Characterization and Exploration of the Flavor Profiles of Green Teas from Different Leaf Maturity Stages of Camellia sinensis cv. Fudingdabai Using E-Nose, E-Tongue, and HS-GC-IMS Combined with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Samples and Chemical Reagents
2.2. Electronic Tongue Sensory Analysis
2.3. Electronic Nose Sensory Analysis
2.4. HS-GC-IMS Analytical Method
2.5. Qualitative and Quantitative Analysis
2.6. Feature Selection and Extraction of Key Flavor Attributes in Green Teas of Different Leaf Maturity Stages
2.7. Data Analysis
3. Results and Discussion
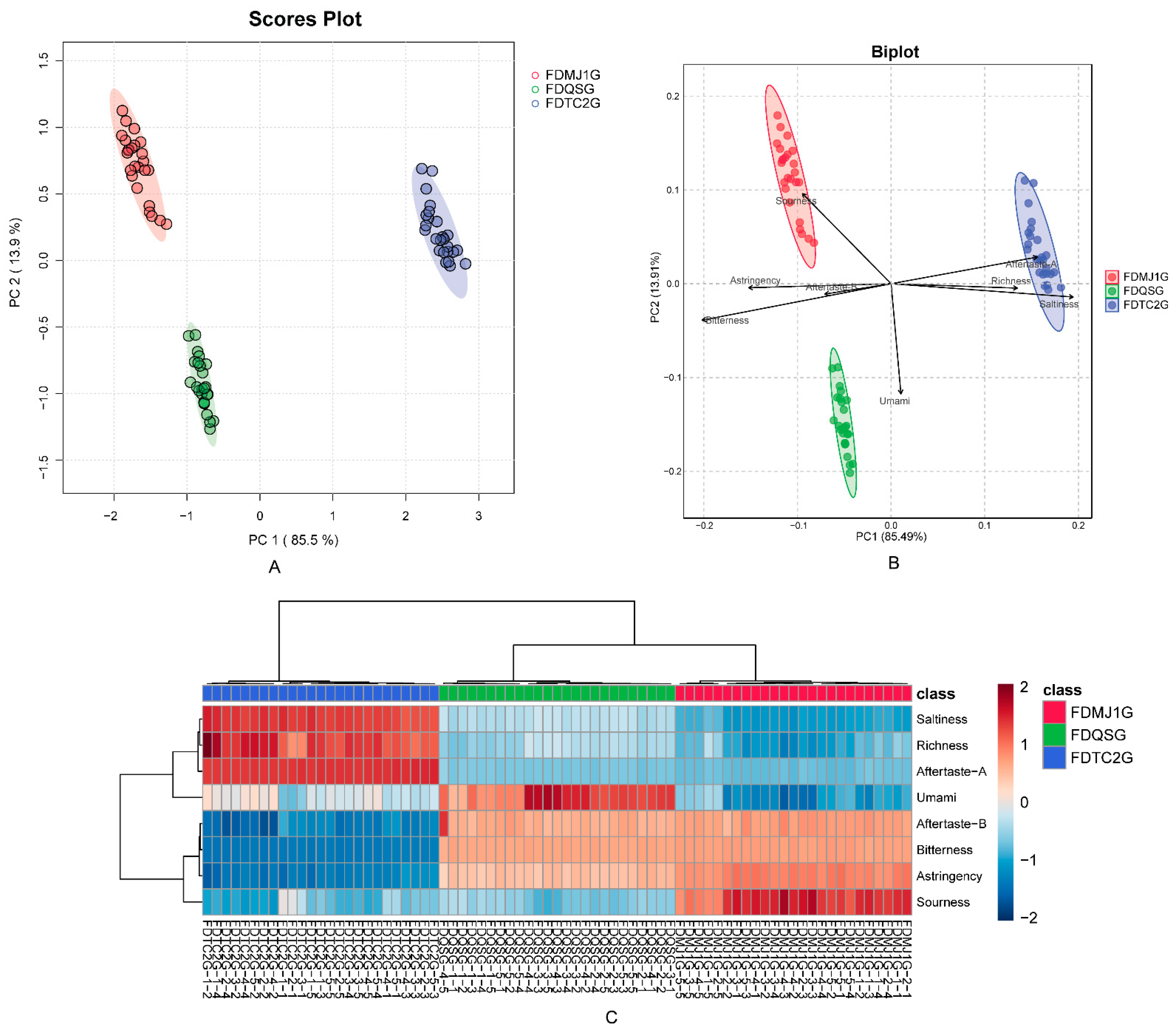
3.1. Electronic Tongue Analysis
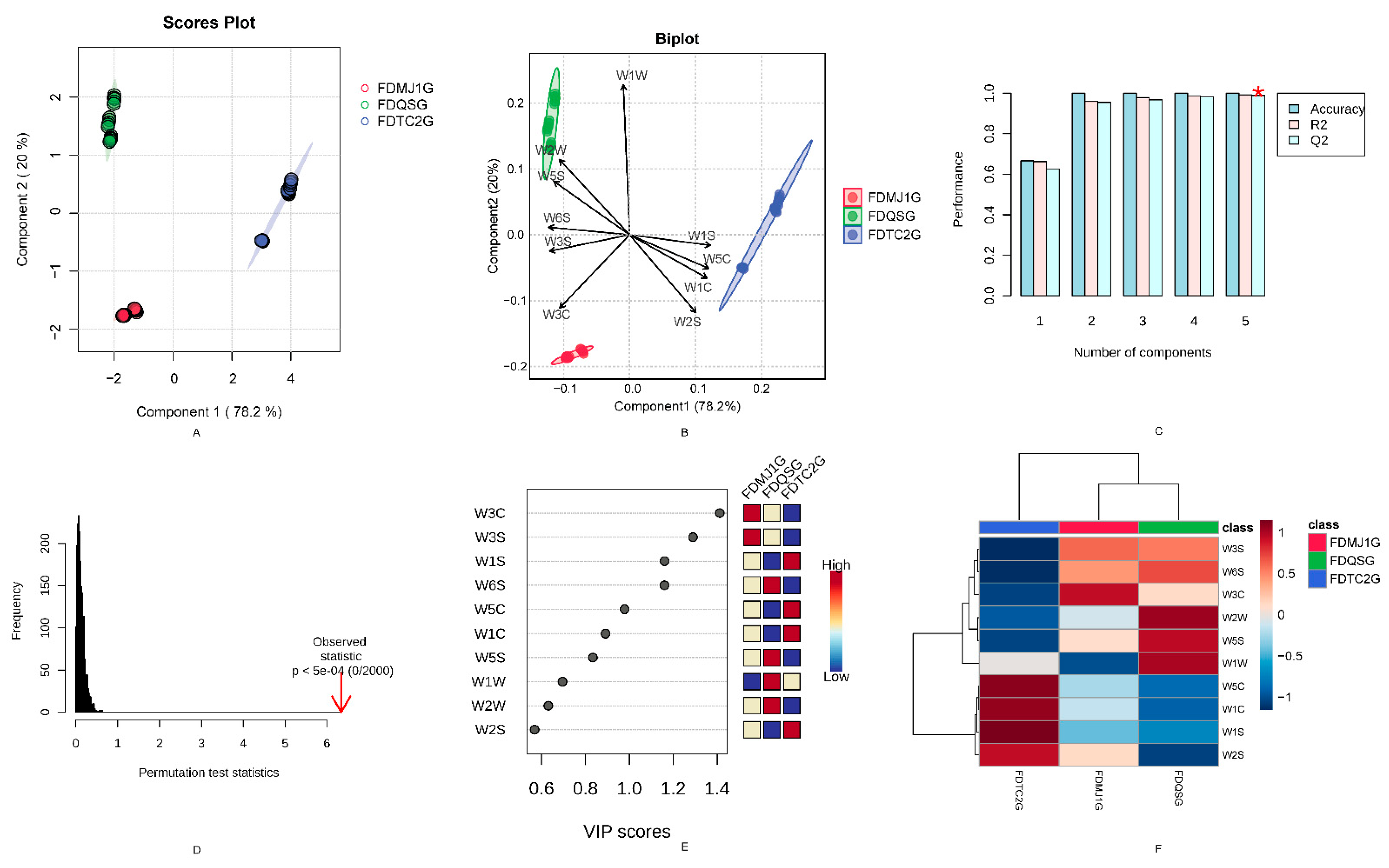
3.2. Electronic Nose Analysis
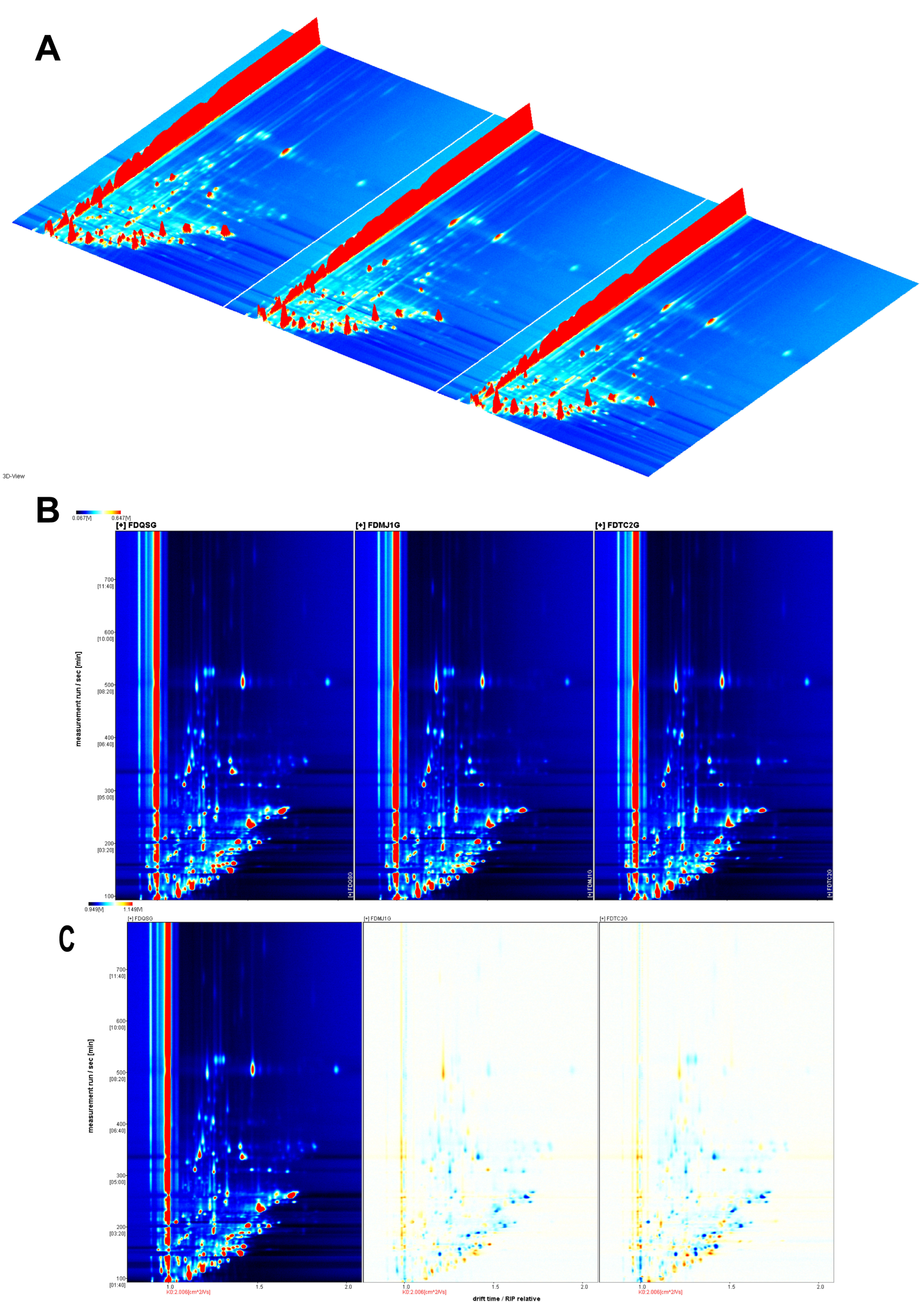
3.3. HS-GC-IMS Analysis
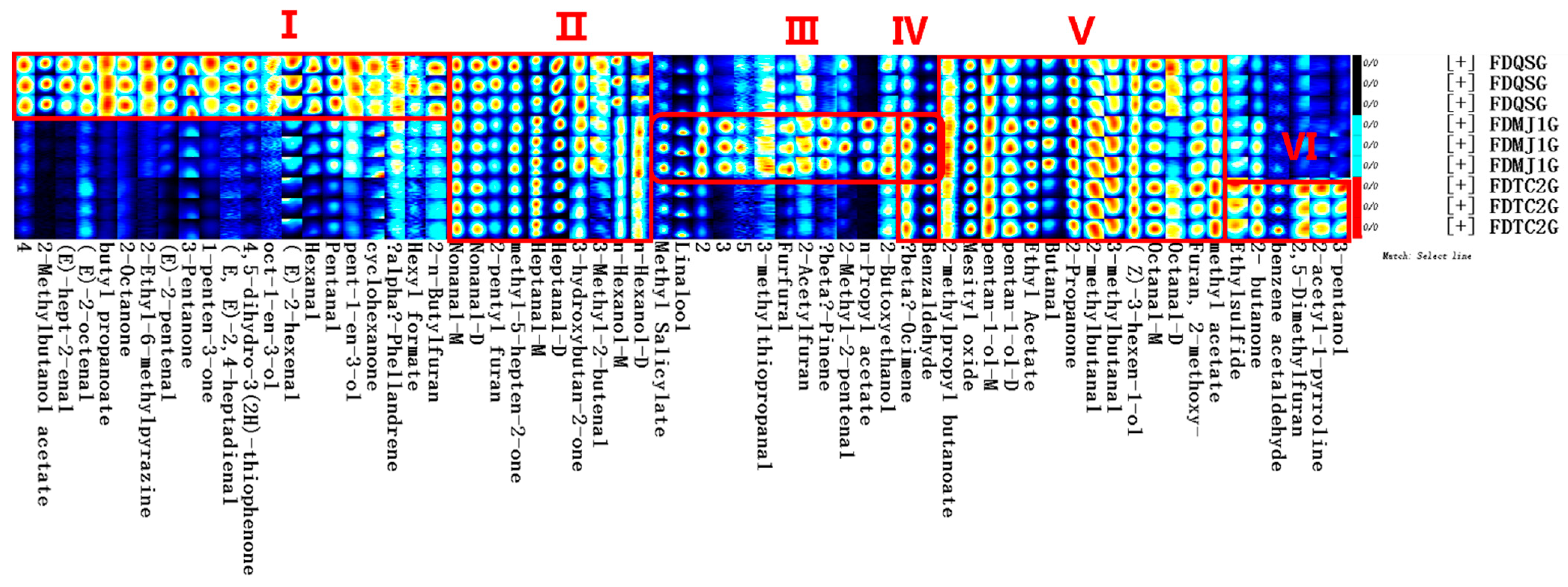
3.3.1. Qualitative and Quantitative VOC Profiling
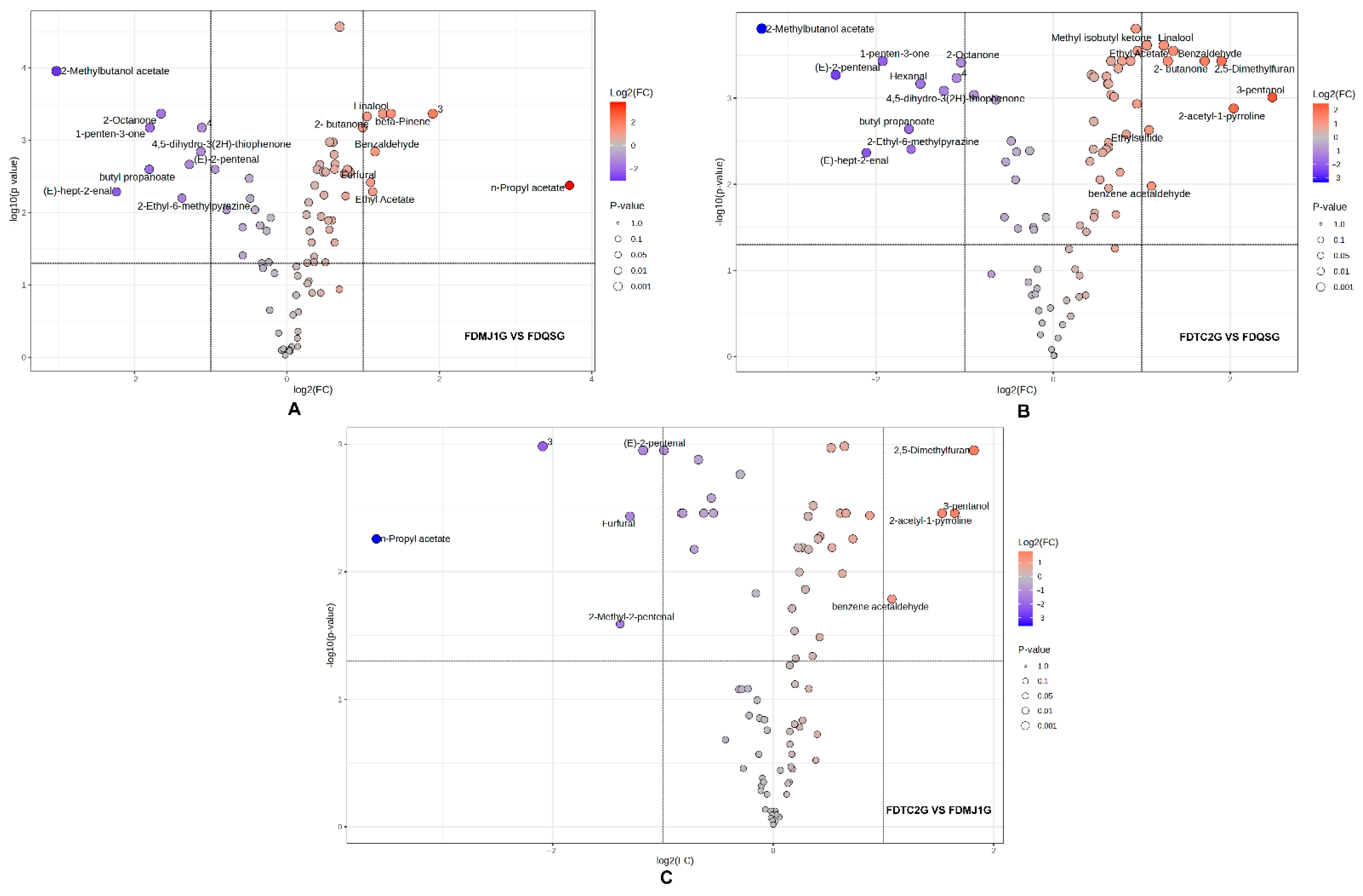
3.3.2. Analysis of VOCs Variation Among FDG of Different Tenderness
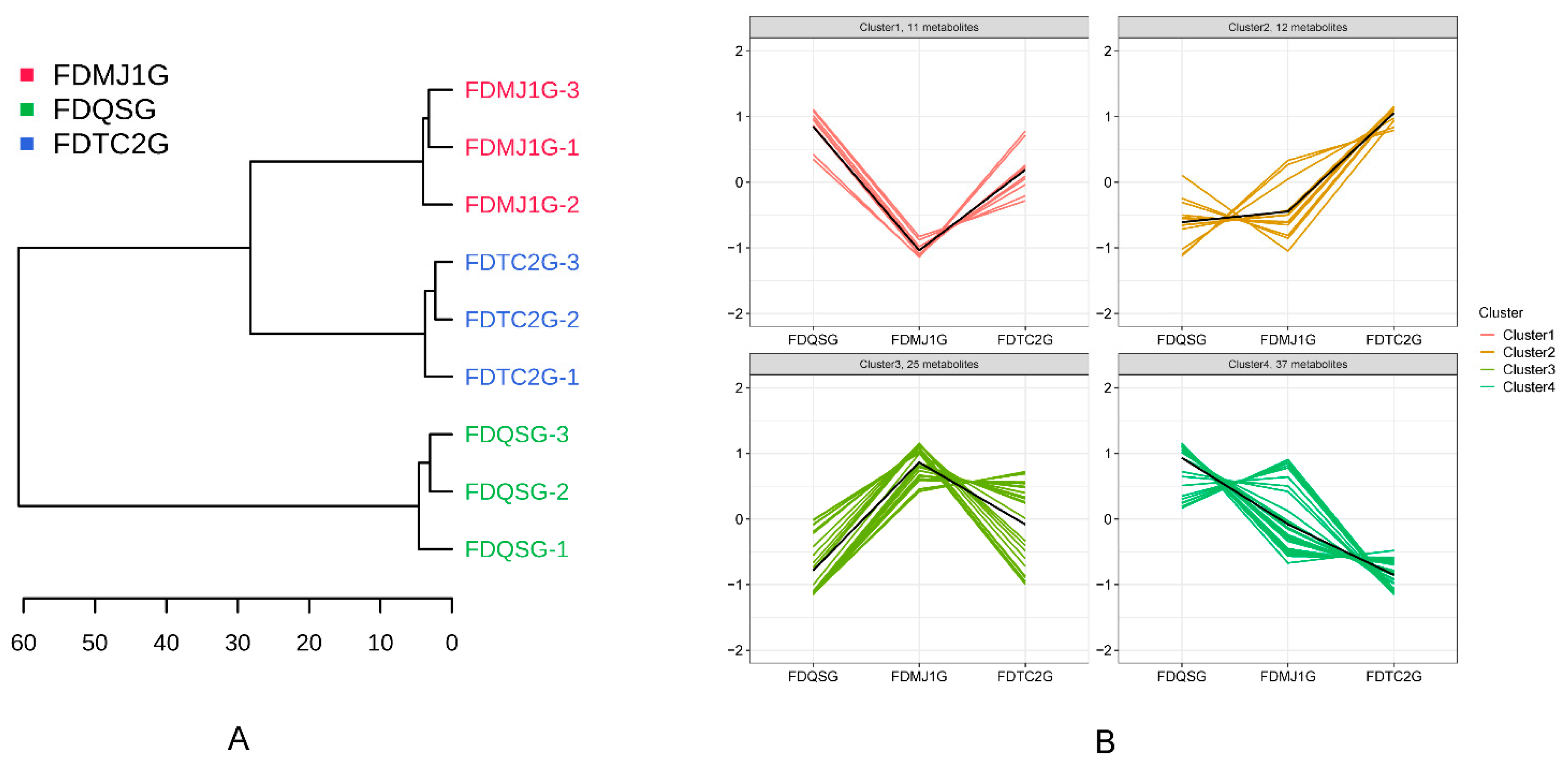
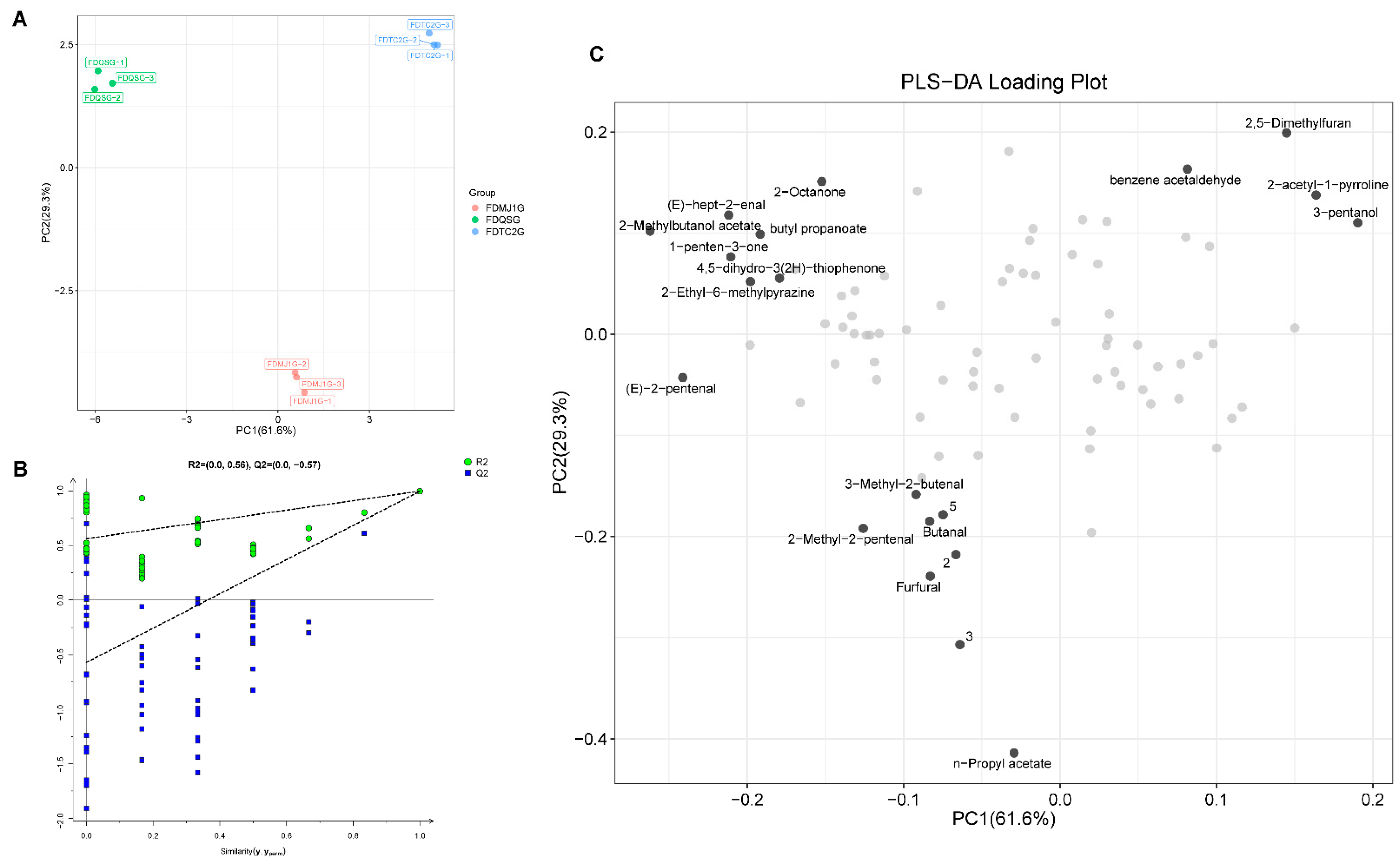
3.3.3. Multivariate Statistical Analysis
3.3.4. rOAV Analysis
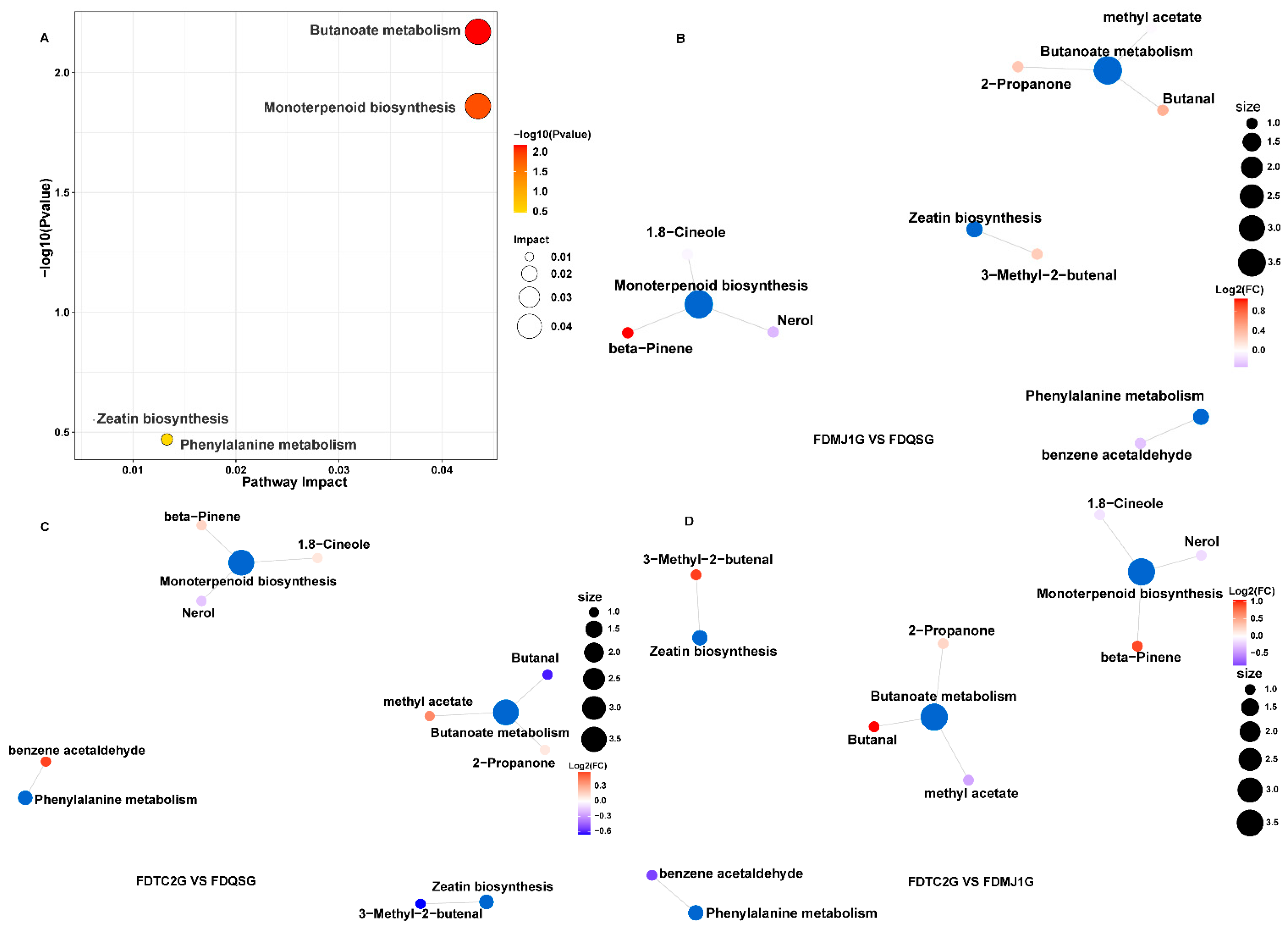
3.3.5. KEGG Functional Annotation and Enrichment of VOCs
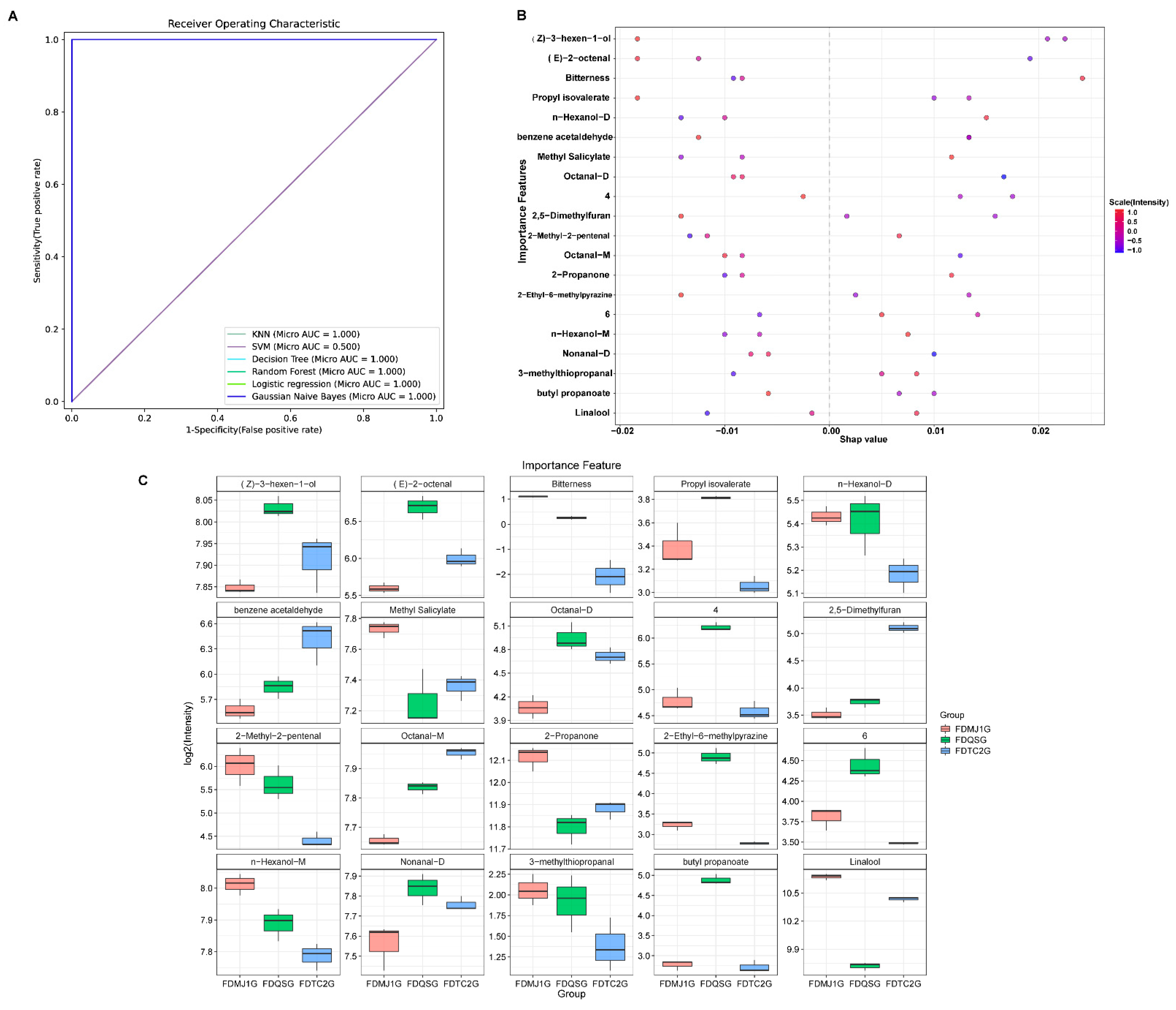
3.4. Identification of Key Flavor Markers of Green Teas of Different Tenderness Based on Six Machine Learning Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDG | Fudingdabai green tea |
| FDQSG | Fuding Que She |
| FDMJ1G | Fuding Maojian |
| FDTC2G | Fuding Taicha |
| ML | machine learning |
| RF | random forest |
| SVM | support vector machine |
| KNN | k-nearest neighbor |
| GNB | Gaussian naïve Bayes |
| LR | logistic regression |
| DT | decision tree |
| VOCs | volatile organic compounds |
| ROC | receiver operating characteristic |
| SHAP | Shapley Additive Explanations |
| AUC | the area under the receiver operating characteristic curve |
| rOAV | relative odor activity value |
References
- Zhou, X.; Hu, J.; Tang, J.; Wang, Q.; Jiang, Y.; Li, J.; Yuan, H.; Yang, Y. A multi-platform approach uncovers the effects of different shaping techniques on the sensory quality and non-volatile metabolites of green tea. Future Foods 2025, 11, 100675. [Google Scholar] [CrossRef]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef]
