Ultrasound-Optimized Extraction and Multi-Target Mechanistic Analysis of Antioxidant and Hypoglycemic Effects of Amomum villosum Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Experimental Methods
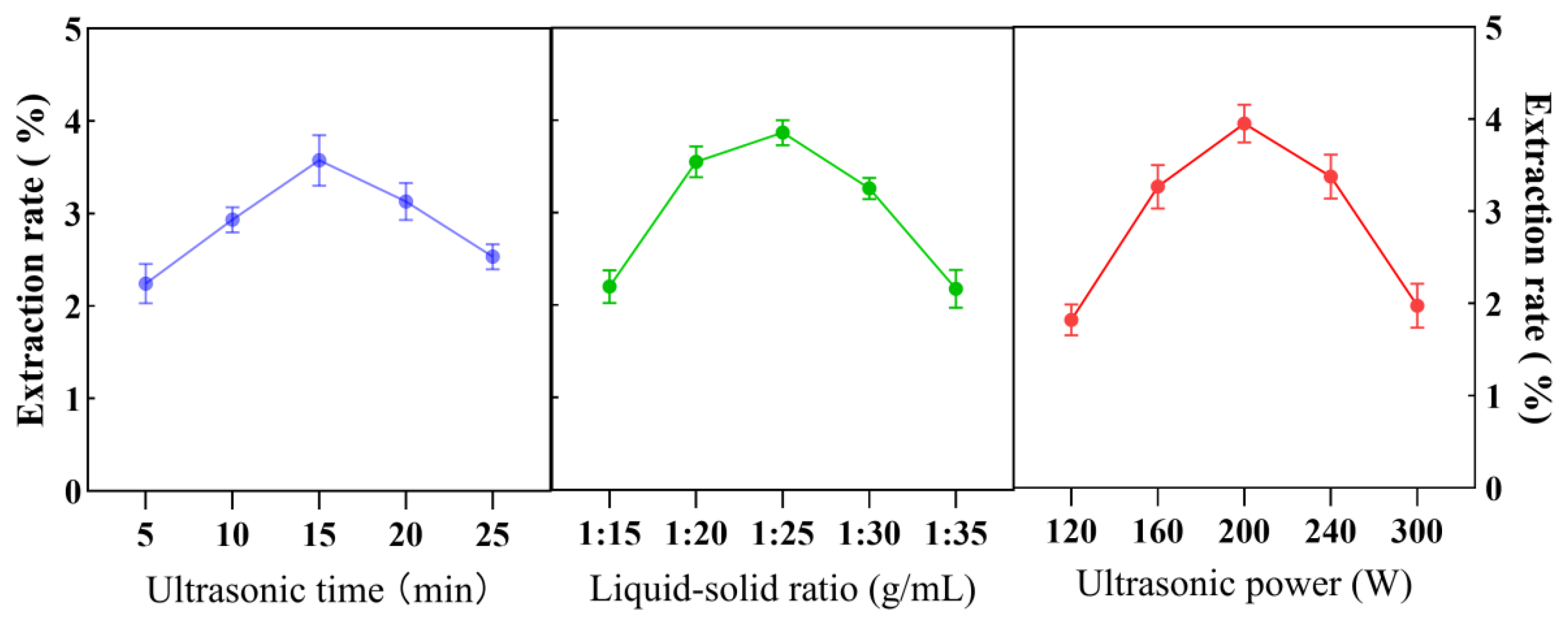
2.3.1. Optimization of Essential Oil Extraction
- Extraction procedure: Essential oil was extracted using a customized ultrasound-essential oil co-extraction system (Model KQ5200DV, Shanghai Precision Instrument Co., Shanghai, China) following Method A of the Chinese Pharmacopoeia (2020 Edition, Part IV). Briefly, 15.0 g of A. villosum powder was mixed with ultrapure water at a preset solvent-to-material ratio and subjected to UAE at 25 ± 1 °C. The extraction yield (Y, %) was calculated using the formula:where V is the volume of extracted essential oil (mL), and m is the mass of the powder sample (g).
- Single-factor experiments: To identify optimal conditions, the following parameters were evaluated individually:
- Ultrasound time: 5, 10, 15, 20, and 25 min under fixed conditions (200 W, 1:20 g/mL).
- Solvent-to-material ratio: 1:15, 1:20, 1:25, 1:30, and 1:35 g/mL after, optimizing the ultrasound time (15 min).
- Ultrasound power: 120, 160, 200, 240, and 280 W (using the optimal time and ratio).
- Each experiment was performed in triplicate.
- Box–Behnken response surface design: Three independent variables—ultrasound time (A: 10, 15, 20 min), solvent-to-material ratio (B: 1:20, 1:25, 1:30 g/mL), and ultrasound power (C: 160, 200, 240 W)—were optimized using Design-Expert 13.0 software (Stat-Ease, Inc., Minneapolis, MN, USA). The design included 17 runs with 5 center points. A quadratic polynomial model was used for data fitting.
2.3.2. Hypoglycemic and Antioxidant Activity Assays
- α-Glucosidase Inhibition:
- Free Radical Scavenging Assays:
- DPPH Assay: 1 mL of AVEO solution (0.2–1.0 mg/mL) was mixed with 1 mL of 0.1 mM DPPH ethanol solution and incubated in the dark for 30 min. Absorbance was measured at 517 nm [34].
