Wild Edible Fungi in the Catalan Linguistic Area: A Scoping Review Linking Nutritional Value to Ethnomycology
Abstract
1. Introduction
- Elaborate a corpus of the WEF from the CLA;
- Conduct a scoping review to determine which WEF from the CLA region have reported nutritional values.
2. Materials and Methods
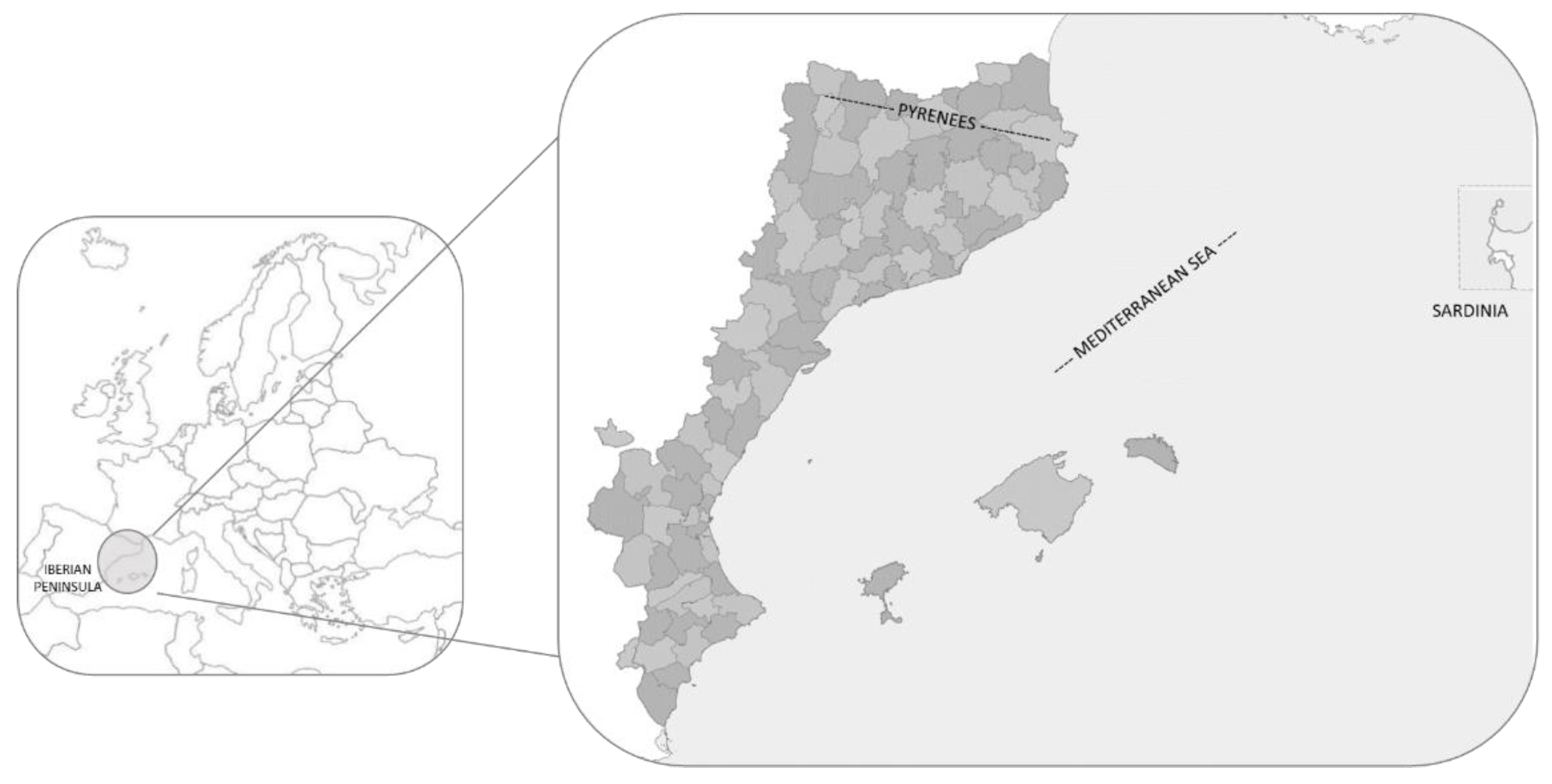
2.1. Study Area
2.2. Study Design and Data Source
2.3. Search Strategy
2.4. Eligibility Criteria
2.5. Study Selection and Data Extraction
2.6. Data Analysis
3. Results and Discussion
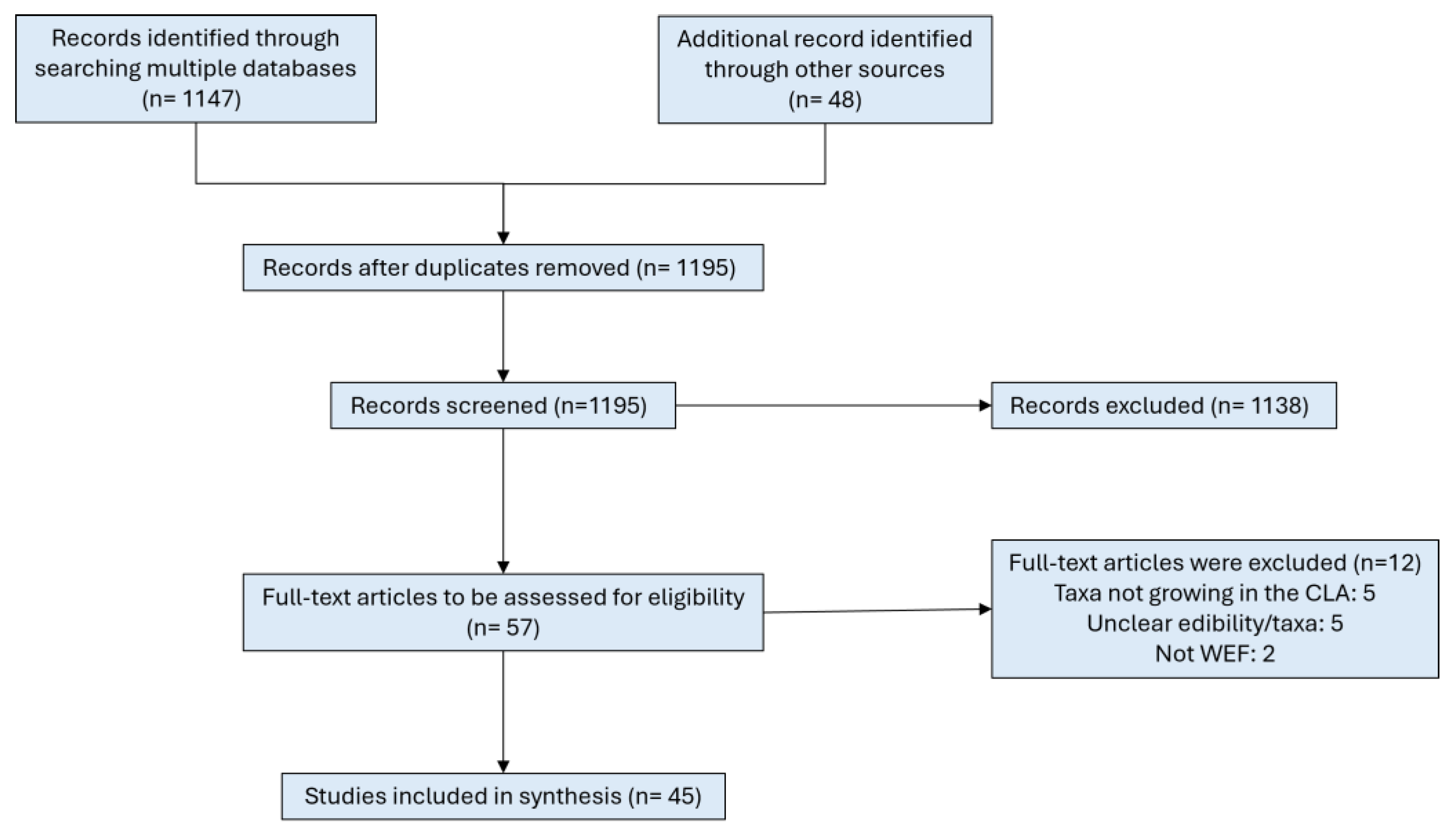
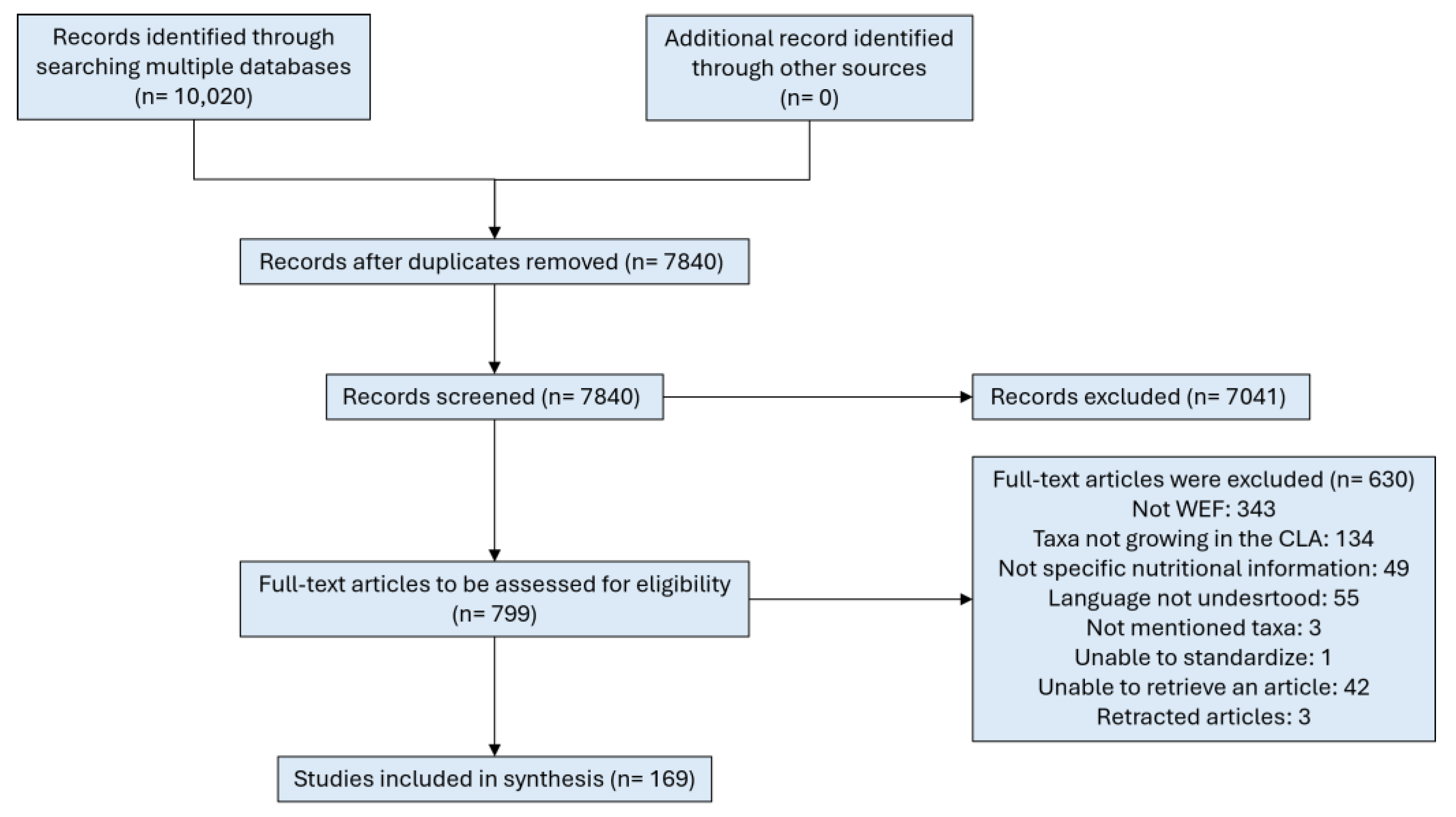
3.1. Study Selection
3.2. WEF Corpus from the CLA
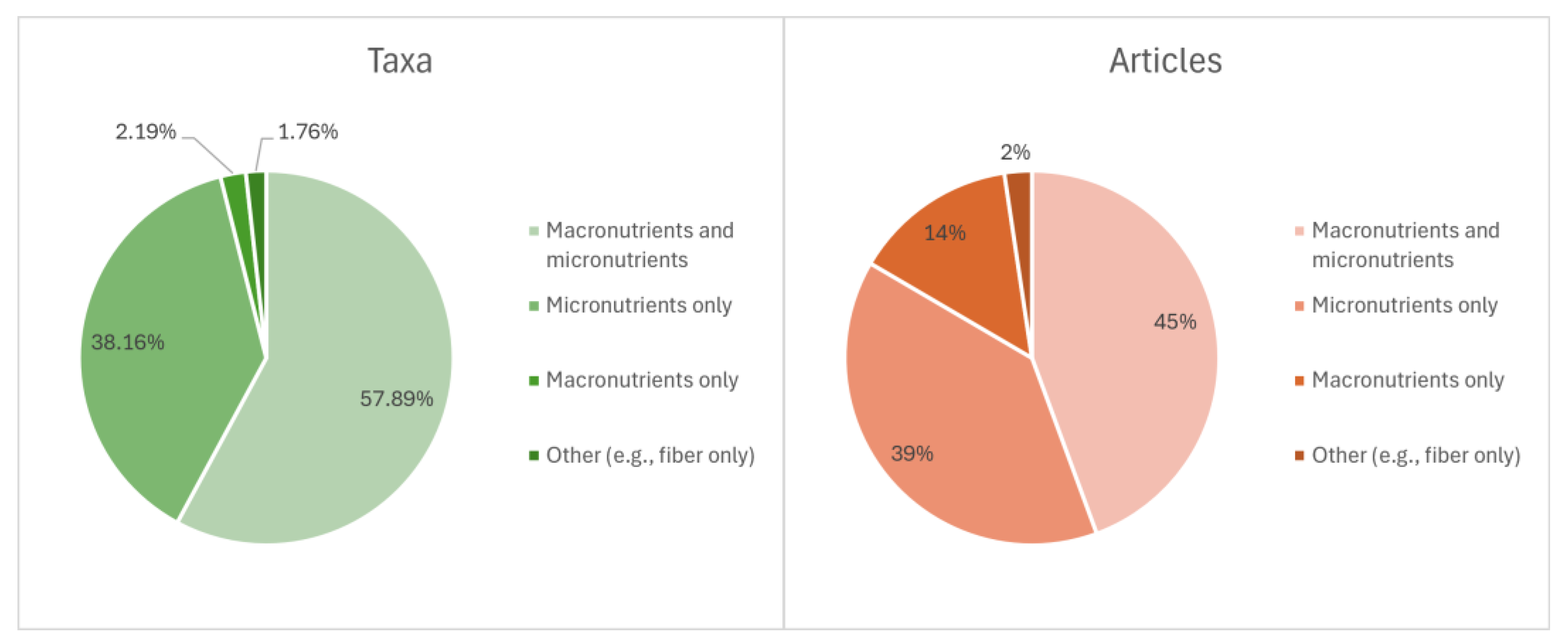
3.3. Nutritional Values of WEF from the CLA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Adequate intake |
| BC | Biblioteca de Catalunya |
| BR | Bibliographic Report |
| CLA | Catalan Linguistic Area |
| CORA | Catalan Open Research Area |
| CRAI | Centre de Recursos per a l’Aprenentatge i la Investigació |
| DM | Dry Matter |
| EI | Ethnobotanicity Index |
| EMI | Ethnomycoticity Index |
| FM | Fluid Matter |
| UB | Universitat de Barcelona |
| WEF | Wild Edible Fungi |
References
- Boa, E.R. Wild Edible Fungi: A Global Overview of Their Use and Importance to People; Non-Wood Forest Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; ISBN 978-92-5-105157-3. [Google Scholar]
- Teke, N.A.; Kinge, T.R.; Bechem, E.; Nji, T.M.; Ndam, L.M.; Mih, A.M. Ethnomycological study in the Kilum-Ijim mountain forest, northwest region, Cameroon. J. Ethnobiol. Ethnomed. 2018, 14, 25. [Google Scholar] [CrossRef]
- Yamin-Pasternak, S. Ethnomycology: Fungi and mushrooms in cultural entanglements. In Ethnomycology: Fungi and Mushrooms in Cultural Entanglements; Anderson, E.N., Pearsall, D., Hunn, E., Turner, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 213–230. [Google Scholar]
- Dutta, A.K.; Acharya, K. Traditional and ethno-medicinal knowledge of mushrooms in West Bengal, India. Asian J. Pharm. Clin. Res. 2014, 7, 36–41. [Google Scholar]
- Frutis-Molina, I.; Valenzuela, R. Macromicetos. In La Diversidad Biológica del Estado de México: Estudio de Estado; Ceballos, G., List, R., Garduño, G., López-Cano, R., Muñozcano-Quintanar, M.J., Collado, E., Eivin San Román, J., Eds.; Gobierno del Estado de México/Biblioteca Mexiquense del Bicentenario: Toluca de Lerdo, Mexico, 2009; pp. 243–249. [Google Scholar]
- Mueller, G.M.; Schmit, J.P.; Leacock, P.R.; Buyck, B.; Cifuentes, J.; Desjardin, D.E.; Halling, R.E.; Hjortstam, K.; Iturriaga, T.; Larsson, K.-H.; et al. Global diversity and distribution of macrofungi. Biodivers. Conserv. 2007, 16, 37–48. [Google Scholar] [CrossRef]
- Mgbekem, M.A.; Lukpata, F.; Ndukaku, N.; Armon, M.; Uka, V.K.; Udosen, G.N.; Pricilla, A.-B. Knowledge and Utilization of mushroom as a food supplement among families in selected local government areas of Cross River State, Nigeria. Food Nutr. Sci. 2019, 10, 1287–1299. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; Kirk, P.M., Cannon, P.F., Minter, D.W., Stalpers, J.A., Eds.; CABI: Wallingford, UK, 2008; ISBN 978-0-85199-826-8. [Google Scholar]
- Krstin, L.; Katanić, Z.; Benčić, K.; Lončar, L.; Pfeiffer, T.Ž. Ethnobotanical survey of culturally important plants and mushrooms in North-Western part of Croatia. Plants 2024, 13, 1566. [Google Scholar] [CrossRef]
- Wasson, V.P.; Wasson, R.G. Mushrooms, Russia, and History; Pantheon Books: New York, NY, USA, 1957. [Google Scholar]
- Fajardo, J.; Verde, A.; Valdés, A.; Rivera, D.; Obón, C. Etnomicología en Castilla-La Mancha (España). Bol. Soc. Micol. Madrid 2010, 34, 19–38. [Google Scholar]
- Illana, C. Robert Gordon Wasson: Un pionero de la etnomicología. Bol. Soc. Micol. Madrid 2007, 31, 273–277. [Google Scholar]
- Pieroni, A.; Nebel, S.; Santoro, R.F.; Heinrich, M. Food for Two Seasons: Culinary Uses of Non-Cultivated Local Vegetables and Mushrooms in a South Italian Village. Int. J. Food Sci. Nutr. 2005, 56, 245–272. [Google Scholar] [CrossRef]
- Yusran, Y.; Erniwati, E.; Khumaidi, A.; Rukmi, R.; Sustri, S. Ethnomycological Study of Macrofungi Utilized by Pamona Community around Lake Poso, Central Sulawesi Province, Indonesia. Jordan J. Biol. Sci. 2024, 17, 77–87. [Google Scholar] [CrossRef]
- Tibuhwa, D.D. Folk taxonomy and use of mushrooms in communities around Ngorongoro and Serengeti National Park, Tanzania. J. Ethnobiol. Ethnomed. 2012, 8, 36. [Google Scholar] [CrossRef]
- Ríos-García, U.; Carrera-Martínez, A.; Martínez-Reyes, M.; Hernández-Santiago, F.; Evangelista, F.R.; Díaz-Aguilar, I.; Olvera-Noriega, J.W.; Pérez-Moreno, J. Traditional knowledge and use of wild mushrooms with biocultural importance in the Mazatec culture in Oaxaca, Mexico, Cradle of the Ethnomycology. For. Syst. 2023, 32, e007. [Google Scholar] [CrossRef]
- Shevchuk, Y.; Kuypers, K.; Janssens, G.E. Fungi as a source of bioactive molecules for the development of longevity medicines. Ageing Res. Rev. 2023, 87, 101929. [Google Scholar] [CrossRef]
- Niell, M.; Girbal, J.; Ribas, L. L’ús dels esclerocis de Claviceps purpurea dins la medicina popular andorrana. Rev. Catalana Micol. 2010, 32, 37–42. [Google Scholar]
- Peintner, U.; Pöder, R. Ethnomycological remarks on the Iceman’s fungi. In The Iceman and His Natural Environment; Bortenschlager, S., Oeggl, K., Eds.; Springer: Vienna, Austria, 2000; pp. 143–150. ISBN 978-3-7091-7403-6. [Google Scholar]
- Peintner, U.; Pöder, R.; Pümpel, T. The Iceman’s Fungi. Mycol. Res. 1998, 102, 1153–1162. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Analysis of traditional knowledge for wild edible mushrooms consumed by residents living in Jirisan National Park (Korea). J. Ethnopharmacol. 2014, 153, 90–97. [Google Scholar] [CrossRef]
- Vallès, J. Etnobotànica: Persones, Plantes, Cultura i Benestar; Institut d’Estudis Catalans: Barcelona, Spain, 2019. [Google Scholar]
- Niveiro, N.; Popoff, O.F.; Albertó, E.O. Hongos comestibles silvestres: Especies exóticas de Suillus (Boletales, Basidiomycota) y Lactarius (Russulales, Basidiomycota) asociadas a cultivos de Pinus elliottil del nordeste argentino. Bonplandia 2009, 18, 65–71. [Google Scholar] [CrossRef]
- Martínez-de Aragón, J.; Riera, P.; Giergiczny, M.; Colinas, C. Value of wild mushroom picking as an environmental service. For. Policy Econ. 2011, 13, 419–424. [Google Scholar] [CrossRef]
- Font-Quer, P. Plantas Medicinales: El Dioscórides Renovado, 11th ed.; Labor: Barcelona, Spain, 1988; ISBN 84-335-6151-0. [Google Scholar]
- Cáceres, F.; Vallès, J.; Gras, A. Exploring ethnobotany in the Catalan linguistic area: Traditional plant-based knowledge for addressing gastrointestinal, metabolic, and nutritional disorders. Plants 2024, 13, 2453. [Google Scholar] [CrossRef]
