Effect of Lactic Acid Bacteria Fermentation Agent on the Structure, Physicochemical Properties, and Digestive Characteristics of Corn, Oat, Barley, and Buckwheat Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fermentation Samples
2.3. pH and TTA Measurements
2.4. Separation of Starch
2.5. SEM
2.6. Particle Size Measurements
2.7. XRD
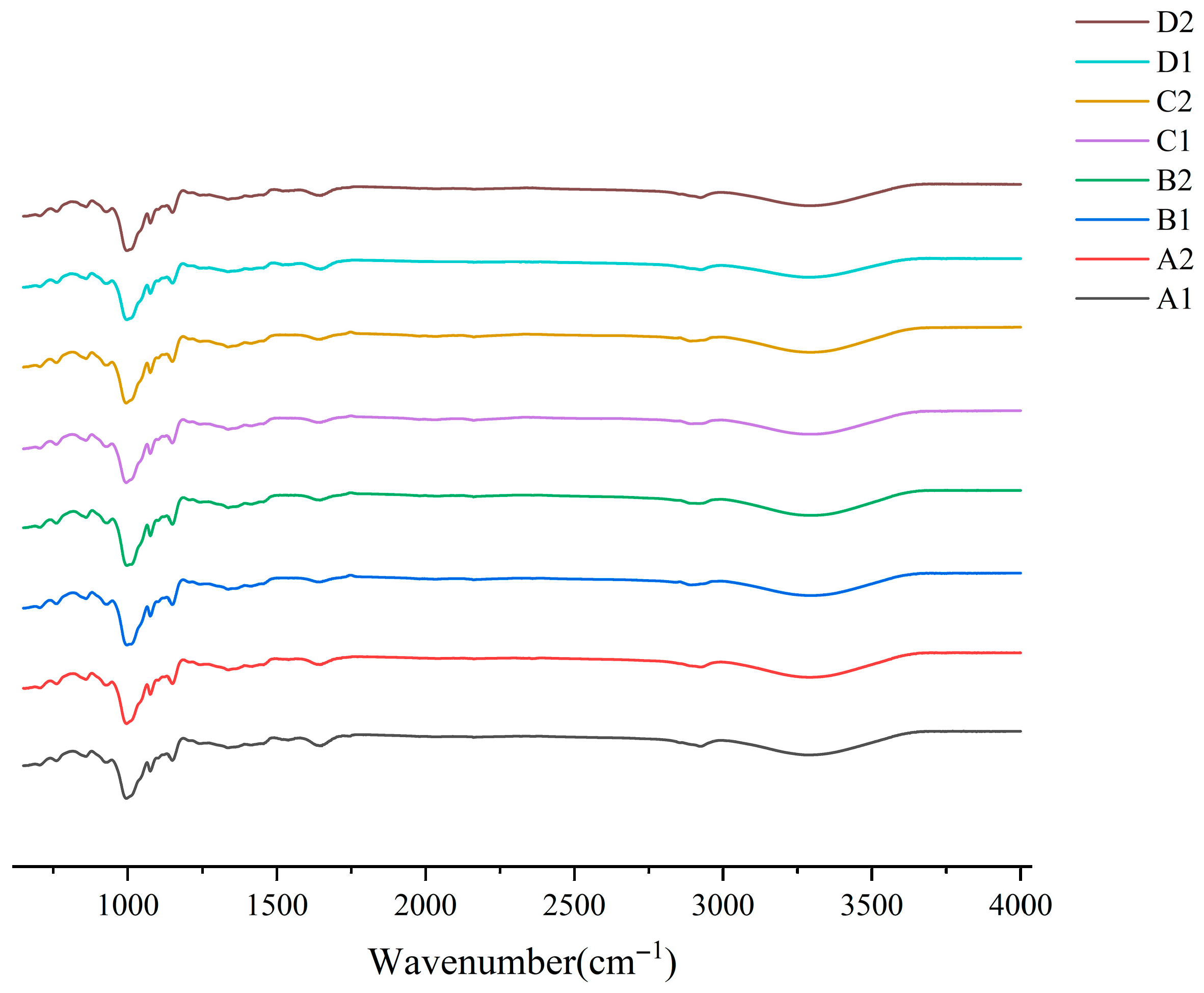
2.8. FT-IR
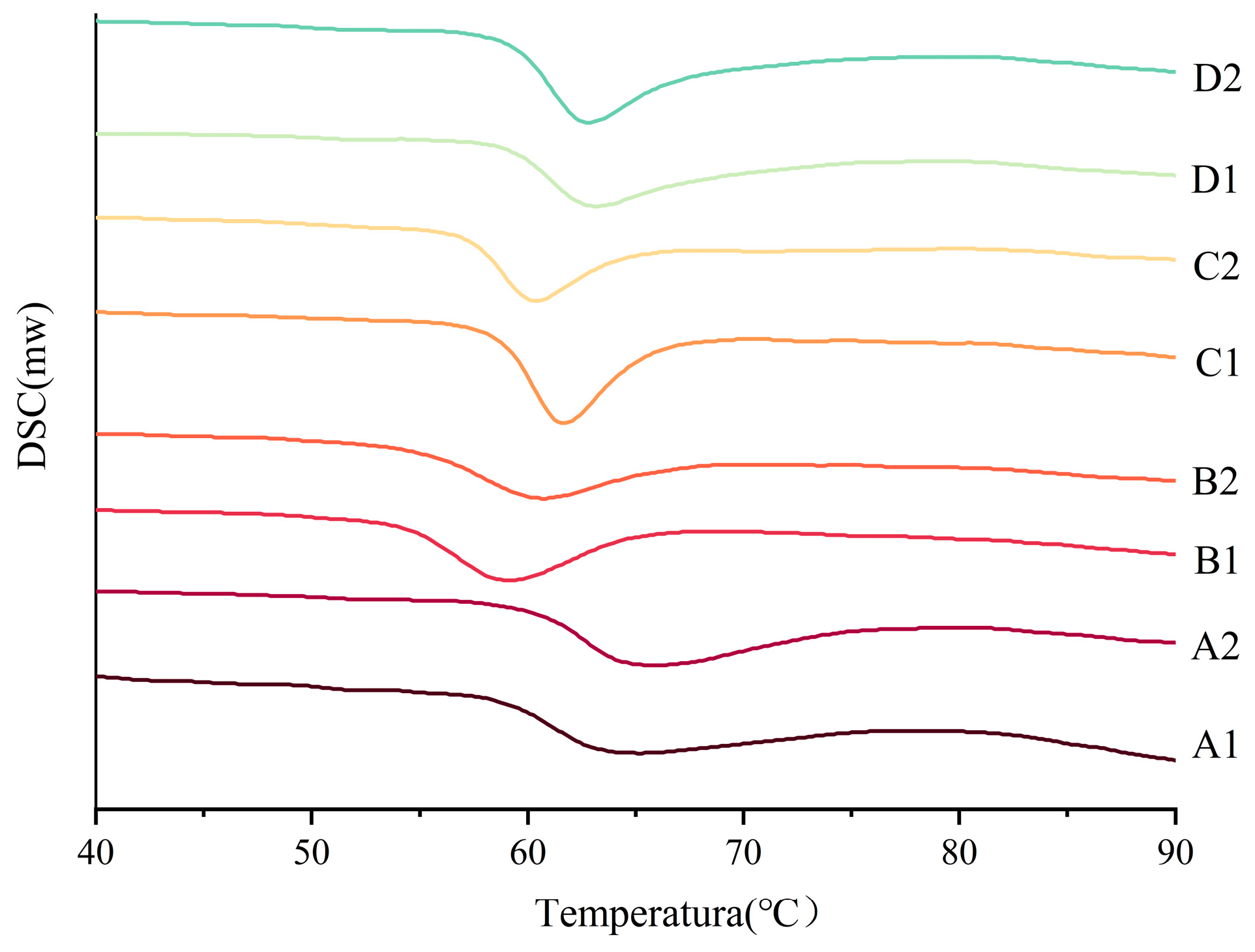
2.9. Thermodynamic Properties
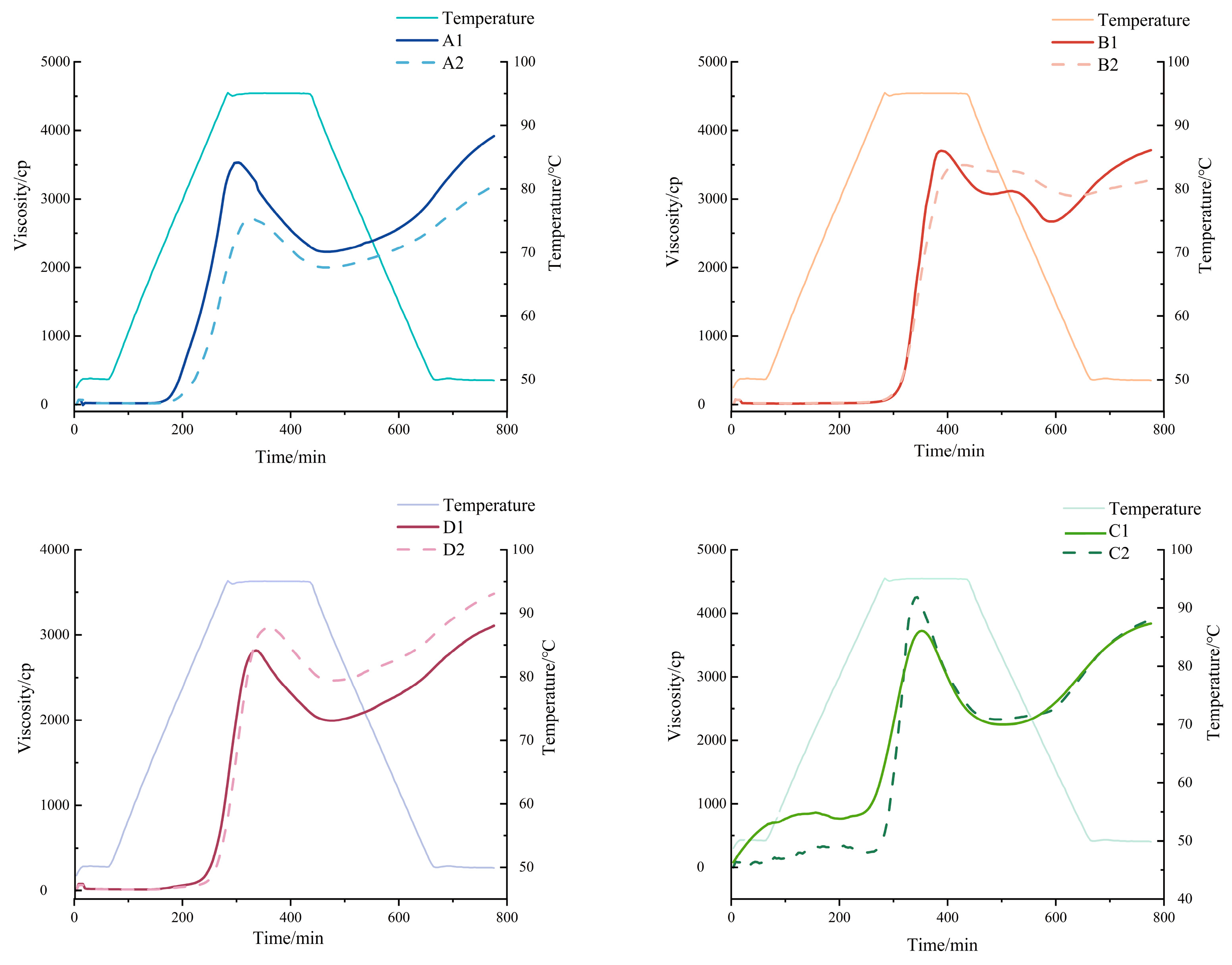
2.10. Pasteurization Properties
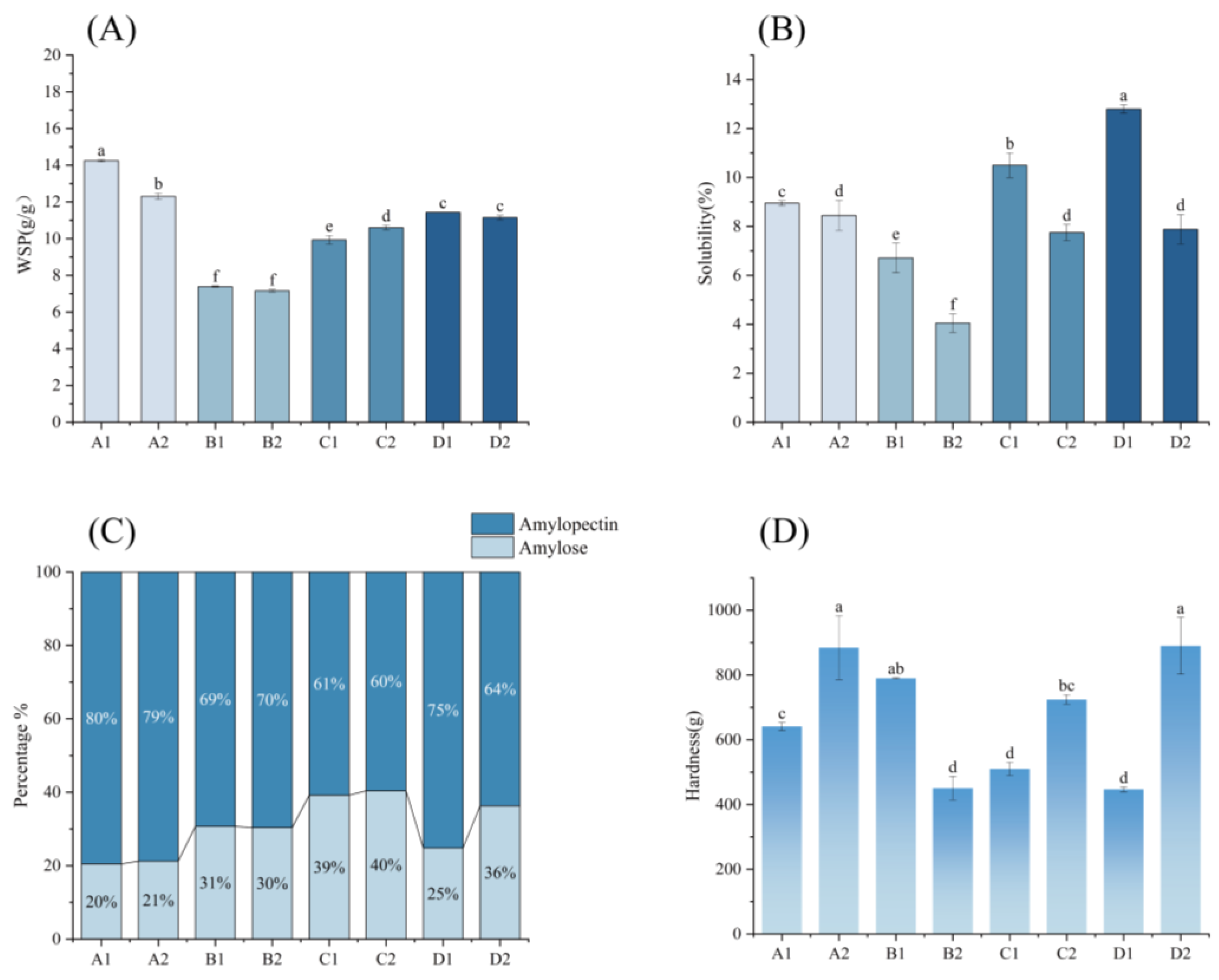
2.11. Solvency and Solubility
2.12. Proportion of Amylose and Amylopectin Measurements
2.13. Starch Gel Hardness Measurements
2.14. Starch Digestibility Measurements
2.15. Statistical Analysis
3. Results
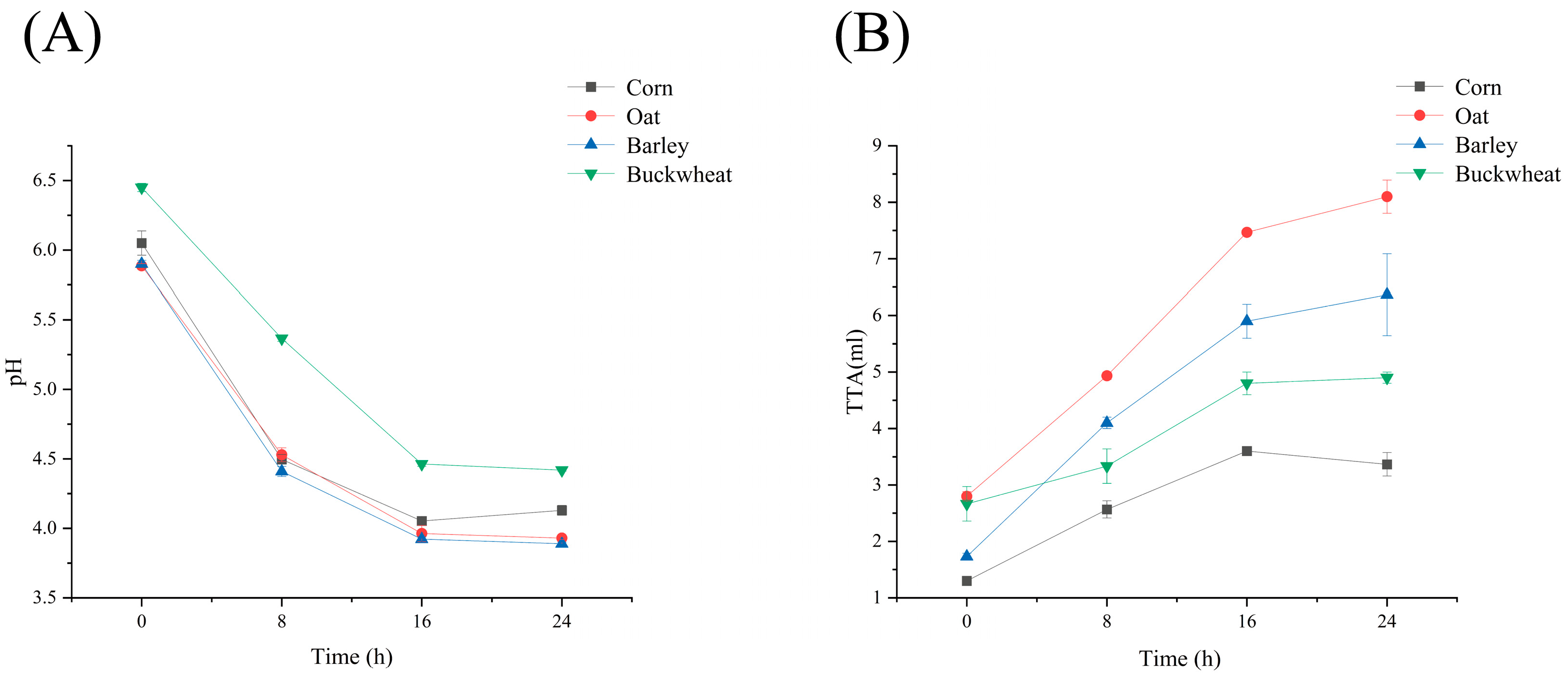
3.1. pH and TTA
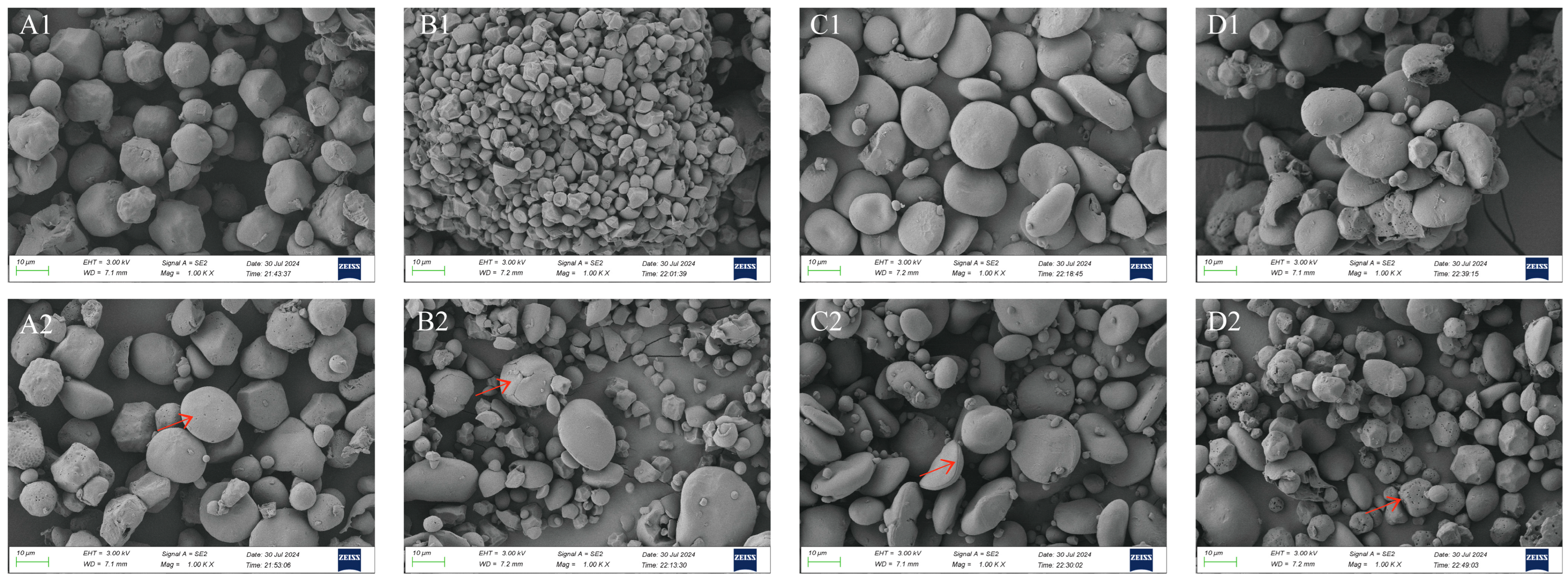
3.2. Starch Morphology
3.3. Particle Size
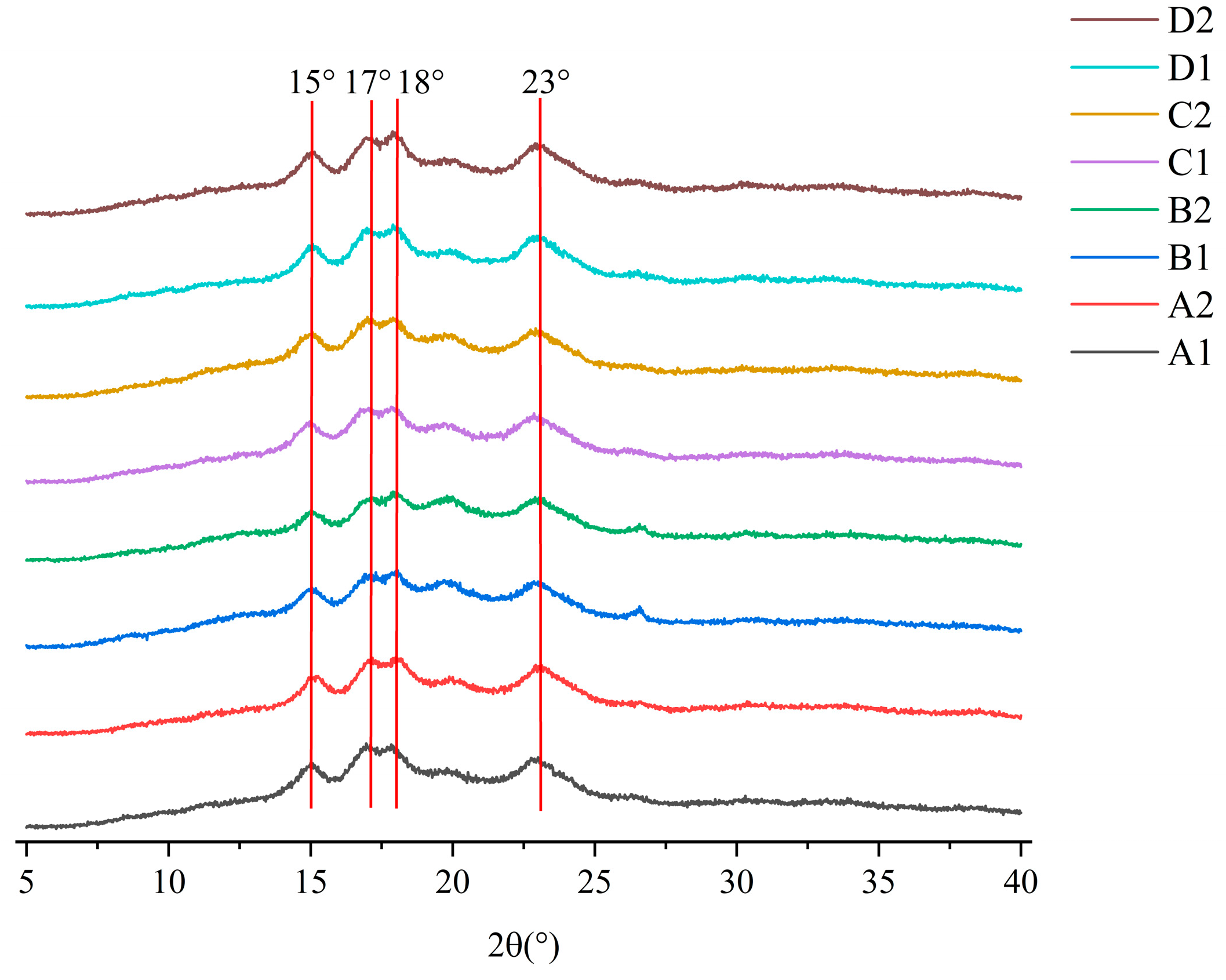
3.4. Long-Range Ordering XRD
3.5. Short-Range Ordering FT-IR
3.6. Pasting Properties of Starches
3.7. Thermodynamic Properties of Starch
3.8. Starch Swelling Power and Solubility
3.9. Proportion of Amylose and Amylopectin
3.10. Starch Gel Hardness
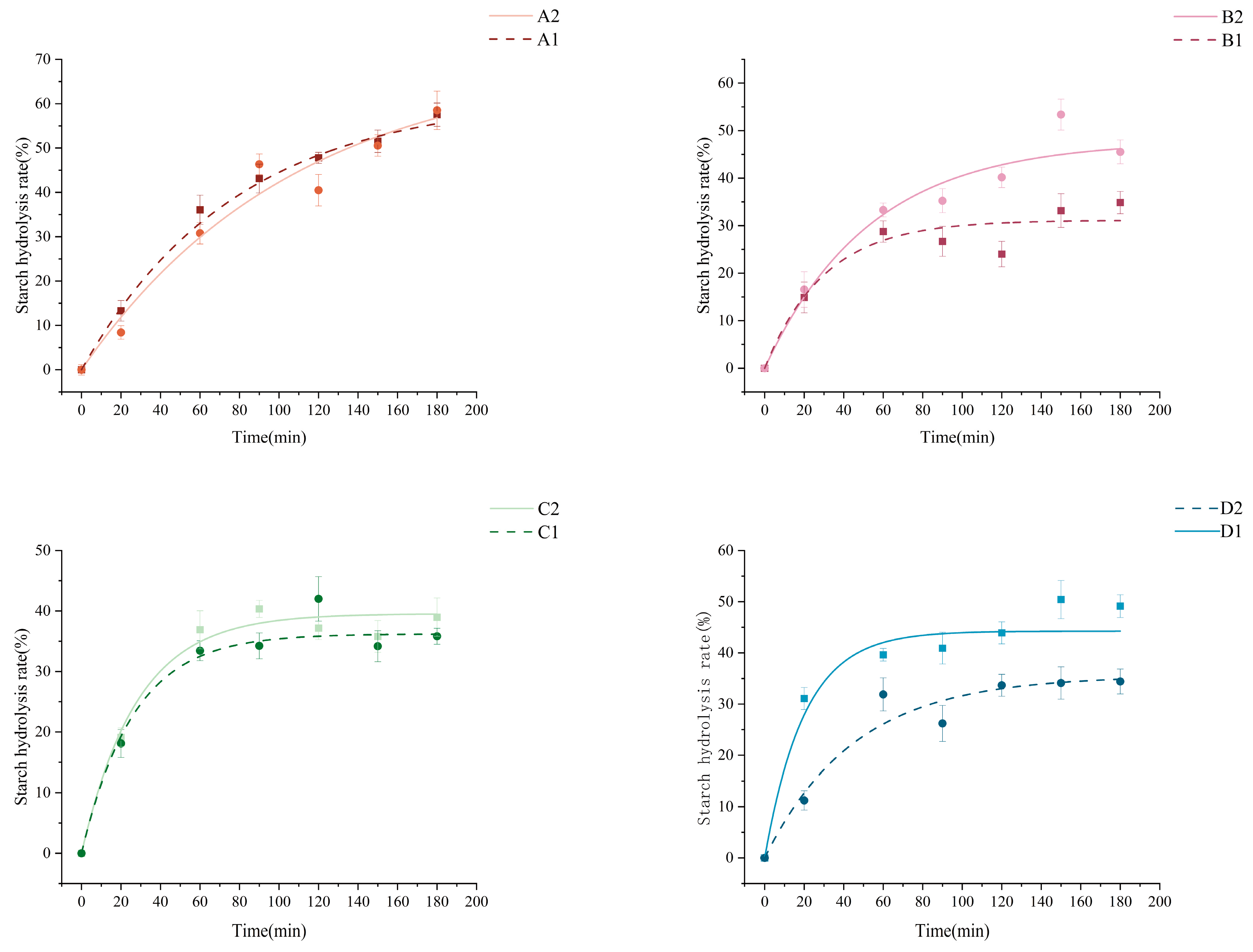
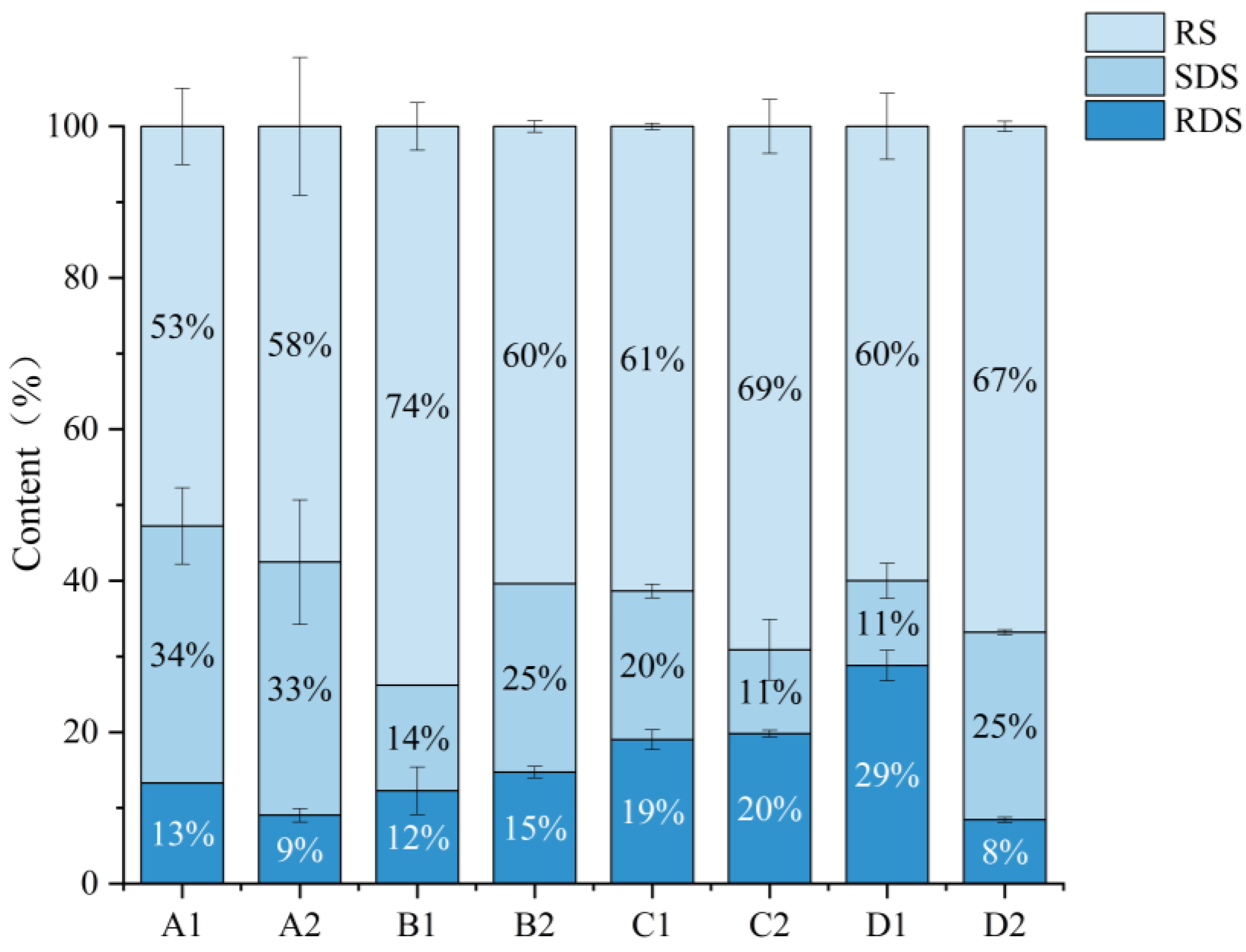
3.11. Starch Digestibility
3.12. Fermentation Effect Analysis
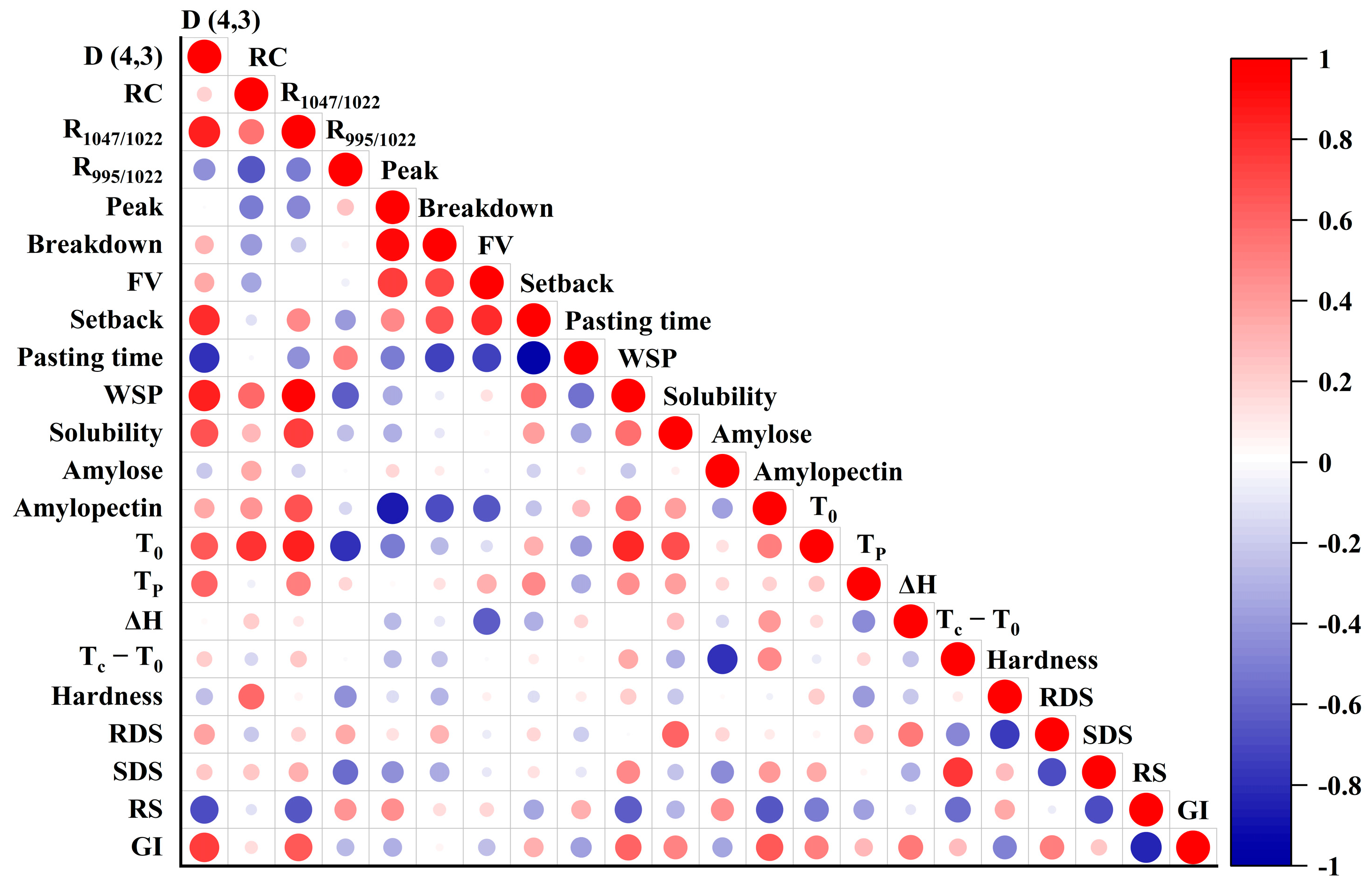
3.13. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | lactic acid bacteria |
| GI | Glycemic Index |
| RS | resistant starch |
| RDS | rapidly digestible starch |
| SDS | slowly digestible starch |
| TTA | total titratable acidity |
| RC | relative crystallinity |
| A1 | unfermented corn starch |
| A2 | corn starch fermented |
| B1 | unfermented oat starch |
| B2 | oat starch fermented |
| C1 | unfermented barley starch |
| C2 | barley starch fermented |
| D1 | unfermented buckwheat starch |
| D2 | buckwheat starch fermented |
References
- Li, C.; Hu, Y.; Gu, F.; Gong, B. Causal Relations among Starch Fine Molecular Structure, Lamellar/Crystalline Structure and in Vitro Digestion Kinetics of Native Rice Starch. Food Funct. 2021, 12, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Julia, C.; Kesse-Guyot, E.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Andreeva, V.A.; Galan, P.; et al. Glycaemic Index, Glycaemic Load and Cancer Risk: Results from the Prospective NutriNet-Santé Cohort. Int. J. Epidemiol. 2021, 51, 250–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, J. Recent Advances in Modification Approaches, Health Benefits, and Food Applications of Resistant Starch. Starch—Stärke 2023, 75, 2100141. [Google Scholar] [CrossRef]
- Arachchi, D.M.; Halim, A.; Fadimu, G.; Farahnaky, A.; Majzoobi, M. Green Starch Modification Using Citric Acid: Quinoa, Chickpea, and Cassava Starches. Foods 2025, 14, 164. [Google Scholar] [CrossRef]
