Structural Characterization of a Novel Pectin Polysaccharide from Mango (Mangifera indica L.) Peel and Its Regulatory Effects on the Gut Microbiota in High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
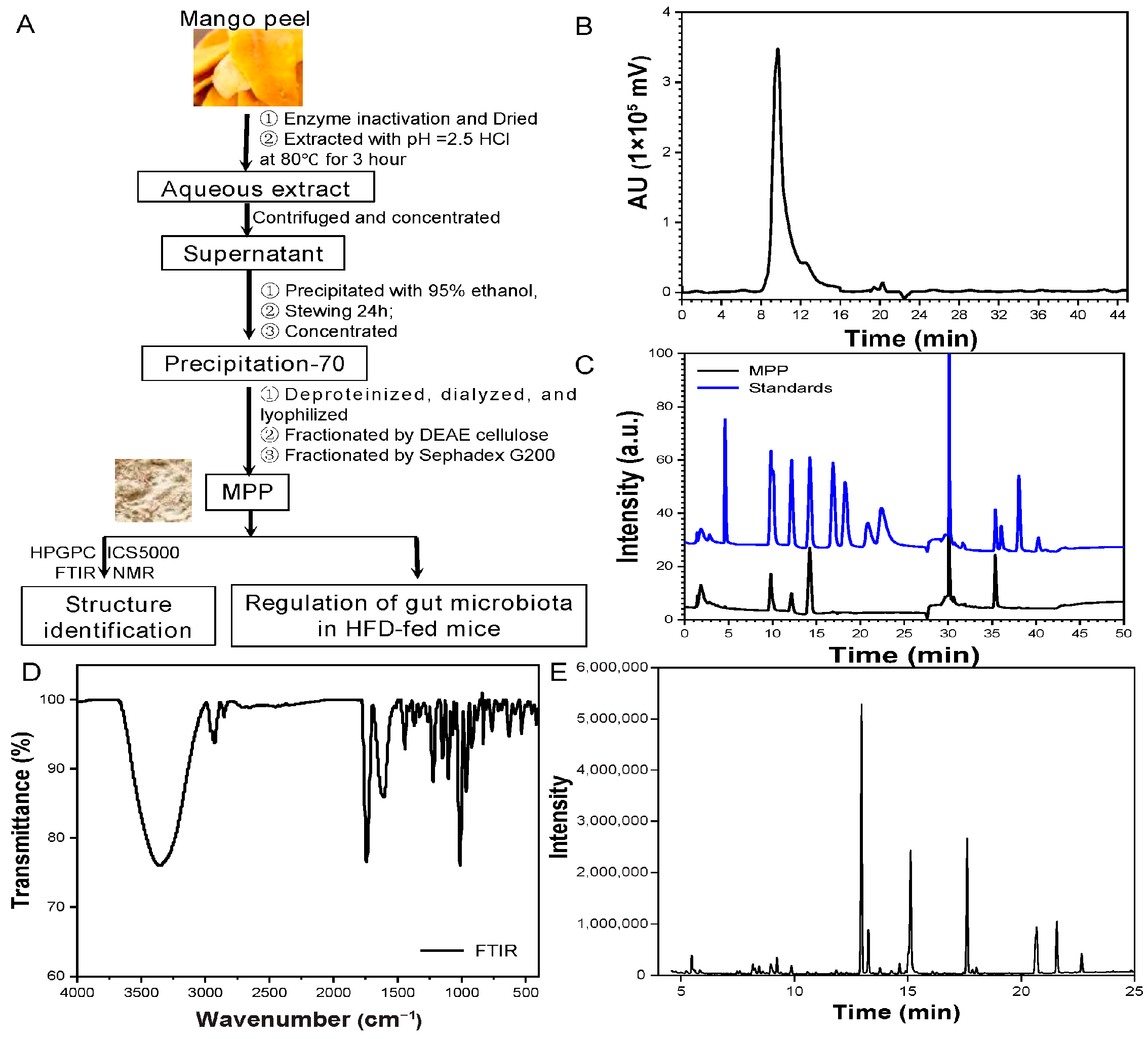
2.2. Extraction and Purification of MPP
2.3. Structural Analysis of MPP
2.3.1. Molecular Weight Analysis of MPP
2.3.2. Monosaccharide Composition Analysis of MPP
2.3.3. FTIR Analysis
2.3.4. Methylation Analysis
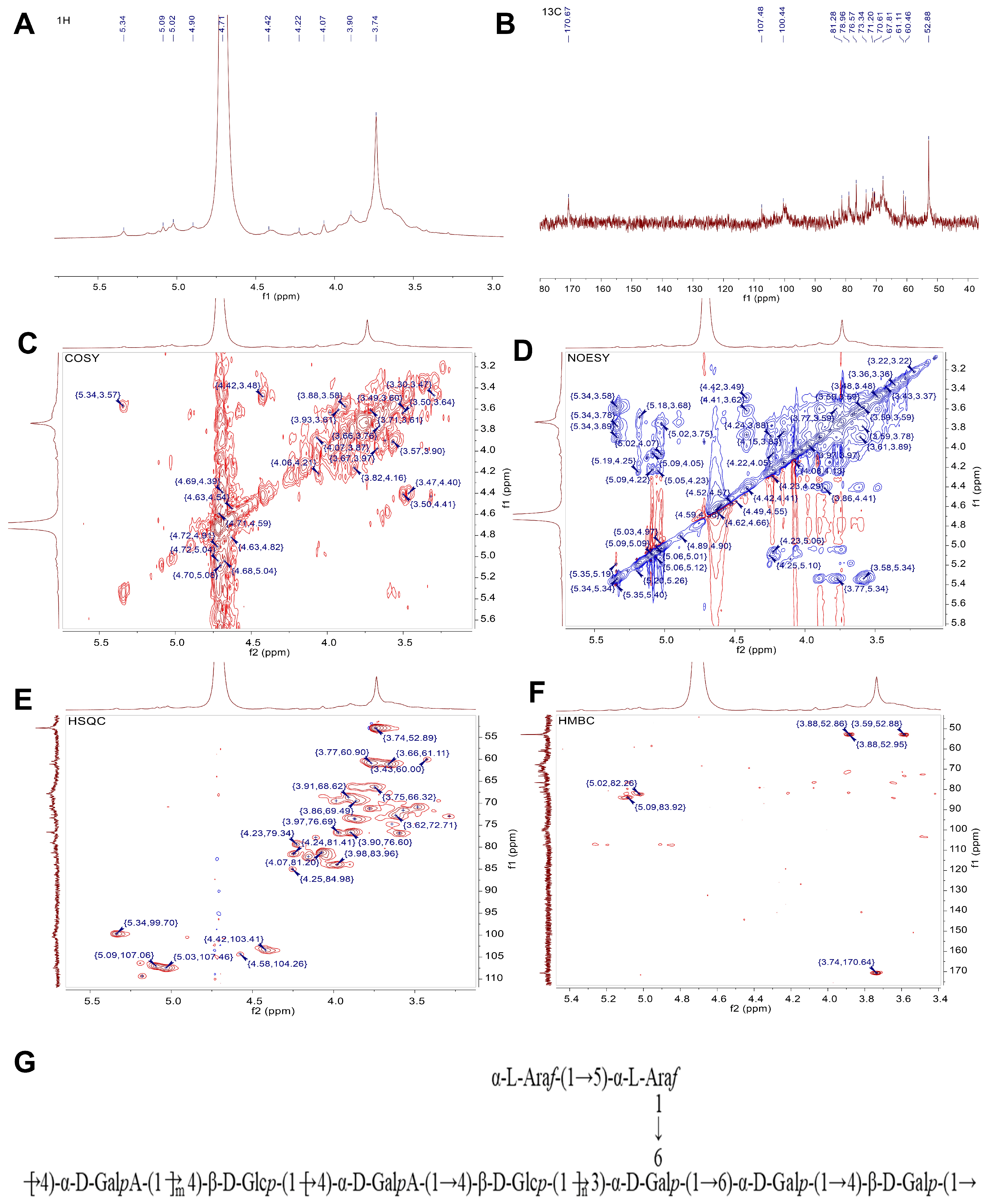
2.3.5. NMR Analysis
2.4. Animal Experiments Design
2.5. Glucose Homeostasis
2.6. Biochemical Parameter Analysis
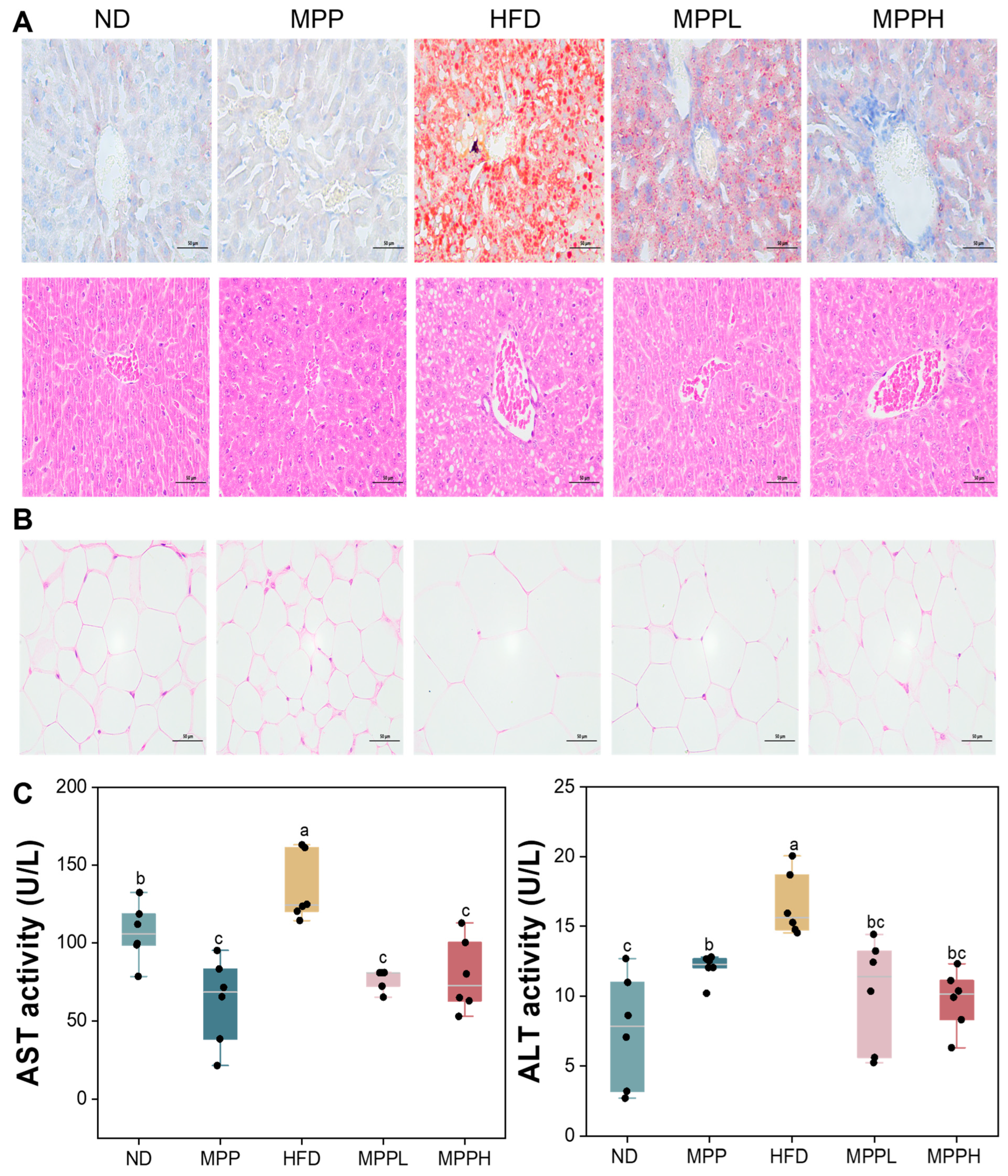
2.7. Histological Analysis
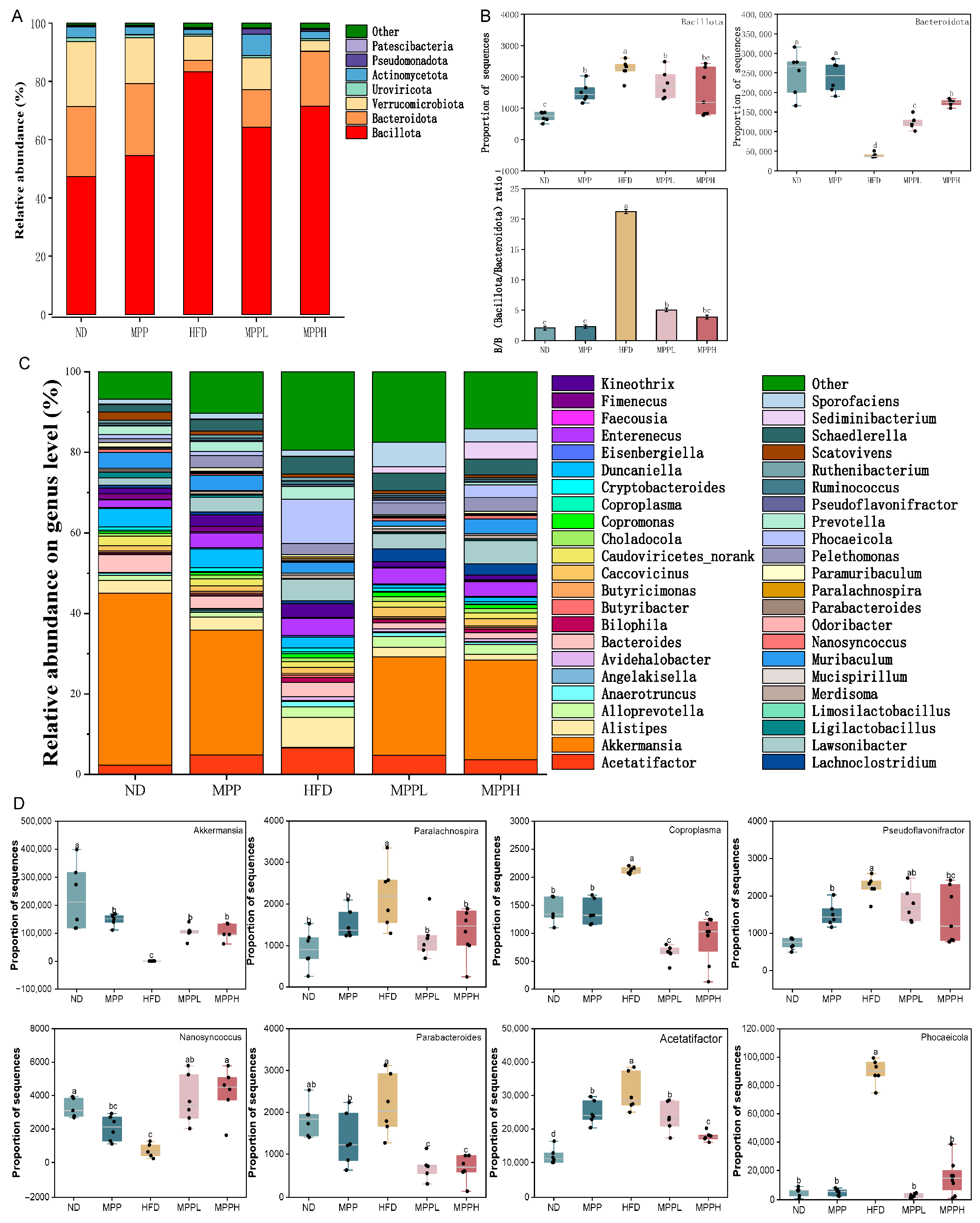
2.8. Metagenomic Sequencing
2.9. Analysis of SCFAs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Subsection Extraction, Purification, and Identification of MPP
3.2. MPP Prevented Obesity and Hepatic Steatosis in HFD-Fed Mice
3.3. MPP Alleviated Abnormal Glucolipid Metabolism in HFD-Induced Obese Mice
3.4. MPP Improved Gut Microbiota Dysbiosis in HFD-Induced Obese Mice
3.5. MPP Regulates SCFAs in HFD-Induced Obese Mice
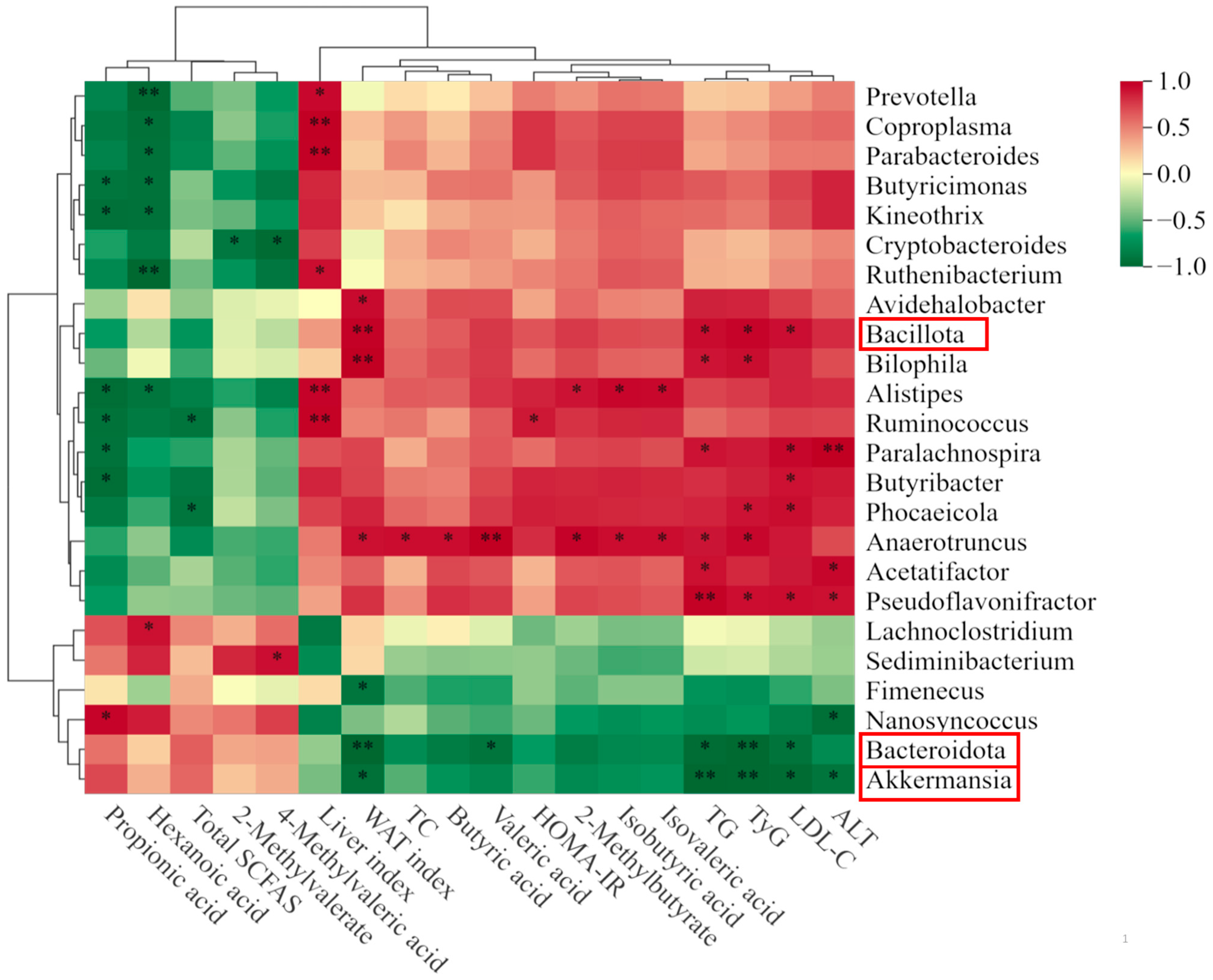
3.6. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| SCFAs | Short-chain fatty acids |
| MPP | Mango peel pectin |
| TG | Triglyceride |
| TC | Total cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| AST | Aspartate transaminase |
| ALT | Alanine aminotransferase |
| Mw | Molecular weight |
| HPGPC | High-performance gel-permeation chromatography |
| FTIR | Fourier Transform Infrared |
| GC-MS | Gas chromatography-mass spectrometry |
| DCM | Dichloromethane |
| NMR | Nuclear magnetic resonance |
| COSY | Correlation spectroscopy |
| HSQC | Heteronuclear single-quantum coherence |
| HMBC | Heteronuclear multiple-bond correlation |
| NOESY | Nuclear Overhauser effect spectroscopy |
| SPF | Specific pathogen free |
| ND | Normal diet |
| HFD | High-fat diet |
| BW | Body weight |
| WAT | White adipose tissue |
| H&E | Hematoxylin and eosin |
| OGTT | Oral glucose tolerance test |