- Cáceres, F.; Vallès, J.; Garnatje, T.; Parada, M.; Gras, A. Gastrointestinal, metabolic, and nutritional disorders: A plant-based ethnoveterinary meta-analysis in the Catalan linguistic area. Front. Vet. Sci. 2022, 9, 908491. [Google Scholar] [CrossRef]
- Bolòs, O.; Vigo, J.; Masalles, R.M.; Ninot, J. Flora Manual dels Països Catalans, 3rd ed.; Editorial Pòrtic: Barcelona, Spain, 2005. [Google Scholar]
- Departament d’Estadística del Govern d’Andorra. Població Total. Estadistica.ad. Available online: https://www.estadistica.ad/portal/apps/sites/#/estadistica-ca/ (accessed on 13 May 2025).
- Institut National de la Statistique et des Etudes Economiques. Populations Légales des Départements. Available online: https://www.insee.fr/fr/accueil (accessed on 13 May 2025).
- Instituto Nacional de Estadística. Población por Comunidades y Ciudades Autónomas y Tamaño de los Municipios. Available online: https://www.ine.es/ (accessed on 13 May 2025).
- Istituto Nazionale di Statistica. Statistiche. ISTAT. Available online: https://esploradati.istat.it/databrowser/#/en (accessed on 13 May 2025).
- Llimona, X. (Universitat de Barcelona and Institut d’Estudis Catalans, Barcelona, Spain). Estimation of macromycetes taxa in the CLA. Personal communication, 2025. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- CRAI Universitat de Barcelona. CRAI Universitat de Barcelona. Available online: https://crai.ub.edu/ (accessed on 16 June 2025).
- Institut d’Estudis Catalans. Etnobotànica dels Països Catalans. Available online: https://etnobotanica.iec.cat/ (accessed on 16 June 2025).
- CRAI Universitat de Barcelona. Cercabib. Available online: https://cercabib.ub.edu (accessed on 16 June 2025).
- Biblioteca de Catalunya. Explora La BC. Available online: https://explora.bnc.cat (accessed on 16 June 2025).
- Index Fungorum Partnership. Index Fungorum. Available online: https://www.indexfungorum.org/ (accessed on 16 June 2025).
- Westerdijk Fungal Biodiversity Institute. MycoBank. Available online: https://www.mycobank.org/ (accessed on 16 June 2025).
- FAO/INFOODS. FAO/INFOODS Databases. Available online: https://www.fao.org/infoods/infoods/tables-and-databases/faoinfoods-databases/en/ (accessed on 16 June 2025).
- Klensin, J.C.; Feskanich, D.; Lin, V.; Truswell, S.; Southgate, D.A.T. Identification of Food Components for INFOODS Data Interchange; United Nations University Press: Tokyo, Japan, 1989; ISBN 92-808-0734-X. [Google Scholar]
- FAO/INFOODS. FAO/INFOODS Guidelines for Checking Food Composition Data Prior to the Publication of a User Table/Database, Version 1.0; FAO: Rome, Italy, 2012; ISBN 978-92-5-107368-1. [Google Scholar]
- Portères, R. Cours D’ethno-Botanique et Ethno-Zoologie (1969–1970) Vol. I: Ethnobotanique Générale; Muséum National d’Histoire Naturelle—Faculté des Lettres: Paris, France, 1970; Volume 1. [Google Scholar]
- Bonet, M.À.; Vallès, J.P. Remeis i Cultura Popular del Montseny. In Etnobotànica D’una Reserva de la Biosfera; de Granollers, M., Ed.; Brau Edicions: Figueres, Spain, 2006; p. 771. [Google Scholar]
- Aloupi, M.; Koutrotsios, G.; Koulousaris, M.; Kalogeropoulos, N. Trace metal contents in wild edible mushrooms growing on serpentine and volcanic soils on the island of Lesvos, Greece. Ecotoxicol. Environ. Saf. 2012, 78, 184–194. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Mitic, V.; Djordjevic, D.; Popovic, G.; Krstic, N.; Nikolic, J.; Stankov-Jovanovic, V. Macroelements versus toxic elements in selected wild edible mushrooms of the Russulaceae family from Serbia. J. Serbian Chem. Soc. 2021, 86, 927–940. [Google Scholar] [CrossRef]
- Haro, A.; Trescastro, A.; Lara, L.; Fernández-Fígares, I.; Nieto, R.; Seiquer, I. Mineral elements content of wild growing edible mushrooms from the southeast of Spain. J. Food Compos. Anal. 2020, 91, 103504. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef] [PubMed]
- López, A.R.; Barea-Sepúlveda, M.; Barbero, G.F.; Ferreiro-González, M.; López-Castillo, J.G.; Palma, M.; Espada-Bellido, E. Essential mineral content (Fe, Mg, P, Mn, K, Ca, and Na) in five wild edible species of Lactarius mushrooms from Southern Spain and Northern Morocco: Reference to Daily Intake. J. Fungi 2022, 8, 1292. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Total, soluble, and insoluble dietary fibre contents of wild growing edible mushrooms. Czech J. Food Sci. 2014, 32, 302–307. [Google Scholar] [CrossRef]
- Martinez-Para, M.D.C.; Torija-Isasa, M.E.; Masoud, T.A. Determination of Cd, Co, Cr, and Ni in edible fungi. Genera Lactarius and Pleurotus. An. Inst. Nac. Investig. Agrar. Ser. Agric. 1983, 23, 65–70. [Google Scholar]
- Alonso, J.; García, M.A.; Pérez-López, M.; Melgar, M.J. The concentrations and bioconcentration factors of copper and zinc in edible mushrooms. Arch. Environ. Contam. Toxicol. 2003, 44, 180–188. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Özel, A.; Col, M.; Duran, C.; Sesli, E.; Colak, A. Nutritional value of some wild edible mushrooms from Black Sea Region (Turkey). Turk. J. Biochem. 2011, 36, 213–221. [Google Scholar]
- Aydin, E.; Gurbuz, I.B.; Karahan, H.; Basdar, C. Effect of different processing technologies on chemical properties of wild-grown edible mushroom Macrolepiota procera var. procera (Scop.). J. Food Process. Preserv. 2017, 41, e12802. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Correia, D.M.; Sá Morais, J.; Ferreira, I.C.F.R. Effects of conservation treatment and cooking on the chemical composition and antioxidant activity of Portuguese wild edible mushrooms. J. Agric. Food Chem. 2007, 55, 4781–4788. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Bucurica, I.A.; Dulama, I.D.; Radulescu, C.; Banica, A.L.; Stanescu, S.G. Heavy metals and associated risks of wild edible mushrooms consumption: Transfer factor, carcinogenic risk, and health risk index. J. Fungi 2024, 10, 844. [Google Scholar] [CrossRef]
- Ćirić, A.; Kruljević, I.; Stojković, D.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M.; Glamočlija, J. Comparative investigation on edible mushrooms Macrolepiota mastoidea, M. rhacodes and M. procera: Functional foods with diverse biological activities. Food Funct. 2019, 10, 7678–7686. [Google Scholar] [CrossRef]
- Dar, A.H.; Wani, A.H.; Talie, M.D.; Bhat, M.Y.; Sheikh, A.R. Nutritional composition and mycolith assessment of some wild mushrooms from Southern Region of Kashmir Himalaya, India. J. Food Compos. Anal. 2024, 132, 106336. [Google Scholar] [CrossRef]
