Functions of Pugionium cornutum (L.) Gaertn Extracts: Investigating the Mechanism of Gastroparesis Amelioration from the Perspective of the Gut Microbiota and Its Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extract Samples
2.3. Determination of the Effect of Improving Gastroparesis
2.4. Exploration Mechanism
2.4.1. Targeted Metabolomics Analysis of Major Metabolites
2.4.2. 16S rRNA High-Throughput Sequencing for Gut Microbiota
2.5. Identification of Chemical Composition
2.6. Prediction and Validation of Active Ingredient
2.7. Statistical Analysis
3. Results and Discussion
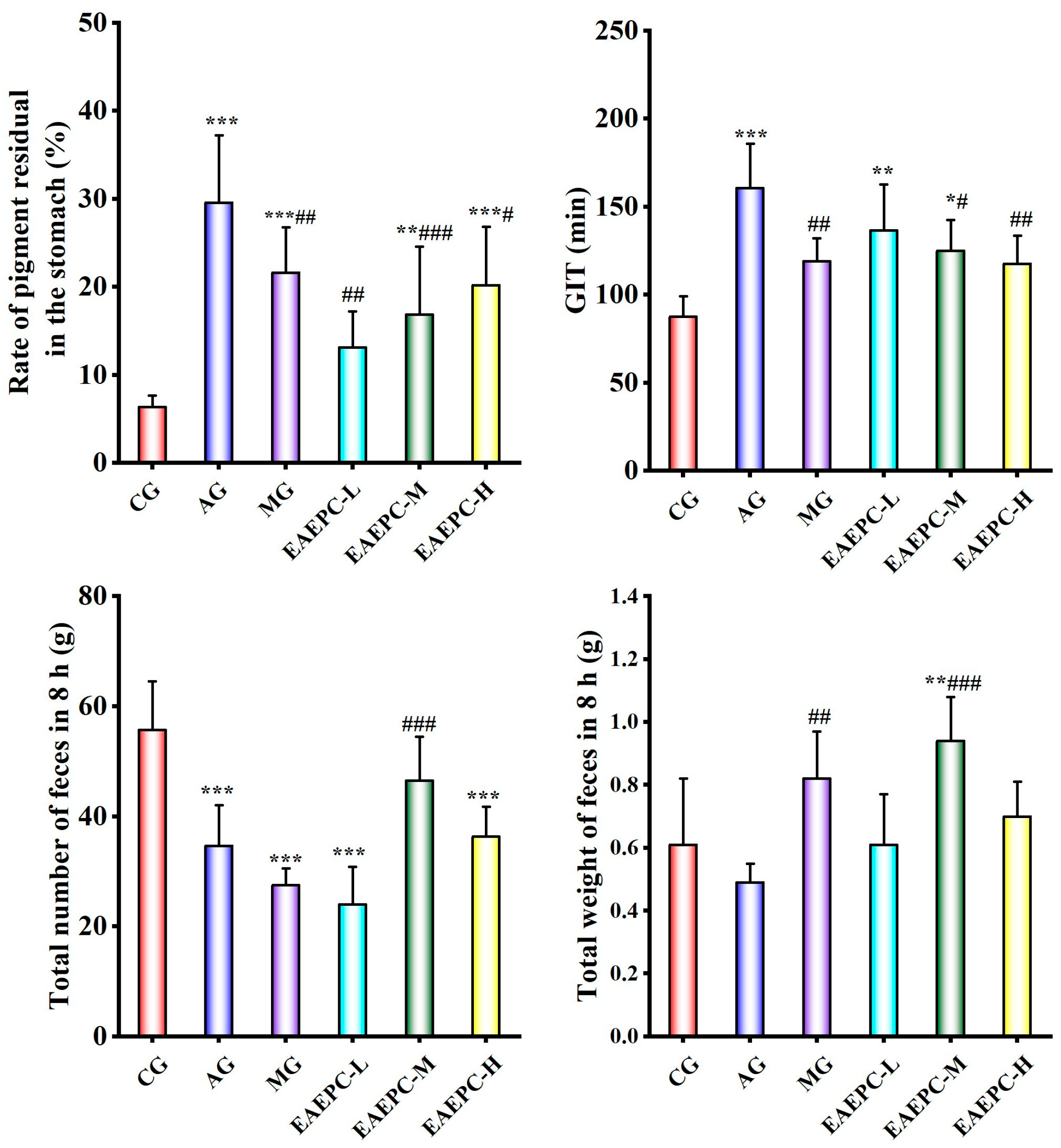
3.1. EAEPC for Gastroparesis Efficacy
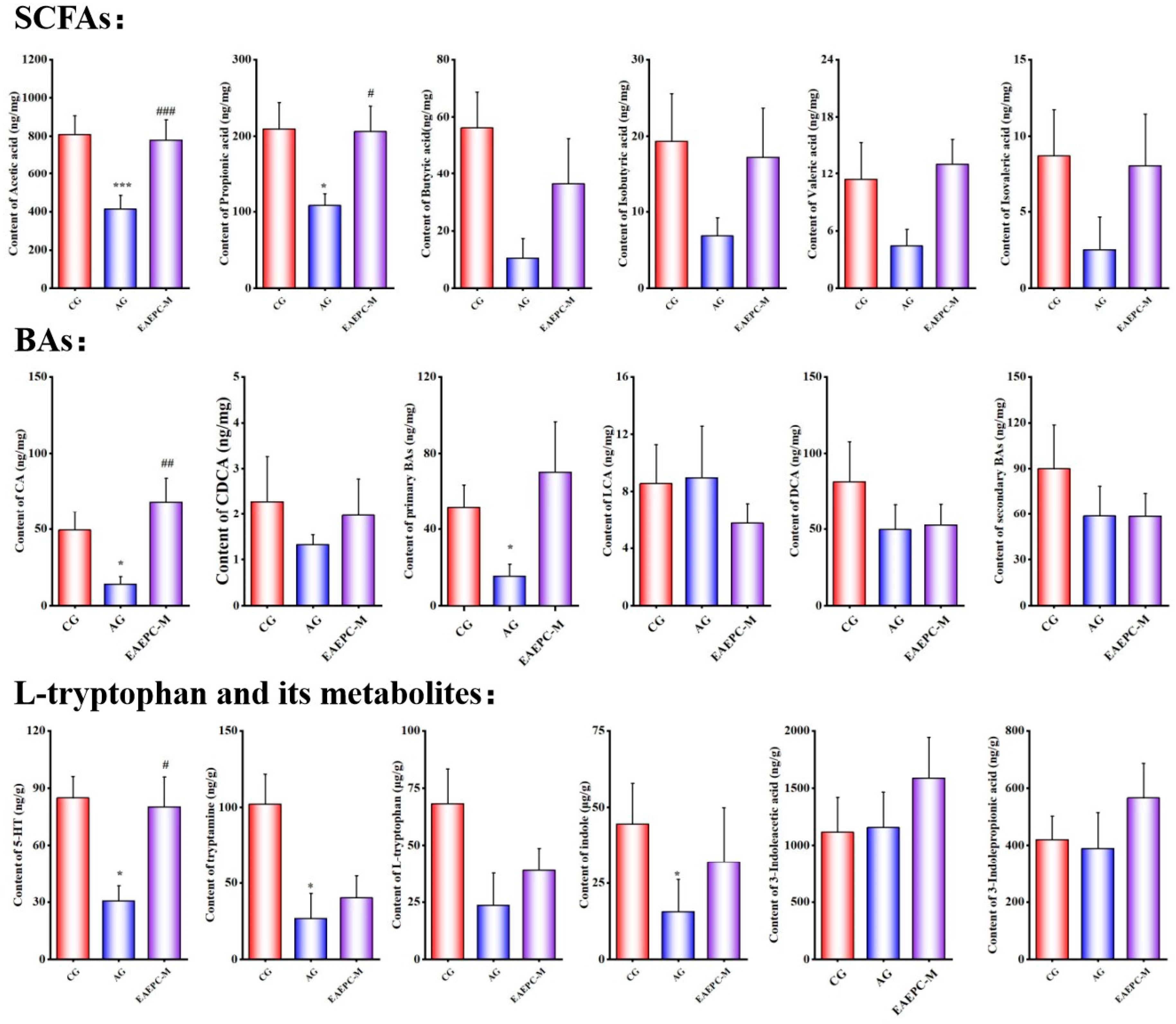
3.2. Alterations in Major Intestinal Metabolites
3.2.1. Short-Chain Fatty Acids
3.2.2. Bile Acids
3.2.3. L-Tryptophan and Its Metabolites
3.3. Effect on the Gut Microbiota
3.3.1. Diversity Analysis
3.3.2. Species Composition and Differential Analysis
3.4. The Correlation and Mechanism of Major Intestinal Metabolites and the Gut Microbiota
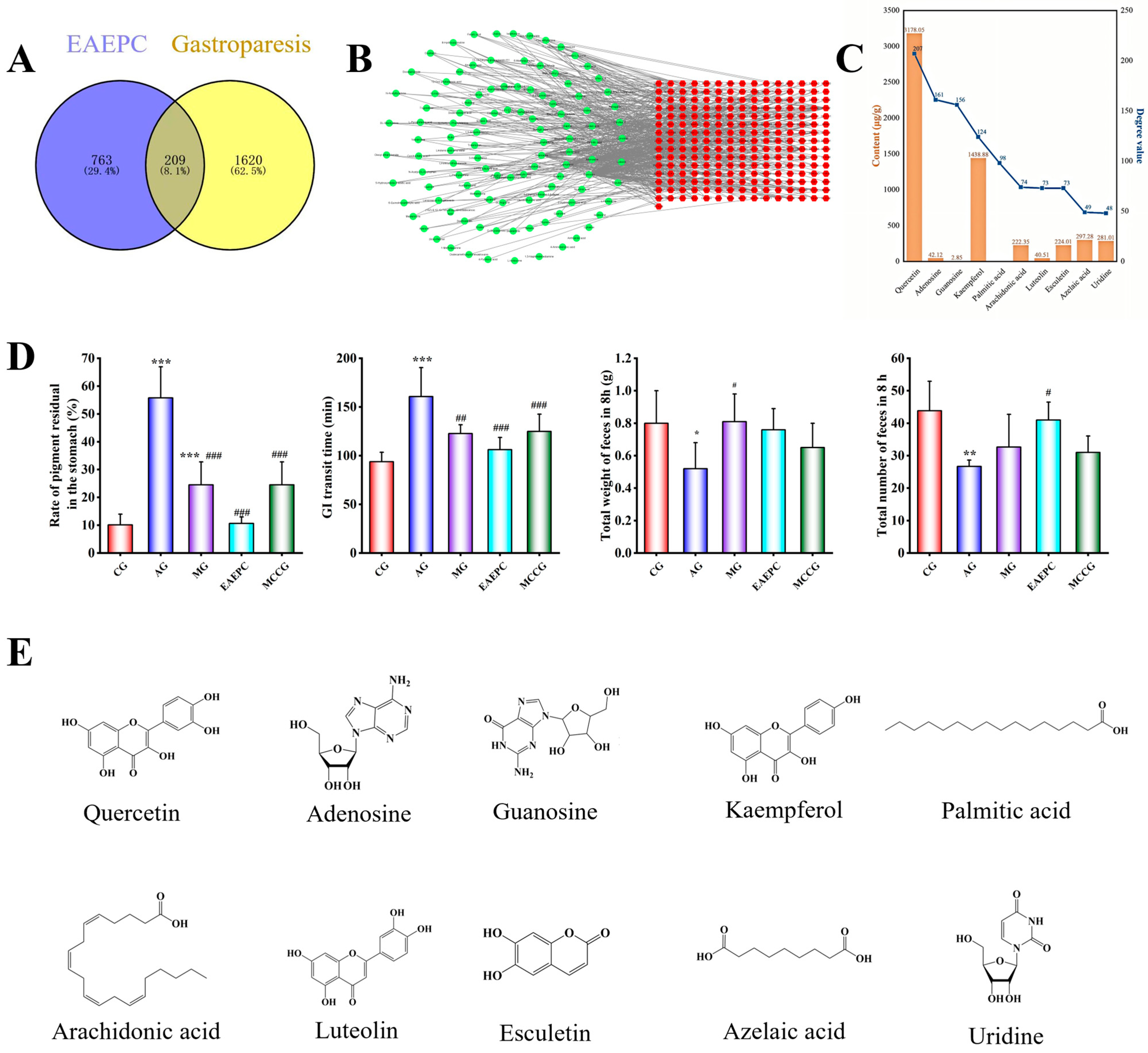
3.5. Identification, Prediction, and Verification of Main Active Ingredients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EAEPC | Extract from Pugionium cornutum (L.) Gaertn |
| SCFAs | Short-chain fatty acids |
| BAs | Bile acids |
| EPC | Ethanolic extract of Pugionium |
| CG | Control group |
| AG | Model group (atropine) |
| EAEPC-H/M/L | EAEPC high, medium, and low concentration |
| GIT | Gastrointestinal transit time |
| MRM | Multi-reaction monitoring |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology |
| TCMID | Traditional Chinese Medicine Integrated Database |
| ETCM | The Encyclopedia of Traditional Chinese Medicine |
| PPI | Protein–protein interaction |
| MCCGs | Multi-component complex granules |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| DCA | Deoxycholic acid |
| LCA | Lithocholic acid |
| 5-HT | 5-hydroxytryptamine |