- Teixeira Oliveira, J.; Machado da Costa, F.; Gonçalvez da Silva, T.; Dotto Simões, G.; dos Santos Pereira, E.; Quevedo da Costa, P.; Andreazza, R.; Cavalheiro Schenkel, P.; Pieniz, S. Green tea and kombucha characterization: Phenolic composition, antioxidant capacity and enzymatic inhibition potential. Food Chem. 2023, 408, 135206. [Google Scholar] [CrossRef]
- Xiao, X.; Erukainure, O.L.; Guo, Y.; Msomi, N.Z.; Chu, M.; Islam, M.S. Jasmine green tea improves glucose homeostasis and antioxidant activities with concomitant hypolipidemic activity in type 2 diabetic rats. Food Biosci. 2025, 69, 107011. [Google Scholar] [CrossRef]
- Lan, S.; Wang, R.; Gao, H.; Zhao, M.; Li, H.; Liu, F.; Geng, R.; Wang, Y.; Liu, X.; Song, C.; et al. Anti-inflammatory and hepatoprotective effects of galactose-rich heteropolysaccharides from Laoshan green tea and its physicochemical properties. Nat. Prod. Res. 2025; 1–7, Advance online publication. [Google Scholar] [CrossRef]
- Schimidt, H.L.; Garcia, A.; Martins, A.; Mello-Carpes, P.B.; Carpes, F.P. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model. Food Res. Int. 2017, 100, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sahjlan, P.; Yadav, S.S. Phytochemistry and anticancer therapeutics of Camellia sinensis (Green tea). Pharmacol. Res. Mod. Chin. Med. 2024, 12, 100484. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bakhaat, G.A.; Tammam, H.G.; Mohamed, R.M.; El-Naggar, S.A. Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: Toxicological, histological and immunohistochemical studies. Biomed. Pharmacother. 2019, 113, 108731. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, S.; Zhou, C.; Tian, C.; Zhu, C.; You, X.; Chen, C.; Lai, Z.; Guo, Y. Analysis of the Genetic Diversity in Tea Plant Germplasm in Fujian Province Based on Restriction Site-Associated DNA Sequencing. Plants 2024, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, M.; Ning, M.; Liao, Y.; Du, X.; Qin, L.; Chen, W.; Liu, X.; Wu, A.; Feng, D.; et al. Cultivar-dependent variation in metabolomic profiles and sensory characteristics of Zhuyeqing green tea (Camellia sinensis). Food Biosci. 2025, 71, 107023. [Google Scholar] [CrossRef]