- Mansur, A.R.; Jeong, G.A.; Lee, C.J. Preparation, Physicochemical Properties, and in Vivo Digestibility of Thermostable Resistant Starch from Malic Acid-Treated Wheat Starch. Food Res. Int. 2022, 162, 112159. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, B.; Dou, Y.; Wei, Y.; Tao, Z.; Zhang, L.; Zhang, T.; Yan, X. Formation and in Vitro Digestion of Sorghum Starch-Resveratrol Complex Nanoparticles and the Corresponding Mechanism. Food Chem. 2025, 492, 145457. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L. Impact of Sourdough Fermentation on Nutrient Transformations in Cereal-Based Foods: Mechanisms, Practical Applications, and Health Implications. Grain Oil Sci. Technol. 2024, 7, 124–132. [Google Scholar] [CrossRef]
- Boscaino, F.; Ionata, E.; De Caro, S.; Sorrentino, A. Non-Conventional Yeasts from Mozzarella Cheese Whey and Artisanal Sourdoughs: Leavening Capacity and Impact on Bread Sensory Profile. Fermentation 2024, 10, 68. [Google Scholar] [CrossRef]
- Semumu, T.; Zhou, N.; Kebaneilwe, L.; Loeto, D.; Ndlovu, T. Exploring the Microbial Diversity of Botswana’s Traditional Sourdoughs. Fermentation 2024, 10, 417. [Google Scholar] [CrossRef]
- Xu, M.; Zou, J.; Zhao, X.; Feng, Y.; Duan, R.; Yang, B. Effect of Lactobacteria Fermentation on Structure and Physicochemical Properties of Chinese Yam Starch (Dioscorea opposita Thunb.). Food Chem. 2022, 387, 132873. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Duan, Z.; Tang, Y.; Shu, W.; Xie, Y.; Liu, Q.; Yuan, Y. Differences in the Chemical Composition and Physicochemical Properties between Brown Rice Kernels and Brown Rice Flours after Lactobacillus fermentation and Their Impact on the Qualities of Brown Rice Noodles. LWT 2025, 215, 117219. [Google Scholar] [CrossRef]
- Cai, X.; Wijesekara, T.; Xu, B. New Insights into Recent Development, Health Benefits, Emerging Technologies, and Future Trends of Cereal-Based Fermented Products. Process Biochem. 2024, 147, 433–439. [Google Scholar] [CrossRef]
- Chang, L.; Dang, Y.; Yang, M.; Liu, Y.; Ma, J.; Liang, J.; Li, R.; Zhang, R.; Du, S. Effects of Lactobacillus plantarum Fermentation on the Structure, Physicochemical Properties, and Digestibility of Foxtail Millet Starches. Int. J. Biol. Macromol. 2024, 270, 132496. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Guo, W.; Chen, P.; Liu, C.; Wei, J.; Zheng, X.; Saeed Omer, S.H. Effects of Bifidobacteria Fermentation on Physico-Chemical, Thermal and Structural Properties of Wheat Starch. Foods 2022, 11, 2585. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, M.; Long, D.; Shi, J.; Guo, J.; Hu, Y.; Liu, S. Revealing the Influence of Oat β-Glucan on the Structural Properties and Digestive Characteristics of Rice Starch: A Perspective on Different Molecular Weights. Int. J. Biol. Macromol. 2025, 306, 141792. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; Kidwai, M.K. Oat Starch: Physico-Chemical, Morphological, Rheological Characteristics and Its Applications—A Review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.; Xu, B. Highland Barley Starch (Qingke): Structures, Properties, Modifications, and Applications. Int. J. Biol. Macromol. 2021, 185, 725–738. [Google Scholar] [CrossRef]
