Isolation, Identification, and Antitumor Activities of Glucosinolates and Isothiocyanates in Chinese Cabbage Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glucosinolate Extraction
2.3. Isolation and Purification of Glucosinolates
2.4. Degradation of Glucosinolates and Derivatization of ITCs with NAC
2.5. Identification and Characterization of NAC-ITCs
2.6. Identification and Characterization of Glucosinolates
2.7. Cell Culture
2.8. Cell Subculture and Counting
2.9. Sample Preparation for In Vitro Cellular Assays
2.10. Antitumor Activity In Vitro
2.11. AO/EB Staining
2.12. Apoptosis Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Purification and Identification of Glucosinolates
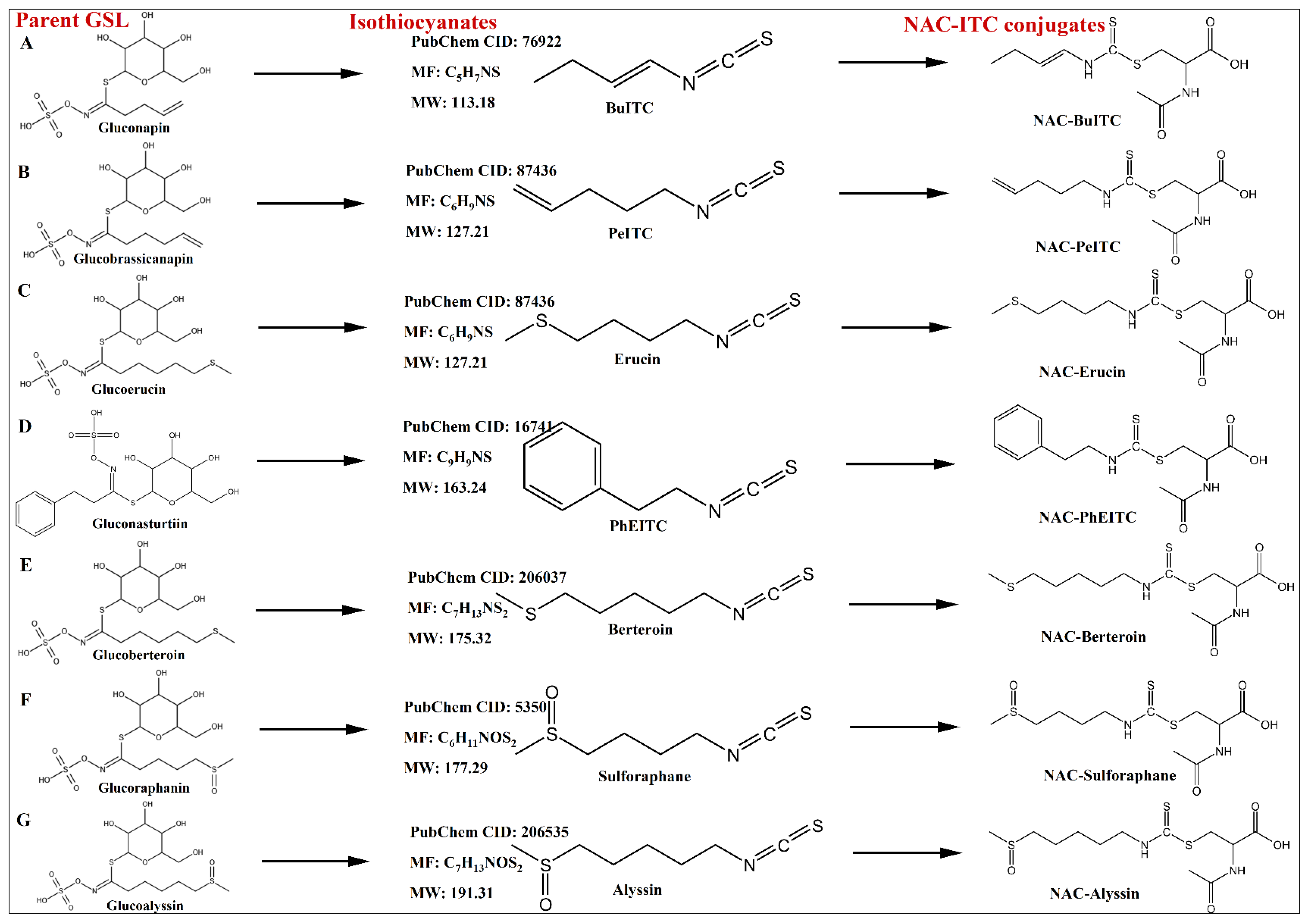
3.2. Identification of NAC-ITCs
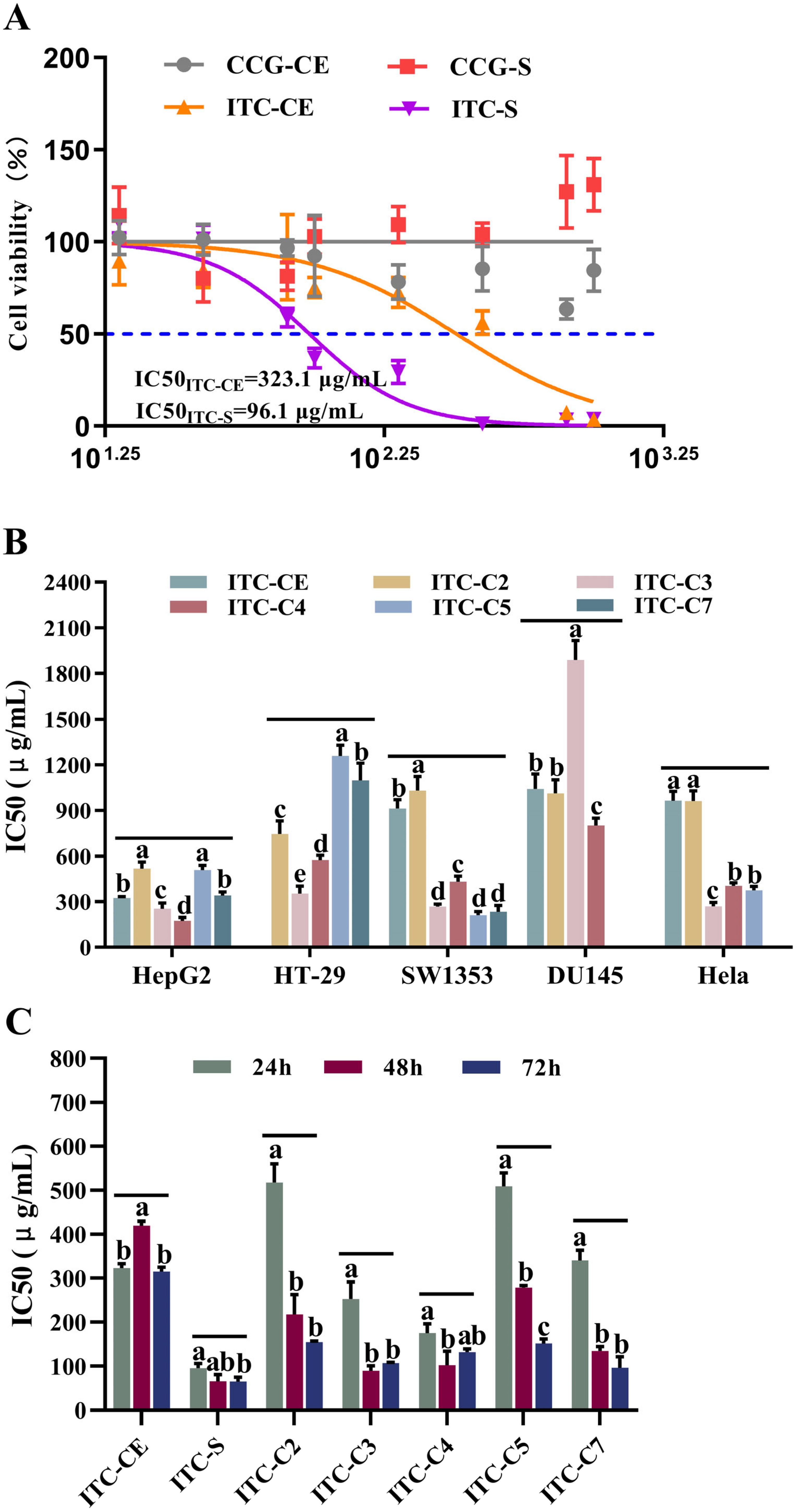
3.3. Antitumor Activity of ITCs on Human Cancer Cells
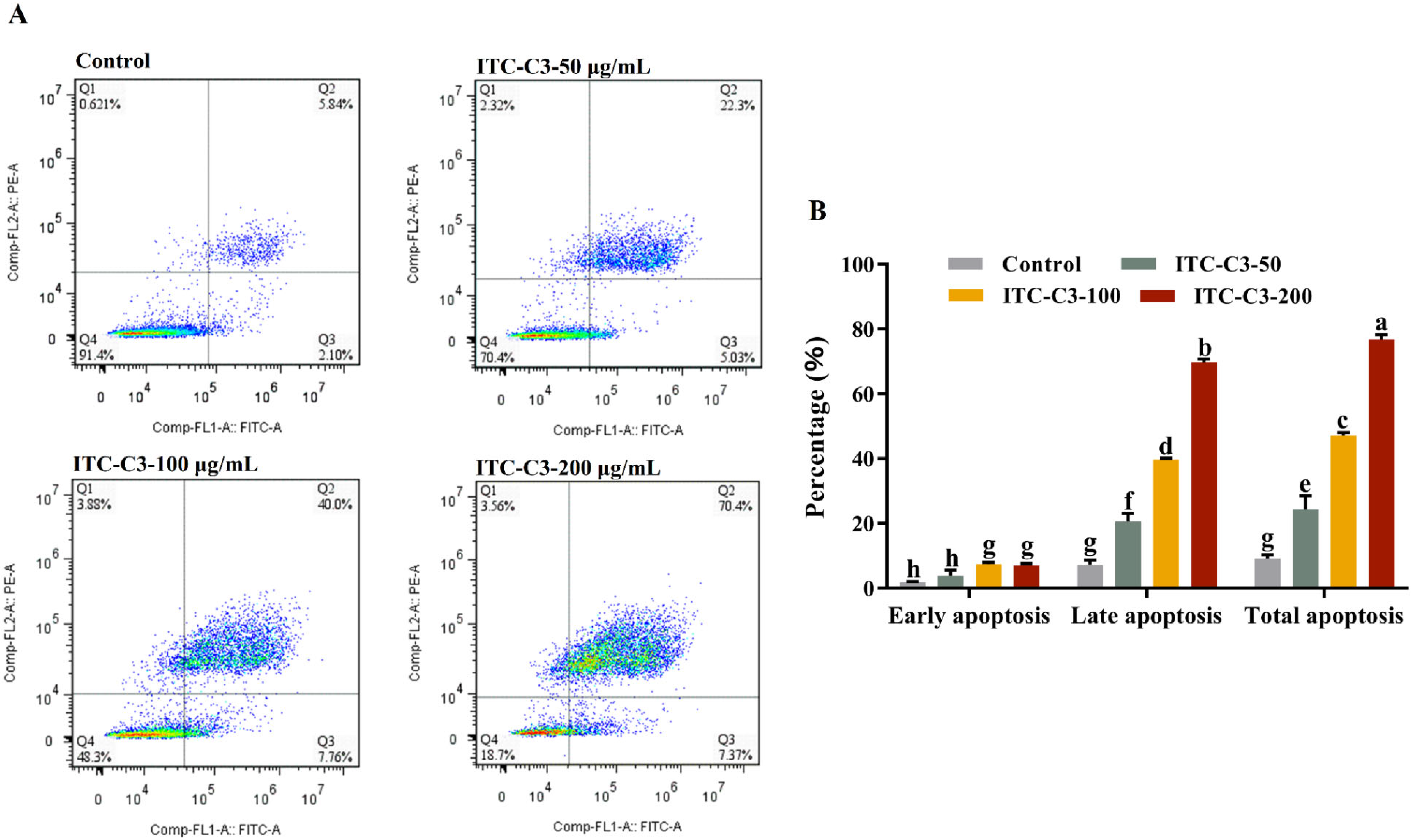
3.4. Effects of ITC-C3 on the Apoptosis of HepG2 Cells
4. Conclusions
5. Chemical Compounds Studied in This Article
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2544–2571. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, H.; Childs, H.; Wu, B.; Yu, L.; Pehrsson, R.P. Glucosinolates in Brassica Vegetables: Characterization and Factors That Influence Distribution, Content, and Intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef]
- Sundaram, M.K.; R, P.; Haque, S.; Akhter, N.; Khan, S.; Ahmad, S.; Hussain, A. Dietary isothiocyanates inhibit cancer progression by modulation of epigenome. Semin. Cancer Biol. 2022, 83, 353–376. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, P.G. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [CrossRef]
- Lam, T.K.; Gallicchio, L.; Lindsley, K.; Shiels, M.; Hammond, E.; Tao, X.G.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184–195. [Google Scholar] [CrossRef]