- Hydroxyl Radical (·OH) Assay: 1 mL of AVEO solution was mixed with 0.5 mL of 6 mM FeSO4 and 0.5 mL of 8.8 mM H2O2. After 10 min, 0.5 mL of 6 mM salicylic acid was added, and absorbance was measured at 510 nm after 30 min at 37 °C [35].
- Superoxide Anion (O2−·) Assay: 0.5 mL of AVEO solution was added to 1.5 mL of 50 mM Tris-HCl (pH 8.2) and incubated at 25 °C for 20 min. Then, 0.5 mL of 3 mM pyrogallol (in 10 mM HCl) was added. After 5 min, the reaction was terminated with HCl, and absorbance was measured at 320 nm [36].
2.3.3. GC-MS Analysis of AVEO Components
2.4. Data Analysis
2.4.1. Validation of Response Surface Model
2.4.2. Statistical Analysis of Bioactivity Data
2.4.3. Network Pharmacology Analysis
3. Results
3.1. Optimization of AVEO
3.2. Hypoglycemic and Antioxidant Properties of AVEO
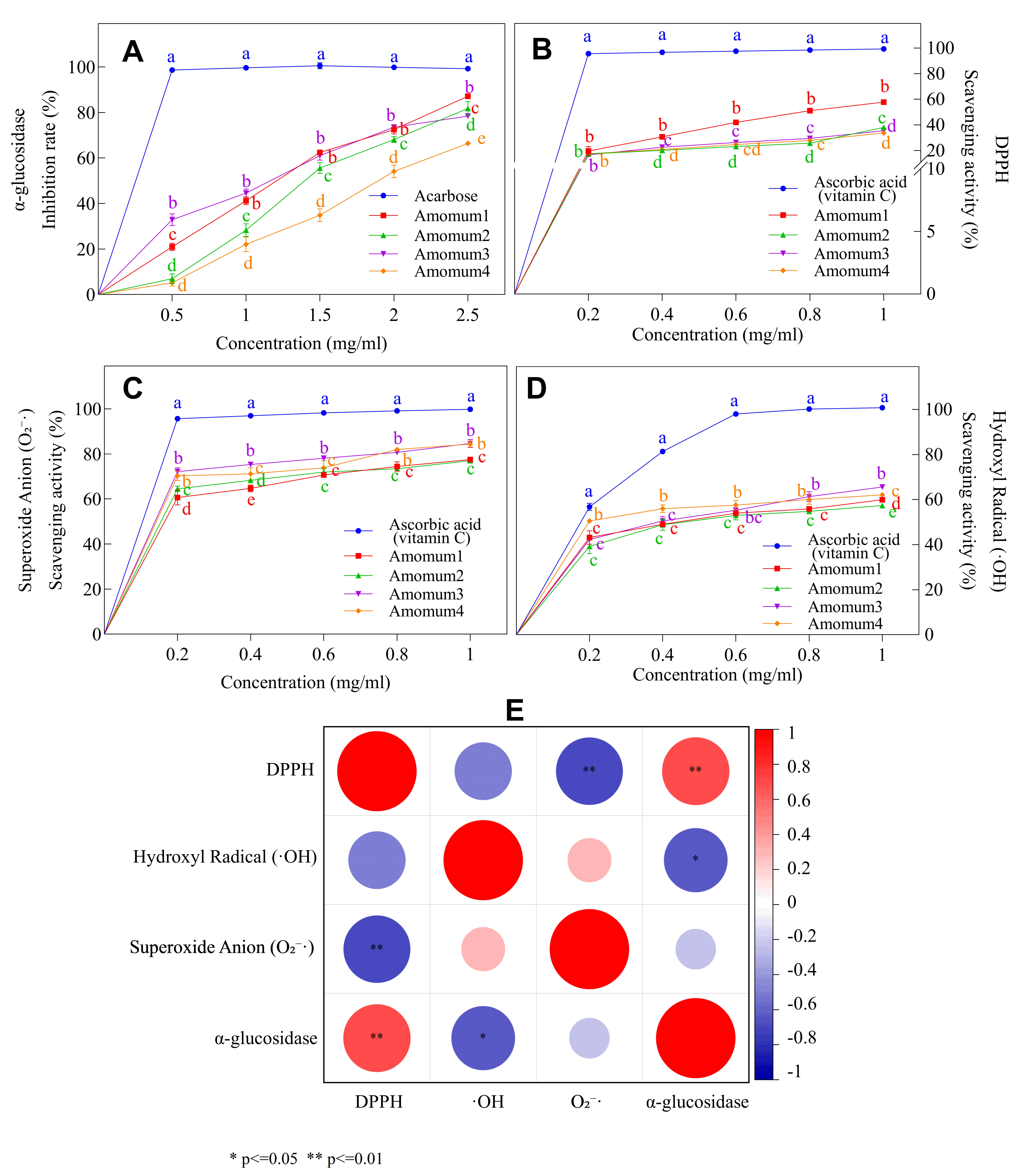
3.2.1. α-Glucosidase Inhibitory Activity
3.2.2. Antioxidant Capacity
3.2.3. Functional Synergy Analysis
3.3. Composition Analysis of AVEO
3.4. Network Pharmacology Analysis of the Antioxidant and Hypoglycemic Mechanisms of AVEO
3.4.1. Screening of Candidate Compounds and Potential Targets
3.4.2. Functional Enrichment
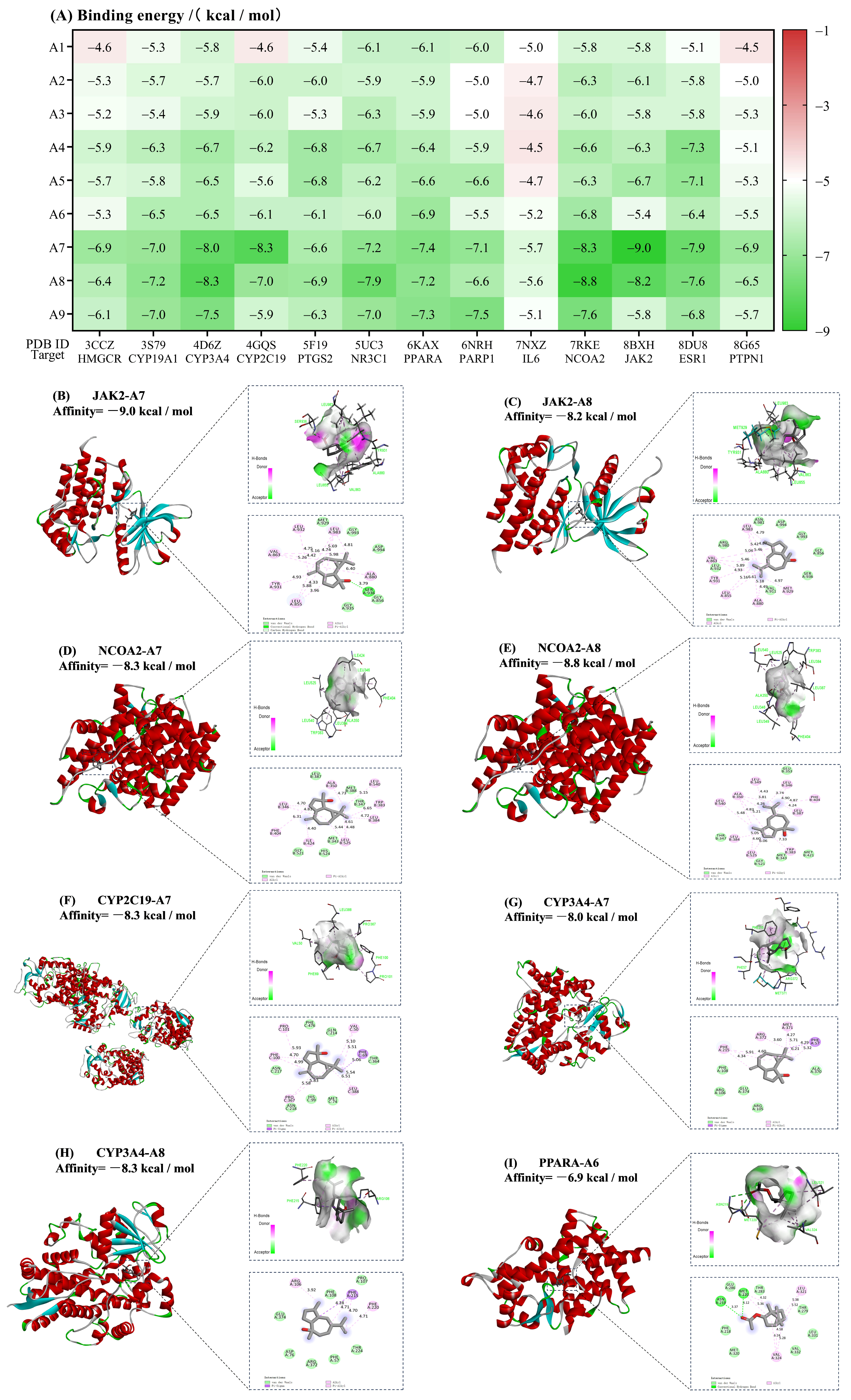
3.5. Molecular Docking Validation
4. Discussion
4.1. Significant Optimization of AVEO Ultrasonic Extraction Process
4.2. AVEO Exhibited Excellent Hypoglycemic and Antioxidant Activities
4.3. Potential Hypoglycemic–Antioxidant Synergistic Mechanisms of AVEO
4.4. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVEO | Amomum villosum essential oil |
| α-GI | α-Glucosidase inhibition |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GC-MS | Gas chromatography–mass spectrometry |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | Protein-protein interaction |
| RNA | Ribonucleic acid |
| DNA | Deoxyribonucleic acid |
| PPAR | Peroxisome proliferator-activated receptor |
| JAK-STAT | Janus kinase–signal transducer and activator of transcription |
| TIC | Total ion chromatogram |
References
- Feng, L.; Wang, Z.; Lei, Z.; Zhang, X.; Zhai, B.; Sun, J.; Guo, D.; Wang, D.; Luan, F.; Zou, J.; et al. Amomum villosum Lour.: An insight into ethnopharmacological, phytochemical, and pharmacological overview. J. Ethnopharmacol. 2024, 335, 118615. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, D.; Xiong, L.; Zhang, Z.; Li, Y.; Liu, K.; Li, H.; Chen, L. Phenolics and terpenoids with good anti-inflammatory activity from the fruits of Amomum villosum and the anti-inflammatory mechanism of active diterpene. Bioorg. Chem. 2024, 145, 107190. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.M.; Zhang, L.X.; Ma, J.; Guan, Z.B. The review of Amomum villosum in Xishuangbanna. China J. Chin. Mater. Med. 2006, 31, 97–101. [Google Scholar]
- Chen, L.; Lai, Y.; Zhang, W.; Cai, J.; Hu, H.; Wang, Y.; Zhao, J.; Li, S. Comparison of volatile compounds in different parts of fresh Amomum villosum Lour. from different geographical areas using cryogenic grinding combined HS–SPME–GC–MS. Chin. Med. 2020, 15, 97. [Google Scholar] [CrossRef]
- Thinh, B.B.; Chac, L.D.; Hanh, D.H.; Korneeva, A.A.; Hung, N.; Igoli, J.O. Effect of extraction method on yield, chemical composition and antimicrobial activity of essential oil from the fruits of Amomum villosum var. xanthioides. J. Essent. Oil-Bear. Plants 2022, 25, 28–37. [Google Scholar] [CrossRef]