- Basharat, Z.; Tufail, T.; Shao, F.; Virk, M.S.; Duan, Y.; Cai, M.; Hu, K.; Basharat, N.; Zhang, H. Sustainable and Contemporary Approaches to Explore the Nutritional and Processing Perspectives of Buckwheat: Current Evidence and Prospects. Food Biosci. 2025, 67, 106312. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, S.; Li, Z.; Pan, Z.; Huang, Z.; Zhao, J.; Ai, Z. Influence of Three Types of Freezing Methods on Physicochemical Properties and Digestibility of Starch in Frozen Unfermented Dough. Food Hydrocoll. 2021, 115, 106619. [Google Scholar] [CrossRef]
- Davoudi, Z.; Azizi, M.H.; Barzegar, M. Porous Corn Starch Obtained from Combined Cold Plasma and Enzymatic Hydrolysis: Microstructure and Physicochemical Properties. Int. J. Biol. Macromol. 2022, 223, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; You, Y.; Liao, X.; Zhang, F.; Kan, J.; Zheng, J. Ultrasonic Modification of Lotus Starch Based on Multi-Scale Structure: Pasting, Rheological, and Thermal Properties. LWT 2023, 184, 115030. [Google Scholar] [CrossRef]
- Zou, J.; Feng, Y.; Xu, M.; Yang, P.; Zhao, X.; Yang, B. The Structure-Glycemic Index Relationship of Chinese Yam (Dioscorea opposita Thunb.) Starch. Food Chem. 2023, 421, 136228. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Y.; Chen, X.; Xu, F.; Zhu, K.; Wang, P.; Zhang, Y. Synergistic Effect of Pectin and the Flavanols Mixture on in Vitro Starch Digestion and the Corresponding Mechanism. Food Hydrocoll. 2025, 158, 110554. [Google Scholar] [CrossRef]
- Hamid, M.G.; Elkhatim, K.A.S.; Idris, Y.M.A.; Elsafy, M.; Rahmatov, M.; Abdelhalim, T.; Muneer, F. Impact of Fermentation Time on the in Vitro Enzymatic Digestibility of Traditionally Extracted Pearl Millet (Pennisetum glaucum) Starch. LWT 2025, 218, 117523. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Zhang, X.; Zhang, J.; Yan, X.; Zhang, Q.; Zhang, B. Structural, Physicochemical and in Vitro Digestibility of White Kidney Bean Protein-Corn Starch Complexes under Various Heat Treatments. Food Res. Int. 2025, 200, 115479. [Google Scholar] [CrossRef]
- Feng, W.; Huang, Z.; Pan, B.; Zhang, T.; Jin, Z.; Miao, M. Regulation of Naked Oat Starch Structure and Thermal Properties: The Key Role of Phosphorus. Food Biosci. 2025, 68, 106673. [Google Scholar] [CrossRef]
- Zhang, R.-Y.; Chen, P.-X.; Liu, A.-B.; Zhu, W.-X.; Jiang, M.-M.; Wang, X.-D.; Liu, H.-M. Effects of Different Isolation Methods on the Structure and Functional Properties of Starch from Tiger Nut (Cyperus esculentus L.) Meal. LWT 2024, 196, 115853. [Google Scholar] [CrossRef]
- Sun, X.; Saleh, A.S.M.; Sun, Z.; Zhao, K.; Zhang, X.; Lu, Y.; Ge, X.; Shen, H.; Li, W. Molecular Structure and Architectural Characteristics of Outer Shells and Inner Blocklets of Normal and Waxy Wheat A- and B- Starch Granules. J. Cereal Sci. 2022, 105, 103477. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Xin, Y.; Yang, J.; Yuan, L.; Huang, X.; Yin, J.; Nie, S.; Xie, M. Comparison on Structure and Physicochemical Properties of Starches from Adzuki Bean and Dolichos Bean. Food Hydrocoll. 2020, 105, 105784. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, S.; Zhu, X.; Guo, J.; Peng, G.; Zhu, X.; Gu, R.; Meng, Z.; Wu, Z.; Gan, H.; et al. Effect of Different Drying Methods on the Structure and Properties of Porous Starch. Heliyon 2024, 10, e31143. [Google Scholar] [CrossRef]