- Demirtas, N.; Sengul, G.F.; Dizeci, N.; Yildirim, O. exploring the chemical composition and nutritional properties of six edible mushroom species from Turkey. J. Food Compos. Anal. 2024, 133, 106477. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Barreira, J.C.M.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of different processing technologies on chemical and antioxidant parameters of Macrolepiota procera wild mushroom. LWT—Food Sci. Technol. 2013, 54, 493–499. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.M.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of gamma irradiation on chemical composition and antioxidant potential of processed samples of the wild mushroom Macrolepiota procera. Food Chem. 2014, 149, 91–98. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.M.; Antonio, A.L.; Morales, P.; Férnandez-Ruiz, V.; Martins, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Exquisite wild mushrooms as a source of dietary fiber: Analysis in electron-beam irradiated samples. LWT—Food Sci. Technol. 2015, 60, 855–859. [Google Scholar] [CrossRef]
- Gençcelep, H.; Uzun, Y.; Tunçtürk, Y.; Demirel, K. Determination of mineral contents of wild-grown edible mushrooms. Food Chem. 2009, 113, 1033–1036. [Google Scholar] [CrossRef]
- Gezer, K.; Kaygusuz, O.; Eyupoglu, V.; Surucu, A.; Doker, S. Determination by ICP/MS of trace metal content in ten edible wild mushrooms from Turkey. Oxid. Commun. 2015, 38, 398–407. [Google Scholar]
- Giannaccini, G.; Betti, L.; Palego, L.; Mascia, G.; Schmid, L.; Lanza, M.; Mela, A.; Fabbrini, L.; Biondi, L.; Lucacchini, A. The trace element content of top-soil and wild edible mushroom samples collected in Tuscany, Italy. Environ. Monit. Assess. 2012, 184, 7579–7595. [Google Scholar] [CrossRef]
- Melgar, M.J.; Alonso, J.; García, M.A. Acumulación de selenio en setas silvestres comestibles: Captación y toxicidad. Selenium accumulation in wild edible mushrooms: Uptake and toxicity. CyTA—J. Food 2009, 7, 217–223. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzyńska, S.; Szostek, M.; Kuczyńska-Kippen, N.; Kalač, P.; Niedzielski, P.; Gąsecka, M.; et al. Toxicological risks and nutritional value of wild edible mushroom species—A half-century monitoring Study. Chemosphere 2021, 263, 128095. [Google Scholar] [CrossRef]
- Palazzolo, E.; Letizia Gargano, M.; Venturella, G. The nutritional composition of selected wild edible mushrooms from Sicily (Southern Italy). Int. J. Food Sci. Nutr. 2012, 63, 79–83. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Akata, I.; Guven, G.; Tepe, B. Metal concentration and health risk assessment of wild mushrooms collected from the Black Sea Region of Turkey. Environ. Sci. Pollut. Res. 2020, 27, 26419–26441. [Google Scholar] [CrossRef]
- Senila, M.; Senila, L.; Resz, M.-A. Chemical composition and nutritional characteristics of popular wild edible mushroom species collected from North-Western Romania. J. Food Compos. Anal. 2024, 134, 106504. [Google Scholar] [CrossRef]
- Titilawo, M.A.; Oluduro, A.O.; Odeyemi, O. Proximate and chemical properties of some underutilized Nigerian wild mushrooms. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 390–397. [Google Scholar] [CrossRef]
- Tuzen, M.; Sesli, E.; Soylak, M. Trace element levels of mushroom species from east Black Sea Region of Turkey. Food Control 2007, 18, 806–810. [Google Scholar] [CrossRef]
- Uzun, Y.; Gençcelep, H.; Tunçtürk, Y.; Demirel, K. Determination of protein and nitrogen fractions of wild edible mushrooms. ASIAN J. Chem. 2009, 21, 2769–2776. [Google Scholar]
- Vetter, J. Phosphorus content of edible wild mushrooms of Hungary. Acta Aliment. 1994, 23, 331–336. [Google Scholar]
- Vetter, J. Data on sodium content of common edible mushrooms. Food Chem. 2003, 81, 589–593. [Google Scholar] [CrossRef]
- Zounr, R.A.; Tuzen, M.; Khuhawar, M.Y. Determination of selenium and arsenic ions in edible mushroom samples by novel chloride–oxalic acid deep eutectic solvent extraction using graphite furnace-atomic absorption spectrometry. J. AOAC Int. 2018, 101, 593–600. [Google Scholar] [CrossRef]
- Arvay, J.; Tomas, J.; Hauptvogl, M.; Massanyi, P.; Harangozo, L.; Toth, T.; Stanovic, R.; Bryndzova, S.; Bumbalova, M. Human exposure to heavy metals and possible public health risks via consumption of wild edible mushrooms from Slovak Paradise National Park, Slovakia. J. Environ. Sci. Health B 2015, 50, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Brzezicha-Cirocka, J.; Grembecka, M.; Grochowska, I.; Falandysz, J.; Szefer, P. Elemental composition of selected species of mushrooms based on a chemometric evaluation. Ecotoxicol. Environ. Saf. 2019, 173, 353–365. [Google Scholar] [CrossRef]
- Gucia, M.; Jarzynska, G.; Kojta, A.K.; Falandysz, J. Temporal variability in 20 chemical elements content of parasol mushroom (Macrolepiota procera) collected from two sites over a few years. J. Environ. Sci. Health B 2012, 47, 81–88. [Google Scholar] [CrossRef]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Riganakos, K.A. Nutritional value and metal content profile of Greek wild edible fungi. Acta Aliment. 2007, 36, 99–110. [Google Scholar] [CrossRef]
- Piepponen, S.; Liukkonenlilja, H.; Kuusi, T. The selenium content of edible mushrooms in Finland. Z. Lebensm. Unters. Forsch. 1983, 177, 257–260. [Google Scholar] [CrossRef]
- López-Vázquez, E.; Prieto-García, F.; Gayosso-Canales, M.; Sánchez, E.M.O.; Ibarra, J.R.V. Phenolic acids, flavonoids, ascorbic acid, β-glucans and antioxidant activity in Mexican wild edible mushrooms. Ital. J. Food Sci. 2017, 29, 766–774. [Google Scholar]
- Ouzouni, P.K.; Petridis, D.; Koller, W.-D.; Riganakos, K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gautam, N. Chemical, bioactive, and antioxidant potential of twenty wild culinary mushroom species. BioMed. Res. Int. 2015, 2015, 346508. [Google Scholar] [CrossRef]
- Bayram, O.F.; Marah, S.; Turkekul, I.; Ozen, T. Phytochemical profile, bioactivity, and molecular docking studies of natural edible mushrooms grown in Tokat and Sivas provinces of Turkey. J. Food Sci. 2024, 89, 5928–5950. [Google Scholar] [CrossRef]
- Mendil, D.; Bardak, H.; Tüzen, M. Trace element concentrations in edible wild mushroom samples from Turkey determined by atomic absorption methods using microwave digestion vs. wet ashing. At. Spectrosc. 2015, 36, 266–272. [Google Scholar] [CrossRef]
- Sesli, E.; Tuzen, M.; Soylak, M. Evaluation of trace metal contents of some wild edible mushrooms from Black Sea Region, Turkey. J. Hazard. Mater. 2008, 160, 462–467. [Google Scholar] [CrossRef]
- Tel, G.; Çavdar, H.; Deveci, E.; Öztürk, M.; Duru, M.E.; Turkoğlu, A. Minerals and Metals in Mushroom Species in Anatolia. Food Addit. Contam. Part B 2014, 7, 226–231. [Google Scholar] [CrossRef]