- Liu, B.; Mao, Q.; Cao, M.; Xie, L. Cruciferous vegetables intake and risk of prostate cancer: A meta-analysis. Int. J. Urol. 2012, 19, 134–141. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Wang, J.; Han, L.; Xiang, Y. Cruciferous vegetable consumption and gastric cancer risk: A meta-analysis of epidemiological studies. Cancer Sci. 2013, 104, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer-Int. J. 2014, 66, 128–139. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Lu, M.D.; Xu, X.W.; Lin, H.D.; Zheng, Z.Q. Cruciferous vegetable consumption and the risk of pancreatic cancer: A meta-analysis. World J. Surg. Oncol. 2015, 13, 44. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Ibrahim, M.D.; Kntayya, S.B.; Ain, N.M.; Lori, R.; Galletti, S.; Loannides, C.; Razis, A.F.A. Induction of Apoptosis by Gluconasturtiin-Isothiocyanate (GNST-ITC) in Human Hepatocarcinoma HepG2 Cells and Human Breast Adenocarcinoma MCF-7 Cells. Molecules 2020, 25, 1240. [Google Scholar] [CrossRef] [PubMed]
- Mitsiogianni, M.; Mantso, T.; Trafails, D.T.; Rupasinghe, H.P.V.; Zoumpourlis, V.; Franco, R.; Botaitis, S.; Pappa, A.; Panayiotidis, M.I. Allyl isothiocyanate regulates lysine acetylation and methylation marks in an experimental model of malignant melanoma. Eur. J. Nutr. 2020, 59, 557–569. [Google Scholar] [CrossRef]
- Núñez-Iglesias, M.; Novio, S.; García-Santiago, C.; Cartea, M.E.; Soengas, P.; Velasco, P.; Freire-Garabal, M. Effects of 3-butenyl isothiocyanate on phenotypically different prostate cancer cells. Int. J. Oncol. 2018, 53, 2213–2223. [Google Scholar] [CrossRef]