- Ma, C.; Ma, B.; Wang, J.; Wang, Z.; Chen, X.; Zhou, B.; Li, X. Geographical origin identification of Chinese white teas, and their differences in tastes, chemical compositions and antioxidant activities among three production regions. Food Chem. X 2022, 16, 100504. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Cai, M.; Zhang, X.; Feng, Z.; Zhang, Q.; Li, L.; Jin, G.; Fan, S.; Lu, L. Impact of spreading time on flavor quality in Duyun Maojian summer green tea. LWT 2024, 214, 117103. [Google Scholar] [CrossRef]
- Xu, S.; Song, L.; Shi, D.; Wu, S.; Ma, F.; Chen, H.; Meng, Q.; Fei, Q.; Meng, L.; Wu, W.; et al. Dynamic variations in flavor profiles of Guizhou high-mountain white tea produced by Eurotium cristatum using solid-state fermentation. Food Biosci. 2025, 68, 106725. [Google Scholar] [CrossRef]
- Wang, C.-M.; Du, X.; Nie, C.-N.; Zhang, X.; Tan, X.-Q.; Li, Q. Evaluation of sensory and safety quality characteristics of “high mountain tea”. Food Sci. Nutr. 2022, 10, 3338–3354. [Google Scholar] [CrossRef]
- Qingyang, W.; Ziwei, Z.; Jihang, H.; Suhui, Z.; Shuling, R.; Xiaochun, L.; Shuirong, Y.; Yun, S. Analysis of aroma precursors in Jinmudan fresh tea leaves and dynamic change of fatty acid volatile during black tea processing. Food Chem. X 2024, 21, 101155. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Zhang, Y.; Li, T.; Zhao, W.; Yu, T.; Wu, Y.; Lu, M.; Ning, J.; Zhang, Z. In vitro bioactivity and metabolomics of green tea processed from raw materials of different. Curr. Res. Food Sci. 2025, 10, 101076. [Google Scholar] [CrossRef]
- Xu, C.; Liang, L.; Li, Y.; Yang, T.; Fan, Y.; Mao, X.; Wang, Y. Studies of quality development and major chemical composition of green tea processed from tea with different shoot maturity. LWT 2021, 142, 111055. [Google Scholar] [CrossRef]
- Chen, R.; Lai, X.; Wen, S.; Li, Q.; Cao, J.; Lai, Z.; Zhang, Z.; Hao, M.; Li, Q.; Sun, S.; et al. Analysis of tea quality of large-leaf black tea with different harvesting tenderness based on metabolomics. Food Control 2024, 163, 110474. [Google Scholar] [CrossRef]
- Qin, C.; Han, Z.; Jiang, Z.; Ke, J.-P.; Li, W.; Zhang, L.; Li, D. Chemical profile and in-vitro bioactivities of three types of yellow teas processed from different tenderness of young shoots of Huoshanjinjizhong (Camellia sinensis var. sinensis). Food Chem. X 2024, 24, 101809. [Google Scholar] [CrossRef]