- Dabbour, I.R.; Takruri, H.R. Protein quality of four types of edible mushrooms found in Jordan. Plant Foods Hum. Nutr. 2002, 57, 1–11. [Google Scholar] [CrossRef]
- Díez, V.A.; Alvarez, A. Compositional and nutritional studies on two wild edible mushrooms from Northwest Spain. Food Chem. 2001, 75, 417–422. [Google Scholar] [CrossRef]
- Erdogan, S.; Yilmaz-Ersela, F.; Merdivan, M. Macro and micro element contents in fruiting bodies of wild edible mushrooms from Mugla in Southwest Anatolia, Turkey. ASIAN J. Chem. 2006, 18, 2159–2167. [Google Scholar]
- Gezer, K.; Kaygusuz, O. An assessment of the heavy metal content of various wild edible mushrooms in the Denizli province, Turkey. J. Environ. Prot. Ecol. 2014, 15, 425–432. [Google Scholar]
- Kaya, A.; Gençcelep, H.; Uzun, Y.; Demirel, K. Analysis of Trace Metal Levels in Wild Mushrooms. ASIAN J. Chem. 2011, 23, 1099–1103. [Google Scholar]
- Demirbas, A. Heavy metal contents in the fruiting bodies of mushrooms growing in Turkey. Dtsch. Lebensm. Rundsch. 2003, 99, 62–67. [Google Scholar]
- Barros, L.; Baptista, P.; Correia, D.M.; Casal, S.; Oliveira, B.; Ferreira, I. Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef]
- Fernandes, Â.; Antonio, A.L.; Barreira, J.C.M.; Botelho, M.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of gamma irradiation on the chemical composition and antioxidant activity of Lactarius deliciosus L. wild edible mushroom. Food Bioprocess Technol. 2013, 6, 2895–2903. [Google Scholar] [CrossRef]
- Jedidi, I.K.; Ayoub, I.K.; Philippe, T.; Bouzouita, N. Chemical composition and nutritional value of three Tunisian wild edible mushrooms. J. Food Meas. Charact. 2017, 11, 2069–2075. [Google Scholar] [CrossRef]
- Ouali, Z.; Chaar, H.; Venturella, G.; Cirlincione, F.; Gargano, M.L.; Jaouani, A. Chemical composition and nutritional value of nine wild edible mushrooms from Northwestern Tunisia. Ital. J. Mycol. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Pająk, M.; Gąsiorek, M.; Jasik, M.; Halecki, W.; Otremba, K.; Pietrzykowski, M. Risk assessment of potential food chain threats from edible wild mushrooms collected in forest ecosystems with heavy metal pollution in Upper Silesia, Poland. Forests 2020, 11, 1240. [Google Scholar] [CrossRef]
- Rasalanavho, M.; Moodley, R.; Jonnalagadda, S.B. Elemental bioaccumulation and nutritional value of five species of wild growing mushrooms from South Africa. Food Chem. 2020, 319, 126596. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T. Macro- and microelement contents in fruiting bodies of wild mushrooms from the Notecka Forest in west-central Poland. Food Chem. 2005, 92, 499–506. [Google Scholar] [CrossRef]
- Xu, Z.; Fu, L.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Zhou, L.; Yang, H.; Ding, C. Chemical composition, antioxidant and antihyperglycemic activities of the wild Lactarius deliciosus from China. Molecules 2019, 24, 1357. [Google Scholar] [CrossRef]
- Alaimo, M.G.; Saitta, A.; Ambrosio, E. Bedrock and soil geochemistry influence the content of chemical elements in wild edible mushrooms (Morchella group) from South Italy (Sicily). Acta Mycol. 2019, 54, 1122. [Google Scholar] [CrossRef]
- Boda, R.H.; Wani, A.H.; Zargar, M.A.; Ganie, B.A.; Wani, B.A.; Ganie, S.A. Nutritional values and antioxidant potential of some edible mushrooms of Kashmir Valley. Pak. J. Pharm. Sci. 2012, 25, 623–627. [Google Scholar]
- Heleno, S.A.; Stojković, D.; Barros, L.; Glamočlija, J.; Soković, M.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Res. Int. 2013, 51, 236–243. [Google Scholar] [CrossRef]
- Vieira, V.; Fernandes, Â.; Barros, L.; Glamočlija, J.; Ćirić, A.; Stojković, D.; Martins, A.; Soković, M.; Ferreira, I.C. Wild Morchella Conica Pers. from different origins: A comparative study of nutritional and bioactive properties. J. Sci. Food Agric. 2016, 96, 90–98. [Google Scholar] [CrossRef]
- Wahid, M.; Sattar, A.; Iqbal, A. Nutrient and heavy metal contents compared among five mushrooms from Pakistan. Karstenia 1992, 32, 1–5. [Google Scholar] [CrossRef]
- Altaf, U.; Lalotra, P.; Sharma, Y.P. Nutritional and mineral composition of four wild edible mushrooms from Jammu and Kashmir, India. Indian Phytopathol. 2020, 73, 313–320. [Google Scholar] [CrossRef]
- Keskin, F.; Sarikurkcu, C.; Akata, I.; Tepe, B. Metal concentrations of wild mushroom species collected from Belgrad Forest (Istanbul, Turkey) with their health risk assessments. Environ. Sci. Pollut. Res. 2021, 28, 36193–36204. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Subbulakshmi, G. Nutritional value of edible wild mushrooms collected from the Khasi Hills of Meghalaya. Food Chem. 2005, 89, 599–603. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Chemical composition and biological properties of Portuguese wild mushrooms: A Comprehensive Study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhou, H.B.; Qiu, G.Z. Chemical composition and antioxidant activity of two edible mycorrhizal fungi from south China. ASIAN J. Chem. 2010, 22, 6867–6878. [Google Scholar]
- Colak, A.; Faiz, Ö.; Sesli, E. Nutritional composition of some wild edible mushrooms. Turk. J. Biochem. 2009, 34, 25–31. [Google Scholar]
- Costa-Silva, F.; Marques, G.; Matos, C.C.; Barros, A.I.R.N.A.; Nunes, F.M. Selenium contents of Portuguese commercial and wild edible mushrooms. Food Chem. 2011, 126, 91–96. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcaș, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Kuka, M.; Cakste, I.; Galoburda, R.; Sabovics, M. Chemical composition of Latvian wild edible mushroom Cantharellus cibarius. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food Consum Well-Being”, Jelgava, Latvia, 8–9 May 2014; Foodbalt: Jelgava, Latvia, 2014; pp. 248–252. [Google Scholar]
- Kumari, D.; Reddy, M.S.; Upadhyay, R.C. Nutritional Composition and Antioxidant Activities of 18 Different Wild Cantharellus mushrooms of northwestern Himalayas. Food Sci. Technol. Int. 2011, 17, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Salihović, M.; Pazalja, M.; Huremović, M.; Ajanović, A.; Tahirović, I. Chemical ingredients of fresh and dry wild mushrooms from Bosnia and Herzegovina. Asian J. Pharm. Res. Health Care 2021, 13, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Resz, M.-A.; Torok, I.; Senila, L. Nutritional composition and health risk of toxic metals of some edible wild mushrooms growing in a mining area of Apuseni Mountains, Western Carpathians. J. Food Compos. Anal. 2024, 128, 106061. [Google Scholar] [CrossRef]
- Sesli, E.; Tuzen, M. Micro- and macroelement contents of edible wild growing mushrooms in Artvin province of Turkey. ASIAN J. Chem. 2006, 18, 1423–1429. [Google Scholar]
