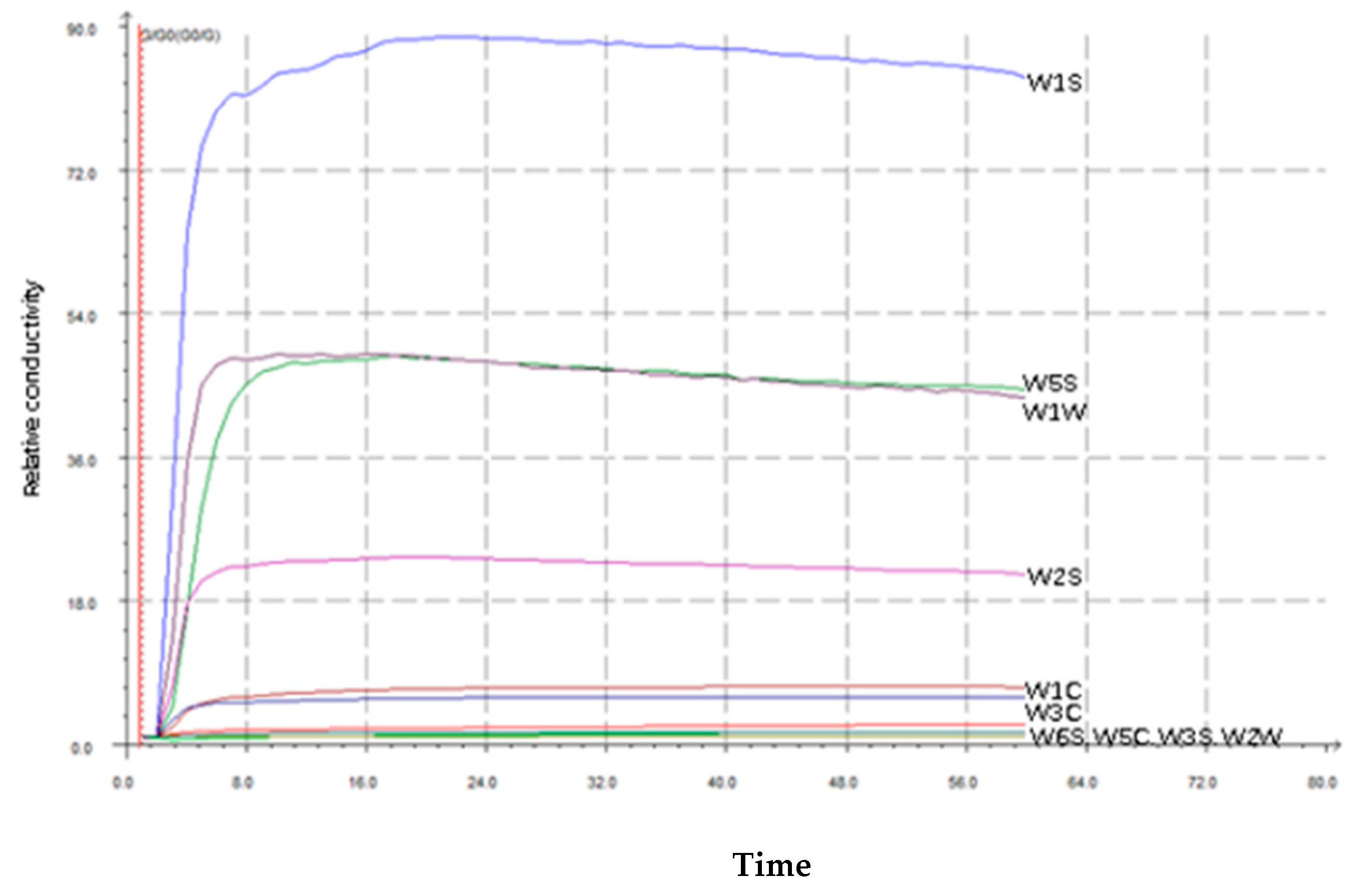
3.2. E-Nose Results
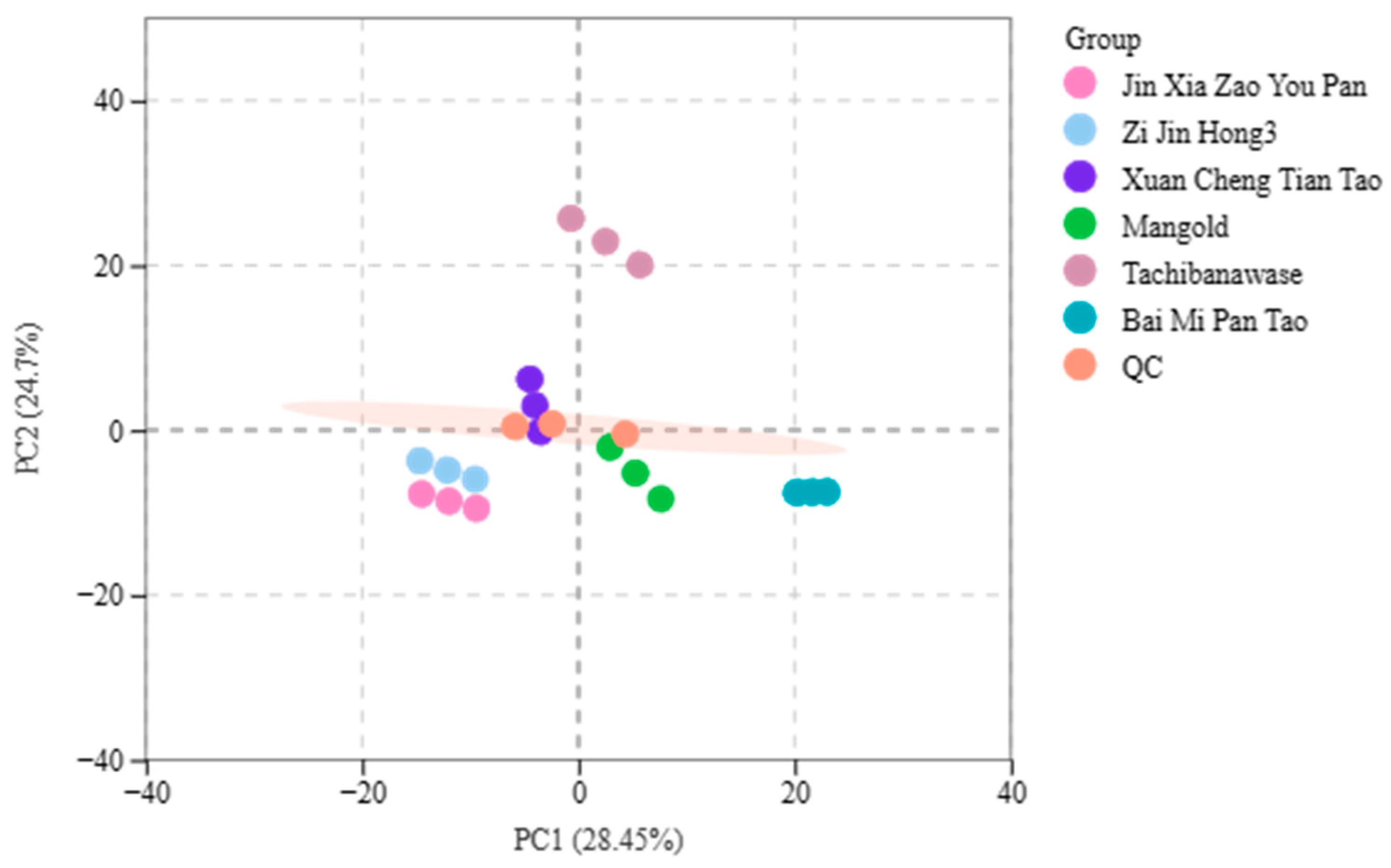
Principal Component Analysis of Population Sample
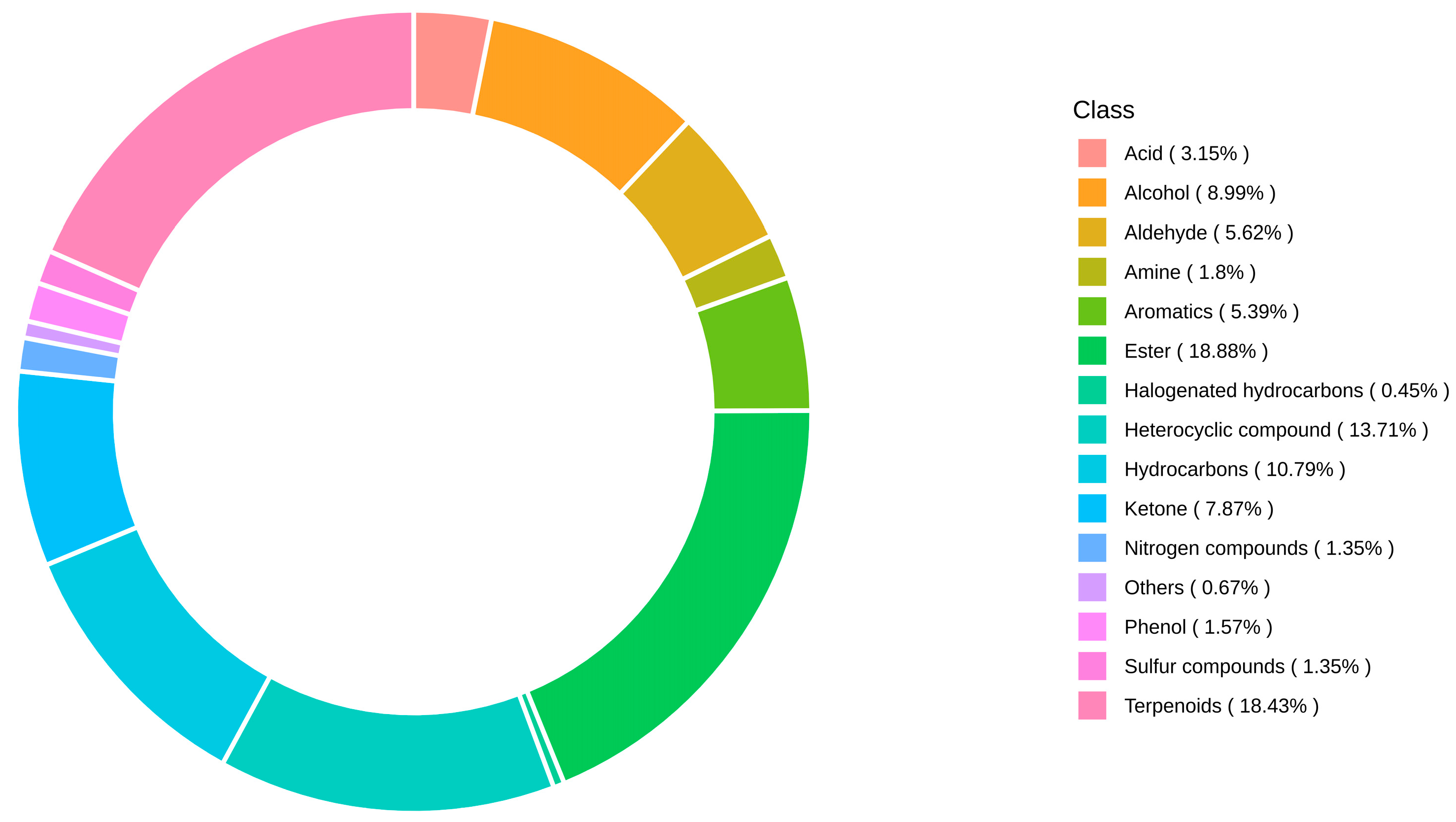
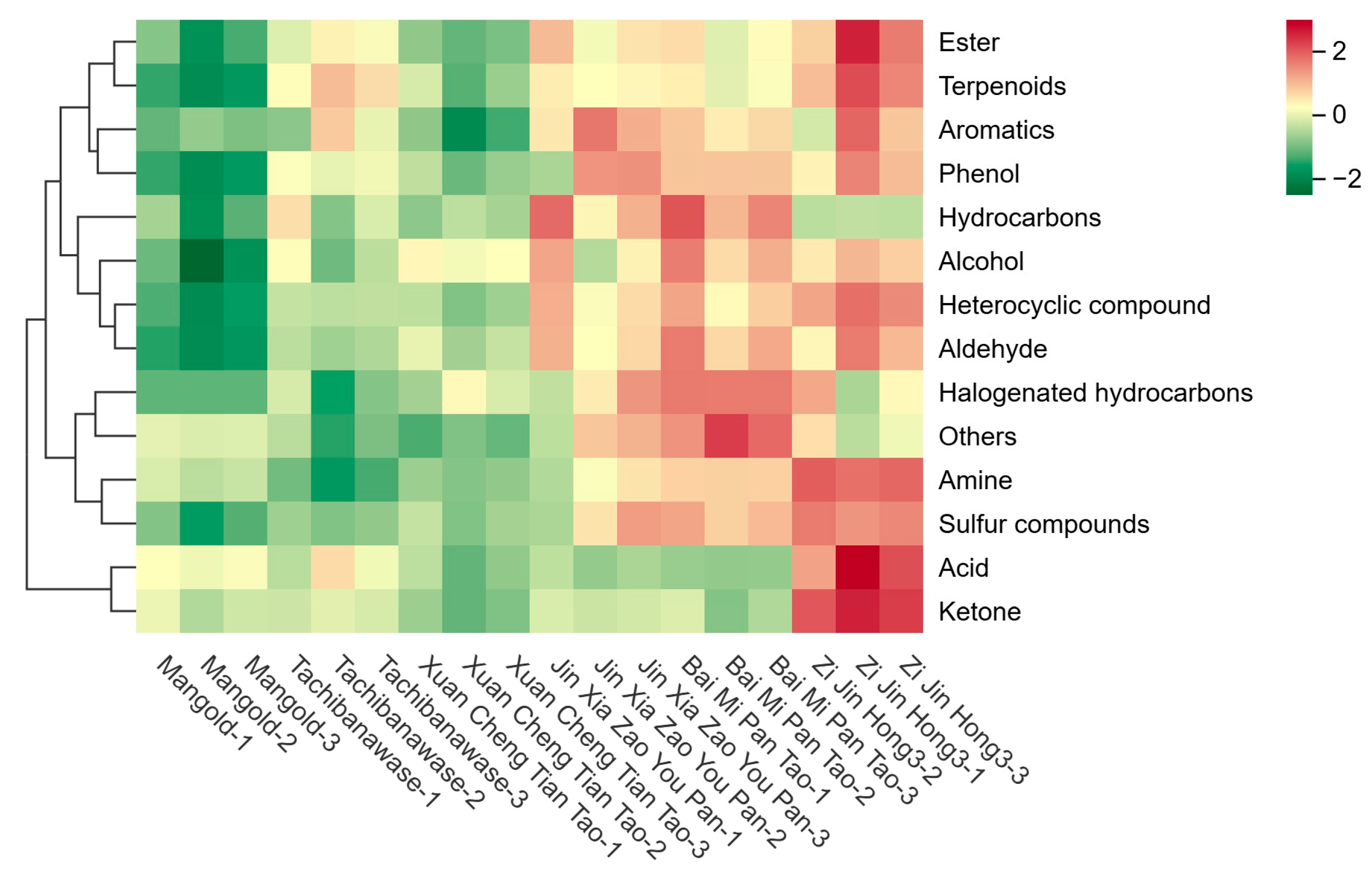
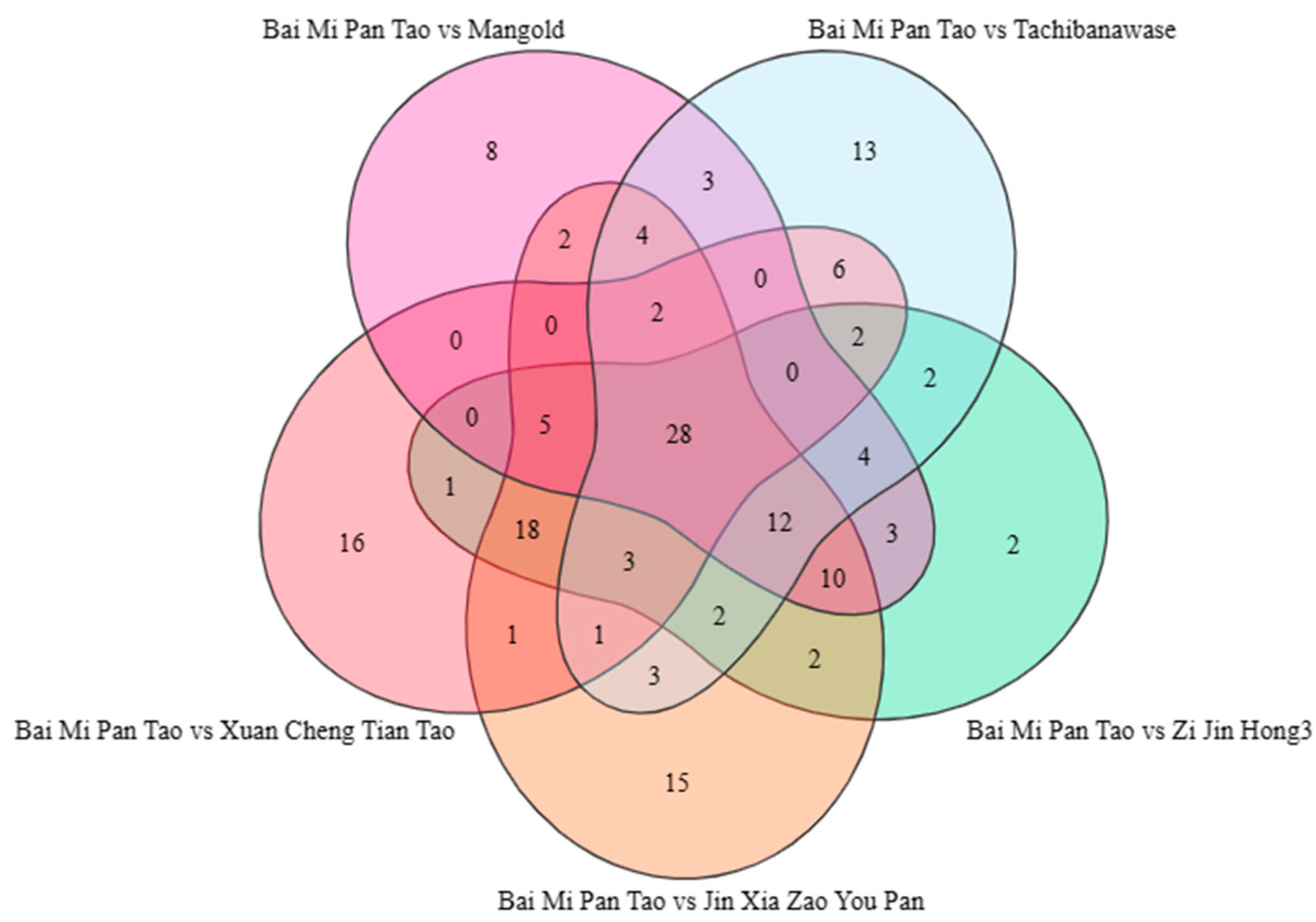
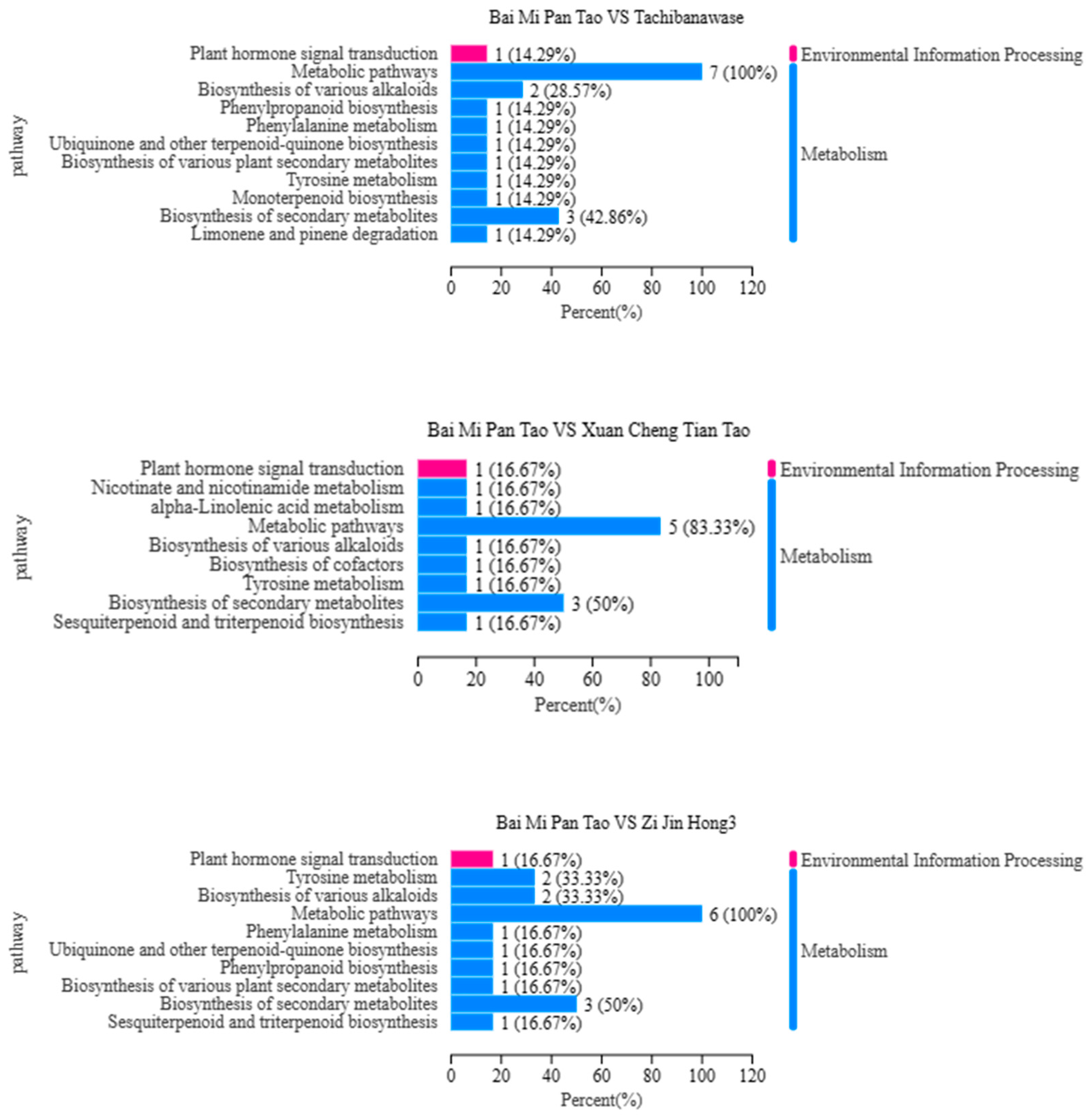
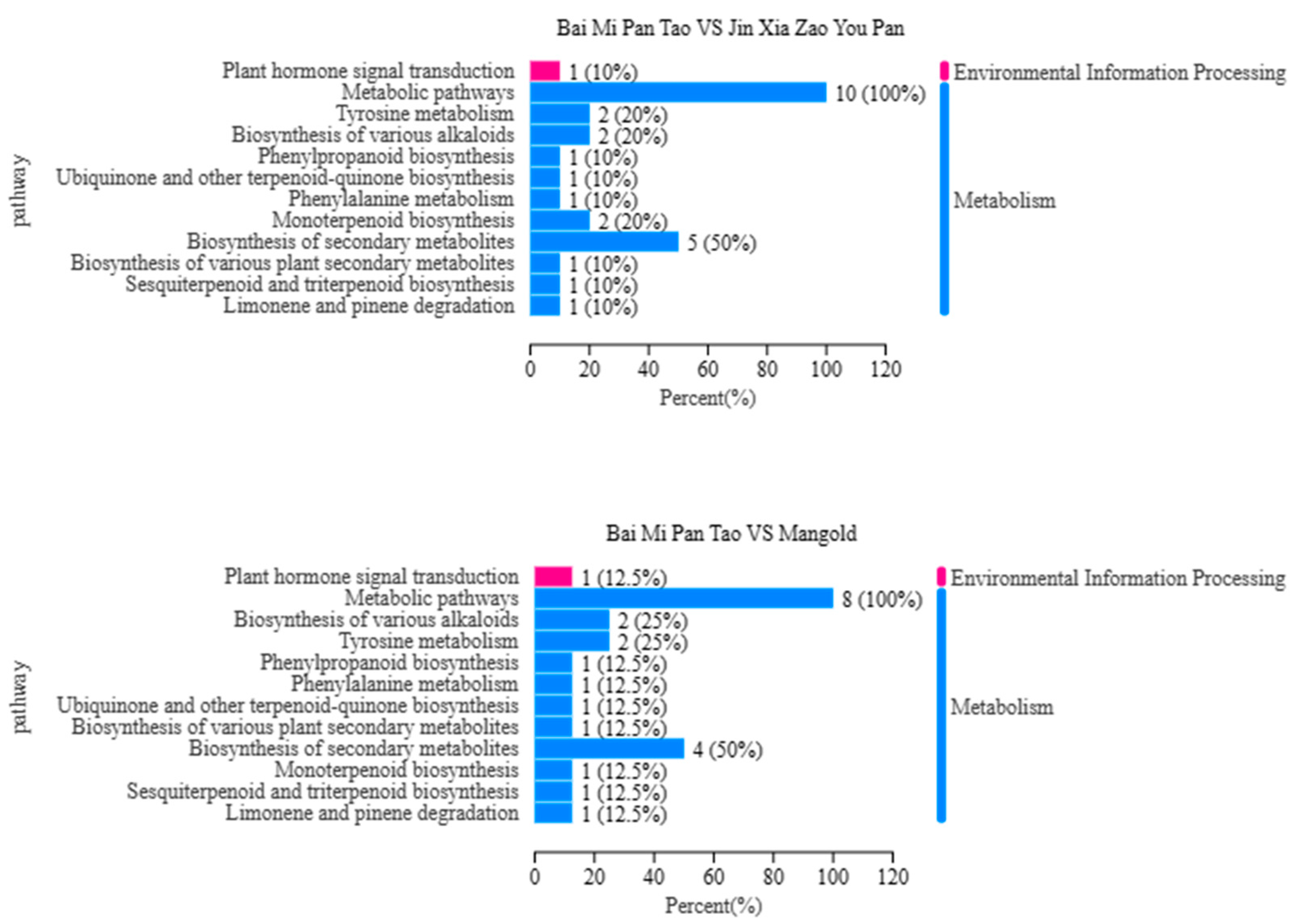
3.3. Category Analysis of Metabolite Composition
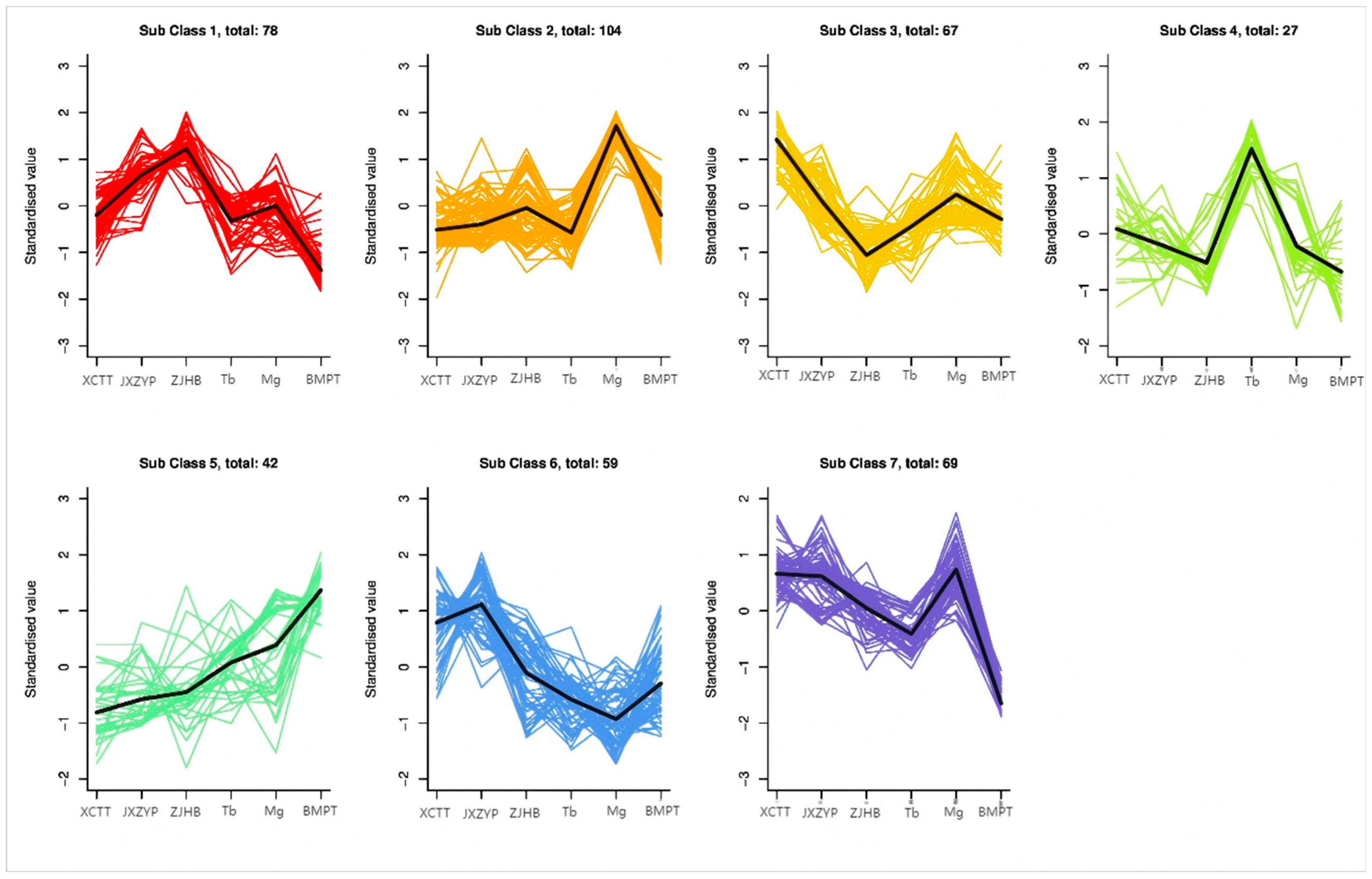
3.4. Metabolite Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.M.; Lu, L.L.; Sun, B.; Ferguson, D.K.; Li, J.F.; Zhou, S.L.; Wang, Y.-F.; Yang, J. Recognizing Prunus persica (peach) and allied Rosaceae by the morphological characteristics of their fruitstones. Veg. Hist. Archaeobotany 2025. [Google Scholar] [CrossRef]