- Han, X.; Wen, H.; Luo, Y.; Yang, J.; Xiao, W.; Ji, X.; Xie, J. Effects of α-Amylase and Glucoamylase on the Characterization and Function of Maize Porous Starches. Food Hydrocoll. 2021, 116, 106661. [Google Scholar] [CrossRef]
- An, D.; Li, H.; Li, D.; Zhang, D.; Huang, Y.; Obadi, M.; Xu, B. The Relation between Wheat Starch Properties and Noodle Springiness: From the View of Microstructure Quantitative Analysis of Gluten-Based Network. Food Chem. 2022, 393, 133396. [Google Scholar] [CrossRef]
- Lei, M.; Yuan, H.; Jia, R.; Huang, Z.; Yang, Y.; Liang, Q.; Liu, X.; Pan, Z. Effects of Different Enzymatic Hydrolysis Times on the Structures and Properties of Corn Microporous Starch Particles and Their Applications in Frozen Dough. Food Hydrocoll. 2025, 164, 111178. [Google Scholar] [CrossRef]
- Li, M.; Daygon, V.D.; Solah, V.; Dhital, S. Starch Granule Size: Does It Matter? Crit. Rev. Food Sci. Nutr. 2023, 63, 3683–3703. [Google Scholar] [CrossRef]
- Wang, K.; Ge, Y.; Jia, Y.; Hou, J.; Lu, F.; Liu, Y. Effect of Exogenous Protein Crosslinking on the Physicochemical Properties and in Vitro Digestibility of Corn Starch. Carbohydr. Polym. 2025, 357, 123428. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, R.; Huo, S.; Li, J.; Wang, M.; Wang, W.; Yuan, Z.; Hu, A.; Zheng, J. Insights into the Changes of Structure and Digestibility of Microwave and Heat Moisture Treated Quinoa Starch. Int. J. Biol. Macromol. 2023, 246, 125681. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Zou, J.; Qin, X.; Lv, Q.; Li, B. Effects of Lactobacillus plantarum Fermentation on the Structure and Digestion of Resistant Starch Type 3 and Properties of Fermented Starch in the Simulated Digestion System. Carbohydr. Polym. 2025, 353, 123264. [Google Scholar] [CrossRef]
- Qi, Q.; Hong, Y.; Zhang, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Combinatorial Effect of Fermentation and Drying on the Relationship between the Structure and Expansion Properties of Tapioca Starch and Potato Starch. Int. J. Biol. Macromol. 2020, 145, 965–973. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Liu, M.; Zhao, Y.; Zhu, Y.; Cui, S.; Xiao, X. Effects of Lactiplantibacillus plantarum Dy-1 Fermentation on Multi-Scale Structure and Physicochemical Properties of Barley Starch. Food Funct. 2024, 15, 1923–1937. [Google Scholar] [CrossRef]
- Liu, Y.; Danial, M.; Liu, L.; Sadiq, F.A.; Wei, X.; Zhang, G. Effects of Co-Fermentation of Lactiplantibacillus Plantarum and Saccharomyces Cerevisiae on Digestive and Quality Properties of Steamed Bread. Foods 2023, 12, 3333. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Khalid, W.; Wang, L.; Li, Y.; Fan, M.; Qian, H. Effects of Lactobacillus plantarum Fermentation on the Retrogradation Behaviors, Physicochemical Properties and Structure of Rice Starch. Carbohydr. Polym. 2025, 368, 124064. [Google Scholar] [CrossRef]
