1. Introduction
The advanced capability of real-time food safety monitoring has positioned intelligent food packaging as a key research focus in recent years [
1,
2]. At present, both at home and abroad, there have been research reports on using plant pigments, such as purple sweet potato [
3], blueberry anthocyanins [
4], and red cabbage [
5], as natural indicators for intelligent food packaging. Among them, grape seed anthocyanins (GSA), as an edible natural pigment with wide sources, excellent water solubility, and strong pH sensitivity [
6,
7], can be used as an indicator for intelligent food packaging. Anthocyanins rank among the most extensively studied bioactive natural compounds. As flavonoid derivatives, they demonstrate well-documented health-promoting properties including safety, antioxidant capacity, and protective effects against diabetes, obesity, cancer, microbial infections, and cardiovascular disorders [
8]. Concurrently, their pH-dependent chromogenic behavior enables applications as colorimetric indicators for tracking metabolic pH shifts in foods or spoilage-induced pH changes mediated by microorganisms [
8], which could enable real-time monitoring of packaged food safety while also delivering information on product response to environmental conditions and shelf-life determination [
9,
10].
This yellow diketone compound (CUR), naturally occurring in the rhizomes of
Zingiberaceae and Araceae species, constitutes a rare botanical pigment [
11]. Curcumin (Cur), a lipophilic diphenolic compound, serves as a natural food additive with coloring, flavoring, and spicing functions. Its recognized safety profile allows high-dose applications, while its bioactive properties—including antioxidant, anti-inflammatory, and antimicrobial effects—enhance its utility [
12]. Notably, Cur’s chromatic transition from orange to red under alkaline conditions results from phenolate anion formation via hydroxyl group deprotonation, enabling its use as a pH-sensitive acid–base indicator [
12,
13]. Single natural pigments have relatively poor stability and functionality and certain limitations. Therefore, GSA can be mixed with CUR to make up for the instability of GSA and the limitation of the color change range of CUR. Zheng et al. [
14] developed a mixed indicator label of anthocyanins and CUR in red cabbage for monitoring the freshness of pork. Zhou et al. [
2] prepared a mixed bilayer indicator film of anthocyanin and CUR for monitoring the freshness of chicken in real time.
In studies such as the above-mentioned ones, since the exposure of meat packaging to storage can lead to a decrease in the stability of natural pigments such as anthocyanins and CUR, the pigments are usually dissolved in natural biopolymers to demonstrate their indicator functions [
15]. In order to better fix natural pigments while considering the safety of food, biodegradable natural macromolecular polymers such as chitosan (CS), carrageenan (CAR), and sodium alginate are often selected as the film base materials for smart packaging films [
16]. Among them, CS is a natural high-molecular polymer obtained by the deacetylation of chitin. It has good film-forming properties, biocompatibility, and biodegradability by itself and is mostly used in the preparation of bioedible membranes [
17]. CAR has good film-forming ability. However, a single CAR film has poor mechanical strength and is hydrophilic. To improve the performance of the indicator film, CAR can be mixed with other biopolymers (such as CS, gelatin (GEL), etc.) for use to enhance its mechanical strength [
18].
Plant-derived cold-pressed oils are recognized as functional enhancers that augment the bioactivity of biopolymeric packaging films [
19]. Moreover, their inherent hydrophobicity impedes moisture transmission through packaging matrices [
20]. Nanoscale encapsulation systems have garnered considerable research interest in food technology due to their distinctive advantages. These systems establish semi-permeable barriers that protect labile oil components against oxidation while enabling controlled release kinetics [
21]. Concurrently, they promote oil droplet uniformity through nano-scale dispersion mechanisms. Flaxseed oil (FO) contains various active substances such as alpha-linolenic acid, phytosterols, vitamin E, and polyphenols. It has high nutritional value and functions such as antioxidation, anti-cancer, anti-inflammation, and blood pressure-lowering. It also has the functions of preventing and improving neurological and immune diseases, diabetes, and cardiovascular diseases, etc. [
22] Tosif et al.’s [
23] research showed that adding FO to the film can increase the moisture resistance of the film and improve its performance. Meanwhile, its emulsion can serve as an emulsified layer to enhance the performance of the film. Many scholars have confirmed [
2,
24,
25] that the method of adding oil emulsification to the membrane can effectively improve the hydrophobicity and UV resistance of the membrane.