- Zavastin, D.E.; Biliută, G.; Dodi, G.; Macsim, A.-M.; Lisa, G.; Gherman, S.P.; Breabăn, I.G.; Miron, A.; Coseri, S. Metal content and crude polysaccharide characterization of selected mushrooms growing in Romania. J. Food Compos. Anal. 2018, 67, 149–158. [Google Scholar] [CrossRef]
- Bak, K.H.; Bauer, S.; Rattner, J.; Wagner, M.; Ludewig, M. Nutritional Properties, Microbial and sensory quality, and formation of biogenic amines in wild-grown mushrooms (Cantharellus cibarius & Boletus edulis) from Austrian local markets. Food Chem. Adv. 2023, 2, 100193. [Google Scholar] [CrossRef]
- Bauer-Petrovska, B.; Jordanoski, B.; Stefov, V.; Kulevanova, S. Investigation of dietary fibre in some edible mushrooms from Macedonia. Nutr. Food Sci. 2001, 31, 242–246. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Sobieralski, K.; Magdziak, Z.; Golinski, P. Accumulation of elements by edible mushroom species II. A comparison of aluminium, barium and nutritional element contents. J. Environ. Sci. Health B 2013, 48, 308–317. [Google Scholar] [CrossRef]
- Saarivirta, M.; Kreula, M. The contents of water-insoluble dietary fibre in Finnish berries and mushrooms. A preliminary study. Z. Lebensm. Unters. Forsch. 1979, 169, 88–89. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.M.; Antonio, A.L.; Santos, P.M.P.; Martins, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Study of chemical changes and antioxidant activity variation induced by gamma-irradiation on wild mushrooms: Comparative study through principal component analysis. Food Res. Int. 2013, 54, 18–25. [Google Scholar] [CrossRef]
- Kalyoncu, F.; Ergönül, B.; Yildiz, H.; Kalmi, E.; Solak, M.H. Chemical composition of four wild edible mushroom species collected from southwest Anatolia. Gazi Univ. J. Sci. 2010, 23, 375–379. [Google Scholar]
- Kaya, A.; Bag, H. Trace element contents of edible macrofungi growing in Adiyaman, Turkey. ASIAN J. Chem. 2010, 22, 1515–1521. [Google Scholar]
- Kostić, M.; Smiljkovic, M.; Petrovic, J.; Glamoclija, J.; Barros, L.; Ferreira, I.C.F.R.; Ciric, A.; Sokovic, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Yildiz, D.; Akata, I.; Tepe, B. Evaluation of the metal concentrations of wild mushroom species with their health risk assessments. Environ. Sci. Pollut. Res. 2021, 28, 21437–21454. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Afiukwa, C.A.; Okechukwu, P.C.; Okoli, S.O.; Idenyi, J.N.; Emmanuel, C. Contents of some vitamins in five edible mushroom varieties consumed in Abakaliki metropolis, Nigeria. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 805–812. [Google Scholar]
- Akyüz, M.; Kirbağ, K. Nutritive value of edible wild and cultured mushrooms. Turk. J. Biol. 2010, 34, 97–102. [Google Scholar] [CrossRef]
- Rugolo, M.; Mascoloti Spréa, R.; Dias, M.I.; Pires, T.C.S.P.; Añibarro-Ortega, M.; Barroetaveña, C.; Caleja, C.; Barros, L. Nutritional composition and bioactive properties of wild edible mushrooms from native Nothofagus patagonian forests. Foods 2022, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, P. In-vitro antioxidant activity and nutritional value of four wild oyster mushroom collected from north-eastern part of Uttar Pradesh. Mycosphere 2017, 8, 592–602. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Fan, W.; Qiao, M.; Hao, H.; Wang, X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan province, China. Environ. Monit. Assess. 2011, 179, 191–199. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical composition of five wild edible mushrooms collected from southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Radović, J.; Leković, A.; Tačić, A.; Dodevska, M.; Stanojković, T.; Marinković, T.; Jelić, Č.; Kundakovic-Vasović, T. Black trumpet, Craterellus Cornucopioides (L.) Pers.: Culinary mushroom with angiotensin converting enzyme inhibitory and cytotoxic activity. Pol. J. Food Nutr. Sci. 2022, 72, 171–181. [Google Scholar] [CrossRef]
- Kula, İ.; Solak, M.H.; Uğurlu, M.; Işıloğlu, M.; Arslan, Y. Determination of mercury, cadmium, lead, zinc, selenium and iron by ICP-OES in mushroom samples from around thermal power plant in Muğla, Turkey. Bull. Environ. Contam. Toxicol. 2011, 87, 276–281. [Google Scholar] [CrossRef]
- Coli, R.; Coli, A.M.; Granetti, B.; Damiani, P. The nutritional value and protein quality of the carpophores of Boletus aereus, Boletus edulis, Boletus pinicola and Boletus reticulatus. Ann. Della Fac. Agrar. Univ. Degli Studi Perugia 1988, 42, 873–898. [Google Scholar]
- Dimitrijevic, M.V.; Mitic, V.D.; Cvetkovic, J.S.; Stankov Jovanovic, V.P.; Mutic, J.J.; Nikolic Mandic, S.D. Update on element content profiles in eleven wild edible mushrooms from family Boletaceae. Eur. Food Res. Technol. 2016, 242, 1–10. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.M.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Feasibility of electron-beam irradiation to preserve wild dried mushrooms: Effects on chemical composition and antioxidant activity. Innov. Food Sci. Emerg. Technol. 2014, 22, 158–166. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Targeted metabolites analysis in wild Boletus species. LWT—Food Sci. Technol. 2011, 44, 1343–1348. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef]
- Lalotra, P. Bioaccumulation of heavy metals in the sporocarps of some wild mushrooms. Curr. Res. Environ. Appl. Mycol. 2016, 6, 159–165. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Li, Y.; Tang, Q.; Han, G.; Liu, A.; Feng, C.; Li, C.; et al. Nutritional composition of Boletus mushrooms from southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 2016, 211, 83–91. [Google Scholar] [CrossRef]
- Malinowski, R.; Sotek, Z.; Stasińska, M.; Malinowska, K.; Radke, P.; Malinowska, A. Bioaccumulation of macronutrients in edible mushrooms in various habitat conditions of NW Poland—Role in the Human Diet. Int. J. Environ. Res. Public. Health 2021, 18, 8881. [Google Scholar] [CrossRef]
- Mena-García, M.; Vanessa-Branco, P.; Dominguez-Olloqui, N.; Fernández-García, D.; Combarros-Fuertes, P.; Miranda-Estevinho, L.; González-Árias, L.; Renes-Bañuelos, E.R.; Fresno-Baro, J.M. Effect of different cooking methods on the total phenolic content, antioxidant activity and sensory properties of wild Boletus edulis mushroom. Int. J. Gastron. Food Sci. 2021, 26, 100416. [Google Scholar] [CrossRef]
- Nikkarinen, M.; Mertanen, E. Impact of geological origin on trace element composition of edible mushrooms. J. Food Compos. Anal. 2004, 17, 301–310. [Google Scholar] [CrossRef]