- Gupta, M.; Arora, R.; Mandal, D. Peach. In Temperate Fruits; Apple Academic Press: Pleasant, NJ, USA, 2021; pp. 247–296. [Google Scholar]
- Li, X.; Gao, P.; Zhang, C.; Xiao, X.; Chen, C.; Song, F. Aroma of peach fruit: A review on aroma volatile compounds and underlying regulatory mechanisms. Int. J. Food Sci. Technol. 2023, 58, 4965–4979. [Google Scholar] [CrossRef]
- Hayat, U.; Ke, C.; Wang, L.; Zhu, G.; Fang, W.; Wang, X.; Chen, C.; Li, Y.; Wu, J. Using quantitative trait locus map** and genomic resources to improve breeding precision in peaches: Current insights and future prospects. Plants 2025, 14, 175. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhang, J.; Wang, Z.; Qi, K.; Li, H.; Tian, R.; Wu, X.; Qiao, X.; Zhang, S.; et al. Comparative analysis of volatile aromatic compounds from a wide range of pear (Pyrus L.) germplasm resources based on HS-SPME with GC–MS. Food Chem. 2023, 418, 135963. [Google Scholar] [CrossRef] [PubMed]
- Ogah, O.; Ene, C.O.; Afiukwa, F.N.; Nwankwo, V.U. Flavor quality in rosaceae fruit and nut crops: “Insights into volatile organic compounds and recent breeding strategies”. Niger. J. Biotechnol. 2024, 41, 191–211. [Google Scholar] [CrossRef]
- Sun, M.; Ma, J.; Cai, Z.; Yan, J.; Ma, R.; Yu, M.; Xie, Y.; Shen, Z. Sensory determination of peach and nectarine germplasms with instrumental analysis. Foods 2023, 12, 4444. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, M.; Cai, Z.; Su, Z.; Li, J.; Shen, Z.; Ma, R.; Yan, J.; Yu, M. Aroma profiling analysis of peach flowers based on electronic nose detection. Horticulturae 2022, 8, 875. [Google Scholar] [CrossRef]
- Valdés, A.; Alvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibanez, E.; Cifuentes, A. Foodomics: Analytical opportunities and challenges. Anal. Chem. 2021, 94, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhan, C.; Yang, C.; Fernie, A.R.; Luo, J. Metabolomics-centered mining of plant metabolic diversity and function: Past decade and future perspectives. Mol. Plant 2023, 16, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Dan, M.; Zhao, G.; Wang, D. Recent advances in chromatography-mass spectrometry and electronic nose technology in food flavor analysis and detection. Food Chem. 2023, 405, 134814. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Hou, X.L.; Jiang, J.M.; Luo, C.Q.; Rehman, L.; Li, X.; Xie, X. Advances in detecting fruit aroma compounds by combining chromatography and spectrometry. J. Sci. Food Agric. 2023, 103, 4755–4766. [Google Scholar] [CrossRef]
- Xu, L.; Zang, E.; Sun, S.; Li, M. Main flavor compounds and molecular regulation mechanisms in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 63, 11859–11879. [Google Scholar] [CrossRef]
- ISO 5495:2005; Sensory Analysis—Methodology—Paired Comparison Test. International Organization for Standardization: Geneva, Switzerland, 2005.