The yellowfin seabream (
Acanthopagrus latus), a warm-water demersal fish species of the Sparidae family, is a commercially significant fish in the coastal regions of southeastern China. Although widely cultivated on a large industrial scale, its high moisture, protein, and fat content [
26] make it highly susceptible to spoilage deterioration during storage, transportation, and marketing. This deterioration stems from endogenous chemical/enzymatic reactions and microbial contamination (including spoilage and pathogenic microorganisms), leading to significant declines in sensory and physicochemical quality. Consequently, developing preservation techniques and quality monitoring methods for yellowfin seabream has become imperative [
26,
27]. However, a review of the literature reveals that reports on the application of intelligent indicator films for preservation and freshness monitoring of yellowfin seabream remain scarce.
Based on this, the present study aimed to develop a novel pH-responsive bilayer indicator film with excellent mechanical strength and water resistance. The indicator layer was fabricated using CAR/GEL and GSA/CUR, while the outer layer was composed of a CS and FO blended emulsion. Through systematic investigation of the effects of CAR/GEL mass ratio, CS concentration, and GSA/CUR pigment mixture dosage on the film’s elongation at break (EAB), tensile strength (TS), water vapor permeability (WVP) and hydroxyl radical clearance (HRC), we optimized the preparation parameters to obtain the best-performing film. The optimally formulated film was subsequently characterized and applied for real-time freshness monitoring of yellowfin seabream (Sparus latus) stored at 25 °C. This study can offer practical guidelines for applying a pH-responsive bilayer indicator film to real-time fish freshness monitoring.
2. Materials and Methods
2.1. Material
The chitosan (CS, N-degree of deacetylation ≥ 90%, the relative molecular mass was about 50–90 kDa), gelatin, derived from fish scales (GEL, 99% purity, relative molecular weight 60 KDa to 130 KDa), carrageenan (CAR, 200 kDa to 1000 kDa), grape seed anthocyanins (GSA, polyphenol content > 95%), and curcumin (purity ≥ 95%) were all food grade and purchased from Zhejiang Yinuo Biotechnology Co., Ltd., Ningbo, China. The flaxseed oil (FO) (Arowana) (the content of linolenic acid is 48–70%) was purchased from RT-Mart Supermarket in Zhangzhou. The glycerin, chloroform, carbinol, anhydrous ethanol, salicylic acid, 2-thiobarbituric acid, ferrous sulfate heptahydrate, et al. were analytically pure and brought from Xilong Scientific Co., Ltd. (Shantou, China). Water was purified with a Milli-Q water purification system (Millipore Co., Ltd., Burlington, MA, USA). Fresh yellowfin seabream (Sparus latus) (about 0.6~1.0 kg weight) was purchased from RT-Mart (Zhangzhou, China) and transported to the laboratory alive.
2.2. Preparation of the CS/FO-CAR/GEL/GSA/CUR Bilayer Indicator Film
2.2.1. Preparation of Mixed Pigment Solution
The mixed pigment solution was prepared according to the method of Zhou et al. [
2]. A total of 0.8 g of GSA powder and 0.8 g of CUR powder were dissolved in 40 mL of 47.5% ethanol. The mixed pigment solution was stored in a dark room at 4 °C until use.
2.2.2. Preparation of the Bilayer Indicator Film
Preparation of hydrophobic film: A certain mass concentration of CS was stirred in deionized water at 60 °C for 30 min by the magnetic stirrer (DF-101S, Shanghai Lichen Bangxi Instrument Technology Co., Ltd., Shanghai, China). Subsequently, FO (1% based on the deionized water content) was added to the CS solution. The mixture was homogenized for 5 min at 480 W and 20 kHz to form a blended emulsion, after which the pH of the emulsion was adjusted to 6.0 ± 0.1. Following degassing, 20 mL of the film-forming emulsion was cast onto a 10 cm diameter polytetrafluoroethylene (PTFE) plate and dried in a 50 °C air-drying oven for 6 h to obtain a hydrophobic single-layer film.