- Ao, H.; Wang, J.; Chen, L.; Li, S.; Dai, C. Comparison of volatile oil between the fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et senjen based on GC-MS and chemometric techniques. Molecules 2019, 24, 1663. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, S.; Cao, J.; Pang, X.; Geng, Z.; Wang, Y.; Zhang, Z.; Du, S. Insecticidal and repellent activity of essential oil from Amomum villosum Lour. and its main compounds against two stored-product insects. Int. J. Food Prop. 2018, 21, 2265–2275. [Google Scholar] [CrossRef]
- Barik, P.; Dagar, K.; Makhija, R.; Singh, A.; Asati, V. A green technology for the extraction of essential oil using microwave and ultrasound-assisted techniques. Curr. Anal. Chem. 2025, 21, 79–105. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Zhou, Q.; Han, J.; Qian, C.; Li, Y.; Meng, X.; Gao, X.; Zhou, T.; Li, P.; et al. Effect of response surface methodology-optimized ultrasound-assisted pretreatment extraction on the composition of essential oil released from tribute citrus peels. Front. Nutr. 2022, 9, 840780. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Wei, M. Development and validation of an ultrasound-assisted supercritical carbon-dioxide procedure for the production of essential oils from Perilla frutescens. LWT 2020, 128, 109503. [Google Scholar] [CrossRef]
- Kazemi, M.; Niazi, A.; Yazdanipour, A. Extraction of Satureja rechingeri volatile components through ultrasound-assisted and microwave-assisted extractions and comparison of the chemical composition with headspace solid-phase microextraction. J. Essent. Oil Res. 2022, 34, 21–35. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Suslick, K.S.; Flannigan, D.J. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Chu, Y.H.; Mousavi Khaneghah, A. Recent advances in orange oil extraction: An opportunity for the valorisation of orange peel waste a review. Int. J. Food Sci. Technol. 2019, 54, 925–932. [Google Scholar] [CrossRef]
- Wang, B.; Chen, H.; Qu, P.; Lin, R.; He, S.; Li, W.; Zhang, C.; Shi, X.; Liu, Y.; Du, H.; et al. Effect of different cultivation patterns on Amomum villosum yield and quality parameters, rhizosphere soil properties, and rhizosphere soil microbes. Horticulturae 2023, 9, 306. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, F.; Zhao, Y.; Wang, H.; Chen, L. Antibacterial Ingredients and modes of the methanol-phase extract from the fruit of Amomum villosum Lour. Plants 2024, 13, 834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Lan, L.; Wang, J.; Guo, P.; Sun, G. Simultaneous determination of eight components in Amomum villosum and its overall qualityconsistency evaluation by four-dimensional fingerprints assisted with antioxidant activity. J. Chromatogr. A 2022, 1674, 463135. [Google Scholar] [CrossRef]
- Ai, Z.; Mowafy, S.; Liu, Y. Comparative analyses of five drying techniques on drying attributes, physicochemical aspects, and flavor components of Amomum villosum fruits. LWT 2022, 154, 112879. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.R.; Antonisamy, P.; Kim, Y.S.; Ryu, D.G.; Lee, G.; Kwon, K.B. Efficacy and safety of Amomum villosum extracts in obese adults: A randomized, double-blind, placebo-controlled trial. J. King Saud Univ. Sci. 2023, 35, 102580. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, S.; Chen, L.; Lu, J.; Zhao, F.; Long, T.; Wen, J.; Huang, J.; Mao, Y.; Qi, Z.; et al. In vivo anti-hyperuricemia and anti-gouty arthritis effects of the ethanol extract from Amomumvillosum Lour. Biomed. Pharmacother. 2023, 161, 114532. [Google Scholar] [CrossRef]