- Li, X.; Wei, S.; Gao, Z.; Zhao, R.; Wang, Z.; Fan, Y.; Cui, L.; Wang, Y. The Influence of Cooperative Fermentation on the Structure, Crystallinity, and Rheological Properties of Buckwheat Starch. Curr. Res. Food Sci. 2024, 8, 100670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, C.; Li, L.; Li, J.; Wang, J.; Fan, X.; Zheng, X. Structural Characteristics and Paste Properties of Wheat Starch in Natural Fermentation during Traditional Chinese Mianpi Processing. Int. J. Biol. Macromol. 2024, 262, 129993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, J.; Ma, S.; Tian, X.; Sun, B.; Huang, J.; Li, L.; Wang, X.; Bao, Q. Effect of Wheat Bran Dietary Fiber on Structural Properties of Wheat Starch after Synergistic Fermentation of Lactobacillus plantarum and Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2021, 190, 86–92. [Google Scholar] [CrossRef]
- Chen, S.; Qin, L.; Chen, T.; Yu, Q.; Chen, Y.; Xiao, W.; Ji, X.; Xie, J. Modification of Starch by Polysaccharides in Pasting, Rheology, Texture and in Vitro Digestion: A Review. Int. J. Biol. Macromol. 2022, 207, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Vamadevan, V.; Bertoft, E. Observations on the Impact of Amylopectin and Amylose Structure on the Swelling of Starch Granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Jia, R.; Cui, C.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Wang, Y.; Xiong, L.; Shi, R.; Sun, Q. A Review of Starch Swelling Behavior: Its Mechanism, Determination Methods, Influencing Factors, and Influence on Food Quality. Carbohydr. Polym. 2023, 321, 121260. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Zhang, Y.; Li, S.; Liu, X.; Zhang, Y.; Zhao, X.; Shen, H.; Xie, F.; Xu, K.; et al. Insights into the Hierarchical Structure and Physicochemical Properties of Starch Isolated from Fermented Dough. Int. J. Biol. Macromol. 2024, 267, 131315. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Jiang, Y.-H.; Xin, W.-G.; Liang, M.; Song, J.-J.; Wang, C.; Chen, X.-Y.; Suo, H.-Y. Effect of Co-Fermentation with Lactiplantibacillus plantarum and Saccharomyces cerevisiae on the Structural, Physicochemical, and Digestibility Properties of Lotus Starch. J. Sci. Food Agric. 2025, 105, 4782–4794. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chen, J.; Yang, Y.; Yu, D.; Ma, Z.; Ren, L.; Wu, N.; Chen, F.; Liu, X.; Wang, B.; et al. Effects of Fermentation on the Structure and Physical Properties of Glutinous Proso Millet Starch. Food Hydrocoll. 2022, 123, 107144. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, W.; Zhu, H.; Yi, C.; Yuan, J.; Liu, Y.; Li, Z.; Cheng, H. Digestibility of Indica Rice and Structural Changes of Rice Starch during Fermentation by Lactobacillus plantarum. LWT 2023, 187, 115392. [Google Scholar] [CrossRef]
- Luo, H.; Dong, F.; Wang, Q.; Li, Y.; Xiong, Y. Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention. Molecules 2021, 26, 3999. [Google Scholar] [CrossRef]
- Ao, W.; Qin, L.; Wu, N.; Ge, P.; Hu, C.; Hu, J.; Peng, Y.; Zhu, Y. Intensification of Rice Flour Gel Structure by Fermenting Corresponding Rice with Lactobacillus plantarum. Curr. Res. Food Sci. 2024, 8, 100743. [Google Scholar] [CrossRef]