- Kim, J.K.; Chu, S.M.; Kim, S.J.; Lee, D.J.; Lee, S.Y.; Lim, S.H.; Ha, S.H.; Kweon, S.; Cho, H. Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. pekinensis. Food Chem. 2010, 119, 423–428. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, W.; Feng, X.; Liu, Q.; Ibrahim, S.A.; Liu, Y. Identification and quantification of intact glucosinolates at different vegetative growth periods in Chinese cabbage cultivars by UHPLC-Q-TOF-MS. Food Chem. 2022, 393, 133414. [Google Scholar] [CrossRef]
- Li, Z.S.; Zheng, S.N.; Liu, Y.M.; Fang, Z.Y.; Yang, L.M.; Zhuang, M.; Zhang, Y.Y.; Lv, H.H.; Wang, Y.; Xu, D.H. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef]
- Wu, Y.F.; Shen, Y.K.; Wu, X.P.; Zhu, Y.; Mupunga, J.; Bao, W.; Huang, J.; Mao, J.W.; Liu, S.W.; You, Y.R. Hydrolysis before Stir-Frying Increases the Isothiocyanate Content of Broccoli. J. Agric. Food Chem. 2018, 66, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Araya-Cloutier, C.; Sanders, M.; Vincken, J.P. Simultaneous Analysis of Glucosinolates and Isothiocyanates by Reversed-Phase Ultra-High-Performance Liquid Chromatography-Electron Spray Ionization-Tandem Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Pilipczuk, T.; Kusznierewicz, B.; Chmiel, T.; Przychodzeń, W.; Bartoszek, A. Simultaneous determination of individual isothiocyanates in plant samples by HPLC-DAD-MS following SPE and derivatization with N-acetyl-L-cysteine. Food Chem. 2017, 214, 587–596. [Google Scholar] [CrossRef]
- Song, L.; Lori, R.; Thornalley, P.J. Purification of major glucosinolates from Brassicaceae seeds and preparation of isothiocyanate and amine metabolites. J. Sci. Food Agric. 2006, 86, 1271–1280. [Google Scholar] [CrossRef]
- Hebert, M.; Serra, E.; Vorobiev, E.; Mhemdi, H. Isolation and Purification of Mustard Glucosinolates by Macroporous Anion-Exchange Resin: Process Optimization and Kinetics’ Modelling. Processes 2022, 10, 191. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Chou, F. Separation and purification of glucosinolates from crude plant homogenates by high-speed counter-current chromatography. J. Chromatogr. A 2003, 996, 85–93. [Google Scholar] [CrossRef]