- Sotek, Z.; Stasińska, M.; Malinowski, R.; Pilarczyk, B.; Pilarczyk, R.; Bąkowska, M.; Malinowska, K.; Radke, P.; Kubus, M.; Malinowska, A.; et al. The role in the human diet of bioaccumulation of selenium, copper, zinc, manganese and iron in edible mushrooms in various habitat conditions of NW Poland—A case study. Sustainability 2023, 15, 13334. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Bao, C.; Zhuang, Y. Metal contents, bioaccumulation, and health risk assessment in wild edible Boletaceae mushrooms. J. Food Sci. 2017, 82, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Zhang, J.; Li, T.; Li, J.-Q.; Wang, Y.-Z.; Liu, H.-G. Variations in element levels accumulated in different parts of Boletus edulis collected from central Yunnan Province, China. J. Chem. 2015, 2015, 372152. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, J.; Li, T.; Wang, Y.-Z.; Liu, H.-G. Content and bioaccumulation of nine mineral elements in ten mushroom species of the genus Boletus. J. Anal. Methods Chem. 2015, 2015, 165412. [Google Scholar] [CrossRef] [PubMed]
- Frankowska, A.; Ziolkowska, J.; Bielawski, L.; Falandysz, J. Profile and bioconcentration of minerals by king bolete (Boletus edulis) from the Pbocka Dale in Poland. Food Addit. Contam. Part B Surveill. 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Mikolajczak, P.; Golinski, P.; Gasecka, M.; Sobieralski, K.; Dawidowicz, L.; Szymanczyk, M. Bioaccumulation of elements in three selected mushroom species from southwest Poland. J. Environ. Sci. Health B 2015, 50, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, J.; Li, J.; Li, T.; Liu, H.; Wang, Y. Determination of mineral contents of wild Boletus edulis mushroom and its edible safety assessment. J. Environ. Sci. Health B 2018, 53, 454–463. [Google Scholar] [CrossRef]
- Glamočlija, J.; Stojković, D.; Nikolić, M.; Ćirić, A.; Reis, F.S.; Barros, L.; Ferreira, I.C.F.R.; Soković, M. A comparative study on edible Agaricus mushrooms as functional foods. Food Funct. 2015, 6, 1900–1910. [Google Scholar] [CrossRef]
- Petrović, J.; Fernandes, Â.; Stojković, D.; Soković, M.; Barros, L.; Ferreira, I.; Shekhar, A.; Glamočlija, J. a step forward towards exploring nutritional and biological potential of mushrooms: A case study of Calocybe gambosa (Fr.) Donk wild growing in Serbia. Pol. J. Food Nutr. Sci. 2022, 72, 17–26. [Google Scholar] [CrossRef]
- Piearce, G.D.; Francis, B.J. Nutritive Potential of the edible mushroom Suillus granulatus (Fries) O. Kuntze, and its utilization prospects in Zambia. Trop. Sci. 1982, 24, 157–164. [Google Scholar]
- Reis, F.S.; Stojković, D.; Barros, L.; Glamočlija, J.; Ćirić, A.; Soković, M.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Can Suillus granulatus (L.) Roussel be classified as a functional food? Food Funct. 2014, 5, 2861–2869. [Google Scholar] [CrossRef]
- Busuioc, G.; Elekes, C.C.; Stihi, C.; Iordache, S.; Ciulei, S.C. The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ. Sci. Pollut. Res. 2011, 18, 890–896. [Google Scholar] [CrossRef]
- Elekes, C.C.; Busuioc, G.; Ionita, G. The bioaccumulation of some heavy metals in the fruiting body of wild growing mushrooms. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 147–151. [Google Scholar]
- Zeng, X.; Suwandi, J.; Fuller, J.; Doronila, A.; Ng, K. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 2012, 18, 367–379. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens Metropolitan Area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Van Griensven, L.J.I.D.; Soković, M.; Ferreira, I.C.F.R. Nutrients and non-nutrients composition and bioactivity of wild and cultivated Coprinus comatus (O.F.Müll.) Pers. Food Chem. Toxicol. 2013, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. study and characterization of selected nutrients in wild mushrooms from Portugal by gas chromatography and high performance liquid chromatography. Microchem. J. 2009, 93, 195–199. [Google Scholar] [CrossRef]
- Álvarez-Puig, C. Etnobotànica d’Andorra: Saber Popular i Vegetals. Bachelor’s Thesis, Universitat de Barcelona, Barcelona, Spain, 2021. [Google Scholar]
- Gras, A.; Garnatje, T.; Marín, J.; Parada, M.; Sala, E.; Talavera, M.; Vallès, J. The power of wild plants in feeding humanity: A meta-analytic ethnobotanical approach in the Catalan linguistic area. Foods 2021, 10, 61. [Google Scholar] [CrossRef]
- Kang, Y.; Łuczaj, Ł.; Kang, J.; Zhang, S. Wild food plants and wild edible fungi in two valleys of the Qinling Mountains (Shaanxi, Central China). J. Ethnobiol. Ethnomed. 2013, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł.; Lamxay, V.; Tongchan, K.; Xayphakatsa, K.; Phimmakong, K.; Radavanh, S.; Kanyasone, V.; Pietras, M.; Karbarz, M. Wild food plants and fungi sold in the markets of Luang Prabang, Lao PDR. J. Ethnobiol. Ethnomed. 2021, 17, 6. [Google Scholar] [CrossRef]
- Martínez-de Aragón, J.; Colinas, C. Producción y aprovechamiento de Lactarius sanguifluus (Paulet ex Fr.) Fr. y Lactarius deliciosus Fr. en los pinares del Prepirineo catalán. In Proceedings of the IV Congreso Forestal Español, Zaragoza, Spain, 26–30 November 1997; Sociedad Española de Ciencias Forestales (SECF): Madrid, Spain, 2005. Available online: https://secforestales.org/publicaciones/index.php/congresos_forestales/article/view/16294 (accessed on 14 August 2025).
- Kalač, P. Proximate composition and nutrients. In Edible Mushrooms; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 7–69. ISBN 978-0-12-804455-1. [Google Scholar]
- Farran, A.; Zamora, R.; Cervera, P. Centre d’Ensenyament Superior de Nutrició i Dietètica (CESNID). In Tablas de Composición de Alimentos del CESNID. Taules de Composició D’aliments del CESNID; Edicions UB: Barcelona, Spain, 2004; ISBN 84-8338-457-4. [Google Scholar]
- Codex Alimentarius Commission. Guidelines for Use of Nutrition and Health Claims; FAO/WHO: Rome, Italy, 2013. [Google Scholar]
- Kalač, P. Nutritional value of wild growing mushrooms. Vyziv. Potravin. 2013, 68, 67–69. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Chowaniak, M.; Niemiec, M.; Paluch, Ł. Bioconcentration of cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) in Lactarius Salmonicolor in the Western Carpathians. J. Elem. 2017, 22, 1537–1547. [Google Scholar] [CrossRef]
- Kalač, P. A Review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.D.C.; Tardío, J. (Eds.) Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3327-3. [Google Scholar]
- Wakchaure, G.C. Production and marketing of mushrooms: Global and national scenario. In Mushrooms-Cultivation, Marketing and Consumption; Directorate of Mushroom Research; Singh, M., Vijay, B., Kamal, S., Eds.; ICAR: Solan, India, 2011; p. 266. [Google Scholar]
- The Periodic Table of Food Initiative (PTFI). Available online: https://foodperiodictable.org (accessed on 29 June 2025).