- Xie, X.; Zheng, M.; Bai, Y.; Zhang, Z.; Zhang, M.; Chen, Z.; Hu, X.; Li, J. Effect of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Fermentation on the Multi-Scale Structure and Physicochemical Properties of Highland Barley Starch. Food Biosci. 2023, 52, 102419. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, C.; Huang, Q.; Guan, Y.; Zhu, Z.; He, Y.; Jiang, G.; Xiong, Y.; Li, X. Correlation between in Vitro Starch Digestibility and Starch Structure/Physicochemical Properties in Rice. Int. J. Biol. Macromol. 2024, 263, 130316. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Liu, X.; Zhang, C.; Ma, M.; Sui, Z.; Corke, H. Lamellar Structure Changes in Rice Starch during α-Amylase Hydrolysis: Effect of Starch Granule Surface and Channel Proteins. Food Biosci. 2024, 61, 104502. [Google Scholar] [CrossRef]
- Lv, X.; Hong, Y.; Zhou, Q.; Jiang, C. Structural Features and Digestibility of Corn Starch with Different Amylose Content. Front. Nutr. 2021, 8, 692673. [Google Scholar] [CrossRef]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough Microbiome Comparison and Benefits. Microorganisms 2021, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.-D.; Sung, J.M. Effects of Fermentation with Lactobacillus plantarum on Rice Flour: The Role of Granular Characteristics. Food Chem. 2025, 464, 141615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-Z.; Zhang, H.; Dai, H.-Q.; Zhao, P.; Mao, Y.-F.; Chen, K.-X.; Chen, Z.-X. Inhibition of Starch Digestion: The Role of Hydrophobic Domain of Both α-Amylase and Substrates. Food Chem. 2021, 341, 128211. [Google Scholar] [CrossRef]
- Liang, W.; Ding, L.; Guo, K.; Liu, Y.; Wen, X.; Kirkensgaard, J.J.K.; Khakimov, B.; Enemark-Rasmussen, K.; Hebelstrup, K.H.; Herburger, K.; et al. The Relationship between Starch Structure and Digestibility by Time-Course Digestion of Amylopectin-Only and Amylose-Only Barley Starches. Food Hydrocoll. 2023, 139, 108491. [Google Scholar] [CrossRef]
- Gupta, R.; Gaur, S. Investigating the Effect of Natural Fermentation in Modifying the Physico-Functional, Structural and Thermal Characteristics of Pearl and Finger Millet Starch. J. Sci. Food Agric. 2024, 104, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ren, F.; Liu, H. Development of Low Glycemic Index Food Products with Wheat Resistant Starch: A Review. Carbohydr. Polym. 2025, 361, 123637. [Google Scholar] [CrossRef] [PubMed]
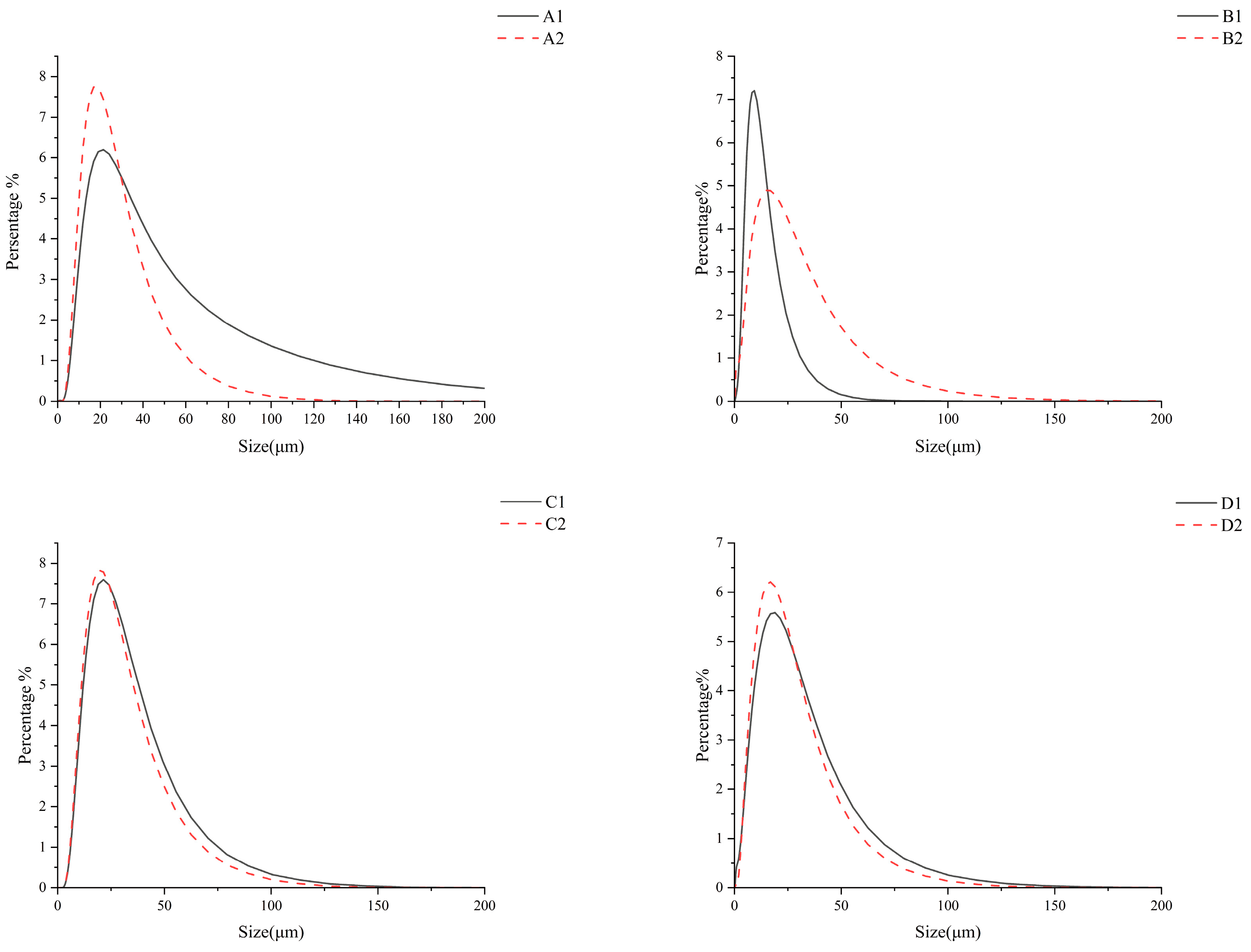
| Sample | D (0.1) | D (0.5) | D (0.9) | D (4,3) |
|---|---|---|---|---|
| A1 | 12.73 ± 1.41 f | 26.96 ± 6.87 a | 68.71 ± 37.11 a | 27.04 ± 5.44 a |
| A2 | 19.74 ± 0.38 e | 16.70 ± 0.23 cd | 36.60 ± 0.81 cd | 16.71 ± 0.24 bc |
| B1 | 58.92 ± 1.11 a | 8.52 ± 0.20 e | 21.06 ± 0.27 e | 8.05 ± 0.41 e |
| B2 | 41.94 ± 2.03 b | 12.24 ± 0.56 de | 37.01 ± 0.67 cd | 10.19 ± 0.70 de |
| C1 | 13.08 ± 0.02 f | 20.08 ± 0.01 bc | 45.06 ± 0.01 b | 20.08 ± 0.01 b |
| C2 | 14.71 ± 0.18 f | 18.77 ± 0.07 bc | 40.74 ± 0.15 bc | 18.78 ± 0.07 b |
| D1 | 25.47 ± 1.07 d | 23.40 ± 1.98 ab | 39.28 ± 9.50 cd | 20.86 ± 0.82 b |
| D2 | 33.61 ± 0.59 c | 14.01 ± 0.13 cde | 35.50 ± 0.12 d | 13.29 ± 0.23 cd |
| Sample | RC % |