| PCoA | Principal Co-ordinate Analysis |
| NMDS | Non-metric Multidimensional Scaling |
References
- Kimura, Y.; Taniguchi, M. Effects of Perilla frutescens var. crispa Herb Extract, the Essential Oil Perillaldehyde, and the Caffeetannin Rosmarinic Acid on Gastric Emptying and Gastrointestinal Motility in Mice. Tradit. Kampo Med. 2021, 8, 194–203. [Google Scholar]
- Ladopoulos, T.; Giannaki, M.; Alexopoulou, C.; Proklou, A.; Pediaditis, E.; Kondili, E. Gastrointestinal Dysmotility in Critically Ill Patients. Ann. Gastroenterol. 2018, 31, 273–281. [Google Scholar] [CrossRef]
- Vy Truong Thuy, N.; Taheri, N.; Chandra, A.; Hayashi, Y. Aging of Enteric Neuromuscular Systems in Gastrointestinal Tract. Neurogastroenterol. Motil. 2022, 34, e14352. [Google Scholar] [CrossRef]
- Gangula, P.R.R.; Sekhar, K.R.; Mukhopadhyay, S. Gender Bias in Gastroparesis: Is Nitric Oxide the Answer? Dig. Dis. Sci. 2011, 56, 2520–2527. [Google Scholar] [CrossRef]
- Hsu, C.-T.; Azzopardi, N.; Broad, J. Prevalence and Disease Burden of Gastroparesis in Asia. J. Gastroenterol. Hepatol. 2024, 39, 649–657. [Google Scholar] [CrossRef]
- Wood, R.J.; Yacob, D.; Levitt, M.A. Surgical Options for the Management of Severe Functional Constipation in Children. Curr. Opin. Pediatr. 2016, 28, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Q.; Liu, Q.; Jin, L.; Chen, B.; Li, C.; Xiao, J.; Shen, Y. Volatile Flavor Compounds of Pugionium cornutum (L.) Gaertn. Before and After Different Dehydration Treatments. Front. Nutr. 2022, 9, 884086. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, C.; Zhang, C.; Chen, B.; Hui, L.; Shen, Y. Compositional and Gastrointestinal Prokinetic Studies of Pugionium (L.). Food Chem. 2015, 186, 285–291. [Google Scholar] [CrossRef]
- Su, C.; Li, H.; Chen, B.; Li, C.; Zhang, C.; Xu, L.; Lan, M.; Shen, Y. Pharmacological Effects of Pugionium cornutum (L.) Gaertn. Extracts on Gastrointestinal Motility Are Partially Mediated by Quercetin. BMC Complement. Med. Ther. 2021, 21, 03395. [Google Scholar] [CrossRef]