- Shao, C.-Y.; Zhang, Y.; Lv, H.-P.; Zhang, Z.-F.; Zeng, J.-M.; Peng, Q.-H.; Zhu, Y.; Lin, Z. Aromatic profiles and enantiomeric distributions of chiral odorants in baked green teas with different picking tenderness. Food Chem. 2022, 388, 132969. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, L.; Wang, J.; Zheng, H.; Yang, S.; Zhou, Q.; Fu, L.; Cai, Z.; Zhang, S.; Wang, C.; et al. Temporal dynamics of bioactive compounds in sweet tea (Lithocarpus litseifolius (Hance) Chun): Linking harvest stages to flavor and health benefits. Food Res. Int. 2025, 218, 116918. [Google Scholar] [CrossRef]
- Deng, J.; Lin, W.; Li, T.; Wei, J.; Cui, J.; Chen, H.; Zhang, W. Maturity-dependent variations in metabolites and taste quality of Hainan mulberry leaf green tea by using metabolomics and e-tongue analysis. Food Biosci. 2025, 68, 106413. [Google Scholar] [CrossRef]
- Deka, H.; Sarmah, P.P.; Nath, B.; Gogoi, M.; Datta, S.; Sabhapondit, S. Unveiling the effect of leaf maturity on biochemical constituents and quality of CTC black tea: Insights from Northeast India’s commercial cultivars. J. Food Meas. Charact. 2024, 18, 9921–9937. [Google Scholar] [CrossRef]
- Feng, J.; Zhuang, J.; Chen, Q.; Lin, H.; Chu, Q.; Chen, P.; Wang, F.; Yu, B.; Hao, Z. The effect of maturity of tea leaves and processing methods on the formation of milky flavor in white tea—A metabolomic study. Food Chem. 2024, 447, 139080. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gao, X.; Shi, B.; Yu, M.; Raza, J.; Sun, H.; Wang, Y.; Song, H.; Xu, Y. Characterize the dynamic changes of volatile compounds during the roasting process of Wuyi rock tea (Shuixian) integrating GC-IMS and GC × GC-O-MS combined with machine learning. Food Chem. 2025, 489, 144931. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, R.; Li, W.; Lang, Y.; Li, X.; Sun, W.; Sun, B. Characterize and explore the dynamic changes in the volatility profiles of sauce-flavor baijiu during different rounds by GC-IMS, GC–MS and GC×GC–MS combined with machine learning. Food Res. Int. 2025, 213, 116568. [Google Scholar] [CrossRef]