Preparation of indicator-layer film: A mixture of 5 g CAR and GEL powder was added to 300 mL of deionized water at 90 °C, along with 1% glycerol (based on the deionized water content), and stirred thoroughly at 90 °C and 1500 rpm for 0.5 h using a magnetic stirrer. After cooling the mixed solution to 60 °C, a predetermined mass concentration (mg/100 mL) of GSA/CUR mixed pigment solution was added and stirred until uniform, followed by pH adjustment to 6.0 ± 0.1. After defoaming, 30 mL of the solution was cast onto the aforementioned PTFE plate and further dried at 45 °C for approximately 6 h to form the bilayer film. Finally, the CS/FO-CAR/GEL/GSA/CUR bilayer indicator film in the PTFE plate was placed in a 25 °C drying oven for equilibration over 6 h before the film could be subjected to physicochemical property testing.
2.2.3. Single-Factor Experiments
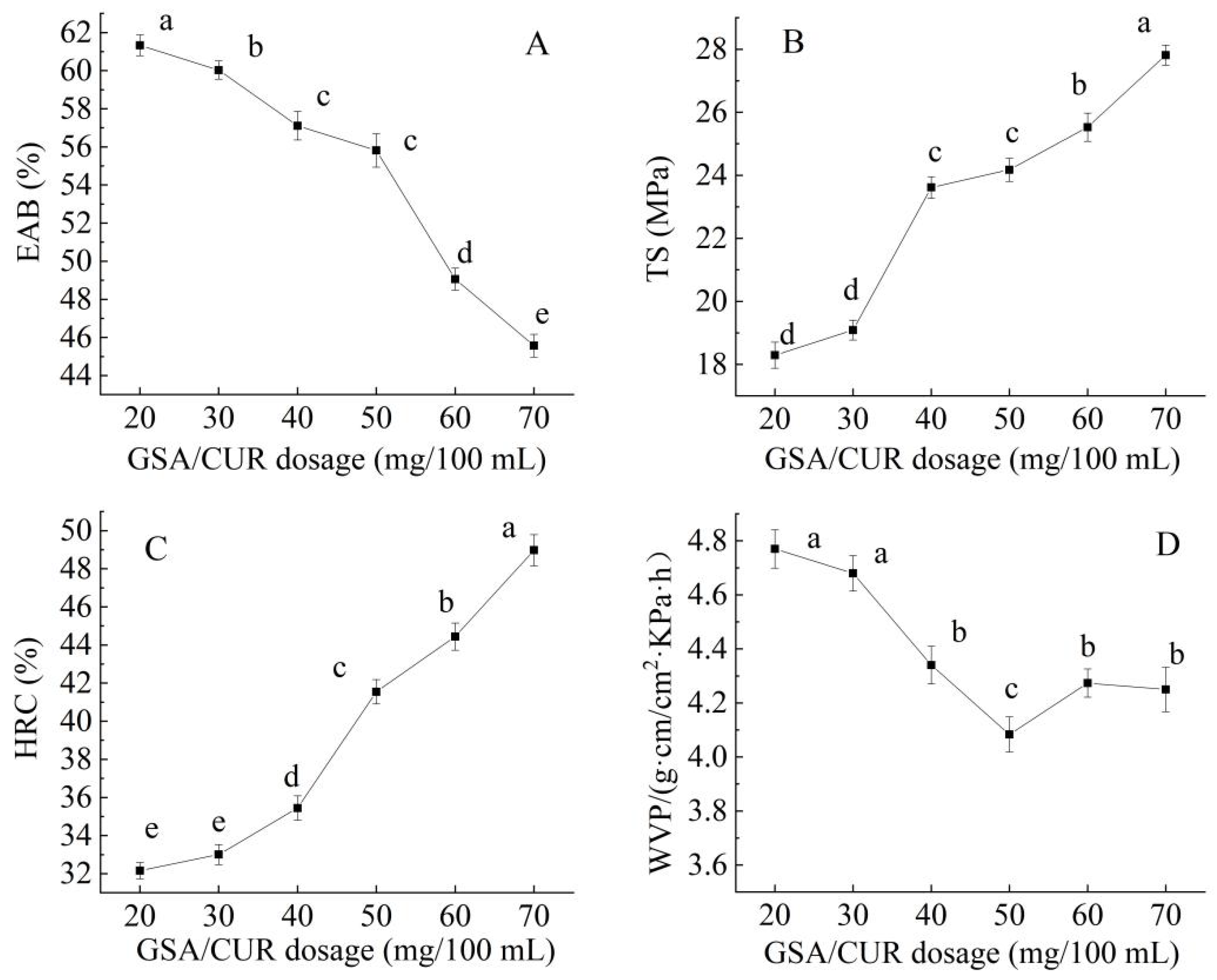
The effects of the CAR/GEL mass ratio (8:2, 7:3, 6:4, 5:5, 4:6, 3:7 g/g), CS concentration (0.5%, 1.0%, 1.5%, 2%, 2.5%, 3.0%), and GSA/CUR dosage (20, 30, 40, 50, 60, 70 mg/100 mL) on the elongation at break (EAB), tensile strength (TS), hydroxyl radical clearance (HRC), and water vapor permeability (WVP) of the CS/FO-CAR/GEL/GSA/CUR bilayer indicator film were systematically investigated.
2.2.4. Response Surface Optimization Test
According to the results of single factor analysis, the effect of CAR/GEL mass ratio, CS concentration, and GSA/CUR dosage on the TS and EAB of the films was detected for further response surface test design in the predetermined range. The factor levels and the codes of response surface test design are shown in
Table 1.
2.2.5. Calculation of the Comprehensive Score of Film
The calculation of the comprehensive score of the CS/FO-CAR/GEL/GSA/CUR bilayer indicator film was based on the method of Jiang et al. [