- Luo, D.; Zeng, J.; Guan, J.; Xu, Y.; Jia, R.B.; Chen, J.; Jiang, G.; Zhou, C. Dietary supplement of Amomum villosum Lour. polysaccharide attenuates ulcerative colitis in BALB/c mice. Foods 2022, 11, 3737. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.S.; Kim, H.G.; Lee, H.W.; Fang, Z.; Kwon, H.H.; Kim, D.W.; Lee, C.M.; Jeong, J.W. Ethyl acetate fraction of Amomum villosum var. xanthioides attenuates hepatic endoplasmic reticulum stress-induced non-alcoholic steatohepatitis via improvement of antioxidant capacities. Antioxidants 2021, 10, 998. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, S.; Zheng, B.; Raza, F.; Luo, Z.; Li, X.; Zhang, Y.; Nie, Q.; Qiu, M. Efficacy and mechanism of active fractions in fruit of Amomum villosum Lour. for gastric cancer. J. Cancer 2021, 12, 5991–5998. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Huang, J.; Zhou, X.; Qian, Z.; Wu, T.; Fan, Q.; Liang, Y.; Cui, G. Network medicine-based analysis of the hepatoprotective effects of Amomum villosum Lour. on alcoholic liver disease in rats. Food Sci. Nutr. 2024, 12, 3759–3773. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Nguyen, T.V.; Piao, C.H.; Shin, H.S.; Song, C.H.; Chai, O.H. Fructus amomi extract attenuates nasal inflammation by restoring Th1/Th2 balance and down-regulation of NF-κB phosphorylation in OVA-induced allergic rhinitis. Biosci. Rep. 2022, 42, BSR20212681. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, Y.; Chen, J.; Zhang, Z.; Wang, H.; Liu, K.; Sun, D.; Li, H.; Chen, L. Antioxidant aromatic compounds from Amomum villosum and target prediction of active ingredients. Bioorg. Chem. 2024, 147, 107375. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yuan, G.; Luo, G.; Guo, X.; Chen, M.; Yang, H.; He, F.; Yang, T.; Zhang, X.; Wu, Q.; et al. Network pharmacology analysis and experimental verification strategies reveal the action mechanism of Danshen decoction in treating ischemic cardiomyopathy. Evid. Based Compl. Alt. Med. 2022, 2022, 7578055. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Liu, X.L.; Zheng, Q.; Kang, Y.J.; Li, W.J.; Xiao, S.; Xiong, Y.F.; Cai, K.Z.; Wu, M.Q.; Yang, M. Prediction of Q-markers of Citri Reticulatae Pericarpium volatile oil and GC-MS based quantitative analysis. China J. Chin. Mater. Med. 2021, 46, 6403–6409. [Google Scholar]
- Zhou, P.; Wang, X.; Zhao, Y.; She, X.; Jia, Y.; Wang, W.; Li, J.; Luo, X. Evaluation of the mechanism of action of rosemary volatile oil in the treatment of Alzheimer’s disease using gas chromatography -mass spectrometry analysis and network pharmacology. Comb. Chem. High Throughput Screen. 2023, 26, 2321–2332. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y.; Liu, L.; Zhang, F.; Ao, H.; Luo, Y. Mechanism of magnolia volatile oil in the treatment of acute pancreatitis based on GC-MS, network pharmacology, and molecular docking. Evid. Based Complement Altern. Med. 2023, 2023, 3503888. [Google Scholar] [CrossRef]
- Yang, J.J.; Yu, H.; Wu, K.; He, D.; Zhang, H.; Cui, Z.X.; Chai, X.; Duan, X. Potential anti-gouty arthritis of citronella essential oil and nutmeg essential oil through reducing oxidative stress and inhibiting PI3K/Akt/mTOR activation-induced NLRP3 activity. Chem. Biodivers. 2024, 21, e202400448. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The emerging role of redox-sensitive Nrf2–Keap1 pathway in diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Huang, Z.; Zhao, Z. Phenolics from Sterculia nobilis Smith pericarp by-products delay carbohydrate digestion by uncompetitively inhibiting α-glucosidase and α-amylase. Food Sci. Technol. 