- Wu, H.; Yao, W.; Ma, S.; Liu, D.; Zhang, M. Evolution of key flavor compounds in sonit sheep lamb during grilling process using HS-GC-IMS, E-nose, E-tongue, and physicochemical techniques. Int. J. Gastron. Food Sci. 2025, 40, 101158. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, S.; Li, T.; Wei, Y.; Gu, Z.; Su, Z.; Ning, J.; Wang, Y.; Hou, Z. Characterization of the key volatile compounds in longjing tea (Camellia sinensis) with different aroma types at different steeping temperatures by GC–MS and GC–IMS. LWT 2024, 200, 116183. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, Z.; Chen, J.; Wu, H.; Jiang, H.; Teng, C.; Dai, Z. Enhanced understanding of dark tea quality through integrated GC-IMS and E-Nose analysis. LWT 2025, 224, 117806. [Google Scholar] [CrossRef]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, M.; Wu, J.; Yu, Y.; Si, W.; Xu, Y.; Yang, J. Flavor characterization of aged Citri Reticulatae Pericarpium from core regions: An integrative approach utilizing GC-IMS, GC–MS, E-nose, E-tongue, and chemometrics. Food Chem. 2025, 490, 144995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.; He, C.; Chen, Y.; Wang, Y.; Hu, L.; Qing, Q.; Zhu, M.; Liu, Z.; Xiao, Y. Characterizing and decoding the dynamic alterations of volatile organic compounds and non-volatile metabolites of dark tea by solid-state fermentation with Penicillium polonicum based on GC–MS, GC-IMS, HPLC, E-nose and E-tongue. Food Res. Int. 2025, 209, 116279. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, G.; Xie, H.; Zhang, J.; Huang, J.; Liu, Z.; Wang, C. Characterization of the key differential aroma compounds in five dark teas from different geographical regions integrating GC–MS, ROAV and chemometrics approaches. Food Res. Int. 2024, 194, 114928. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Y.; Wang, L.; Zhang, Y.; Wang, F.; Ou, Y.; Li, H.; Yang, L.; Qiu, S.; Lu, J. Machine learning-enhanced flavoromics: Identifying key aroma compounds and predicting sensory quality in sauce-flavor baijiu. Food Chem. 2025, 475, 143328. [Google Scholar] [CrossRef]