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Afkari-Sayyah, A.H.; Khorramifar, A.; Karami, H. Investigation of the possibility of detecting peach fruit cultivars by electronic nose. J. Environ. Sci. Stud. 2023, 8, 6351–6357. [Google Scholar]
- Zhang, Y.; Liu, W.; Zhang, B.; Zhang, Y.; Cai, Z.; Song, H.; Ma, R.; Yu, M. Analysis of volatile compounds and their potential regulators in four high-quality peach (Prunus persica L.) cultivars with unique aromas. LWT 2022, 160, 113195. [Google Scholar] [CrossRef]
- Cariou, S.; Chaignaud, M.; Montreer, P.; Fages, M.; Fanlo, J.L. Odor concentration prediction by gas chromatography and mass spectrometry (GC-MS): Importance of VOCs quantification and odor detection threshold accuracy. Chem. Eng. Trans. 2016, 54, 67–72. [Google Scholar]
- Qin, Q.; Wang, L.; Wang, Q.; Wang, R.; Li, C.; Qiao, Y.; Liu, H. Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review. Plants 2025, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Syed, N.; Singh, S.; Chaturvedi, S.; Nannaware, A.D.; Khare, S.K.; Rout, P.K. Production of lactones for flavoring and pharmacological purposes from unsaturated lipids: An industrial perspective. Crit. Rev. Food Sci. Nutr. 2023, 63, 10047–10078. [Google Scholar] [CrossRef]
- Yang, C.; Chen, T.; Shen, B.R.; Sun, S.; Song, H.; Chen, D.; Xi, W. Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L.) fruit. Food Sci. Nutr. 2019, 7, 3635–3643. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, B.; Niu, Y.; Xiong, J.; Zhang, J. Characterization of key aroma-active compounds in yellow peach (Prunus persica L.‘Jinxiu’) based on GC–MS–O, OAV, aroma recombination and omission experiment. J. Food Meas. Charact. 2023, 17, 4448–4461. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Wang, Y.; Liu, X.; Zhang, Y.; Fu, H. Non-Destructive Detection of Fruit Quality: Technologies, Applications and Prospects. Foods 2025, 14, 2137. [Google Scholar] [CrossRef] [PubMed]
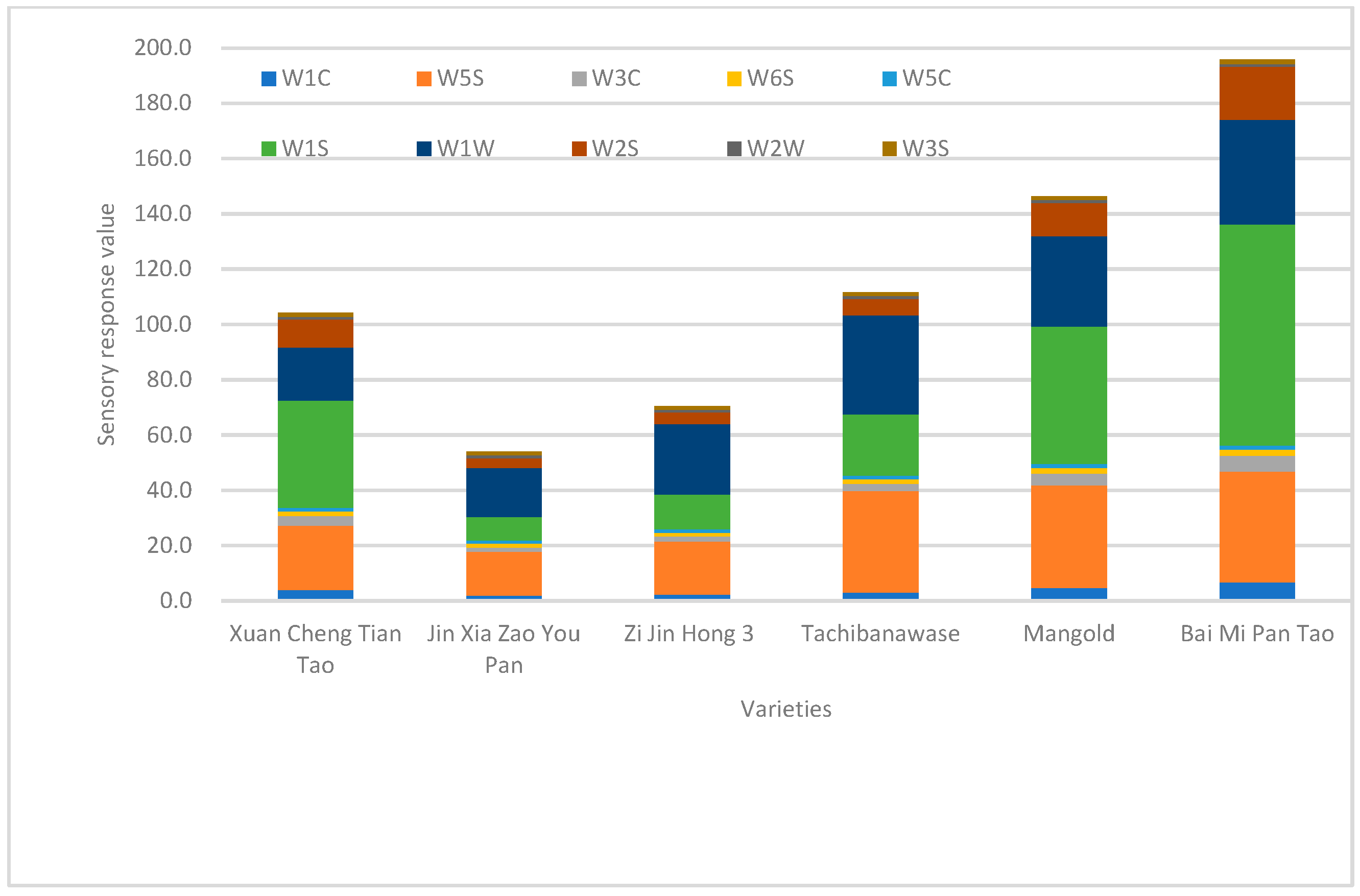
| No. | Name | Harvest Date | Species or Variety | Country of Origin | Type | Color of the Flesh |
|---|---|---|---|---|---|---|
| 1. | Xuan Cheng Tian Tao (XCTT) | 7 June | P. persica (L.) Batsch | China | Peach | Red |
| 2. | Jin Xia Zao You Pan (JXZYP) | 15 June | P. persica var. nectarina (Ait.) Maxim | China | Nectarine | Yellow |
| 3. | Zi Jin Hong 3 (ZJH3) | 23 June | P. persica var. nectarina (Ait.) Maxim | China | Nectarine | Yellow |
| 4. | Tachibanawase (Tb) | 1 July | P. persica (L.) Batsch | Japan | Peach | White |
| 5. | Mangold (Mg) | 5 July | P. persica (L.) Batsch | USA | Peach | Yellow |