|---|---|
| A1 | 35.55 ± 0.91 cd |
| A2 | 41.18 ± 1.61 ab |
| B1 | 32.57 ± 0.99 d |
| B2 | 32.99 ± 0.053 d |
| C1 | 34.26 ± 3.07 d |
| C2 | 38.11 ± 0.089 bc |
| D1 | 37.823 ± 0.33 c |
| D2 | 41.72 ± 0.60 a |
| Sample | R1047/1022 cm−1 | R995/1022 cm−1 |
|---|---|---|
| A1 | 0.699167091 ± 0.000457073 a | 0.90404789 ± 0.007000453 b |
| A2 | 0.688026327 ± 0.00068372 b | 0.88342442 ± 0.004103075 c |
| B1 | 0.660990101 ± 0.000470347 c | 0.92633817 ± 0.002279967 a |
| B2 | 0.657804596 ± 0.001815707 d | 0.92027157 ± 0.000052704 a |
| C1 | 0.678004337 ± 0.00031932 e | 0.89634106 ± 0.003683401 b |
| C2 | 0.674796404 ± 0.000475477 f | 0.90516304 ± 0.00478207 b |
| D1 | 0.694524633 ± 0.00040991 g | 0.91774859 ± 0.00002986 a |
| D2 | 0.682641434 ± 0.00045467 h | 0.90101114 ± 0.002253363 b |
| Sample | Peak Viscosity | Trough 1 | Breakdown | Final Visc | Setback | Peak Time | Pasting Temp |
|---|---|---|---|---|---|---|---|
| A1 | 3492.5 ± 143.54 c | 2110 ± 33.94 cd | 1382.5 ± 109.60 bc | 3901.5 ± 99.70 a | 1791.5 ± 65.76 a | 5.035 ± 0.05 f | 51.175 ± 1.38 e |
| A2 | 2709.5 ± 19.09 e | 2054.5 ± 75.66 d | 655 ± 94.75 f | 3178 ± 19.80 d | 1123.5 ± 95.46 c | 5.57 ± 0.14 e | 77.525 ± 1.10 d |
| B1 | 3706 ± 5.66 b | 2649 ± 28.28 a | 1057 ± 33.94 de | 3699.5 ± 17.68 b | 1050.5 ± 10.61 cd | 6.47 ± 0.01 b | 94.775 ± 0.04 a |
| B2 | 3502 ± 25.46 c | 2324.5 ± 210.01 bc | 1177.5 ± 235.47 cd | 3218 ± 84.85 d | 893.5 ± 125.16 d | 6.935 ± 0.09 a | 94.775 ± 0.04 a |
| C1 | 3807 ± 42.43 b | 2220.5 ± 34.65 bcd | 1586.5 ± 77.07 b | 3905.5 ± 88.39 a | 1685 ± 123.04 ab | 5.835 ± 0.05 cd | 50.25 ± 0.02 e |
| C2 | 4374.5 ± 171.83 a | 2297.5 ± 43.13 bc | 2077 ± 214.96 a | 3806 ± 134.35 ab | 1508.5 ± 91.22 b | 5.7 ± 0.04 de | 51.875 ± 1.66 e |
| D1 | 2833.5 ± 20.51 e | 2039 ± 63.64 d | 794.5 ± 43.13 ef | 3149.5 ± 60.10 d | 1110.5 ± 3.54 c | 5.6 ± 0.01 e | 85.6 ± 0.07 c |
| D2 | 3067 ± 28.28 d | 2415.5 ± 71.42 b | 651.5 ± 43.13 f | 3471 ± 18.38 c | 1055.5 ± 53.03 cd | 5.935 ± 0.09 c | 88.025 ± 0.11 b |
| Sample | To | TP | TC | Tc − T0 | ∆H |
|---|---|---|---|---|---|
| A1 | 58.535 ± 0.12021 c | 65.295 ± 0.03536 a | 74.8425 ± 1.34704 a | 16.3075 ± 1.22683 a | −8.0275 ± 0.27931 de |
| A2 | 60.2383 ± 0.09192 a | 57.1 ± 0.32527 f | 72.415 ± 0.90745 b | 12.1767 ± 0.99938 b | −4.8833 ± 1.09366 b |
| B1 | 54.2225 ± 0.07425 f | 59.1 ± 0.04243 e | 64.9371 ± 0.11373 f | 10.7146 ± 0.03948 b | −7.1367 ± 0.17913 cde |
| B2 | 54.6733 ± 0.06128 e | 60.58 ± 0.11314 d | 66.6983 ± 0.24749 de | 12.025 ± 0.30877 b | −6.3517 ± 0.0165 bcd |
| C1 | 58.6667 ± 0.00471 c | 61.835 ± 0.13435 c | 65.89 ± 0.12728 ef | 7.2233 ± 0.12257 d | −7.57 ± 0.32527 de |
| C2 | 57.3217 ± 0.08721 d | 60.505 ± 0.17678 d | 64.5417 ± 0.29463 f | 7.22 ± 0.38184 d | −5.58 ± 0.53269 bc |
| D1 | 59.1838 ± 0.20683 b | 63.21 ± 0.07071 b | 67.6067 ± 0.59868 cd | 8.4229 ± 0.80551 cd | −4.3713 ± 0.06894 a |
| D2 | 59.3025 ± 0.01061 b | 62.94 ± 0.07071 b | 68.301 ± 0.39739 c | 8.9985 ± 0.38679 c | −8.4033 ± 1.80548 e |
| Sample | C∞ (%) | K × 10−2 (min−1) | R2 | AUC | eGI |
|---|---|---|---|---|---|