| SD | Standard deviation |
| AUC | Area under the curve |
| HOMA-IR | Homeostasis model assessment |
| FBG | Fasting blood glucose |
| PCA | Principal component analysis |
| DM | Dispersity |
| RG-I | Rhamenogalacturonan-I |
| RG-II | Rhamenogalacturonan-II |
| D-GalA | D-galacturonic acid |
| D-Gal | D-galactose |
| D-Glu | D-glucose |
| l-Ara | L-arabinose |
| TyG | Triglyceride–glucose index |
| ANOVA | Analysis of variance |
| pCOA | Principal co-ordinate analysis |
| LefSe | Linear discriminant analysis effect size |
| OPLS-DA | Orthogonal projections to latent structures discriminant analysis |
| NMDS | Nonmetric multidimensional scaling |
References
- Coutinho, W.; Halpern, B. Pharmacotherapy for obesity: Moving towards efficacy improvement. Diabetol. Metab. Syndr. 2024, 16, 6. [Google Scholar] [CrossRef]
- Smith, K.B.; Smith, M.S. Obesity statistics. Prim. Care Clin. Off. Pract. 2016, 43, 121–135. [Google Scholar] [CrossRef]
- Silva, S.C.D.A.; Lemos, M.D.T.D.; Junior, O.H.D.S. Overweight during development dysregulates cellular metabolism and critical genes that control food intake in the prefrontal cortex. Physiol. Behav. 2024, 276, 114453. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, X.; Wu, C. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, J.; Yi, J. Dose-dependent effects of apple pectin on alleviating high fat-induced obesity modulated by gut microbiota and SCFAs. Food Sci. Hum. Well. 2022, 11, 143–154. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y. Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice. Mol. Nutr. Food Res. 2018, 62, e1800446. [Google Scholar] [CrossRef]
- Sommer, F.; Baeckhed, F. The gut microbiota-masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Cao, S.Y.; Zhao, C.N.; Xu, X.Y. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Arora, T.; Backhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. JAMA Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.L.; Zhang, X. Polyphenols from Fu Brick tea reduce obesity via modulation of gut microbiota and gut microbiota-related intestinal oxidative stress and barrier