- Rochfort, S.; Caridi, D.; Stinton, M.; Trenerry, V.C.; Jones, R. The isolation and purification of glucoraphanin from broccoli seeds by solid phase extraction and preparative high performance liquid chromatography. J. Chromatogr. A 2006, 1120, 205–210. [Google Scholar] [CrossRef]
- Chiang, W.; Pusateri, D.J.; Leitz, R. Gas chromatography mass spectrometry method for the determination of sulforaphane and sulforaphane nitrile in broccoli. J. Agric. Food Chem. 1998, 46, 1018–1021. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef]
- Greer, M.A. Isolation from Rutabaga seed of progoitrin, the precursor of the naturally occurring antithyroid compound, goitrin (L-5-Vinyl-2-Thiooxazolidone). J. Am. Chem. Soc. 1956, 78, 1260–1261. [Google Scholar] [CrossRef]
- Agerbirk, N.; Vos, M.D.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Hauder, J.; Winkler, S.; Bub, A.; Rüfer, C.E.; Pignitter, M.; Somoza, V. LC-MS/MS Quantification of Sulforaphane and Indole-3-carbinol Metabolites in Human Plasma and Urine after Dietary Intake of Selenium-Fortified Broccoli. J. Agric. Food Chem. 2011, 59, 8047–8057. [Google Scholar] [CrossRef]
- Mcdanell, R.; McLean, A.; Hanley, A.; Heaney, R.; Fenwick, G. Chemical and biological properties of indole glucosinolates (Glucobrassicins)—A Review. Food Chem. Toxicol. 1988, 26, 59–70. [Google Scholar] [CrossRef]