| A1 | 58.94 ± 2.94 b | 1.45 ± 0.12 e | 0.95791 ± 0.30 a | 68.33 ± 0.80 ab | 67.10 ± 0.69 ab |
| A2 | 69.45 ± 2.23 a | 0.95 ± 0.03 e | 0.96314 ± 0.43 a | 65.08 ± 0.71 b | 64.30 ± 0.60 b |
| B1 | 29.99 ± 1.39 f | 2.99 ± 0.27 c | 0.93754 ± 0.21 a | 44.00 ± 2.95 d | 46.12 ± 2.54 d |
| B2 | 49.19 ± 1.68 c | 1.63 ± 0.04 de | 0.95433 ± 0.42 a | 60.05 ± 2.65 c | 59.96 ± 2.28 c |
| C1 | 40.1035 ± 1.956 de | 3.01 ± 0.49 c | 0.98713 ± 0.53 a | 58.65 ± 0.68 c | 58.75 ± 0.58 c |
| C2 | 37.977 ± 0.98 e | 4.24 ± 0.49 b | 0.98191 ± 0.64 a | 59.35 ± 0.48 c | 59.35 ± 0.41 c |
| D1 | 44.23 ± 2.06 d | 5.11 ± 0.13 a | 0.9585 ± 0.46 a | 70.97 ± 3.09 a | 69.37 ± 2.66 a |
| D2 | 35.63 ± 1.44 e | 2.23 ± 0.36 d | 0.95122 ± 0.20 a | 48.27 ± 0.42 d | 49.81 ± 0.36 d |
| Corn Starch | Oat Starch | Barley Starch | Buckwheat Starch | |
|---|---|---|---|---|
| D(4,3) | −38.21% | +26.58% | −6.47% | −36.31% |
| R1047/1022 cm−1 | −1.60% | −0.48% | −0.47% | −1.71% |
| R995/1022 cm−1 | −2.30% | −0.60% | +0.98% | −1.82% |
| Peak viscosity | −22.48% | −5.53% | +14.38% | +8.23% |
| Breakdown | −52.68% | +11.40% | +30.87% | −18.00% |
| Final visc | −19.06% | −12.52% | −2.55% | +10.22% |
| Pasting temp | +51.51% | 0% | +3.23% | +2.83% |
| RC% | +15.56% | +1.26% | +11.23% | +10.30% |
| To | +2.91% | +0.83% | −2.29% | +0.20% |
| TP | −12.60% | +2.50% | −2.15% | −0.43% |
| TC | −3.25% | +2.72% | −2.05% | +1.03% |
| Tc − T0 | −25.26% | +12.24% | +0.04% | +6.83% |
| DH | −39.15% | +1.12% | +100.66% | 91.06% |
| WSP | −13.63% | −3.13 | +6.73% | −2.44% |
| Solubility | −5.59% | −39.71% | −26.19% | −38.42% |
| Amylose | +5% | −3.32% | +2.56% | +44% |
| Amylopectin | −1.25% | +1.45% | −1.64% | −15.33% |
| Gel hardness | +37.94% | −42.95% | +42.05% | 94.68% |
| RS | +5% | −6% | +8% | +7% |
| SDS | −1% | +11% | −9% | +14% |
| RDS | −4% | +3% | +1% | −21% |
| GI | −4.17% | +29.99% | +1.02% | −28.19% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Z.; Wang, J.; Teng, W.; Wang, Y.; Zhang, Y.; Cao, J. Effect of Lactic Acid Bacteria Fermentation Agent on the Structure, Physicochemical Properties, and Digestive Characteristics of Corn, Oat, Barley, and Buckwheat Starch. Foods 2025, 14, 2904. https://doi.org/10.3390/foods14162904
You Z, Wang J, Teng W, Wang Y, Zhang Y, Cao J. Effect of Lactic Acid Bacteria Fermentation Agent on the Structure, Physicochemical Properties, and Digestive Characteristics of Corn, Oat, Barley, and Buckwheat Starch. Foods. 2025; 14(16):2904. https://doi.org/10.3390/foods14162904
Chicago/Turabian StyleYou, Ziyi, Jinpeng Wang, Wendi Teng, Ying Wang, Yuemei Zhang, and Jinxuan Cao. 2025. "Effect of Lactic Acid Bacteria Fermentation Agent on the Structure, Physicochemical Properties, and Digestive Characteristics of Corn, Oat, Barley, and Buckwheat Starch" Foods 14, no. 16: 2904. https://doi.org/10.3390/foods14162904
APA StyleYou, Z., Wang, J., Teng, W., Wang, Y., Zhang, Y., & Cao, J. (2025). Effect of Lactic Acid Bacteria Fermentation Agent on the Structure, Physicochemical Properties, and Digestive Characteristics of Corn, Oat, Barley, and Buckwheat Starch. Foods, 14(16), 2904. https://doi.org/10.3390/foods14162904