- Hu, Y.; Sheng, W.; Adade, S.Y.-S.S.; Wang, J.; Li, H.; Chen, Q. Comparison of machine learning and deep learning models for detecting quality components of vine tea using smartphone-based portable near-infrared device. Food Control 2025, 174, 111244. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, M.; Gu, J.; Deng, Z.; Zhang, W.; Pan, Z.; Luo, G.; Wu, R.; Qin, J.; Gomi, K. Machine learning-assisted aroma profile prediction in Jiang-flavor baijiu. Food Chem. 2025, 478, 143661. [Google Scholar] [CrossRef]
- MetaboAnalyst. MetaboAnalyst 6.0—From Raw Spectra to Biomarkers, Patterns, Functions and Systems Biology. Available online: https://www.metaboanalyst.ca (accessed on 25 June 2025).
- Shan, X.; Jiang, Y.; Zhang, S.; Chen, L.; Niu, L.; Zhang, Q.; Zhou, Q.; Wang, Y.; Yuan, H.; Li, J. Key umami taste contributors in Longjing green tea uncovered by integrated means of sensory quantitative descriptive analysis, metabolomics, quantification analysis and taste addition experiments. Food Chem. 2024, 453, 139628. [Google Scholar] [CrossRef]
- Wang, J.J.; Yuan, H.B.; Hua, J.J.; Yang, Y.Q.; Shen, S.; Pang, D.D.; Jiang, Y.W.; Zhu, J.Y.; Yu, L.Y. Comparison on Main Flavoring Substances in New Shoots of Large Leaf Tea Cultivars with Different Varieties and Tenderness Based on Multivariate Statistical Analysis. Sci. Technol. Food Ind. 2022, 43, 81–92. [Google Scholar] [CrossRef]
- Kun, J.; Yang, Y.; Sun, J.; Dai, H.; Luo, Z.; Tong, H. Characterization of potential aroma compounds in five aroma types of green tea using the sensomics approach. LWT 2025, 215, 117177. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Hua, J.; Deng, Y.; Jiang, Y.; Li, J.; Wang, J.; Yuan, H.; Dong, C. Quantitation of pyrazines in roasted green tea by infrared-assisted extraction coupled to headspace solid-phase microextraction in combination with GC-QqQ-MS/MS. Food Res. Int. 2020, 134, 109167. [Google Scholar] [CrossRef]
- Liao, X.; Yan, J.; Wang, B.; Meng, Q.; Zhang, L.; Tong, H. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ‘Zhonghuang 1’. Food Res. Int. 2020, 136, 109355. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ho, C.-T.; Lin, Z.; Zhu, Y.; Feng, Z.; Ni, D.; Zeng, S.; Zeng, X.; Wang, Y.; Ning, J.; et al. Sensomics-Assisted Characterization of Key Flowery Aroma Compounds in Lu’an Guapian Green Tea Infusion (Camellia sinensis). J. Agric. Food Chem. 2023, 71, 6120–6132. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.-P.; Shao, C.-Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.-D.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, M.; Zhang, C. Vascular macrophages sense octanal and drive athero-inflammation. Cell. Mol. Immunol. 2022, 19, 1077–1078. [Google Scholar] [CrossRef]