- Núñez-Iglesias, M.J.; Novío, S.; García, C.; Pérez-Muñuzuri, E.; Soengas, P.; Cartea, E.; Velasco, P.; Manuel Freire-Garabal, M. Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. Int. J. Mol. Sci. 2019, 20, 4977. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Zhou, Z.G.; Smith, A.J.O.; Bowater, R.P.; Wormstone, L.M.; Chen, Y.Q.; Bao, Y.P. Chemopreventive activities of sulforaphane and its metabolites in human hepatoma HepG2 cells. Nutrients 2018, 10, 585. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Khan, M.A.; Alalami, U.; Somvanshi, P.; Bhardwaj, T.; Pramodh, S.; Raina, R.; Shekfeh, Z.; Haque, S.; Hussain, A. Phytochemicals induce apoptosis by modulation of nitric oxide signaling pathway in cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11827–11844. [Google Scholar]
- Peng, X.H.; Zhou, Y.; Tian, H.; Yang, G.X.; Li, C.L.; Geng, Y.; Wu, S.; Wu, W. Sulforaphane inhibits invasion by phosphorylating ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer DU145 cells. Oncol. Rep. 2015, 34, 1565–1572. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Jyotsana Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Nie, J.; Qin, X.; Li, Z. Revealing the anti-melanoma mechanism of n-BuOH fraction from the red kidney bean coat extract based on network pharmacology and transcriptomic approach. Food Res. Int. 2021, 140, 109880. [Google Scholar] [CrossRef]
- Yuan, J.; Stepanov, I.; Murphy, S.E.; Wang, R.W.; Allen, S.; Jensen, J.; Strayer, L.; Adams-Haduch, J.; Upadhyaya, P.; Chap Le, C.; et al. Clinical Trial of 2-Phenethyl Isothiocyanate as an Inhibitor of Metabolic Activation of a Tobacco-Specific Lung Carcinogen in Cigarette Smokers. Cancer Prev. Res. 2016, 9, 396–405. [Google Scholar] [CrossRef]
- Batra, S.; Sahu, R.P.; Kandala, P.K.; Srivastava, S.K. Benzyl Isothiocyanate-Mediated Inhibition of Histone Deacetylase Leads to NF-KB Turnoff in Human Pancreatic Carcinoma Cells. Mol. Cancer Ther. 2010, 9, 1596–1608. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, J.X.; Wang, Q.; Zhang, H.Y.; Yang, P. Inhibitory Effect of Benzyl Isothiocyanate on Proliferation in vitro of Human Glioma Cells. Asian Pac. J. Cancer Prev. 2013, 14, 2607–2610. [Google Scholar] [CrossRef]