2023, 173, 114339. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. LWT-Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, H.; Zou, Y. Application of network pharmacology, bioinformatics, computational molecular docking, and experimental validation to study the anticancer effects of oleanolic acid in oral squamous carcinoma cells. Acta Pharm. 2025, 75, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. Swissadme: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. Swisstargetprediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. Genecards version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef]
- Wu, F.; Lin, B.; Chen, J.; Zheng, F.; Yang, Y.; Rasheed, U.; Chen, G. Mechanistic insights into the antioxidant potential of sugarcane vinegar polyphenols: A combined approach of DPPH-UPLC-MS, network pharmacology and molecular docking. Foods 2024, 13, 3379. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. David: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The string database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Flachsenberg, F.; Ehrt, C.; Gutermuth, T.; Rarey, M. Redocking the pdb. J. Chem. Inf. Model. 2024, 64, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. Pubchem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef] [PubMed]
- Paggi, J.M.; Pandit, A.; Dror, R.O. The art and science of molecular docking. Annu. Rev. Biochem. 2024, 93, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Murshed, A.; Sayaf, A.M.; Khan, A.; Khan, S.A.; Tricarico, P.M.; Moltrasio, C.; Agouni, A.; Yeoh, K.K.; Marzano, A.V.; et al. Exploring global natural product databases for NLRP3 inhibition: Unveiling novel combinatorial therapeutic strategy for hidradenitis suppurativa. J. Infect. Public Health 2025, 18, 102697. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, B.; Ke, L.; Fan, X.; Huang, L.; Peng, Z. Exploring the antitumor potential of cucurbitacin B in hepatocellular carcinoma through network pharmacology, molecular docking, and molecular dynamics simulations. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–19. [Google Scholar] [CrossRef]
- Shady, N.H.; Zayed, A.; Alaaeldin, R.; Hisham, M.; Gawesh, M.; Mohammed, R.; Elrehany, M.A.; Abdelmohsen, U.R. Plant and endophyte-derived anti-hyperlipidemics: A comprehensive review with in silico studies. South Afr. J. Bot. 2023, 163, 105–120. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.; Shu, Y.; Cao, H. Research progress on chemical constituents and pharmacological effects of Amomum villosum. Guangdong Chem. Ind. 2022, 49, 111–114. [Google Scholar]
- Ramanathan, A.; Thangarasu, V. Effect of high-frequency microwave irradiation on Aegle Marmelos Correa oil extraction: Kinetic and thermodynamic study. Energy Procedia 2019, 158, 1046–1051. [Google Scholar] [CrossRef]
- Hussein, F.; Antonescu, C.; Karshafian, R. Ultrasound and microbubble induced release from intracellular compartments. BMC Biotechnol. 2017, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xun, Z.; Xu, L.; Li, Y.; Wang, Y.; Guo, S. Constructed the metabolic compound accumulation profiles of daylily at different main producing areas using multi-omics techniques. Food Biosci. 2024, 59, 104128. [Google Scholar] [CrossRef]