function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L. The interaction between flavonoids and intestinal microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Y.; Chen, D. Natural polysaccharides protect against diet-induced obesity by improving lipid metabolism and regulating the immune system. Food Res. Int. 2023, 172, 113192. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, M.; Zhang, L. Dietary Pectin from Premna microphylla Turcz leaves prevents obesity by regulating gut microbiota and lipid metabolism in mice fed high-fat diet. Foods 2024, 13, 2248. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Wang, X. Mangiferin alleviated poststroke cognitive impairment by modulating lipid metabolism in cerebral ischemia/reperfusion rats. Eur. J. Pharmacol. 2024, 977, 176724. [Google Scholar] [CrossRef]
- Rojas, R.; Alvarez-Perez, O.B.; Contreras-Esquivel, J.C. Valorisation of mango peels: Extraction of pectin and antioxidant and antifungal polyphenols. Waste Biomass Valorization 2020, 11, 89–98. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Wang, R. Extraction of pectins from renewable grapefruit (Citrus paradisi) peels using deep eutectic solvents and analysis of their structural and physicochemical properties. Int. J. Biol. Macromol. 2024, 254, 127785. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Zhao, H. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, S.; Li, H. Structural characterization of Alpiniae oxyphyllae fructus polysaccharide 2 and its activation effects on RAW264.7 macrophages. Int. Immunopharmacol. 2021, 97, 107708. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Ding, J. Structural features and emulsifying stability of a highly branched arabinogalactan from immature peach (Prunus persica) exudates. Food Hydrocoll. 2020, 104, 105721. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y. A review of isolation, chemical properties, and bioactivities of polysaccharides from Bletilla striata. Biomed. Res. Int. 2020, 2020, 5391379. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Lopusiewicz, L. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Inngjerdingen, K.T.; Patel, T.R.; Chen, X. Immunological and structural properties of a pectic polymer from Glinus oppositifolius. Glycobiology 2007, 17, 1299–1310. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Drusch, S.; Acevedo, F. Structure, controlled release mechanisms and health benefits of pectins as an encapsulation material for bioactive food components. Food Funct. 2022, 13, 10870–10881. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Sun, L. The inhibitory effects of a rhamnogalacturonan I (RG-I) domain from ginseng pectin on galectin-3 and its structure-activity elationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Xu, Y. Structural characterization of a pectic polysaccharide from Rubus chingii Hu. unripe fruits and its efficacy in inhibiting intestinal lipid absorption in vivo. Carbohydr. Polym. 2025, 363, 123728. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Hu, X.; Chen, L.; Shi, S. Antioxidant capacity and phenolic compounds of Lonicerae macranthoides by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2016, 124, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Hou, C.; Yan, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Ji, X.; Yan, Y.; Hou, C. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef]
- Dranca, F.; Talon, E.; Vargas, M.; Oroian, M. Microwave vs. conventional extraction of pectin from Malus domestica ‘Falticeni’ pomace and its potential use in hydrocolloid-based films. Food Hydrocoll. 2021, 121, 107026. [Google Scholar] [CrossRef]
- Munoz-Almagro, N.; Vendrell-Calatayud, M.; Mendez-Albinana, P. Extraction optimization and structural characterization of pectin from persimmon fruit (Diospyros kaki Thunb. var. Rojo brillante). Carbohydr. Polym. 2021, 272, 118411. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Halambek, J.; Djakovic, S. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C.S. Red pomelo peel pectin based edible composite films: Effect of pectin incorporation on mechanical, structural, morphological and thermal properties of composite films. Food Hydrocoll. 