- Wu, J.; Han, J.L.; Hou, B.X.; Deng, C.W.; Wu, H.L.; Shen, L.F. Sulforaphane inhibits TGF-β-induced epithelial-mesenchymal transition of hepatocellular carcinoma cells via the reactive oxygen species-dependent pathway. Oncol. Rep. 2016, 35, 2977–2983. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Trafalis, D.T.; Franco, R.; Zoumpourlis, V.; Pappa, A.; Panayiotidis, M.I. Sulforaphane and iberin are potent epigenetic modulators of histone acetylation and methylation in malignant melanoma. Eur. J. Nutr. 2021, 60, 147–158. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, L.M.; Kim, H.; Khor, T.O.; Wu, R.Y.; Li, W.J.; Kong, A.T. Phenethyl isothiocyanate (PEITC) suppresses prostate cancer cell invasion epigenetically through regulating microRNA-194. Mol. Nutr. Food Res. 2016, 60, 1427–1436. [Google Scholar] [CrossRef] [PubMed]

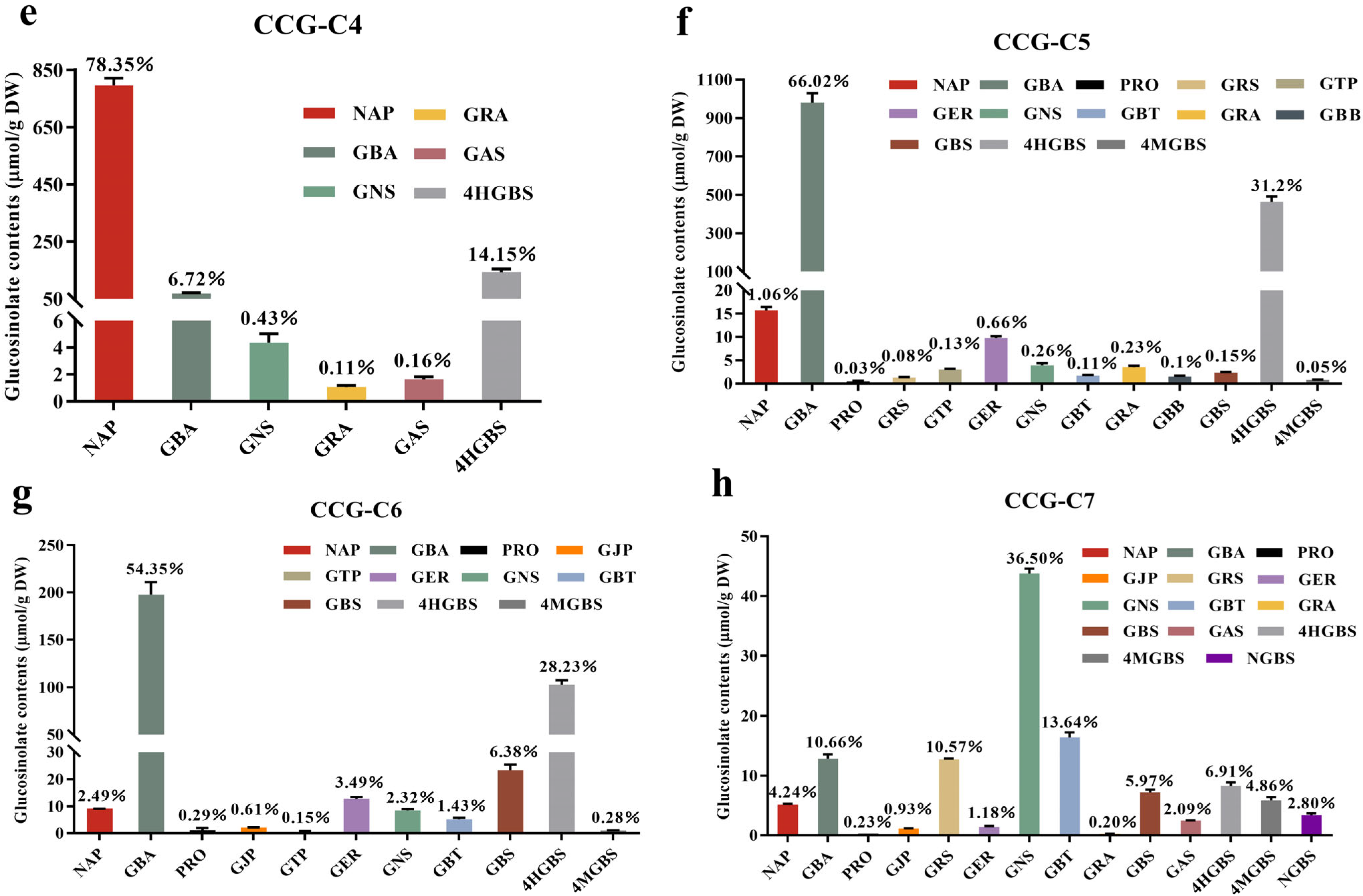


| Compounds | Chemical Formula | Precursor Ions, m/z | Retention Time/min | Mass Error/ppm | Fragments, m/z |
|---|---|---|---|---|---|
| Gluconapin (NAP) | C11H19NO9S2 | 372.0425 | 1.56 | −0.9 | 372.04, 274.99, 259.01, 241.00, 96.96 |
| Glucobrassicanapin (GBA) | C12H21NO9S2 | 386.0586 | 2.7 | 0.2 | 386.05, 274.99, 259.01, 241.00, 96.96 |
| Progoitrin (PRO) | C11H19NO10S2 | 388.0374 | 0.97 | −0.9 | 388.03, 274.99, 259.01, 241.00, 96.96 |
| Glucojiaputin (GJP) | C12H23NO9S2 | 388.073 | 2.99 | −3 | 388.07, 259.01, 96.96 |
| Gluconapoleiferin (GNF) | C12H21NO10S2 | 402.0533 | 1.17 | −0.2 | 402.05, 274.99, 259.01, 96.96 |
| Glucoraphasativusain (GRS) | C13H25NO9S2 | 402.0894 | 4.20 | −1.1 | 402.09, 259.01, 96.96 |