- Wen, B.; Ren, S.; Zhang, Y.; Duan, Y.; Shen, J.; Zhu, X.; Wang, Y.; Ma, Y.; Zou, Z.; Fang, W. Effects of geographic locations and topographical factors on secondary metabolites distribution in green tea at a regional scale. Food Control 2020, 110, 106979. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Chen, X.; Bayanheshig; Jiao, Q.; Tan, X.; Wang, W. Numerical simulation of ultrasonic enhancement by acoustic streaming and thermal effect on mass transfer through a new computation model. Int. J. Heat Mass Transf. 2021, 171, 121074. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef]
- Zabot, G.L.; Viganó, J.; Silva, E.K. Low-frequency ultrasound coupled with high-pressure technologies: Impact of hybridized techniques on the recovery of phytochemical compounds. Molecules 2021, 26, 5117. [Google Scholar] [CrossRef]
- Asakura, Y.; Yasuda, K. Frequency and power dependence of the sonochemical reaction. Ultrason. Sonochem. 2021, 81, 105858. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Antonisamy, P.; Kim, Y.S.; Lee, G.; Ham, H.D.; Kwon, K.B. Inhibitory effect of Amomum villosum water extracts on α-glucosidase activity. Physiol. Mol. Plant Pathol. 2022, 117, 101779. [Google Scholar] [CrossRef]
- Khatib, S.; Mahdi, I.; Drissi, B.; Fahsi, N.; Bouissane, L.; Sobeh, M. Tetraclinis articulata (Vahl) Mast.: Volatile constituents, antioxidant, antidiabetic and wound healing activities of its essential oil. Heliyon 2024, 10, e24563. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wu, S.L.; Hu, J.; He, X.F.; Huang, X.Y.; Li, T.Z.; Ma, Y.B.; Zhang, X.M.; Geng, C.A. Norlignans as potent GLP-1 secretagogues from the fruits of Amomum villosum. Phytochemistry 2022, 199, 113204. [Google Scholar] [CrossRef]
- Rongjie, Z.; Zhenglin, Z.; Meihong, J.; Sangchan, K.; Zaixia, Y. Hypoglycemic effects of extract of Amomum xanthoides on experimental diabetes in rats. J. Yanbian Med. Coll. 2006, 29, 97–99. [Google Scholar]
- Sriramavaratharajan, V.; Murugan, R. Screening of chemical composition, in vitro antioxidant, α-amylase and α-glucosidase inhibitory activities of the leaf essential oils of Cinnamomum wightii from different populations. Nat. Prod. Commun. 2018, 13, 1539–1542. [Google Scholar] [CrossRef]
- Sallau, A.B.; Yakubu, R.N.; Aliyu, S.M.; Salihu, A.; Boniface, B.Y. In vitro effect of terpenoids-rich extract of Momordica charantia on alpha glucosidase activity. Vitae 2018, 25, 148–153. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Dai, Y.; Zhao, L.; Wang, W.; Ding, X. Identification and molecular binding mechanism of novel α-glucosidase inhibitory peptides from hot-pressed peanut meal protein hydrolysates. Foods 2023, 12, 663. [Google Scholar] [CrossRef]
- Nafiu, M.; Tom Ashafa, A. Antioxidant and inhibitory effects of saponin extracts from Dianthus basuticus Burtt Davy on key enzymes implicated in type 2 diabetes in vitro. Pharmacogn. Mag. 2017, 13, 576. [Google Scholar] [CrossRef]
- Mehmood, M.F.U.; Marvi, H.A.; Abuelizz, H.A.; Aziz, N.; Bano, R.; Wazir, A.; Ahmad, I.; Abbas, K.; Ishtiaq, S.; Amin, A. Advance glycation end products inhibition by citrus paradisi peel extract; characterization, LCMS-QTOF analysis, and biological evaluation. Food Sci. Nutr. 2024, 12, 10655–10665. [Google Scholar] [CrossRef]
- Hao, W.; Wang, M.; Lv, M. The inhibitory effects of Yixing black tea extracts on α-glucosidase. J. Food Biochem. 2017, 41, e12269. [Google Scholar] [CrossRef]