| 6. | Bai Mi Pan Tao (BMPT) | 2 August | P. persica var. compressa Bean. | China | Peach | White |
| Sensor Name | Sensor Sensitives |
|---|---|
| W1C | Sensitive to aromatic benzene |
| W3C | Sensitive to ammonia and aromatic compounds |
| W5C | Sensitive to nitrogen oxides |
| W1S | Sensitive to short-chain alkanes such as methane |
| W2S | Sensitive to alcohols, ethers, aldehydes and ketones |
| W3S | Sensitive to long-chain alkanes |
| W5S | Sensitive to hydrocarbons and aromatic compounds |
| W6S | Sensitive to hydrogen |
| W1W | Sensitive to terpenes and organosulfur compounds |
| W2W | Sensitive to aromatic compounds and sulphur and chlorine compounds |
| Name | Aroma | Sweetness | Sourceness | Astrigency | Bitterness | Persistnece | Overall Impression | Special Flavour |
|---|---|---|---|---|---|---|---|---|
| Xuan Cheng Tian Tao (XCTT) | 3.8 | 3.2 | 0 | 3.1 | 0 | 1.8 | 2.2 | Peach |
| Jin Xia Zao You Pan (JXZYP) | 5 | 7.2 | 3 | 0 | 0 | 3.2 | 6.8 | Gardenia |
| Zi Jin Hong 3 (ZJH3) | 7.1 | 7.2 | 0 | 0 | 0 | 4.3 | 6.9 | Gardenia; passion fruit |
| Tachibanawase (Tb) | 6.9 | 3.2 | 7.2 | 0 | 0 | 6 | 0.4 | |
| Mangold (Mg) | 6.8 | 4.3 | 6.8 | 0 | 0 | 4.8 | 1.8 | Peach; honeydew |
| Bai Mi Pan Tao (BMPT) | 5.2 | 7.2 | 0 | 0 | 0 | 2.1 | 8.1 | Peach; moderate milk; violet |
| Group | R2X | R2Y | Q2 |
|---|---|---|---|
| Bai Mi Pan Tao vs. Xuan Cheng Tian Tao | 0.888 | 1.000 | 0.998 |
| Bai Mi Pan Tao vs. Jin Xia Zao You Pan | 0.933 | 1.000 | 0.997 |
| Bai Mi Pan Tao vs. Zi Jin Hong 3 | 0.922 | 1.000 | 0.997 |
| Bai Mi Pan Tao vs. Tachiwanawase | 0.899 | 1.000 | 0.995 |
| Bai Mi Pan Tao vs. Mangold | 0.856 | 1.000 | 0.995 |
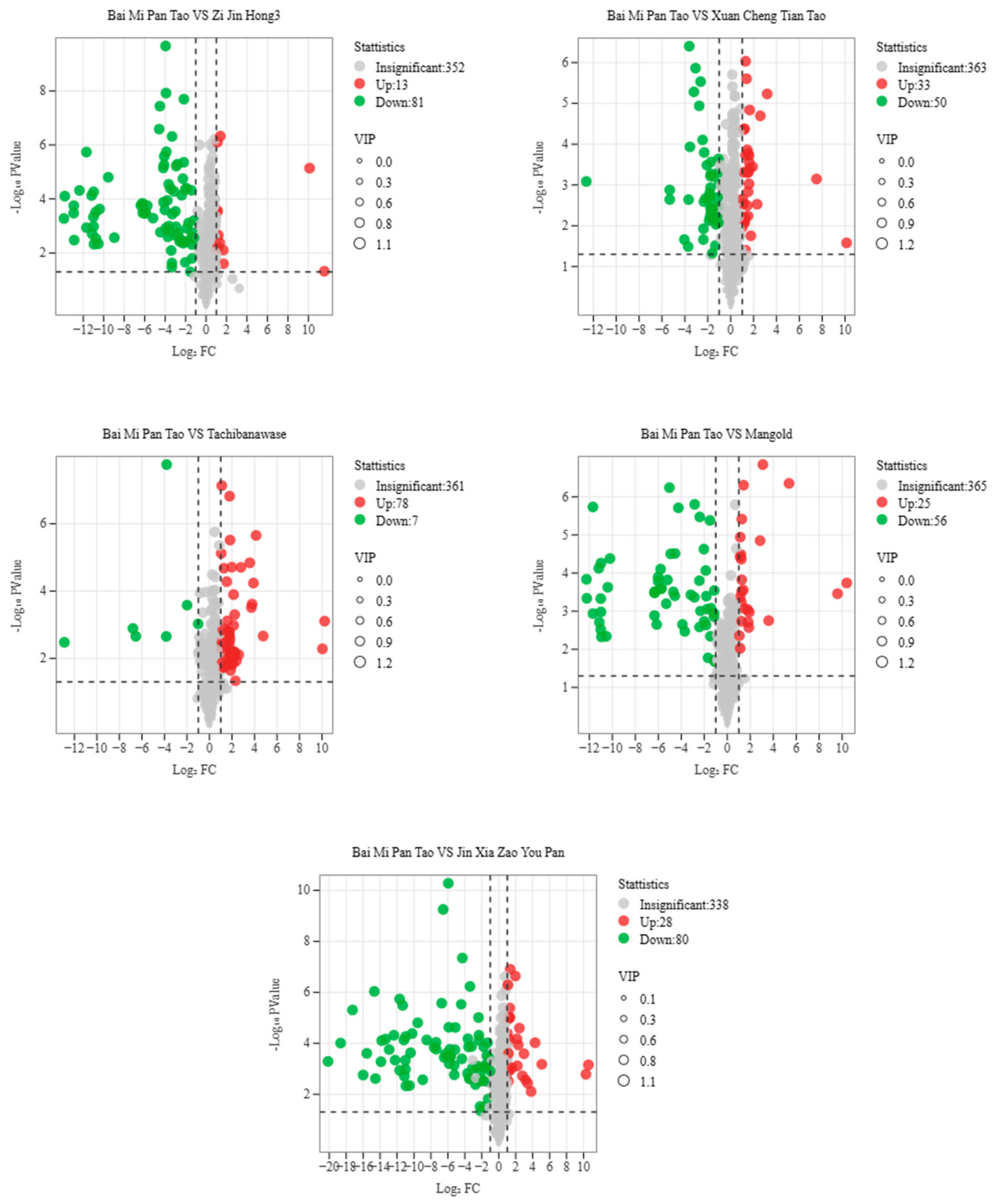
| Group Name | All Sig Diff | Down Regulated | Up Regulated |
|---|---|---|---|
| Bai Mi Pan Tao vs. Xuan Cheng Tian Tao | 83 | 50 | 33 |
| Bai Mi Pan Tao vs. Jin Xia Zao You Pan | 108 | 80 | 28 |
| Bai Mi Pan Tao vs. Zi Jin Hong 3 | 94 | 81 | 13 |
| Bai Mi Pan Tao vs. Tachiwanawase | 85 | 7 | 78 |
| Bai Mi Pan Tao vs. Mangold | 81 | 56 | 25 |
| “Bai Mi Pan Tao” vs. “Xuan Cheng Tian Tao” | “Bai Mi Pan Tao” vs. “Jin Xia Zao You Pan” | “Bai Mi Pan Tao” vs. “Zi Jin Hong 3” | “Bai Mi Pan Tao” vs. “Tachiwanawase” | “Bai Mi Pan Tao” vs. “Mangold” |
|---|---|---|---|---|
| Octanal | 2,7-Octadien-4-ol, 2-methyl-6-methylene-, (S)- | (R)-(+)-1-(p-Tolyl) ethylamine | Isomaltol | 1-Octanol |
| Isobutyl isovalerate | Ethanone, 1-(2-thienyl)- | 2-Furanmethanol, tetrahydro-α, α,5-trimethyl-5-(4-methyl-3-cyclohexen-1-yl)-, [2S-[2α,5β (R*)]]- | Azulene | Butanoic acid, 2-methyl-, 3-methylbutyl ester |
| Acetic acid, hexyl ester | Hotrienol | 2-Cyclopentylethanol | 2-Nonanol | |