9]. Nine groups of experimental data were randomly selected from three groups of single-factor experiments. The data were standardized using SPSS 17.0 software, and the weights were obtained through factor analysis of dimension reduction processing. The positive effect indicators are referred to in Equation (1), the negative effect indicators are referred to in Equation (2), and the comprehensive score is referred to in Equation (3).
where
P: Membership degree value;
Rmax: The maximum value in the determination of the index;
Rmin: The minimum value in the index determination;
S: Comprehensive score;
P1, P2, P3, P4: Membership degree values corresponding to the indicators;
a, b, c, d: The weights of the corresponding indicators.
2.3. Determination of Physical and Chemical Properties of Film
2.3.1. The Thickness of the Film
The film was sectioned into specimens measuring 4 mm in width and 20 mm in length. Thickness measurements were taken at six randomly selected points across the film cross-section, covering upper, middle, and lower regions, using a digital micrometer (Mitutoyo BMD-25D) with an accuracy of 0.001 mm. The average value was reported as the final result.
2.3.2. The Test of TS and EAB
The test of TS and EAB was referred to the method of XUE et al. [
28]. Using a texture analyzer (CT3-10K, Brookfield AMETEK, Middleboro, MA, USA), tensile strength (TS) and elongation at break (EAB) were measured. Dumbbell-shaped film strips (30 mm × 10 mm) were clamped vertically between fixtures. A tensile test was initiated at constant speed until rupture, during which the initial gauge length (L0), rupture length (L1), and peak force (F) were recorded. Each sample was measured 3 times and averaged. The mechanical properties of the film were calculated according to Equations (4) and (5).
where
TS: tensile strength of the films, Mpa;
F: the maximum tension of the films, N;
b: width of the films, mm;
d: Thickness of the films, mm.
where
EAB: elongation at break of film, %;
L1—length of the film at fracture, mm;
L0—original length of film, mm.