- Zhang, M.; Shuai, X.X.; Wei, Z.; Dai, T.T.; Wei, C.B.; Li, Y.; He, J.J.; Du, L.Q. Characterization, antioxidant and antitumor activities of phenolic compounds from Amomum villosum Lour. Front. Nutr. 2024, 11, 1327164. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes–A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic efficacy of lactoferrin in type 2 diabetic pediatrics; controlling impact on PPAR-γ, SIRT-1, and TLR4 downstream signaling pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, L.N.; Alshammari, G.M.; Al-Dossari, A.M.; Subash-Babu, P.; Binobead, M.A.; Alhussain, M.H.; AlSedairy, S.A.; Al-Nouri, D.M.; Shamlan, G. Beta vulgaris L. (Beetroot) methanolic extract prevents hepatic steatosis and liver damage in T2DM rats by hypoglycemic, insulin-sensitizing, antioxidant effects, and upregulation of PPARα. Biology 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, N.S.; AlSedairy, S.A.; Al-Harbi, L.N.; Alshammari, G.M.; Yahya, M.A. Carthamus tinctorius L. (safflower) flower extract attenuates hepatic injury and steatosis in a rat model of type 2 diabetes mellitus via nrf2-dependent hypoglycemic, antioxidant, and hypolipidemic effects. Antioxidants 2024, 13, 1098. [Google Scholar] [CrossRef]
- Alwadani, A.H.; Almasri, S.A.; Aloud, A.A.; Albadr, N.A.; Alshammari, G.M.; Yahya, M.A. The synergistic protective effect of γ-oryzanol (OZ) and N-acetylcysteine (NAC) against experimentally induced NAFLD in rats entails hypoglycemic, antioxidant, and PPARα stimulatory effects. Nutrients 2023, 15, 106. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Ruan, X. Bornyl acetate: A promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated fromCryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef]
- Abdelsamia, E.M.; Khaleel, S.A.; Balah, A.; Abdel Baky, N.A. Curcumin augments the cardioprotective effect of metformin in an experimental model of type I diabetes mellitus; Impact of Nrf2/HO-1 and JAK/STAT pathways. Biomed. Pharmacother. 2019, 109, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, X. Aronia melanocarpa anthocyanin extracts improve hepatic structure and function in high-fat diet-/streptozotocin-induced T2DM mice. J. Agric. Food Chem. 2022, 70, 11531–11543. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Zhang, Q.; Wang, X.; Kitakaze, T.; Yamashita, Y.; Kohno, M.; Ashida, H. Mung bean peptides promote glucose uptake via Jak2 activation in L6 myotubes. Food Funct. 2023, 14, 5375–5390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef]
- Gao, J.; Cang, X.; Liu, L.; Lin, J.; Zhu, S.; Liu, L.; Liu, X.; Zhu, J.; Xu, C. Farrerol alleviates insulin resistance and hepatic steatosis of metabolic associated fatty liver disease by targeting PTPN1. J. Cell. Mol. Med. 2024, 28, e70096. [Google Scholar] [CrossRef]
- Du, R.; Wu, N.; Bai, Y.; Tang, L.; Li, L. circMAP3K4 regulates insulin resistance in trophoblast cells during gestational diabetes mellitus by modulating the miR-6795-5p/PTPN1 axis. J. Transl. Med. 2022, 20, 180. [Google Scholar] [CrossRef]
- Wu, J.; Williams, D.; Walter, G.A.; Thompson, W.E.; Sidell, N. Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp. Cell Res. 2014, 328, 351–360. [Google Scholar] [CrossRef]




| Sample | Collection Site | Planting Year | Collection Year | Planting Density (Plants/m2) | Annual Output (kg/ha) |
|---|---|---|---|---|---|
| Amomum1 | Leyi Village, Wangdian Yao Ethnic Township, Youjiang District, Baise City, Guangxi, China | 2019 | 2023 | 8.60 ± 0.55 a | 1233.30 ± 60.00 a |
| Amomum2 | 2019 | 2022 | 8.20 ± 0.45 a | 1224.60 ± 149.90 a | |
| Amomum3 | Shangmeng Village, Pingshan Township, Long’an County, Nanning City, Guangxi, China | 2019 | 2023 | 8.80 ± 0.84 a | 1248.00 ± 106.00 a |