- Kour, D.; Negi, R.; Khan, S.S.; Kumar, S.; Kaur, S.; Kaur, T.; Sharma, B.; Dasila, H.; Kour, H.; Ramniwas, S.; et al. Microbes mediated induced systemic response in plants: A review. Plant Stress 2024, 11, 100334. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Al-Mijalli, S.H.; Mrabti, H.N.; Abdnim, R.; Abdallah, E.M.; Jeddi, M.; Bnouham, M.; Lee, L.-H.; Ardianto, C.; et al. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed. Pharmacother. 2023, 164, 114937. [Google Scholar] [CrossRef]
- Oliveira-Fernandes, J.; Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Sousa, R.M.O.F.; Santos, C. Satureja montana and Mentha pulegium essential oils’ antimicrobial properties against Pseudomonas syringae pv. actinidiae and elicitor potential through the modulation of kiwifruit hormonal defenses. Microbiol. Res. 2023, 277, 127490. [Google Scholar] [CrossRef]
- Hu, Z.; Bakry, A.M.; Shi, L.; Zhan, P.; He, W.; Eid, W.A.M.; Ferweez, H.; Hamed, Y.S.; Ismail, H.A.; Tian, H.; et al. Mechanistic insights into cross-modal aroma-taste interactions mediating sweetness perception enhancement in Fu brick tea. Food Chem. 2025, 489, 144933. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Q.; Tian, T.; Yu, H.; Xu, Z.; Tian, H.; Yuan, H. Interactions among key aroma compounds and the influence of taste substances on aroma perception in Gouda cheese. LWT 2024, 207, 116670. [Google Scholar] [CrossRef]
- Wang, B.-H.; Huang, P.-H.; Lo, C.-Y.; Chang, W.-C. Metabolomic analysis elucidates the dynamic changes in aroma compounds and the milk aroma mechanism across various portions of tea leaves during different stages of Oolong tea processing. Food Res. Int. 2025, 209, 116203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, J.; An, J.; Yan, D.; Shi, X.; Jin, X. Predicting open-plan office window operating behavior using the random forest algorithm. J. Build. Eng. 2021, 42, 102514. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: https://www.thegoodscentscompany.com/ (accessed on 9 June 2025).