2022, 123, 107135. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, Y. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Pacheco-Jimenez, A.A.; Lizardi-Mendoza, J.; Heredia, J.B. Physicochemical characterization of pectin and mango peel (Mangifera indica L.) from Mexican cultivars. Heliyon 2024, 10, e35184. [Google Scholar] [CrossRef]
- Zoghi, A.; Vedadi, S.; Esfahani, Z.H. A review on pectin extraction methods using lignocellulosic wastes. Biomass Convers. Biorefinery 2023, 13, 5577–5589. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Y.; Chen, X. Water extract of shepherd’s purse prevents high-fructose induced-liver injury by regulating glucolipid metabolism and gut microbiota. Food Chem. 2021, 342, 128536. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.Y. Beneficial effects of dietary polyphenols on high-fat diet-induced obesity linking with modulation of gut microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, X. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Kurita, O.; Miyake, Y.; Yamazaki, E. Chemical modification of citrus pectin to improve its dissolution into water. Carbohydr. Polym. 2012, 87, 1720–1727. [Google Scholar] [CrossRef]
- Logan, K.; Wright, A.J.; Goff, H.D. Correlating the structure and in vitro digestion viscosities of different pectin fibers to in vivo human satiety. Food Funct. 2015, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Zhou, Y. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013, 61, 8565–8572. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Hao, J. Lycopene, amaranth, and sorghum red pigments counteract obesity and modulate the gut microbiota in high-fat diet fed C57BL/6 mice. J. Funct. Foods 2021, 87, 104816. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G. Fucosylated chondroitin sulfate from Isostichopus badionotus alleviates metabolic syndromes and gut microbiota dysbiosis induced by high-fat and high-fructose diet. Int. J. Biol. Macromol. 2019, 124, 377–388. [Google Scholar] [CrossRef]