2.3.3. Determination of Hydroxyl Radical Scavenging Rate (HRC)
The hydroxyl radical scavenging rate (HRC) was measured according to the method of Xue et al. [
28]. In a test tube, 0.2 mL of the membrane solution was added and dissolved with deionized water to a total volume of 12 mL, while 12 mL of distilled water was used as the blank group. To both the experimental and blank group tubes, 1 mL of FeSO
4 solution (6 mmol/L) and 1 mL of H
2O
2 solution (6 mmol/L) were added and mixed well. Subsequently, 1 mL of salicylic acid solution (6 mmol/L) was added, followed by incubation in a water bath for 30 min to equilibrate. The absorbance was then measured at 510 nm. The HRC was calculated according to Equation (6).
where
Ai: Absorbance of the experimental group;
A0: Absorbance of the blank group.
2.3.4. Water Vapor Permeability (WVP)
The measurement was performed according to the method of Mali [
29]. First, 3 g of anhydrous calcium chloride was weighed into a small glass jar. An intact film was selected, its thickness measured using a vernier caliper, and then the film was securely fixed to cover the jar opening. After recording the initial weight, the sample was placed in a desiccator at 100% relative humidity. The weight was measured and recorded every 2 h. The WVP was calculated according to Equation (7)
where
Δm: Weight difference before and after the 2-h interval (g);
d: Film thickness (cm);
S: Area of the bottle opening (cm2);
t: Duration of the interval (h);
ΔP: Water vapor pressure difference (kPa).
2.3.5. FTIR Analysis
The CS/FO-CAR/GEL/GSA/CUR bilayer indicator film was trimmed to a suitable dimension (typically circular, 10–15 mm diameter) to guarantee flat, wrinkle-free surfaces devoid of contaminants. Against a blank reference background, samples were mounted on the holder and subjected to infrared transmittance measurements.
2.3.6. SEM Analysis
Microstructural features of the CS/FO-CAR/GEL/GSA/CUR bilayer indicator were examined using scanning electron microscopy (Hitachi SU-8000, Tokyo, Japan) at 20 kV accelerating voltage. Post-drying, specimens were sectioned to optimal dimensions, adhered to stainless steel substrates with conductive carbon tape, mounted on copper stages for gold sputter-coating, and imaged to document surface/cross-sectional morphology [
30].
2.4. Determination of pH Color Sensitivity of Film
The indicator film, packaged in transparent sealed bags, was placed in a desiccator maintained at a relative humidity of 50 ± 1%. Color measurements were taken every 1 day for a total of 10 days. To study the color sensitivity of the film, pH values of 1, 3, 5, 7, 8, 9, and 11 were selected. The indicator films were immersed in buffer solutions of the corresponding pH values, and their color changes were observed and recorded using a colorimeter after 6 min. The ΔE value was calculated according to Equation (8).
where
L, a, b: Values of the film;
L0, a0, b0: Initial values, respectively.
2.5. The Application of Film in the Preservation and Freshness Detection of Yellowfin Seabream (Sparus latus)
The yellowfin seabream (Sparus latus) was humanely euthanized via immersion in an ice–water slurry (2.4 kg ice: 3.6 L water: 1.5 kg fish) for 20 min. Subsequently, specimens were promptly decapitated, descaled, eviscerated, and rinsed under tap water. All samples underwent ice storage ≤ 24 h prior to processing. Before experimentation, fish tissues were sectioned into 2 cm3 cubic portions. The fish meat (10 g per group) was put into triangular glass bottles and were sealed with film. The pH indicator layer of the film was downward. The fish preserved with the double-layer indicator film (comprising a hydrophobic layer and an indicator layer) served as the experimental group (EG), while the fish preserved with a single-layer film (without the hydrophobic layer) functioned as the control group (CG). The fish samples were stored at 25 °C for 0, 12, 24, 36, and 48 h, respectively. At each time point, changes in physicochemical indicators of the samples were measured, while corresponding color changes of the indicator film were observed and recorded.
2.5.1. Determination of Moisture Content in Fish
A 5.0 g sample of fish was placed in a pre-dried weighing bottle (m
0), and the initial weight (m
1) was recorded. The sample was then placed in a drying oven at 105 °C for 2 h. After removal, the weighing bottle was cooled before the weight (m
2) was weighed and recorded. The drying process was repeated (30 min per cycle after the first drying) until the weight difference between consecutive measurements was less than 2 mg. The moisture content was calculated according to Equation (9).
where
m0: Weight of undried sample (g);
m1: Initial mass of sample and the bottle (g);
m2: Constant mass of weighing bottle + sample after drying (g).