| Taxa | BR | Vernacular Names (In Catalan, Unless Another Language Is Indicated) | Macro-Nutrients | Micro-Nutrients | Num. Art. | References |
|---|---|---|---|---|---|---|
| Lactarius sanguifluus (Paulet) Fr. | 115 | Bolet de sang, esclata-sang, esclata-sang blanc, esclata-sang de migjornet, esclata-sang de murta, esclata-sang de pi, esclata-sang de sivina, esclata-sang de xipell, esclata-sang d’hivern, esclata-sang mascle, esclata-sang murta, esclata-sang sanguinós, esteper, gallufer, lleterola, mare del rovelló, pebràs, pebre, pinatell, putifler, rovelló, rovelló blanc, rovelló de llistó, rovelló de plana, rovelló de sang, rovelló de solell, rovelló d’espígol, rovelló esclata-sang, rovelló vinader, rovelló vinagrer, seta (Spanish), vinader | 🞬 | 🞬 | 7 | [47,48,49,50,51,52,53] |
| Macrolepiota procera (Scop.) Singer | 110 | Apagallums, bolet de frare, calceta, cama-sec, cama-seca, capell de senyor, cogoma, cogombre, cogomella, cogomella vera, cohoma, coloma, farinosa, maneta, massa, mazza di tamburo (Italian), paloma, pamperol, pampinella, paraigua, para-sol, pimpinella, pimpinella farinosa, pota d’ase, senyal d’alzina, sombrilla (Spanish) | 🞬 | 🞬 | 33 | [49,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] |
| Amanita caesarea (Scop.) Pers. | 108 | Bolet d’or, cocou, monjola, oriol, oronja, ou de monjola, ou de rei, ou de reig, quicou, rei, reig, rovell d’ou | 🞬 | 🞬 | 4 | [72,86,87,88] |
| Marasmius oreades (Bolton) Fr. | 108 | Cama-sec, cama-sec de prat, caramanyola, carmanyola, carrereta, carrerola, carretera, correjola, correola (spanish), correrola, corretjola, corriola, corrioleta, esclata-sang caminer, fals moixerdó, fals moixernó, moixeriga, moixernó, moixina, paraigüet | 🞬 | 🞬 | 10 | [54,66,76,79,80,85,89,90,91,92] |
| Tricholoma terreum (Schaeff.) P. Kumm. | 107 | Bolet de rosada, bolet negre, brunet, bruneta, esteperol, fraret, fredeluc, fredolí, fredolic, fredolic mascle, fredolís, fredoluc, gírgola d’estepa, gírgola d’estèpera, griset, moreneta, morro d’ovella, negrentí, negret, negric, negrito (Spanish), orella d’estèpera, palometa | 🞬 | 🞬 | 10 | [75,77,83,92,93,94,95,96,97,98] |
| Lactarius deliciosus (L.) Gray | 102 | Bolet comestible, esclata-sang, esclata-sang de bruc, esclata-sang de bruc femella, esclata-sang foraster, esclata-sang vermell, esteper, paratge, peratxe, pinatell, pinenc, pinenca, pinetell, pinetenc, pinetenca, rodelló, rovelló, rovelló de boixera, rovelló de bruc, rovelló de llicorella, rovelló de monte, rovelló d’obaga, rovelló d’ombria, rovelló pinetell, rovellola, vermell | 🞬 | 🞬 | 30 | [47,48,49,50,51,52,53,54,57,69,70,78,79,80,81,83,85,92,95,96,97,99,100,101,102,103,104,105,106,107] |
| Morchella esculenta (L.) Pers. | 94 | Aligany, aragall, arigany, brújola, greixot, marúgola, mírgola rossa, morga, morúgola, murga, murga de campana, murga de cap rodó, múrgara, múrgola, múrgola cònica, múrgola de cap rodó, múrgola de rec, múrgola de ribera, múrgola fosca, múrgola grisa, múrgola rossa, murguela grisa, murguela rossa, múrmola, múrmola blanca, múrmola de freixe, múrmola rossa, rabassola, vírgula | 🞬 | 🞬 | 12 | [49,52,66,76,83,96,108,109,110,111,112,113] |
| Hygrophorus russula (Schaeff. ex Fr.) Bataille | 93 | Bolet d’alzina, cabrit roig, carlet, carlet de mura, carlet vermell, carlí, carlista, carló, carlot, cruelda, escarlet, escarlet ver, escarlet vermell, escarlot, escarlot vermell, llenega vermella, rovelló d’alzina, vermella, vinassa, vinós, vinosa | 🞬 | 🞬 | 2 | [87,114] |
| Cantharellus cibarius Fr. | 89 | Agerola, cama-seca, cama-seca de mata, carn de gallina, cresta de gall, espicatornell, gerola, ginesterola, girola, girolle (French), picanell, picornell, picornell d’ullastre, picornell foraster, rossinyol, rossinyol comú, torrendó, torrentó, vaqueta | 🞬 | 🞬 | 32 | [52,54,58,66,72,74,76,79,80,81,85,86,87,88,91,103,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] |
| Craterellus lutescens (Fr.) Fr. | 87 | Cama tarongina, camagroc, camagroga, cama-roja de bruc, cama-seca de bruc, cama-seca d’hivern, ginesterola de pi, indi, misto, moixernó de bosc, peu de rei, picornell de càrritx, picornell de pi, pota de perdiu, rossinyol de pi, rossinyolic, rossinyolic camagroc, sip, tarongina, trompeta, vaqueta de pi | 🞬 | 🞬 | 1 | [103] |
| Hydnum repandum L. | 84 | Agulla, agulles, agulleta, agulletes, blanquet, boïna, boixet, bolet de prat, buina, dent de rata, llémena, llengo de bou, llengua boïna, llengua bovina, llengua de bou, llengua de vaca, llengua de vedella, llengüeta, peu de cabrit, peu tort, picaronell, picarronell, picornell, picornell blanc, picornell pelut, pied-de-mouton (French), punxeguda, vaquet, vaqueta | 🞬 | 🞬 | 15 | [52,54,55,69,72,85,92,98,102,103,118,123,125,130,131] |
| Armillaria mellea (Vahl) P. Kumm. | 82 | Alzinó, alzinoi, aulinell, bolet d’ametler, bolet de roure, bolet de soca, bolet de soca d’alzina, bolet de soca d’aulina, cama-sec de soca, cama-seca de soca, flora d’arbre, flota d’alzina, flota d’arbre, flota de noguera, flota de pollancre, flota de roure, flotona, gírbola d’alzina, gírbola d’aulina, gírbola de roure, gírgola d’alzina, mata de roure, olinell, pollancó, pollancró, pollarenca, polletó, rourenc, rouró, socada d’alzina | 🞬 | - | 20 | [55,59,77,78,79,81,85,87,91,97,98,114,118,125,126,132,133,134,135,136] |
| Pleurotus ostreatus (Jacq.) P. Kumm. | 79 | Auriana, bolet de ventall, clopí, clopissa, clopissó, flora d’orella, flota d’arbre, giragola, gírbola, gírbola d’arbre, gírgola, gírgola d’arbre, gírgola de beç, gírgola de poll, gírgola de pollancre, orella, orellana, orellana comuna, orellana d’arbre, orellana de poll, orellana de polla, orellana de pollancre, orelleta, oriana de polla, pollancró, vimequer | 🞬 | 🞬 | 22 | [53,54,58,66,76,77,78,83,84,90,93,96,97,98,109,120,133,137,138,139,140,141] |
| Craterellus cornucopioides (L.) Pers. | 73 | Alzinoia, corn, corn de l’abundància, corneta, orella d’ase, orella de burro, orella de ruc, rossinyol negra, rossinyol negre, trompeta, trompeta de la mort, trompeta de perdiu, trompeta dels morts, trompeta negra, ull de perdiu, vaqueta negra | 🞬 | 🞬 | 13 | [58,67,72,78,79,85,91,103,118,119,130,142,143] |
| Chroogomphus rutilus (Schaeff.) O.K. Mill. | 70 | Bec de perdiu, bitxac, cabridet, cama de perdiu, cama-roja, carnero (Spanish), fetge, fetget, pebrasset de moro, peu de perdiu, pota de perdiu, ull de perdiu | - | 🞬 | 4 | [72,90,97,144] |
| Boletus edulis Bull. | 69 | Bolet de bou, bolet de porcino (Italian), bolet porcí, cep, cep de bordeus, cepa, cèpe (French), ceperó, cigró, corball blanc, porcino (Italian), siureny, siureny de baga, siurenya, siuró, siurol, surenc, sureny, sureny de baga, trompellot | 🞬 | 🞬 | 47 | [52,54,55,58,59,65,68,69,70,73,75,77,79,80,81,84,85,86,105,109,119,120,123,124,125,126,127,128,129,131,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161] |
| Agaricus campestris L. | 61 | Bola de neu, bolet, bolet blanc, bolet blanc matós, bolet blanc salvatge, bolet de camí, bolet de porc, camperol, camperol comú, comaga, culblanc, fumat, gírgola blanca, pamperol, pexigà, rovellol, rubiol, rubirol, terrerol, terronenc, xampinyó, xampinyó de bosc, xampinyó silvestre | 🞬 | 🞬 | 15 | [49,54,58,59,66,67,69,73,76,79,88,90,112,133,162] |