| Amomum4 | 2019 | 2022 | 8.60 ± 1.14 a | 1319.40 ± 143.20 a |
| Source of Variation | Sum of Squares | Degrees of Freedom (df) | Mean Square | F | p |
|---|---|---|---|---|---|
| Model | 2.02 | 9 | 0.2249 | 13.89 | 0.0011 ** |
| A—Ultrasound Time | 0.0005 | 1 | 0.0005 | 0.0278 | 0.8723 |
| B—Solvent-to-Material Ratio | 0.0925 | 1 | 0.0925 | 5.71 | 0.0482 * |
| C—Ultrasound Power | 0.0085 | 1 | 0.0085 | 0.522 | 0.4934 |
| AB | 0.0552 | 1 | 0.0552 | 3.41 | 0.1072 |
| AC | 0.042 | 1 | 0.042 | 2.6 | 0.1512 |
| BC | 0.0182 | 1 | 0.0182 | 1.13 | 0.3239 |
| A2 | 0.6104 | 1 | 0.6104 | 37.71 | 0.0005 *** |
| B2 | 0.2543 | 1 | 0.2543 | 15.71 | 0.0054 ** |
| C2 | 0.7632 | 1 | 0.7632 | 47.15 | 0.0002 *** |
| Residual | 0.1133 | 7 | 0.0162 | ||
| Lack of Fit | 0.01 | 3 | 0.0033 | 0.129 | 0.9379 |
| Pure Error | 0.1033 | 4 | 0.0258 | ||
| Total | 2.14 | 16 | |||
| R2 = 0.9470 | R2adj = 0.8788 |
| No. | Retention Time (min) | Component | Molecular Formula | CAS | Match Factor (NIST20.L,%) | Relative Peak Area (%) | GI Absorption | No. of Active Ingredients |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.457 | p-Cymene | C10H14 | 99-87-6 | 87.06 | 0.35 | Low | / |
| 2 | 8.74 | Cyclohexane-1-butenylidene- | C10H16 | 36144-40-8 | 86.49 | 5.21 | Low | / |
| 3 | 10.42 | Linalool | C10H18O | 78-70-6 | 87.37 | 0.59 | High | A1 |
| 4 | 11.463 | (+)-2-Bornanone | C10H16O | 464-49-3 | 88.93 | 15.91 | High | A2 |
| 5 | 11.907 | Isoborneol | C10H18O | 124-76-5 | 86.13 | 0.16 | High | A3 |
| 6 | 12.169 | 5,5-Dimethyl-1,3-hexadiene | C8H14 | 1515-79-3 | 86.78 | 3.52 | Low | / |
| 7 | 12.495 | Terpinen-4-ol | C10H18O | 562-74-3 | 85.85 | 0.43 | High | A4 |
| 8 | 12.811 | α-Terpineol | C10H18O | 98-55-5 | 86.15 | 0.19 | High | A5 |
| 9 | 15.71 | Bornyl acetate | C12H20O2 | 5655-61-8 | 87.68 | 52.1 | High | A6 |
| 10 | 18.785 | (+)-Cyclosativene | C15H24 | 22469-52-9 | 88.06 | 1.43 | Low | / |
| 11 | 22.422 | α-Cubebene | C15 H24 | 17699-14-8 | 91.85 | 0.25 | Low | / |
| 12 | 24.075 | β-Sesquiphellandrene | C15H24 | 20307-83-9 | 86.66 | 0.66 | Low | / |
| 13 | 25.974 | (−)-Spathulenol | C15H24O | 77171-55-2 | 86.82 | 0.8 | High | A7 |
| 14 | 26.691 | (−)-Pogostol | C15H26 O | 21698-41-9 | 93.13 | 0.18 | High | A8 |
| 15 | 30.681 | α-Bisabolol | C15H26 O | 515-69-5 | 85.83 | 0.16 | High | A9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Liao, Y.; Wei, L.; Feng, X.; Dai, Y.; Liu, Q.; Feng, S. Ultrasound-Optimized Extraction and Multi-Target Mechanistic Analysis of Antioxidant and Hypoglycemic Effects of Amomum villosum Essential Oil. Foods 2025, 14, 2772. https://doi.org/10.3390/foods14162772
Wu W, Liao Y, Wei L, Feng X, Dai Y, Liu Q, Feng S. Ultrasound-Optimized Extraction and Multi-Target Mechanistic Analysis of Antioxidant and Hypoglycemic Effects of Amomum villosum Essential Oil. Foods. 2025; 14(16):2772. https://doi.org/10.3390/foods14162772
Chicago/Turabian StyleWu, Wenxiang, Yining Liao, Lixia Wei, Xuezhen Feng, Yan Dai, Qingrong Liu, and Shuzhen Feng. 2025. "Ultrasound-Optimized Extraction and Multi-Target Mechanistic Analysis of Antioxidant and Hypoglycemic Effects of Amomum villosum Essential Oil" Foods 14, no. 16: 2772. https://doi.org/10.3390/foods14162772
APA StyleWu, W., Liao, Y., Wei, L., Feng, X., Dai, Y., Liu, Q., & Feng, S. (2025). Ultrasound-Optimized Extraction and Multi-Target Mechanistic Analysis of Antioxidant and Hypoglycemic Effects of Amomum villosum Essential Oil. Foods, 14(16), 2772. https://doi.org/10.3390/foods14162772