- Qin, Y.; Fan, R.; Liu, Y. Exploring the potential mechanism of Rubus corchorifolius L. fruit polyphenol-rich extract in mitigating non-alcoholic fatty liver disease by integration of metabolomics and transcriptomics profiling. Food Funct. 2023, 14, 9295–9308. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Piao, X.; Mahfuz, S. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cao, D. Integrated multi-omics reveals the relationship between growth performance, rumen microbes and metabolic status of Hu sheep with different residual feed intakes. Anim. Nutr. 2024, 18, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.R.; Colli, C.; Alvares, E.P.; Filisetti, T.M.C.C. Effects of fructans-containing yacon (Smallanthus sonchifolius Poepp and Endl.) flour on caecum mucosal morphometry, calcium and magnesium balance, and bone calcium retention in growing rats. Br. J. Nutr. 2007, 97, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.W.; Tunsjo, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Borthakur, A.; Saksena, S.; Gill, R.K. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: Involvement of NF-kappaB pathway. J. Cellular. BioChem. 2008, 103, 1452–1463. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid. Reseach. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, C.; Gao, Z. Anti-obesity mechanism of Ganpu tea revealed by microbiome, metabolome and transcriptome analyses. Food Chem. 2023, 412, 135048. [Google Scholar] [CrossRef]
- Yan, J.K.; Chen, T.T.; Li, L.Q. The anti-hyperlipidemic effect and underlying mechanisms of barley (Hordeum vulgare L.) grass polysaccharides in mice induced by a high-fat diet. Food Funct. 2023, 14, 7066–7081. [Google Scholar] [CrossRef]
- Li, T.T.; Huang, Z.R.; Jia, R.B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef] [PubMed]
- Dubin, K.; Callahan, M.K.; Ren, B. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, Z.; Kit Yeah, Y. Combing fecal microbial community data to identify consistent obesity-specific microbial signatures and shared metabolic pathways. iScience 2023, 26, 106476. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X. Roles of intestinal parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Liu, L. Interplay between dietary intake, gut microbiota, and metabolic profile in obese adolescents: Sex-dependent differential patterns. Clinial. Nutr. 2022, 41, 2706–2719. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Han, L. Leucine-restricted diet ameliorates obesity-linked cognitive deficits: Involvement of the microbiota-gut-brain axis. J. Agric. Food Chem. 2023, 71, 9404–9418. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M. IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Minamoto, Y.; Kawas, J.R. Akkermansia and microbial degradation of mucus in cats and dogs: Implications to the growing worldwide epidemic of pet obesity. Vet. Sci. 2020, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.W.; Li, G.X.; Zeng, L. Gynostemma pentaphyllum saponins alleviate non-alcoholic fatty liver disease in rats by regulating intestinal flora and short-chain fatty acid metabolism. China J. Chin. Mater. Medica 2022, 47, 2500–2508. [Google Scholar]



| Linkage Types | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio |
|---|---|---|---|
| t-Ara(f) | 1,4-di-O-acetyl-2,3,5-tri-O-methyl arabinitol | 279 | 9.92 |
| t-Glc(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl glucitol | 323 | 3.43 |
| t-Gal(p)-UA | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl galactitol | 325 | 8.93 |
| 5-Ara(f) | 1,4,5-tri-O-acetyl-2,3-di-O-methyl arabinitol | 307 | 5.18 |
| 4-Gal(p)-UA | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl galactitol | 353 | 32.13 |
| 4-Gal(p) | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl galactitol | 351 | 8.08 |
| 4-Glc(p) | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 351 | 22.24 |
| 6-Gal(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl galactitol | 351 | 4.12 |
| 2,4-Glc(p) | 1,2,4,5-tetra-O-acetyl-3,6-di-O-methyl glucitol | 379 | 1.41 |
| 4,6-Gal(p) | 1,4,5,6-tetra-O-acetyl-2,3-di-O-methyl galactitol | 379 | 1.65 |
| 3,6-Gal(p) | 1,3,5,6-tetra-O-acetyl-2,4-di-O-methyl galactitol | 379 | 2.90 |
| Glycosyl Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| A | →4)-α-D-GalpA-OMe-(1→ | 5.34 | 3.57 | 3.78 | 3.89 | 3.87 | / |