| Glucotropaeolin (GTP) | C14H19NO9S2 | 408.041 | 2.24 | −4.5 | 408.04, 96.96 |
| Glucoerucin (GER) | C12H23NO9S3 | 420.0469 | 2.93 | 1.5 | 420.05, 96.96 |
| Gluconasturtiin (GNS) | C15H21NO9S2 | 422.0583 | 3.78 | −0.6 | 422.05, 274.99, 259.01, 96.96 |
| Glucoberteroin (GBT) | C13H25NO9S2 | 434.0615 | 3.74 | −0.8 | 434.06, 274.99, 259.01, 96.95 |
| Glucoraphanin (GRA) | C12H23NO10S3 | 436.0409 | 0.92 | −0.5 | 436.04, 421.02, 388.04, 372.04, 178.01 |
| Glucobarbarin (GBB) | C15H21NO10S2 | 438.0533 | 3.38 | −0.2 | 438.05, 274.99, 96.96 |
| Glucobrassicin (GBS) | C16H20N2O9S2 | 447.0537 | 3.35 | −0.1 | 447.05, 274.99, 259.01, 96.96 |
| Glucoalyssin (GAS) | C13H25NO10S3 | 450.0564 | 1.06 | −0.7 | 450.06, 386.05, 274.99, 259.01, 241.00, 96.96 |
| 4-Hydroxyglucobrassicin (4HGBS) | C16H20N2O10S2 | 463.0485 | 2.21 | −0.3 | 463.05, 274.99, 259.01, 241.00, 96.95 |
| 4-Methoxyglucobrassicin (4MGBS) | C17H22N2O10S2 | 477.0644 | 3.98 | 0.2 | 477.06, 96.96 |
| Neoglucobrassicin (NGBS) | C17H22N2O10S2 | 477.0644 | 4.62 | 0.2 | 477.06, 446.04, 96.96 |
| NAC-ITC Conjugates | Isothiocyanates | Chemical Formula | Precursor Ions, m/z | Retention Time/min | Mass Error/10−6 | Fragments, m/z | Detected Fractions | ||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Name | Mw | Parent GSL | |||||||
| NAC-BuITC | BuITC | 113.18 | NAP | C10H16N2O3S2 | 277.0675 | 29.68 | −0.1 | 299.0487, 277.0675, 164.0366, 130.0494, 122.0262, 76.0213 | ITC-CE, ITC-S, ITC-C3, ITC-C4, ITC-C5, ITCC6, ITC-C7 |
| NAC-PeITC | PeITC | 127.21 | GBA | C11H18N2O3S2 | 291.0828 | 34.53 | −1.4 | 313.0646, 291.0828, 164.0372, 130.0495, 122.0266, 76.0208 | ITC-CE, ITC-S, ITC-C4, ITC-C5, ITC-C6, ITC-C7 |
| NAC-Erucin | Erucin | 161.29 | GER | C11H20N2O3S3 | 325.0704 | 34.27 | −1.6 | 347.0518, 325.0704, 130.0495, | ITC-C4, ITC-C5, ITC-C6 |