| Boletus aereus Bull. | 61 | Cap de negre, cep, cep negre, cigró, mollerol, padrina, pixacà, siurac, siureny, siureny fosc, siuró, siurol, surenc, sureny, sureny fosc | 🞬 | 🞬 | 8 | [54,87,119,145,148,151,156,158] |
| Calocybe gambosa (Fr.) Donk | 60 | Bolet de Sant Jordi, cama-seca blanca, moixern de primavera, moixernó, moixernó de prat, moixernó de primavera, moixernó de Sant Jordi, moixernó ver, moixeró, moixeró blanc, moixeró de primavera, moixerró, moscardó, perretxico (Basque) | 🞬 | 🞬 | 5 | [58,97,128,136,163] |
| Infundibulicybe geotropa (Bull.) Harmaja | 59 | Barretet de frare, campana, candela, candela d’arboç, candela de bruc, caperons, frare, gírgola de bruc, moixernó de candela, moixernó de Sant Miquel, moixernó de tardor, moixeró de Sant Miquel, moixeró de tardor, orella de frare, orellana de bruc, pampa, porrosa, tassa de bruc | 🞬 | 🞬 | 3 | [71,89,132] |
| Hygrophorus latitabundus Britzelm. | 58 | Bateó, bavallosa, bavós, bavós rabassut, bavosa, cabrit, caramellosa, gírgola de llim, gírgola de mata, gomera, llenega, llenega negra, llenegall, mocosa, mocosa grisa, mocosa negra, pegalosa, senyoreta | - | - | 0 | - |
| Suillus granulatus (L.) Roussel | 57 | Bolet de bou, bolet de pi, bolet de vaca, cabreta, esponja, groguet, mataparent, moixí, moixí granellut, moixina, molleric, molleric granellut, molleric ver, mollic, pebrada, pinetell, pixacà, vaca, vacassa | 🞬 | 🞬 | 12 | [62,66,71,75,76,77,83,84,92,128,164,165] |
| Hypomyces lateritius (Fr.) Tul. and C. Tul. | 56 | Esclata-sang de tot l’any, esclata-sang mascle, esclata-sang putifler, mare de la pinenca, mare de rovelló, mare del pinetell, mare del rovelló, pinenca tofonera, pinetell boixat, pinetella, rovelló mascle, rovellola, rovellona, rovellonera, tofana | - | - | 0 | - |
| Russula cyanoxantha (Schaeff.) Fr. | 55 | Blava, blavet, blaveta, camadolça, cualbra, cualbra blava, cualbra llora, cualbra morada, cualgra llora, llaurell, llora, llora aspra, llora blava, llora carbonera, llora verda de peltereau, llorell, palomí, puagra, puagra llora, raurell, terradolça, terrandolça | 🞬 | 🞬 | 7 | [54,69,73,77,98,119,166] |
| Tricholoma equestre (L.) P. Kumm. | 54 | Camagroc, canari, carbonera groga, carboneta groga, carlet groc, escarlet groc, fredolic, fredolic groc, groguet, groguí, groguillo, negret, pixaconill, rentiscle, rosteta, taverniscle, verderol, xamardiscle, xavarnol, xaverniscle, xaverníscola | 🞬 | 🞬 | 4 | [49,54,102,167] |
| Lyophyllum decastes (Fr.) Singer | 53 | Agret, bolet de bruc, bolet de pila, carner, flota carnera, flota de bruc, flota de bruc cendrosa, flota de mòdega, flota d’olina, flotona de bruc, flotona de carbonera, gírbola de bruc, gírgola de bruc, gírgola de mòdega, gírgola d’estepa, moixernó d’estepa, pom de terra, soca de bruc, soca de carnera | - | 🞬 | 3 | [130,141,144] |
| Suillus luteus (L.) Roussel | 52 | Bolet de bou, bolet de vaca, mataparent anellat, moix, moixí, moixí de calceta, molleric, molleric calçat, molleric de calceta, mollic, pinatell de calçeta, pinetell, pinetell de calceta, vaca | 🞬 | 🞬 | 15 | [49,66,76,81,83,85,90,95,96,97,104,106,129,133,168] |
| Lycoperdon perlatum Pers. | 51 | Bufa, bufa de bou, bufa de jai, bufa de jaia, bufa de monja, bufa del dimoni, esclatabufa, fum de terra, fumosa, llufa de llop, pet de bou, pet de ca, pet de frare, pet de llop, pet de llop perlat, pet de monja, pet de moro, pet de vella, pet del diable | 🞬 | 🞬 | 11 | [61,66,76,78,79,81,91,116,118,135,139] |
| Coprinus comatus (O.F. Müll.) Pers. | 50 | Aglà d’alzina, bolet de femer, bolet de fems, bolet de merda, bolet de tinta, bolet negre, coprí menut, coprí pelut, paraigua, pixacà barbut | 🞬 | 🞬 | 13 | [49,54,61,69,85,90,92,97,133,136,141,169,170] |
| Russula delica Fr. | 50 | Blanqueta, blava, bolet blanc, bolet fort, campanilla (Spanish), cogoma, cualbra blanca, esclata-sang blanc, esclata-sang d’alzina, forta, llora blanca, pebràs, pebràs comú, pebràs ver, pebrassa blanca, pebrotasso, terlandòs, terrandòs | 🞬 | 🞬 | 11 | [47,50,66,76,89,97,98,125,144,147,171] |
| Food Components | Units | BR | Range |
|---|---|---|---|
| Energy kJ | kJ (original)/100 g DM 1 | 52 | 74.07–1736.59 |
| Energy kcal | kcal (original)/100 g DM | 194 | 20.38–700.96 |
| Water/Moisture | g/100 g FM 2 | 306 | 2.17–96.60 |
| Protein: total | g/100 g DM | 423 | 1.21–83.40 |
| Fat: total | g/100 g DM | 331 | 0.09–89.70 |
| Carbohydrates: available | g/100 g DM | 338 | 0.85–89.80 |
| Fibre: total dietary | g/100 g DM | 98 | 0.28–83 |
| Insoluble fibre | g/100 g DM | 57 | 3.92–50.60 |
| Soluble fibre | g/100 g DM | 23 | 0.75–24 |
| Ash | g/100 g DM | 366 | 0.01–38.90 |
| Ca | mg/100 g DM | 518 | 0–19,100 |
| Cu | mg/100 g DM | 753 | 0–340 |
| Fe | mg/100 g DM | 682 | 0–15,100 |
| K | mg/100 g DM | 489 | 0.25–32,789.17 |
| Mg | mg/100 g DM | 528 | 0.01–9180 |
| Mn | mg/100 g DM | 619 | 0–315.07 |
| Na | mg/100 g DM | 460 | 0–16,100 |
| P | mg/100 g DM | 352 | 0.03–782,000 |
| Se | mg/100 g DM | 247 | 0–450 |
| Zn | mg/100 g DM | 772 | 0–740 |
| Al | µg/100 g DM | 232 | 160–1,510,000 |
| As | µg/100 g DM | 134 | 0–280 |
| Cd | µg/100 g DM | 501 | 0.31–6,128,000 |
| Co | µg/100 g DM | 251 | 0.40–1,180,000 |
| Cr | µg/100 g DM | 356 | 0.16–20,300 |
| Ni | µg/100 g DM | 367 | 1–17,415 |
| Pb | µg/100 g DM | 456 | 2–1,050,000 |
| Thiamine | mg/100 g DM | 16 | 0.14–17.03 |
| Riboflavin | mg/100 g DM | 13 | 0.06–4.97 |
| Niacin | mg/100 g DM | 17 | 0.66–77.64 |
| Vitamin B6 | mg/100 g DM | 8 | 0–1.13 |
| Vitamin B12 | µg/100 g DM | 5 | 3.59–23.84 |
| Ascorbic acid (vitamin C) | mg/100 g DM | 76 | 0.33–5320 |
| Folate | µg/100 g DM | 2 | 87.10–159 |
| Vitamin A | µg/100 g DM | 6 | 0–38,360 |
| β-carotene | µg/100 g DM | 36 | 0.27–4835 |
| Vitamin D2 (ergosterol) | µg/100 g DM | 15 | 740–240,900 |
| Vitamin D3 (cholecalciferol) | µg/100 g DM | 6 | 2.07–1520 |
| Vitamin E (as total tocopherols) | µg/100 g DM | 75 | 0–47,900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Puig, C.; Casamartina, J.; Garnatje, T.; Niell, M.; Gras, A.; Vallès, J. Wild Edible Fungi in the Catalan Linguistic Area: A Scoping Review Linking Nutritional Value to Ethnomycology. Foods 2025, 14, 2897. https://doi.org/10.3390/foods14162897
Álvarez-Puig C, Casamartina J, Garnatje T, Niell M, Gras A, Vallès J. Wild Edible Fungi in the Catalan Linguistic Area: A Scoping Review Linking Nutritional Value to Ethnomycology. Foods. 2025; 14(16):2897. https://doi.org/10.3390/foods14162897
Chicago/Turabian StyleÁlvarez-Puig, Canòlich, Joan Casamartina, Teresa Garnatje, Manel Niell, Airy Gras, and Joan Vallès. 2025. "Wild Edible Fungi in the Catalan Linguistic Area: A Scoping Review Linking Nutritional Value to Ethnomycology" Foods 14, no. 16: 2897. https://doi.org/10.3390/foods14162897
APA StyleÁlvarez-Puig, C., Casamartina, J., Garnatje, T., Niell, M., Gras, A., & Vallès, J. (2025). Wild Edible Fungi in the Catalan Linguistic Area: A Scoping Review Linking Nutritional Value to Ethnomycology. Foods, 14(16), 2897. https://doi.org/10.3390/foods14162897