- Khuituan, P.; Huipao, N.; Jeanmard, N.; Thantongsakul, S.; Promjun, W.; Chuthong, S.; Tipbunjong, C.; Peerakietkhajorn, S. Sargassum Plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice. Nutrients 2022, 14, 496. [Google Scholar] [CrossRef]
- Cai, T.; Dong, Y.; Feng, Z.; Cai, B. Ameliorative Effects of the Mixed Aqueous Extract of Aurantii Fructus Immaturus and Magnoliae Officinalis Cortex on Loperamide-Induced STC Mice. Heliyon 2024, 10, e33705. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, L.; Jin, X.; Chen, Z.; Qiao, X.; Cui, X.; Gao, J.; Zhang, L. Ethanol Extract of Mao Jian Green Tea Attenuates Gastrointestinal Symptoms in a Rat Model of Irritable Bowel Syndrome with Constipation via the 5-Hydroxytryptamine Signaling Pathway. Foods 2023, 12, 1101. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Wang, Q.; Abbott, R.J.; Yu, Q.-S.; Lin, K.; Liu, J.-Q. Pleistocene Climate Change and the Origin of Two Desert Plant Species, Pugionium cornutum and Pugionium dolabratum (Brassicaceae), in Northwest China. New Phytol. 2013, 199, 277–287. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, J.; Hu, Y.; Zhang, W. Role of Gut Microbiota-Derived Signals in the Regulation of Gastrointestinal Motility. Front. Med. 2022, 9, 961703. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of an Atractylodes Lancea Rhizome Extract and a Volatile Component β-Eudesmol on Gastrointestinal Motility in Mice. J. Ethnopharmacol. 2012, 141, 530–536. [Google Scholar] [CrossRef]
- Omolaso, B.O.; Oluwole, F.S.; Odukanmi, O.A.; Adesanwo, J.K.; Ishola, A.A.; Adewole, K.E. Evaluation of the Gastrointestinal Anti-Motility Effect of Anacardium Occidentale Stem Bark Extract: A Mechanistic Study of Antidiarrheal Activity. J. Pharm. Anal. 2021, 11, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An Isotope-Labeled Chemical Derivatization Method for the Quantitation of Short-Chain Fatty Acids in