| NAC-PhEITC | PhEITC | 163.24 | GNS | C14H18N2O3S2 | 327.0824 | 38.08 | −2.4 | 349.0643, 327.0824, 164.0374, 130.0647, 122.0265, 76.0209 | ITC-S, ITC-C4, ITC-C5, ITC-C6, ITC-C7 |
| NAC-Berteroin | Berteroin | 175.32 | GBT | C12H22N2O3S3 | 339.086 | 37.86 | −1.7 | 361.0675, 339.0860, 164.0373, 130.0495, 122.0266, 76.0209 | ITC-CE, ITC-C4, ITC-C5, ITC-C6, ITC-C7 |
| NAC-Sulforaphane | Sulforaphane | 177.29 | GRA | C11H20N2O4S3 | 341.0651 | 16.15 | −2.0 | 363.0464, 341.0651 | ITC-CE, ITC-S, ITC-C2, ITC-C4, ITC-C5, ITC-C6 |
| NAC-Alyssin | Alyssin | 191.31 | GAS | C12H22N2O4S3 | 355.0812 | 21.92 | −0.8 | 377.0625, 355.0812 | ITC-CE, ITC-S, ITC-C2, ITC-C3, ITC-C4, ITC-C5, ITC-C6, ITC-C7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Liu, Y.; Feng, X.; Liu, Q.; Ibrahim, S.A.; Huang, W. Isolation, Identification, and Antitumor Activities of Glucosinolates and Isothiocyanates in Chinese Cabbage Seeds. Foods 2025, 14, 2808. https://doi.org/10.3390/foods14162808
Zhou B, Liu Y, Feng X, Liu Q, Ibrahim SA, Huang W. Isolation, Identification, and Antitumor Activities of Glucosinolates and Isothiocyanates in Chinese Cabbage Seeds. Foods. 2025; 14(16):2808. https://doi.org/10.3390/foods14162808
Chicago/Turabian StyleZhou, Bei, Ying Liu, Xi Feng, Qian Liu, Salam A. Ibrahim, and Wen Huang. 2025. "Isolation, Identification, and Antitumor Activities of Glucosinolates and Isothiocyanates in Chinese Cabbage Seeds" Foods 14, no. 16: 2808. https://doi.org/10.3390/foods14162808
APA StyleZhou, B., Liu, Y., Feng, X., Liu, Q., Ibrahim, S. A., & Huang, W. (2025). Isolation, Identification, and Antitumor Activities of Glucosinolates and Isothiocyanates in Chinese Cabbage Seeds. Foods, 14(16), 2808. https://doi.org/10.3390/foods14162808