2.5.2. Determination of pH of Fish
Weigh 10.0 g of fish meat sample and homogenize with 90 mL of distilled water using a blender. Allow the mixture to stand for 10 min, then filter the supernatant. Measure the pH value of the filtrate using a calibrated pH meter (Testo735-2, Testo AG, Titisee-Neustadt, Germany) at room temperature (25 ± 1 °C). Record triplicate measurements for each sample.
2.5.3. Measurement of TVB-N
The TVB-N quantification was performed according to Xue et al. [
31]. Samples (2.0 g) were treated with MgO (0.2 g) in a Kjeldahl distillation apparatus (Kjeltec 8400, FOSS, Hillerød, Denmark), followed by steam distillation analysis. Results were expressed as mg nitrogen per 100 g sample (mg N/100 g).
2.5.4. Measurement of TBARS
The TBARS quantification followed Xue et al. [
31]. Briefly, 5 g minced fish was homogenized with 50 mL of 7.5% trichloroacetic acid (TCA) containing 0.1% EDTA. The mixture was incubated at 50 °C (water bath) for 30 min, then filtered through Whatman No. 1 filter paper. A 3 mL aliquot of filtrate was reacted with 3 mL of 20 mmol/L 2-thiobarbituric acid (TBA) solution at 100 °C for 45 min. After cooling, the solution was centrifuged (5000×
g, 4 °C, 10 min), mixed with 3 mL chloroform, and vortexed for 30 s. Following phase separation, supernatant absorbance was recorded at 532 nm and 600 nm against a blank (3 mL TCA + 3 mL TBA + 3 mL chloroform). Results were expressed as mg malondialdehyde (MDA) per kg sample according to Equation (10).
where
c: The concentration of malondialdehyde obtained from the standard curve/(μg/ mL);
V: Volume of sample liquid (mL);
m: Sample mass (g).
2.5.5. Analysis of Fatty Acid Composition in Fish
The fatty acid composition of fish meat was analyzed through a standardized procedure involving lipid extraction followed by gas chromatographic determination [
32]. For lipid extraction, 5.0 g of fish sample was homogenized with 60 mL of chloroform–methanol (2:1,
v/
v) and stirred at 45 °C for 2.5 h. After filtration, the extract was washed with 15 mL saturated NaCl solution in a separatory funnel, with the lower chloroform phase collected and dehydrated using anhydrous sodium sulfate. The purified lipid extract was obtained by rotary evaporation at 45 °C and quantitatively recorded. For fatty acid methyl ester (FAME) preparation, the extracted lipids were dissolved in benzene–petroleum ether (1:1,
v/
v) and derivatized with 14% BF
3–methanol solution through 2.5 h incubation at 45 °C followed by 12 h refrigeration at 4 °C. The FAMEs were then extracted with n-hexane for subsequent GC analysis.
Chromatographic separation was performed on an SH-Rt™-2560 capillary column (100 m × 0.25 mm ID, 0.20 μm film thickness) using a temperature program starting at 140 °C (1 min hold), ramping at 4 °C/min to 240 °C (19 min hold). The GC system operated with nitrogen carrier gas at 20 mL/min, 10:1 split ratio, and 1 μL injection volume, with injector and detector temperatures maintained at 250 °C and 260 °C, respectively. Fatty acids were identified by comparing retention times with a 37-component FAME standard mixture and quantified using the area normalization method, with results expressed as relative percentages of total fatty acids.
2.6. Statistical Analysis
Statistical analyses were performed as follows: ANOVA with Tukey’s post hoc test (p < 0.05) using Microsoft Excel® 2010 to determine inter-group differences; principal component analysis (PCA) executed in SPSS 17.0; optimization design implemented via Design-Expert 8.0.6; correlation analyses conducted with Excel®.