Human Feces by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Bassett, C.M.C.; McCullough, R.S.; Edel, A.L.; Maddaford, T.G.; Dibrov, E.; Blackwood, D.P.; Austria, J.A.; Pierce, G.N. Trans–Fatty Acids in the Diet Stimulate Atherosclerosis. Metab. Clin. Exp. 2009, 58, 1802–1808. [Google Scholar] [CrossRef]
- Estadella, D.; da Penha Oller do Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B.; Damaso, A.R.; de Piano, A. Lipotoxicity: Effects of Dietary Saturated and Transfatty Acids. Mediat. Inflamm. 2013, 2013, 137579. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-R.; Yu, J.-B.; Fu, J.; Pan, L.-B.; Yu, H.; Han, P.; Zhang, Z.-W.; Peng, R.; Xu, H.; Wang, Y. Determination and Application of Nineteen Monoamines in the Gut Microbiota Targeting Phenylalanine, Tryptophan, and Glutamic Acid Metabolic Pathways. Molecules 2021, 26, 1377. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Martinot, E.; Sèdes, L.; Baptissart, M.; Lobaccaro, J.-M.; Caira, F.; Beaudoin, C.; Volle, D.H. Bile Acids and Their Receptors. Mol. Asp. Med. 2017, 56, 2–9. [Google Scholar] [CrossRef]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel Functions, Fecal Unconjugated Primary and Secondary Bile Acids, and Colonic Transit in Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal Programming by Microbiota Regulates Intestinal Physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signalling in the Gut-Functions, Dysfunctions and Therapeutic Targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Buey, B.; Forcen, A.; Grasa, L.; Layunta, E.; Mesonero, J.E.; Latorre, E. Gut Microbiota-Derived Short-Chain Fatty Acids: Novel Regulators of Intestinal Serotonin Transporter. Life 2023, 13, 1085. [Google Scholar] [CrossRef]