| 99.7 | 71.47 | 71.15 | 76.58 | 73.28 | 170.67 | ||
| B | →4)-β-D-Glcp-(1→ | 4.42 | 3.48 | 3.86 | 3.61 | 3.87 | 3.66, 3.76 |
| 103.41 | 70.87 | 69.44 | 76.76 | 73.63 | 61.07 | ||
| C | →4)-β-D-Galp-(1→ | 4.58 | 3.62 | 3.98 | 4.22 | n.d | 3.78, 3.6 |
| 104.26 | 72.71 | 69.46 | 79.35 | n.d | 60.47 | ||
| D | →6)-α-D-Galp-(1→ | 5.09 | 4.05 | n.d | n.d | n.d | 3.74, 3.83 |
| 107.06 | 80.87 | n.d | n.d | n.d | 66.32 | ||
| E | →5)-α-L-Araf-(1→ | 5.03 | 4.09 | n.d | 4.15 | 3.89 | / |
| 107.46 | 81.08 | n.d | 82.18 | 66.51 | / | ||
| F | α-L-Araf-(1→ | 5.18 | 4.11 | n.d | 4.25 | 3.43 | / |
| 109.31 | 77.75 | n.d | 81.42 | 60.04 | / | ||
| G | →3,6)-α-D-Galp-(1→ | 4.9 | 3.66 | 3.98 | n.d | n.d | 3.91,4.07 |
| 100.6 | 74.82 | 76.66 | n.d | n.d | 68.46 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Zhang, W.; Wang, L.; Fei, T.; Xiao, J.; Wang, L. Structural Characterization of a Novel Pectin Polysaccharide from Mango (Mangifera indica L.) Peel and Its Regulatory Effects on the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Foods 2025, 14, 2910. https://doi.org/10.3390/foods14162910
Fan R, Zhang W, Wang L, Fei T, Xiao J, Wang L. Structural Characterization of a Novel Pectin Polysaccharide from Mango (Mangifera indica L.) Peel and Its Regulatory Effects on the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Foods. 2025; 14(16):2910. https://doi.org/10.3390/foods14162910
Chicago/Turabian StyleFan, Ruyan, Wenting Zhang, Lang Wang, Tao Fei, Jianbo Xiao, and Lu Wang. 2025. "Structural Characterization of a Novel Pectin Polysaccharide from Mango (Mangifera indica L.) Peel and Its Regulatory Effects on the Gut Microbiota in High-Fat Diet-Induced Obese Mice" Foods 14, no. 16: 2910. https://doi.org/10.3390/foods14162910
APA StyleFan, R., Zhang, W., Wang, L., Fei, T., Xiao, J., & Wang, L. (2025). Structural Characterization of a Novel Pectin Polysaccharide from Mango (Mangifera indica L.) Peel and Its Regulatory Effects on the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Foods, 14(16), 2910. https://doi.org/10.3390/foods14162910