- Flavor Ingredient Library. Available online: https://www.femaflavor.org/flavor-library (accessed on 10 June 2025).
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media, 2011st ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]

| NO. | Volatile Compounds | Odor Type | Chemical Classes | OT (μg/L) | rOAV | ||
|---|---|---|---|---|---|---|---|
| FDQSG | FDMJ1G | FDTC2G | |||||
| 1 | Linalool | floral | Terpenes | 0.22 | 3.8 × 103 | 7.4 × 103 | 6.3 × 103 |
| 2 | trans-linalool oxide | floral | Terpenes | 60 | 4.2 | 3.5 | 4.0 |
| 3 | Nonanal-M | aldehydic | Aldehydes | 1.1 | 9.1 × 102 | 8.1 × 102 | 8.6 × 102 |
| 4 | Nonanal-D | aldehydic | Aldehydes | 1.1 | 2.1 × 102 | 1.7 × 102 | 2.0 × 102 |
| 5 | (E)-2-octenal | fatty | Aldehydes | 3.0 | 35 | 16 | 21 |
| 6 | Octanal-M | aldehydic | Aldehydes | 0.587 | 389 | 343 | 422 |
| 7 | Heptanal-M | green | Aldehydes | 2.80 | 135 | 166 | 171 |
| 8 | Heptanal-D | green | Aldehydes | 2.80 | 736 | 514 | 423 |
| 9 | (E)-hept-2-enal | green | Aldehydes | 13 | 5.6 | 0.95 | 0.89 |
| 10 | Octanal-D | aldehydic | Aldehydes | 0.587 | 52.7 | 28.6 | 44.8 |
| 11 | Pentanal | fermented | Aldehydes | 12 | 66 | 45 | 25 |
| 12 | 2-methylbutanal | cocoa | Aldehydes | 1.0 | 1.1 × 103 | 1.3 × 103 | 1.4 × 103 |
| 13 | 3-methylbutanal | aldehydic | Aldehydes | 1.1 | 5.8 × 102 | 7.3 × 102 | 6.9 × 102 |
| 14 | Butanal | chocolate | Aldehydes | 2.0 | 69 | 94 | 45 |
| 15 | Hexanal | green | Aldehydes | 2.4 | 1.7 × 102 | 71 | 41 |
| 16 | (E)-2-hexenal | green | Aldehydes | 88.7 | 5.96 | 3.43 | 2.62 |
| 17 | methyl-5-hepten-2-one | citrus | Ketones | 68 | 8.5 | 8.8 | 7.6 |
| 18 | 2-Octanone | earthy | Ketones | 50.2 | 2.74 | 0.71 | 0.92 |
| 19 | 3-hydroxybutan-2-one | buttery | Ketones | 14 | 9.7 | 9.5 | 7.0 |
| 20 | 2-Propanone | solvent | Ketones | 832 | 4.28 | 5.33 | 4.53 |
| 21 | 1-penten-3-one | spicy | Ketones | 23 | 5.9 | 1.4 | 1.1 |
| 22 | 3-Pentanone | ethereal | Ketones | 40 | 3.0 | 1.9 | 1.5 |
| 23 | n-Hexanol-M | herbal | Alcohols | 5.6 | 42.3 | 46.1 | 39.4 |
| 24 | (Z)-3-hexen-1-ol | green | Alcohols | 3.9 | 67 | 59 | 62 |
| 25 | n-Hexanol-D | herbal | Alcohols | 5.6 | 7.6 | 7.7 | 6.5 |
| 26 | 1.8-Cineole | herbal | Alcohols | 1.10 | 11.7 | 11.4 | 12.5 |
| 27 | oct-1-en-3-ol | earthy | Alcohols | 1.5 | 33 | 22 | 17 |
| 28 | pentan-1-ol-M | fermented | Alcohols | 150.2 | 2.30 | 2.40 | 2.41 |
| 29 | pentan-1-ol-D | fermented | Alcohols | 150.2 | 1.56 | 1.66 | 1.66 |
| 30 | 2-Methylbutanol acetate | fruity | Esters | 5.00 | 25.9 | 2.58 | 1.83 |
| 31 | Propyl isovalerate | fruity | Esters | 8.70 | 1.61 | 1.21 | 0.96 |
| 32 | Ethyl Acetate | ethereal | Esters | 5.00 | 54.5 | 95.9 | 92.3 |
| 33 | methyl acetate | ethereal | Esters | 1500 | 1.080 | 1.070 | 1.430 |
| 34 | 2-pentyl furan | fruity | Furans | 5.80 | 19.0 | 13.8 | 11.6 |
| 35 | 2-n-Butylfuran | spicy | Furans | 5.0 | 5.8 | 3.4 | 3.0 |
| 36 | Methyl Salicylate | minty | Aromatics | 40 | 3.8 | 5.3 | 4.1 |
| 37 | benzene acetaldehyde | green | Aromatics | 6.3 | 9.2 | 7.6 | 14 |
| 38 | 3-methylthiopropanal | vegetable | Sulfur-containing Compounds | 0.45 | 8.5 | 9.3 | 5.9 |
| 39 | Ethylsulfide | coffee, meat | Sulfur-containing Compounds | 4.8 | 3.6 | 3.7 | 5.2 |
| 40 | 2-Ethyl-3,5-dimethylpyrazine | nutty | Pyrazines | 0.040 | 1.42 × 103 | 1.44 × 103 | 1.35 × 103 |
| 41 | 2-acetyl-1-pyrroline | popcorn | Other Heterocyclics | 0.12 | 57 | 66 | 1.6 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Huang, M.; Tang, W.; Li, Y.; Li, L.; Xie, J.; Li, X.; Dong, F.; Wang, M. Characterization and Exploration of the Flavor Profiles of Green Teas from Different Leaf Maturity Stages of Camellia sinensis cv. Fudingdabai Using E-Nose, E-Tongue, and HS-GC-IMS Combined with Machine Learning. Foods 2025, 14, 2861. https://doi.org/10.3390/foods14162861
Liu X, Huang M, Tang W, Li Y, Li L, Xie J, Li X, Dong F, Wang M. Characterization and Exploration of the Flavor Profiles of Green Teas from Different Leaf Maturity Stages of Camellia sinensis cv. Fudingdabai Using E-Nose, E-Tongue, and HS-GC-IMS Combined with Machine Learning. Foods. 2025; 14(16):2861. https://doi.org/10.3390/foods14162861
Chicago/Turabian StyleLiu, Xiaohui, Mingzheng Huang, Weiyuan Tang, Yucai Li, Lun Li, Jinyi Xie, Xiangdong Li, Fabao Dong, and Maosheng Wang. 2025. "Characterization and Exploration of the Flavor Profiles of Green Teas from Different Leaf Maturity Stages of Camellia sinensis cv. Fudingdabai Using E-Nose, E-Tongue, and HS-GC-IMS Combined with Machine Learning" Foods 14, no. 16: 2861. https://doi.org/10.3390/foods14162861
APA StyleLiu, X., Huang, M., Tang, W., Li, Y., Li, L., Xie, J., Li, X., Dong, F., & Wang, M. (2025). Characterization and Exploration of the Flavor Profiles of Green Teas from Different Leaf Maturity Stages of Camellia sinensis cv. Fudingdabai Using E-Nose, E-Tongue, and HS-GC-IMS Combined with Machine Learning. Foods, 14(16), 2861. https://doi.org/10.3390/foods14162861