- Lotstedt, B.; Boyer, D.; Visner, G.; Freiberger, D.; Lurie, M.; Kane, M.; DiFilippo, C.; Lundeberg, J.; Narvaez-Rivas, M.; Setchell, K.; et al. The Impact of Gastrointestinal Dysmotility on the Aerodigestive Microbiome of Pediatric Lung Transplant Recipients. J. Heart Lung Transplant. 2021, 40, 210–219. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the Gut Microbiota in Host Appetite Control: Bacterial Growth to Animal Feeding Behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, Y.; Ke, X. Interaction between the Gut Microbiota and Intestinal Motility. Evid. BASED Complement. Altern. Med. 2022, 2022, 3240573. [Google Scholar] [CrossRef]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Effects of GLP-1 and Incretin-Based Therapies on Gastrointestinal Motor Function. Exp. Diabetes Res. 2011, 2011, 279530. [Google Scholar] [CrossRef]
- Xie, T.; Jin, F.; Jia, X.; Mao, H.; Xu, Y.; Zhang, S. High Cellulose Diet Promotes Intestinal Motility through Regulating Intestinal Immune Homeostasis and Serotonin Biosynthesis. Biol. Chem. 2022, 403, 279–292. [Google Scholar] [CrossRef]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT Cells—A Major Target for GLP-1 and Gut Microbial Metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Benech, N.; Rolhion, N.; Sokol, H. Tryptophan Metabolites Get the Gut Moving. Cell Host Microbe 2021, 29, 145–147. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, M.R.; Park, J.J.; Choi, J.Y.; Song, B.R.; Son, H.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Quercetin Promotes Gastrointestinal Motility and Mucin Secretion in Loperamide-Induced Constipation of SD Rats through Regulation of the mAChRs Downstream Signal. Pharm. Biol. 2018, 56, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Dicarlo, G.; Autore, G.; Izzo, A.A.; Maiolino, P.; Mascolo, N.; Viola, P.; Diurno, M.V.; Capasso, F. Inhibition of Intestinal Motility and Secretion by Flavonoids in Mice and Rats: Structure-Activity Relationships. J. Pharm. Pharmacol. 1993, 45, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, H.; Wang, L.; Gan, H.; Xiao, X.; Huang, L.; Li, W.; Li, Z. Luteolin Ameliorates Loperamide-Induced Functional Constipation in Mice. Braz. J. Med. Biol. Res. 2023, 56, e12466. [Google Scholar] [CrossRef]