| 3-Hexene, 1-(1-methoxyethoxy)-, (Z)- | Benzaldehyde, 4-methyl- | Di-epi-.α.-cedrene-(I) | Acetic acid, heptyl ester | |
| Acetic Acid, (acetyloxy)- | 7-Octen-4-ol, 2-methyl-6-methylene-, (S)- | 2,3-Dehydro-1,8-cineole | Undecane | |
| 3-Hexen-1-ol, acetate, (Z)- | Benzenemethanol, 4-methyl- | cis-2,6-Dimethyl-2,6-octadiene | 2-Isopropyl-5-methylhex-2-enal | |
| Isospathulenol | Lilac Alcohol C | Acetophenone, 4′-hydroxy- | Pyrazine, 2,3-dimethyl-5-(1-methylpropyl)- | |
| 2,6,11,15-Tetramethyl-hexadeca-2,6,8,10,14-pentaene | Benzene, (1-methoxypropyl)- | 2,4-Heptadien-1-ol, (E,E)- | 2,4-Dimethyl-2-oxazoline-4-methanol | |
| N,N-Dimethylhexanamide | Benzyl angelate | (1R,3aS,4aS,8aS)-1,4,4,6-Tetramethyl-1,2,3,3a,4,4a,7,8-octahydrocyclopenta [1,4]cyclobuta [1,2]benzene | ||
| Benzene, 1,2,4-trimethyl- | L-α-Terpineol | Panaxene | ||
| 2-Hydroxyfluorene | Butanoic acid, 4-hexenyl ester, (Z)- | Cyclohexene, 3-(1,5-dimethyl-4-hexenyl)-6-methylene-, [S-(R*,S*)]- | ||
| 5-Isoxazolecarboxylic acid, 4,5-dihydro-5-methyl-, methyl ester, (R)- | Benzenemethanethiol | 1,7-Octadiene, 2-methyl-6-methylene- | ||
| trans-3-Methyl-4-octanolide | 1-Hepten-6-one, 2-methyl- | 2,6-Dodecadien-1-al | ||
| Thiazol-2-Amine, N-(4-dimethylaminobenzyl)- | Hexanoic acid, propyl ester | |||
| Niacinamide | Benzoic acid, 1-methylethyl ester | |||
| Phenanthrene, 7-ethenyl-1,2,3,4,4a,4b,5,6,7,9,10,10a-dodecahydro-1,1,4a,7-tetramethyl-, [4aS-(4a.α.,4b.β,7 β,10a.β)]- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Ma, J.; Cai, Z.; Yan, J.; Ma, R.; Yu, M.; Xie, Y.; Shen, Z. Integrating Sensory Evaluation, Electronic Nose, and Metabolomics to Characterize Aroma in Peach and Nectarine Varieties. Foods 2025, 14, 3087. https://doi.org/10.3390/foods14173087
Sun M, Ma J, Cai Z, Yan J, Ma R, Yu M, Xie Y, Shen Z. Integrating Sensory Evaluation, Electronic Nose, and Metabolomics to Characterize Aroma in Peach and Nectarine Varieties. Foods. 2025; 14(17):3087. https://doi.org/10.3390/foods14173087
Chicago/Turabian StyleSun, Meng, Julin Ma, Zhixiang Cai, Juan Yan, Ruijuan Ma, Mingliang Yu, Yinfeng Xie, and Zhijun Shen. 2025. "Integrating Sensory Evaluation, Electronic Nose, and Metabolomics to Characterize Aroma in Peach and Nectarine Varieties" Foods 14, no. 17: 3087. https://doi.org/10.3390/foods14173087
APA StyleSun, M., Ma, J., Cai, Z., Yan, J., Ma, R., Yu, M., Xie, Y., & Shen, Z. (2025). Integrating Sensory Evaluation, Electronic Nose, and Metabolomics to Characterize Aroma in Peach and Nectarine Varieties. Foods, 14(17), 3087. https://doi.org/10.3390/foods14173087