- Wallace, J.L. Eicosanoids in the Gastrointestinal Tract. Br. J. Pharmacol. 2019, 176, 1000–1008. [Google Scholar] [CrossRef]



| Coupling Coordination Degree Method | ||||
| Group | Coupling C-value | Harmonization Index T-value | Coupling Coordination Degree D-value | Coordination level |
| EAEPC-L | 0.193 | 0.448 | 0.294 | 3 |
| EAEPC-M | 0.462 | 0.536 | 0.498 | 5 |
| EAEPC-H | 0.245 | 0.425 | 0.323 | 4 |
| Weighted RSR Method | ||||
| Group | RSR value | RSR rank | RSR Fitting value | Staging Level |
| EAEPC-L | 0.667 | 2 | 0.665 | 2 |
| EAEPC-M | 0.704 | 1 | 0.704 | 3 |
| EAEPC-H | 0.630 | 3 | 0.630 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, H.; Wu, Q.; Chen, B.; Xu, K.; Li, C.; Shen, Y. Functions of Pugionium cornutum (L.) Gaertn Extracts: Investigating the Mechanism of Gastroparesis Amelioration from the Perspective of the Gut Microbiota and Its Metabolites. Foods 2025, 14, 2800. https://doi.org/10.3390/foods14162800
Gao Y, Li H, Wu Q, Chen B, Xu K, Li C, Shen Y. Functions of Pugionium cornutum (L.) Gaertn Extracts: Investigating the Mechanism of Gastroparesis Amelioration from the Perspective of the Gut Microbiota and Its Metabolites. Foods. 2025; 14(16):2800. https://doi.org/10.3390/foods14162800
Chicago/Turabian StyleGao, Yangzu, Haoyu Li, Qian Wu, Bang Chen, Kangzhen Xu, Cong Li, and Yehua Shen. 2025. "Functions of Pugionium cornutum (L.) Gaertn Extracts: Investigating the Mechanism of Gastroparesis Amelioration from the Perspective of the Gut Microbiota and Its Metabolites" Foods 14, no. 16: 2800. https://doi.org/10.3390/foods14162800
APA StyleGao, Y., Li, H., Wu, Q., Chen, B., Xu, K., Li, C., & Shen, Y. (2025). Functions of Pugionium cornutum (L.) Gaertn Extracts: Investigating the Mechanism of Gastroparesis Amelioration from the Perspective of the Gut Microbiota and Its Metabolites. Foods, 14(16), 2800. https://doi.org/10.3390/foods14162800






